Abstract
Patient: Male, 82-year-old
Final Diagnosis: A rare case of persistently depressed T lymphocyte subsets post COVID-19 infection
Symptoms: Shortness of breath
Medication:—
Clinical Procedure: —
Specialty: Immunology • Infectious Diseases
Objective:
Unusual clinical course
Background:
On rare occasions, viral infections are known to also depress immune cell lines, further worsening clinical outcomes. We describe a patient who presented 3 weeks after recovery from mild COVID-19 disease with clinical features of an atypical pneumonia and was found to have a low CD4+ T-cell count.
Case Report:
An 82-year-old man with a past medical history of coronary artery disease, rheumatoid arthritis, gout, hyper-tension, and atrial fibrillation presented with a 1-week history of progressively worsening shortness of breath and cough. He was noted to have recovered from mild SARS-CoV-2 infection 3 weeks prior to his current presentation and had been at his baseline level of health following infection. A T cell subset panel was obtained, which revealed an absolute CD3 count of 92 (reference range 840–3060), absolute CD4 count of 52 (reference range 500–1400), absolute CD8 count of 37 (reference range 180–1170), and a normal CD4: CD8 ratio. He was subsequently started on atovaquone for pneumocystis jiroveci pneumonia prophylaxis.
Conclusions:
This case highlights the need for a high index of suspicion for lymphocyte depletion in older patients with multiple comorbidities who present during or after SARS-CoV-2 infection with atypical symptoms that are suggestive of immunosuppression. In such instances, there should be a low threshold to start prophylactic therapy for possible opportunistic infections.
Keywords: Lymphopenia, COVID-19, Immunosuppression
Background
The clinical spectrum of COVID-19 disease ranges from mild symptoms, including upper respiratory symptoms, cough, and malaise, to critical illness requiring invasive ventilatory support [1,2]. Compared with other coronavirus pandemics, COVID-19 has proven to be more fatal, leading to the loss of millions of lives and the shattering of economies, indeed changing the world order [1]. T-cell immunity plays a major role in the body’s response to viral illnesses [3]. In some instances, an exaggerated response to infections leads to a hyperinflammatory state, which can be detrimental [4,5]. On rare occasions, viral infections are also known to depress immune cell lines [6], further worsening clinical outcomes. The lymphocyte count has been shown to predict disease outcomes in patients with COVID-19 [7].
In this case, we describe a patient who presented 3 weeks after recovery from mild COVID-19 disease with clinical features of an atypical pneumonia and was found to have a low CD4+ T-cell count.
Case Report
We present the case of an 82-year-old man with a past medical history of coronary artery disease, rheumatoid arthritis, gout, hypertension, and atrial fibrillation who presented with a 1-week history of progressively worsening shortness of breath and cough. He was noted to have recovered from mild COVID-19 disease 3 weeks before his current presentation and had been at his baseline level of health following the SARS-CoV-2 infection.
The clinical examination on presentation was significant for tachycardia, with an irregular rhythm, bilateral crackles in all lung fields on auscultation, and bilateral lower extremity pitting edema. Initial laboratory investigations were significant for a white blood cell (WBC) count of 14.7×109 (reference range 4.5–11.0×109), hyponatremia, with a sodium level of 130 mmol/L (reference range 136–145 mmol/L), mild hypocalcemia, with a calcium level of 8.5 mg/dL (reference range 8.7–10.4 mg/dL), and elevated B-natriuretic peptide level of 951 pg/mL (reference range 2.00–100.00 pg/mL) (Table 1). Cardiac biomarkers were negative, and renal function and hepatic function testing were within the reference range. Arterial blood gas showed hypoxemic respiratory failure. SARS-CoV-2 PCR, influenza A, and influenza B tests that were done on admission were also negative. A chest X-ray demonstrated multifocal airspace opacity, consistent with pneumonia (Figure 1C). An electrocardiogram done on admission showed atrial fibrillation, with a rapid ventricular response, at a rate of 141 beats/min. The patient was admitted to the Coronary Care Unit for the management of decompensated heart failure, rapid atrial fibrillation, and possible atypical pneumonia. He was started on intravenous diuresis with furosemide, metoprolol for rate control, anticoagulation treatment with apixaban, and antibiotic treatment with intravenous ceftriaxone and azithromycin.
Table 1.
Laboratory values over the course of disease.
| Normal range | Day 1 | Day 3 | Day 10 | Day 16 | |
|---|---|---|---|---|---|
| CBC | |||||
| WBC (×109/L) | 4.5–11.0 | 14.2 (H) | 22.5 (H) | 8.5 | 26.8 (H) |
| Hgb (g/dL) | 14–18 | 12.6 | 13.4 | 10.9 (L) | 9.8 (L) |
| Platelets (×106/L) | 130–400 | 206 | 211 | 167 | 143 |
| Manual differential | |||||
| Segments (%) | 42–75 | 94 (H) | 90 (H) | 97 (H) | 99 (H) |
| Band (%) | 0–9 | 1 | 4 | ||
| Lymphocyte manual (%) | 20–44 | 3 | 2 (L) | 2 (L) | 0 (L) |
| Monocyte manual (%) | 0–12 | 2 | 3 | 1 | 1 |
| Eosinophil manual (%) | 0–5 | 0 | 0 | 0 | 0 |
| Neutrophil absolute (%) | 1.9–9.2 | 13.5 (H) | 21.4 (H) | 8.2 | 26.5 (H) |
| Lymphocyte absolute (%) | 1.3–4.5 | 0.4 (L) | 0.4 (L) | 0.2 (L) | 0.0 (L) |
| Monocytes absolute (%) | 0.0–1.3 | 0.3 | 0.7 | 0.1 | 0.3 |
| Eosinophil absolute (%) | 0.0–0.6 | 0.0 | 0.0 | 0.0 | 0.0 |
(H) – above the upper limit of normal; (L) – below the normal range.
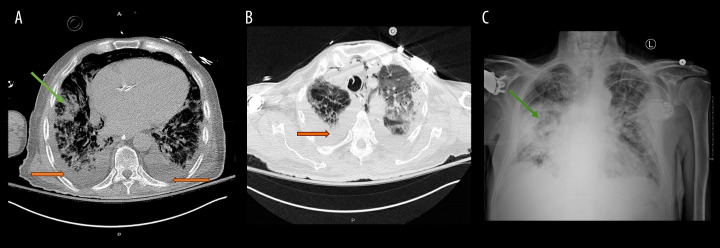
Figure 1.
(A, B) Computed tomography of chest done on day 3 of admission showing diffuse infiltrates consistent with acute respiratory distress syndrome (green arrow) and small bilateral pleural effusions (orange arrow). (C) Multifocal airspace opacity, consistent with pneumonia, is shown.
While in the Coronary Care Unit, he developed worsening respiratory failure and subsequently required intubation and ventilator support due to impending respiratory failure on day 3 of admission. An echocardiogram performed 3 days after admission revealed a moderately reduced left ventricular systolic function, with diffuse hypokinesis and moderately dilated left atrium.
From days 3 to 7, we noticed worsening leukocytosis despite antibiotic therapy, and attempts to wean ventilatory support were unsuccessful. A computed tomography scan done at that time showed diffuse infiltrates, consistent with acute respiratory distress syndrome, and small bilateral pleural effusions (Figure 1A, 1B).
Results of the lower respiratory sputum panel were unremarkable. Blood and urine cultures taken owing to worsening leukocytosis were also within normal limits. Repeat SARS-CoV-2 testing was negative. HIV testing and HIV viral load testing were negative. Per a consultation with the Infectious Disease team, a T cell subset panel was obtained, which revealed an absolute CD3 count of 92 (reference range 840–3060), absolute CD4 count of 52 (reference range 500–1400), absolute CD8 count of 37 (reference range 180–1170), and a normal CD4: CD8 ratio (Table 2). He was subsequently treated with atovaquone for pneumocystis jiroveci pneumonia (PJP) prophylaxis.
Table 2.
T lymphocyte subset panel.
| T cell subset panel | Results | Normal range |
|---|---|---|
| CD3% | 66 | 57–86 |
| CD3 absolute | 92 (L) | 840–3060 |
| CD4% | 37 | 30–61 |
| CD4 absolute | 52 | 490–1740 |
| CD8% | 26 | 12–42 |
| CD8 absolute | 37 (L) | 180–1170 |
| CD19% (B cell) | 17 | 6–29 |
| CD19 absolute | 24 (L) | 110–660 |
| CD16 + CD56+ (NK)% | 16 | 4–25 |
| CD16 + CD56+ | 22 (L) | 70–760 |
| Lymphocyte absolute | 140 (L) | 850–3900 |
(L) – below the normal range.
There was clinical deterioration over the following week, and the Palliative Care team was consulted. Upon discussion among the family and the medical team, the patient’s family opted for no further escalation of care on day 12 of admission, and the patient subsequently died on day 16 of admission.
Discussion
Here we present a patient who recovered from COVID-19 disease and presented a few weeks later with worsening pulmonary infection and depression of the T lymphocyte panel. He was admitted to the hospital for deteriorating respiratory function, worsening leukocytosis, and progressive worsening of radiographic findings, despite appropriate antimicrobial therapy. Repeat SARS-CoV-2 testing was negative, as was an upper respiratory microbial panel, including negative influenza A and B testing. Despite worsening leukocytosis, absolute lymphocyte counts on the manual differential were noted to be low, thus prompting testing for T cell subtypes, which revealed decreased numbers of CD4+ T cells and CD8+ T cells.
The major role of CD4+ T cells in the setting of a viral infection is the activation of the innate immune system and the promotion of the function of B cells and CD8+ T cells [8]. After the resolution of an active immune response to a viral infection, such as SARS-CoV-2, many of the effector CD4+ T cells die, resulting in a smaller number of memory CD4+ T cells, which may persist for a long duration [9]. In the absence of this expected response in T cells, a patient becomes susceptible to secondary infections, which portends bad outcomes.
SARS-CoV-2 infection has been associated with a decrease in lymphocyte counts in the peripheral blood, including CD4+ T cells and CD8+ T cells, with little to no effect on B lymphocyte counts. This phenomenon is more pronounced in patients with severe COVID-19 disease [10]. However, the exact mechanism by which SARS-CoV-2 infection can lead to lymphopenia, particularly targeting the T cell population, is unclear [10]. It has been postulated that COVID-19-associated lymphopenia may be related to the sequestration of immune cells in other organs, such as the lungs and gastrointestinal tract, leading to an overall reduction in the peripheral lymphocyte count [11,12].
It was thought that the low CD4+ T cell count following SARSCoV-2 infection increased our patient’s susceptibility to infections, which was believed to have contributed considerably to his rapid decline. Indeed, because of the critical role of T cells in the response to viral infections, a decrease in T cell populations may render the patient relatively immunocompromised during and after infection with SARS-CoV-2, thereby increasing susceptibility to infections.
Although it is well known and documented, there are no specific guidelines on the management of lymphopenia that developed or is associated with SARS-CoV-2 infection [13]. Most treatment is directed at specific infections, and in severe cases like ours, it might be considered reasonable to start prophylaxis for opportunistic infections, such as PJP [14]. Therefore, atovaquone was started for PJP, based on the worsening respiratory clinical features and radiographic findings in the presence of low lymphocyte count, particularly the CD4+ T cells of 200.
The prognoses in most patients with COVID-19 disease and lymphopenia, particularly those with low CD4+ T cells is poor [15]. Older age, presence of comorbidities, and cardiac conditions were all significantly associated with longer hospital stays and death in hospitalized patients with COVID-19 and lymphopenia [8], as was the case here.
Several case reports highlight the association between lymphopenia and SARS-CoV-2 infection. However, most of those cases were reported in patients with active SARS-CoV-2 infection. Our case is unique because it describes a patient who had recovered from SARS-CoV-2 infection several weeks prior, subsequently developed low CD4+ T cell count, and eventually succumbed to an atypical lower respiratory tract infection due to his acquired immunocompromised state.
Conclusions
In conclusion, there should be a high clinical suspicion for lymphocyte depletion in older patients and in those with comorbidities who present with nonspecific clinical features during or following SARS-CoV-2 infection, with consideration for the measurement of a lymphocyte subset panel and for the initiation of prophylactic therapy for possible opportunistic infections, which may occur in this setting. In our case, despite the initiation of prophylactic treatment for the possibility of underlying PJP, given the clinical presentation and radiographic imaging, our patient had an ultimately fatal course.
Footnotes
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Petersen E, Koopmans M, Go U, et al. Comparing SARS-CoV-2 with SARSCoV and influenza pandemics. Lancet Infect Dis. 2020;20(9):e238–44. doi: 10.1016/S1473-3099(20)30484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barth RF, Buja LM, Parwani AV. The spectrum of pathological findings in coronavirus disease (COVID-19) and the pathogenesis of SARS-CoV-2. Diagn Pathol. 2020;15(1):85. doi: 10.1186/s13000-020-00999-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Candia P, Prattichizzo F, Garavelli S, Matarese G. T Cells: Warriors of SARS-CoV-2 Infection. Trends Immunol. 2021;42(1):18–30. doi: 10.1016/j.it.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gustine JN, Jones D. Immunopathology of hyperinflammation in COVID-19. Am J Pathol. 2021;191(1):4–17. doi: 10.1016/j.ajpath.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pereira M, Shivdasani D, Roy D, et al. Post-COVID-19 unusual inflammatory syndromes detected on 18F-FDG PET/CT scan. Clin Nucl Med. 2022;47(4):e363–65. doi: 10.1097/RLU.0000000000004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westmeier J, Paniskaki K, Karaköse Z, et al. Impaired cytotoxic CD8+ T cell response in elderly COVID-19 patients. mBio. 2020;11(5):e02243–20. doi: 10.1128/mBio.02243-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): Systematic review and meta-analysis. J Intensive Care. 2020;8(1):36. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen XS, Jiang D, Gao L, Zhou JZ, et al. Clinical characteristics and predictive value of lower CD4+T cell level in patients with moderate and severe COVID-19: A multicenter retrospective study. BMC Infect Dis. 2021;21(1):57. doi: 10.1186/s12879-020-05741-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan S, Wu G. Is lymphopenia different between SARS and COVID-19 patients? FASEB J. 2021;35(2):e21245. doi: 10.1096/fj.202002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altmann DM, Boyton RJ. SARS-CoV-2 T cell immunity: Specificity, function, durability, and role in protection. Sci Immunol. 2020;5(49):eabd6160. doi: 10.1126/sciimmunol.abd6160. [DOI] [PubMed] [Google Scholar]
- 12.Signore A, Lauri C, Colandrea M, et al. Lymphopenia in patients affected by SARS-CoV-2 infection is caused by margination of lymphocytes in large bowel: An [18F]FDG PET/CT study. Eur J Nucl Med Mol Imaging. 2022;49(10):3419–29. doi: 10.1007/s00259-022-05801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fathi N, Rezaei N. Lymphopenia in COVID-19: Therapeutic opportunities. Cell Biol Int. 2020;44(9):1792–97. doi: 10.1002/cbin.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdoli A, Falahi S, Kenarkoohi A. COVID-19-associated opportunistic infections: A snapshot on the current reports. Clin Exp Med. 2022;22(3):327–46. doi: 10.1007/s10238-021-00751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurra N, Woodard PI, Gandrakota N, et al. Opportunistic infections in COVID-19: A systematic review and meta-analysis. Cureus. 2022;14(3):e23687. doi: 10.7759/cureus.23687. [DOI] [PMC free article] [PubMed] [Google Scholar]