Abstract
Background:
Early detection of imported multidrug-resistant tuberculosis (MDR-TB) is crucial, but knowledge gaps remain about migration- and travel-associated MDR-TB epidemiology. The aim was to describe epidemiologic characteristics among international travellers and migrants with MDR-TB.
Methods:
Clinician-determined and microbiologically confirmed MDR-TB diagnoses deemed to be related to travel or migration were extracted from GeoSentinel, a global surveillance network of travel and tropical medicine clinics, from January 2008 through December 2020. MDR-TB was defined as resistance to both isoniazid and rifampicin. Additional resistance to either a fluoroquinolone or a second-line injectable drug was categorized as pre-extensively drug-resistant (pre-XDR) TB, and as extensively drug-resistant (XDR) TB when resistance was detected for both. Sub-analyses were performed based on degree of resistance and country of origin.
Results:
Of 201 patients, 136 had MDR-TB (67.7%), 25 had XDR-TB (12.4%), 23 had pre-XDR TB (11.4%) and 17 had unspecified MDR- or XDR-TB (8.5%); 196 (97.5%) were immigrants, of which 92 (45.8%) originated from the former Soviet Union. The median interval from arrival to presentation was 154 days (interquartile range [IQR]: 10–751 days); 34.3% of patients presented within 1 month after immigration, 30.9% between 1 and 12 months and 34.9% after ≥1 year. Pre-XDR- and XDR-TB patients from the former Soviet Union other than Georgia presented earlier than those with MDR-TB (26 days [IQR: 8–522] vs. 369 days [IQR: 84–827]), while patients from Georgia presented very early, irrespective of the level of resistance (8 days [IQR: 2–18] vs. 2 days [IQR: 1–17]).
Conclusions:
MDR-TB is uncommon in traditional travellers. Purposeful medical migration may partly explain differences in time to presentation among different groups. Public health resources are needed to better understand factors contributing to cross-border MDR-TB spread and to develop strategies to optimize care of TB-infected patients in their home countries before migration.
Keywords: Travel, epidemiology, immigration, migrant, medical migration, extensively drug-resistant tuberculosis, MDR-TB
Introduction
Tuberculosis (TB) continues to threaten public health globally. Although the reported incidence of drug-sensitive TB is declining in most regions of the world, TB remains one of the top 10 overall causes of death in low- and low-middle income countries.1 Multidrug-resistant (MDR) TB is among the major obstacles to elimination of the disease. Despite recent advances in diagnostics and shortened treatment regimens, difficulties in providing universal access to diagnostics, drug susceptibility testing, effective treatment, considerable treatment-related toxicity and excessive treatment costs contribute to inferior outcomes for drug-resistant TB.2–4 Consequently, mortality in individuals with MDR-TB is increased compared to drug-sensitive TB.1
Migrants, in particular refugees and asylum seekers, are a difficult-to-reach subpopulation of travellers to middle- and high-income countries who may import TB and who have important implications for clinical care.5 Among patients with MDR-TB, the proportion of migrants is higher than among patients with drug-susceptible TB. In fact, in many high-income countries, most patients presenting with MDR-TB are foreign-born.6,7 In low-incidence countries of Western Europe, for example, 57 to 100% of patients with MDR-TB were foreign-born in 2017. Nevertheless, the proportion of MDR-TB among all TB cases in this population remains low at 0.7 to 4.5%.8 Domestically acquired MDR-TB occurs less frequently than imported cases, and clusters are seen mainly within groups who migrated from the same country.9,10 Asylum seeking for MDR-TB healthcare has been studied, but no robust evidence exists on the prevalence of this putative pattern of migration.6 Tuberculin skin test conversion rates have been studied in long-term travellers (travel longer than 3–6 months).10,11 However, evidence of acquisition of MDR-TB during long-term travel such as tourism, business travel and volunteerism remains anecdotal.12,13
The crucial importance of the prevention of cross-border spread as well as the early detection and efficient management of imported MDR-TB is recognized by stakeholders in low-incidence countries, but considerable knowledge gaps remain about the epidemiology of migration and travel-associated TB.14,15 The aim of this analysis was to describe epidemiologic characteristics and migration history among international travellers and migrants with MDR-TB reported to the GeoSentinel Surveillance Network.
Methods
Patient records were extracted from GeoSentinel, a global surveillance network of 68 travel and tropical medicine sites in 28 countries on six continents.16 Patient records from January 2008 through December 2020 were reviewed. Records were included when there was a clinician-determined and microbiologically confirmed diagnosis of MDR-TB deemed related to travel or migration from a high-incidence country (annual TB incidence of ≥20/100 000 population). Immigration was defined as any entry of a foreign-born individual to a reporting state with the goal to remain (as opposed to short- or long-term travellers and expatriates). This definition thus comprised immigrants holding a visa, refugees and asylum seekers. Standard WHO definitions for drug-resistant TB were used: MDR-TB was defined as resistance to at least isoniazid and rifampicin. MDR-TB with additional resistance was categorized as (1) pre-extensively drug resistant TB (pre-XDR), defined as MDR-TB plus resistance to either any fluoroquinolone or second line injectable and (2) extensively drug resistant (XDR-) TB, defined as MDR-TB plus resistance to any fluoroquinolone and any second-line injectable drug (capreomycin, kanamycin and amikacin). This definition was determined for all cases after 2015 and for most cases before 2015 using supplemental antimicrobial susceptibility testing data. Traveller or migrant demographics, trip details and migration details (if available) were analysed. For foreign-born patients (those not born in the country of the GeoSentinel site they presented to), descriptive analyses of the time interval from immigration to the date of the first presentation to a GeoSentinel site and the date of symptom onset were performed. Sub-analyses were performed for three groups based on country of origin due to a large proportion of patients from the Republic of Georgia (Georgia) in the data especially from the Paris GeoSentinel site17: Georgia, the former Soviet Union (FSU) excluding Georgia, and other countries (countries not in the FSU). Temporal analyses related to the time point of travel were performed in relation to the degree of resistance by grouping pre-XDR and XDR-TB cases into a high-grade resistance group referred to as pre-XDR/XDR, comparing it with the remaining MDR patients. Drug-sensitive (DS) TB cases reported to the GeoSentinel database for the same time period were analysed for length of time from immigration to presentation at a GeoSentinel site and compared by frequency to MDR-TB cases.
Data analysis was descriptive. For data analysis, STATA® 16 (StataCorp, College Station, TX, USA) was used. GeoSentinel’s data collection protocol has been reviewed by a human subjects advisor for the Centers for Disease Control and Prevention’s National Center for Emerging and Zoonotic Infectious Diseases and is classified as public health surveillance and not human subjects research. For sites located in countries where regulations require it, ethical clearance was obtained.
Results
Demographics
Of 275 778 records in the database during January 2008 to December 2020, 4067 had a diagnosis of active TB; 3865 (95.0%) with DS-TB and 202 (5.0%) with MDR-TB, the latter of which this report focuses on. One record (1/202) was excluded a priori as the patient’s diagnosis was not deemed to be related to travel or migration and the only reported cross-border travel was to another low incidence Western European country. In total, 184 of 201 (90.0%) patients’ TB isolates could be definitively classified by their resistance profiles: 136 were MDR-TB (67.7%), 23 were pre-XDR-TB (11.4%) and 25 were XDR-TB (12.4%) (Table 1). A total of 17 patients did not have detailed isolate resistance profiles reported. Patients were reported from 19 GeoSentinel sites, with centres in France (Paris, Bordeaux and Marseille) and Norway (Oslo) accounting for 143 (70.8%) of the patients. Other countries that reported more than five cases were Sweden (Stockholm), Australia (Melbourne), Italy (Brescia), Canada (Vancouver and Ottawa) and the UK (Cambridge). Most patients were male (123; 61.2%). There were six pregnant patients among the 78 females (7.7%). An extrapulmonary focus as part of the clinical syndrome was reported in 28 individuals (13.9%).
Table 1.
Demographics of patients with multidrug-resistant tuberculosis reported to GeoSentinel, 2008–2020 (n = 201)
| Patient characteristics | n (%) |
|---|---|
| Male sex | 123 (61.2%) |
| Median age, years [IQR] | 31.5 [26–39] |
| HIV | 8 (4.0%) |
| Insulin-dependent diabetes mellitus | 3 (1.5%) |
| Pregnancy | 6 (3.0%) |
| Degree of TB resistance | |
| MDR | 136 (67.7%) |
| Pre-XDR | 23 (11.4%) |
| XDR | 25 (12.4%) |
| Unspecified MDR/XDRa | 17 (8.5%) |
| Localization of disease | |
| Pulmonary | 173 (86.1%) |
| Extrapulmonary | 28 (13.9%) |
| Foreign-born (immigrant) | 196 (97.5%) |
| VFR last 12 months | 14 (7.1%) |
| WHO region and country of origin b | |
| African | 31 (15.4%) |
| Congo (DRC) | 2 (1.0%) |
| Côte d’Ivoire | 2 (1.0%) |
| Eritrea | 15 (7.5%) |
| Ethiopia | 4 (2.0%) |
| Mali | 3 (1.5%) |
| Senegal | 2 (1.0%) |
| Eastern Mediterranean | 22 (10.9%) |
| Pakistan | 4 (2.0%) |
| Somalia | 15 (7.5%) |
| European | 107 (53.2%) |
| Belarus | 3 (1.5%) |
| France | 4 (2.0%) |
| Georgia | 47 (23.4%) |
| Lithuania | 15 (7.5%) |
| Moldova | 7 (3.5%) |
| Romania | 7 (3.5%) |
| Russia | 12 (6.0%) |
| Ukraine | 4 (2.0%) |
| Uzbekistan | 3 (1.5%) |
| South-East Asia | 11 (5.5%) |
| India | 6 (3.0%) |
| Myanmar | 2 (1.0%) |
| Nepal | 2 (1.0%) |
| Western Pacific | 28 (13.9%) |
| China | 6 (3.0%) |
| Mongolia | 5 (2.5%) |
| Philippines | 8 (4.0%) |
| Viet Nam | 7 (3.5%) |
Note. Data are numbers (n) and percentages (%) unless indicated otherwise. Abbreviations. n: number of patients; IQR: interquartile range; HIV: human immunodeficiency virus; TB: tuberculosis; MDR: multidrug-resistant; XDR: extensively drug resistant; VFR: visiting friends and relatives; DRC: Democratic Republic of Congo.
17 of 201 cases recorded from 2008–2014 could not be further classified.
Only countries with two or more cases are shown. The denominator is the total number of patients (n = 201). When countries with only one case are included all WHO regions are represented.
Of 201 patients, 196 (97.5%) were immigrants born outside the reporting country (foreign-born). Five patients (2.5%) were born in one of the reporting low incidence countries—three reported potential risk exposure during long-term business-related travel, one while studying abroad, and one while visiting friends and relatives (VFR). Birthplaces of patients included all WHO regions. However, only two patients originated from the Americas (Guadeloupe (French West Indies) and Peru) (Table 1). A total of 47 patients were born in Georgia (47 of 201, 23.4%), 41 (87.2%) of which were reported by sites in France. An additional 45 patients (22.4%) originated from other FSU states. Among patients with detailed information on drug susceptibility, pre-XDR and XDR-TB were seen more frequently among patients from Georgia (26 of 45, 57.7%) and other republics of the FSU (12 of 41, 29.2%) when compared to patients from other countries (7 of 82, 8.5%) (Table 2). Among patients with pre-XDR and XDR-TB, 81% (21 of 26) of Georgian patients were male compared to 54 and 56% (7 of 13 and 5 of 9) of patients from the FSU and other countries, respectively.
Table 2.
Drug resistance profile and time interval from immigration to presentation analysed by region of origin among 175 foreign-born individuals with drug-resistant TB reported to GeoSentinel, 2008–2020
| Region of origin | Time to presentation from immigration | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Median | IQR | <1 month | 1–12 months | 1–5 years | >5 years | |||||||
|
|
|
|
|
|
|
|||||||
| n | % | [days] | [days] | n | % | n | % | n | % | n | % | |
| FSU (excl. Georgia) | 41 | 23.4 | 227 | 23–825 | 13 | 31.7 | 10 | 24.4 | 10 | 24.4 | 8 | 19.5 |
| MDR | 29 | 369 | 84–827 | 6 | 20.7 | 8 | 27.6 | 9 | 31.0 | 6 | 20.7 | |
| Pre-XDR/XDR | 12 | 26 | 8–522 | 7 | 58.3 | 2 | 16.7 | 1 | 8.3 | 2 | 16.7 | |
| Georgia | 45 | 25.7 | 5 | 1–17 | 37 | 82.2 | 5 | 11.1 | 1 | 2.2 | 2 | 4.4 |
| MDR | 19 | 2 | 1–17 | 15 | 78.9 | 2 | 10.5 | 1 | 5.3 | 1 | 5.3 | |
| Pre-XDR/XDR | 26 | 8 | 2–18 | 22 | 84.6 | 3 | 11.5 | 0 | 0 | 1 | 3.8 | |
| Other | 89 | 50.9 | 307 | 94–1266 | 10 | 11.2 | 39 | 43.8 | 25 | 28.1 | 15 | 16.9 |
| MDR | 82 | 290 | 94–1266 | 8 | 9.8 | 38 | 46.3 | 21 | 25.6 | 15 | 18.3 | |
| Pre-XDR/XDR | 7 | 485 | 4–1266 | 2 | 28.6 | 1 | 14.3 | 4 | 57.1 | 0 | 0 | |
| Total | 175 | 100 | 154 | 10–751 | 60 | 34.3 | 54 | 30.9 | 36 | 20.6 | 25 | 14.3 |
| MDR | 130 | 220 | 43–827 | 29 | 22.3 | 48 | 36.9 | 31 | 23.8 | 22 | 16.9 | |
| Pre-XDR/XDR | 45 | 10 | 4–67 | 31 | 68.9 | 6 | 13.3 | 5 | 11.1 | 3 | 6.7 | |
Note. Only foreign-born patients with specified drug resistance status and known arrival date were included (n = 175): The median time to arrival was calculated for patients with a known arrival date (n = 175). Arrival date missing for one patient from the FSU (pre-XDR/XDR) and three from other regions (two MDR, one pre-XDR/XDR); drug resistance not further classified into MDR or Pre-XDR/XDR-TB in 17 of 201 cases from 2008 to 2014.
Abbreviations. n: number of patients; IQR: interquartile range; FSU: former Soviet Union; XDR: extensively drug resistant; MDR: multidrug-resistant.
Fourteen and 11 patients were reported to have travelled back to their country of birth as VFRs during the last 12 months and 1–5 years before presentation, respectively. An additional three had a potential exposure in a high-incidence country other than their country of birth: a Syrian patient spent 5 years in Russia for studies before immigrating to Northern Europe; a patient from Ivory Coast lived in China immediately before immigrating to Europe; and a Moldovan patient spent 2 months in Russia before immigrating to Central Europe.
Analyses related to the time point of immigration
The arrival date in the country of the GeoSentinel site was known for 175 of 196 (89.3%) foreign-born individuals. The median time interval from arrival to presentation to a GeoSentinel site was 154 days (interquartile range [IQR]: 10–751 days); 60 (34%) presented within 1 month after entering the country, 54 (31.8%) presented between 1 month and 1 year, and 61 (35%) presented more than a year after immigration (Table 2). In contrast, DS-TB cases had a median interval of 1292 days (IQR: 292–3583 days) from immigration to presentation at a GeoSentinel site. When analysed by region of origin, median time to presentation was 5 days (IQR: 1–17 days) for MDR-TB patients from Georgia, 227 days (IQR: 23–825 days) for patients from the FSU other than Georgia and 307 days (IQR: 94–1266 days) for patients from other countries. Among patients from FSU countries other than Georgia, the median time to presentation for patients with pre-XDR/XDR-TB was shorter than for patients with MDR-TB (26 days [IQR: 8–522] vs. 369 days [IQR: 84–827], respectively). In patients from Georgia, median time to presentation was very short in both MDR and pre-XDR/XDR groups (Table 2, Figures 1 and 2).
Figure 1.

Dropped-line plot depicting the individual temporal relation between symptom onset, immigration and clinical presentation among foreign-born patients with known symptom onset (n = 87). One outlier from the group ‘Other’ with a presentation almost three decades after immigration was removed for better scaling. Every horizontal bar represents an individual patient. The intercept (y = 0) represents the time point of immigration. The red dots depict the time point of symptom onset, and the black dots represent the time point of presentation at the reporting site. High colour intensity of the bar represents ongoing symptoms, and low colour intensity represents the asymptomatic interval since immigration
Figure 2.
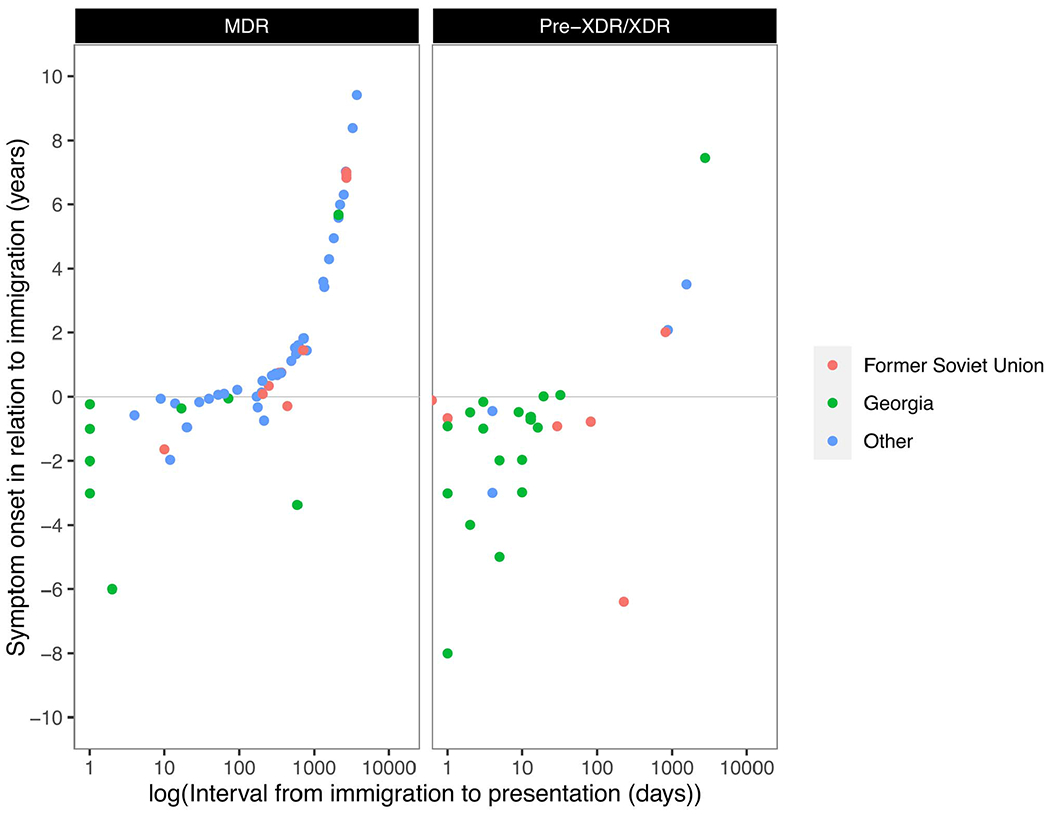
Correlation of time intervals from immigration to presentation (x, logarithmic) and from symptom onset to immigration (y, linear) for patients with MDR-TB (left) and higher degree of resistance (pre-XDR/XDR, right). Intercept (y = 0) = time point of immigration
Among foreign-born individuals with detailed resistance information, 87 of 175 (49.7%) had a recorded date of symptom onset. Of these, 42 (48.3%) reported symptom onset before immigration, 34 of which (81%) presented to the clinic within 1 month. Patients originating from Georgia clustered as early presenters with long-standing symptoms in relation to the time point of immigration and 37 of 45 (82%) presented within 1 month (Figures 1 and 2). No similar subcohort of early presenters with long-standing symptoms was observed among DS-TB patients. When analysed by resistance, 22 of 29 (75.8%) patients with pre-XDR and XDR-TB reported symptom onset before immigration, while 19 of 58 (32.8%) patients with MDR-TB reported symptom onset before immigration.
Discussion
This descriptive analysis of patients presenting with MDR-TB at GeoSentinel travel and tropical medicine sites showed that almost all cases occurred among foreign-born individuals. Given the known median incubation period of a few months to 2 years for active TB, the observed lag time between arrival and presentation in our cohort suggests that at least half of our patients were likely exposed in their country of origin.18 However, it cannot be ascertained whether exposure occurred before immigration, during transit, while living in the new country of residence or while visiting friends or relatives back in the country of origin. More than half of TB cases in migrants occur five or more years after immigration, underlining the importance of latent TB infection screening and treatment programs.19 A recent comprehensive whole-genome-sequencing-based outbreak analysis highlights the complexity of transmission dynamics and the potential use of whole genome sequencing to narrow down the region of acquisition via phylogenetic markers.20 Only 7.1% of patients reported VFR-travel to high-incidence countries in the past year, which is lower than the 20% to 55% of all TB cases in VFRs reported in the literature.21,22 This difference may be explained by different travel patterns of patients attending our GeoSentinel clinics.
None of the cases in our analysis were short- or long-term tourists or missionaries/volunteers; three occurred among business travellers and one in a student. This finding is in line with previous reports of a relatively low absolute risk of acquiring tuberculosis during short-term and long-term travel.10,23 Specifically, a prospective trial from The Netherlands reported an increased relative risk of infection with a tuberculin skin test conversion rate of 2.8 per 1000 person-months of travel in non-healthcare workers spending 3 to 12 months in high-endemicity countries (incidence >1000 cases per 100 000), thus approaching the estimated infection rate of the host country.11,24 The US Centers for Disease Control and Prevention and others have issued detailed recommendations for the prevention of TB exposure for international travellers anticipating prolonged TB exposure during their travels.25,26
Our analysis included a large number of patients from Georgia, who mainly presented to French sites with active TB and high levels of resistance (pre-XDR and XDR-TB). Median time from immigration to presentation was extremely short for this group, and almost all patients had symptom onset preceding immigration. In 2018, 31% of all MDR-TB cases in Georgia were reported among patients with previous anti-TB treatment as per the WHO.27 The high proportion of Georgian patients with pre-XDR and XDR-TB in our cohort suggests a high rate of previous treatment. In fact, 67% (42 of 63) of all MDR/XDR-TB patients treated at Pitié-Salpêtrière Hospital in Paris between 2011 and 2016 reported previous treatment and 63.5% were from Georgia (40 of 63).28 High rates of person-to-person spread of XDR-TB strains in Georgia could also explain this finding, but there is no evidence in the literature to date to support this. A common perception among Georgian patients is that the tailored treatment regimens available in France are more efficient and less toxic than the treatment offered by the national treatment program in Georgia, which may explain the focused migration to France for primary or, in most cases, repeated courses of treatment (Eric Caumes, personal communication—9 June 2020).
Differences were identified in temporal presentation patterns between patients from different regions of origin, with a short time to presentation among XDR-TB patients originating from the FSU. Similar to our findings, in a study from France reporting data from 2006–2012, 34.4% of patients from the FSU (for whom the date of arrival to France was known) were hospitalized within a month of arrival and subsequently diagnosed with MDR-TB.17 In another case series of 20 XDR-TB patients arriving from the FSU to France, the median time from arrival to hospitalization was 2 days (IQR: 1–7 days).29 It is unclear why XDR-TB patients from the FSU and specifically Georgia present for care within such a short time interval in comparison to other groups, but this may be due to differences in TB screening protocols in destination countries or purposeful medical migration for TB care. Of note, DS-TB patients reported to GeoSentinel presented much later after immigration compared to MDR-TB (median 1292 days [IQR: 292–3583 days] vs. 154 days [IQR: 10–751 days]) and no subcohorts of patients with long-standing symptoms before immigration were identified. However, no statistical comparisons can be made between MDR-TB and DS-TB since GeoSentinel data are not systematically reported and the observed difference may partly be a result of referral bias to GeoSentinel’s specialized infectious disease centres, e.g. in the event of difficult-to-treat TB.
National treatment programs in high-burden countries may fail to provide true patient-centred care, leading to loss of faith in treatment success, fear of catastrophic costs and persistent stigma, which may create a potential driving force for medical migration.30 The international public health community should continue to work with high TB-burden countries to identify patients at high risk and provide both robust treatment regimens and directly observed treatment to reduce both the incidence of MDR-TB and medical migration.
This study has several limitations. The majority of cases were reported from only two countries, France and Norway, and few cases were reported from sites in North America and Asia, thus decreasing generalizability. The few cases reported from US sites are likely a reflection of the use of specialized TB health department clinics upon migration that are not affiliated with GeoSentinel, and the same may apply in other locations. A general limitation of GeoSentinel is that the majority of the network’s sites are found in Western Europe and North America (Northern Hemisphere dominance). Although a third of patients in this study show a pattern suggestive of medical migration, we were unable to determine the relative contribution of medical migration to the MDR-TB incidence in low-burden countries. By virtue of migration patterns, populations originating from specific countries might be over-represented at certain GeoSentinel sites. Given the long incubation period of tuberculosis, the causal link to travel or migration cannot be ascertained with certainty, as previously mentioned. In addition, time to presentation after immigration may have been overestimated in patients who might have been seen at another clinic before presentation at the GeoSentinel site. This may apply to DS-TB patients in particular. Finally, GeoSentinel is not designed to collect detailed clinical data such as molecular epidemiological data, or outcome information. It also does not collect past treatment received, which is an important risk factor for MDR-TB acquisition.
In conclusion, traditional travel was very rarely associated with the acquisition of MDR-TB in this multinational study. Purposeful medical migration for MDR-TB treatment and retreatment may partly explain the variation in time to presentation among different groups of migrants. Public health resources are needed to better understand factors contributing to the cross-border spread of MDR-TB and to develop strategies to optimize care of TB-infected patients in their home countries before migration.
Acknowledgements
We would like to thank Ashley Brown, MPH, for assisting with data extraction.
Sources of Funding
This work was supported by a Cooperative Agreement (U50CK00189) from the Centers for Disease Control and Prevention (CDC), as well as funding from the International Society of Travel Medicine (ISTM) and the Public Health Agency of Canada (PHAC).
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC.
Footnotes
Conflict of Interest
The authors have declared no conflicts of interest.
References
- 1.World Health Oragnization (WHO). Global Tuberculosis Report 2019, Geneva: World Health Organization, 2019. [Google Scholar]
- 2.Dorman SE, Schumacher SG, Alland D et al. Xpert MTB/RIF ultra for detection of mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis 2018; 18:76–84. doi: 10.1016/S1473-3099(17)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conradie F, Diacon A, Howell P et al. Sustained high rate of successful treatment outcomes: interim results of 75 patients in the nix-TB clinical study of pretomanid, bedaquiline and linezolid. N Engl J Med. 2020; 382:893–902.32130813 [Google Scholar]
- 4.Günther G, Gomez GB, Lange C, Rupert S, van Leth F. Availability, price and affordability of anti-tuberculosis drugs in Europe: a TBNET survey. Eur Respir J 2015; 45:1081–8. doi: 10.1183/09031936.00124614. [DOI] [PubMed] [Google Scholar]
- 5.Heuvelings CC, de Vries SG, Greve PF et al. Effectiveness of interventions for diagnosis and treatment of tuberculosis in hard-to-reach populations in countries of low and medium tuberculosis incidence: a systematic review. Lancet Infect Dis 2017; 17:e144–58. doi: 10.1016/S1473-3099(16)30532-1. [DOI] [PubMed] [Google Scholar]
- 6.Lönnroth K, Mor Z, Erkens C et al. Tuberculosis in migrants in low-incidence countries: epidemiology and intervention entry points. Int J Tuberc Lung Dis 2017; 21:624–37. doi: 10.5588/ijtld.16.0845. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC). Reported Tuberculosis in the United States, 2017 Atlanta, GA: US Department of Health and Human Services, CDC, Published online, 2018. [Google Scholar]
- 8.Surveillance Atlas of Infectious Diseases. https://atlas.ecdc.europa.eu/
- 9.Lafeuille E, Veziris N, Sougakoff W et al. XDR-tuberculosis in France: community transmission due to non-compliance with isolation precautions. Médecine Mal Infect 2016; 46:52–5. doi: 10.1016/j.medmal.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Freeman RJ, Mancuso JD, Riddle MS, Keep LW. Systematic review and meta-analysis of TST conversion risk in deployed military and long-term civilian travelers. J Travel Med 2010; 17:233–42. doi: 10.1111/j.1708-8305.2010.00424.x. [DOI] [PubMed] [Google Scholar]
- 11.Cobelens FG, van Deutekom H, Draayer-Jansen IW et al. Risk of infection with mycobacterium tuberculosis in travellers to areas of high tuberculosis endemicity. The Lancet 2000; 356:461–5. doi: 10.1016/S0140-6736(00)02554-X. [DOI] [PubMed] [Google Scholar]
- 12.Salazar-Austin N, Ordonez AA, Hsu AJ et al. Extensively drug-resistant tuberculosis in a young child after travel to India. Lancet Infect Dis 2015; 15:1485–91. doi: 10.1016/S1473-3099(15)00356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salzer HJF, Terhalle E, Lange C. Extensively drug-resistant tuberculosis in long-term travellers. Lancet Infect Dis 2016; 16:642–3. doi: 10.1016/S1473-3099(16)30068-8. [DOI] [PubMed] [Google Scholar]
- 14.Matteelli A, Centis R, Sulis G, Tadolini M. Crossborder travel and multidrugresistant tuberculosis (MDRTB) in Europe. Travel Med Infect Dis 2016; 14:588–90. doi: 10.1016/j.tmaid.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Jackson C, Abubakar I. Ending tuberculosis in risk groups in Europe: challenges from travel and population movement. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull 2017; 22:30489. doi: 10.2807/1560-7917.ES.2017.22.12.30489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kira Harvey M, Esposito DH, Pauline Han MA et al. Surveillance for travel-related disease — GeoSentinel surveillance system, United States, 1997–2011. MMWR Morb Mortal Wkly Rep 2013; 62:1–23 Published online July 19. [PubMed] [Google Scholar]
- 17.Bernard C, Brossier F, Sougakoff W et al. A surge of MDR and XDR tuberculosis in France among patients born in the former Soviet Union. Eurosurveillance 2013; 18:20555. doi: 10.2807/1560-7917.ES2013.18.33.20555. [DOI] [PubMed] [Google Scholar]
- 18.Behr MA, Edelstein PH, Ramakrishnan L. Revisiting the timetable of tuberculosis. BMJ. Published online August 2018; 23:k2738. doi: 10.1136/bmj.k2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenaway C, Castelli F. Infectious diseases at different stages of migration: an expert review. J Travel Med 2019; 26:taz007. doi: 10.1093/jtm/taz007. [DOI] [PubMed] [Google Scholar]
- 20.Walker TM, Kohl TA, Omar SV et al. Whole-genome sequencing for prediction of mycobacterium tuberculosis drug susceptibility and resistance: a retrospective cohort study. Lancet Infect Dis 2015; 15:1193–202. doi: 10.1016/S1473-3099(15)00062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kik SV, Mensen M, Beltman M et al. Risk of travelling to the country of origin for tuberculosis among immigrants living in a low-incidence country. Int J Tuberc Lung Dis 2011; 15:38–43. [PubMed] [Google Scholar]
- 22.McCarthy OR. Asian immigrant tuberculosis—the effect of visiting Asia. Br J Chest 1984; 78:248–53. doi: 10.1016/0007-0971(84)90136-0. [DOI] [PubMed] [Google Scholar]
- 23.Brown ML, Henderson SJ, Ferguson RW, Jung P. Revisiting tuberculosis risk in peace corps volunteers, 2006–13. J Travel Med 2016; 23:2–7. doi: 10.1093/jtm/tav005. [DOI] [PubMed] [Google Scholar]
- 24.Rieder HL. Risk of travel-associated tuberculosis. Clin Infect Dis 2001; 33:1393–6. doi: 10.1086/323127. [DOI] [PubMed] [Google Scholar]
- 25.Denholm JT, Thevarajan I. Tuberculosis and the traveller: evaluating and reducing risk through travel consultation. J Travel Med 2016; 23:taw008. doi: 10.1093/jtm/taw008. [DOI] [PubMed] [Google Scholar]
- 26.International Travelers| TB in Specific Populations | TB | CDC. Published December 12, 2018. https://www.cdc.gov/tb/topic/populations/travelers/default.htm (24 April 2021, date last accessed).
- 27.WHO. Tuberculosis country profile: Georgia. Published 2018. https://www.who.int/tb/country/data/profiles/en/ (20 January 2021, date last accessed).
- 28.Caumes E Literature that could change travel medicine practice: Post-travel. Presented at the: Conference of the International Society of Travel Medicine 15; May 17, 2017; International Society of Travel Medicine: Barcelona. https://www.istm.org/cistm15 [Google Scholar]
- 29.Henry B, Revest M, Dournon N et al. Preliminary Favorable outcome for medically and surgically managed extensively drug-resistant tuberculosis, France, 2009–2014. Emerg Infect Dis 2016; 22:518–21. doi: 10.3201/eid2203.151130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furin J, Isaakidis P, Reid AJ, Kielmann K. “I’m fed up”: experiences of prior anti-tuberculosis treatment in patients with drug-resistant tuberculosis and HIV. Int J Tuberc Lung Dis 2014; 18:1479–84. doi: 10.5588/ijtld.14.0277. [DOI] [PubMed] [Google Scholar]


