Abstract
Alcohol-associated stimuli contribute to relapse risk; therefore, understanding the behavioral and neural mechanisms underlying the ability of such stimuli to promote alcohol-seeking is important for developing effective treatments for alcohol use disorders. The Pavlovian instrumental transfer (PIT) paradigm can be used to study the influence of Pavlovian cues on independently-trained instrumental responses earning reward. The effects can be either general, increasing the vigor of reward related behaviours, or specific to responses that earn a common outcome. These different forms of PIT are mediated by distinct neural circuits involving the nucleus accumbens (NAC) core and shell, respectively. Here we examined the effects of pharmacological inactivation of either NAC core or shell on PIT generated by alcohol- and sucrose-predictive stimuli. We find that presentations of a stimulus predicting sucrose enhanced responding for sucrose but not alcohol, suggesting an outcome-specific effect. In contrast, presentations of an alcohol-predictive stimulus enhanced responding for both alcohol and sucrose suggesting a generally-arousing effect. Inactivation of the NAC core reduced PIT and in particular, the effect of the alcohol stimulus. Inactivation of the NAC shell reduced the specificity of the stimulus effects but left the ability of the stimuli to non-specifically invigorate responding intact consistent with a role in mediating the specificity of PIT. Together, these results suggest that the NAC core plays a particularly important role in mediating the influence of alcohol-predictive cues on reward seeking behaviors.
Keywords: stimulus, incentive, motivation, ventral striatum, learning
Alcohol-associated stimuli contribute to craving, compulsive drug use and relapse even following substantial periods of abstinence (O’Brien et al, 1998; Mann et al., 2005; Sinha et al., 2009). Understanding the mechanisms by which conditioned stimuli elicit responding for reward is critical to understanding addiction pathology. Environmental stimuli can gain control of drug and alcohol-seeking through Pavlovian conditioning when they are repeatedly paired with the pharmacological effects of the drug and can subsequently act to maintain high rates of responding, reinstate responding, or increase response vigor and bias response selection (Krank, 1989; Le & Shaham, 2002; Nie & Janak, 2003; Corbit & Janak, 2007; Chaudhri et al., 2008). The Pavlovian instrumental transfer paradigm (PIT) is unique in that Pavlovian and instrumental training are conducted independently, thus preventing opportunity for the direct association between stimuli and responses. It therefore provides a relatively pure assessment of the influence of Pavlovian incentive effects on instrumental performance, and increased responding in the presence of the stimuli reflects the conditioned invigorating effects of the stimuli rather than stimulus-response association or conditioned reinforcement process that may be acquired in other paradigms (LeBlanc et al., 2012).
Previous experiments using a Pavlovian Instrumental Transfer (PIT) paradigm have demonstrated that reward-related cues can influence performance of instrumental actions either through non-specific arousal acquired through association with rewards generally or through expectancy of a specific rewarding event (Corbit & Balleine, 2005). For example, when two unique stimuli are paired with distinct rewards, and their effects tested on performance of two responses that have previously been paired with those same rewards, the effects tend to be quite selective. That is, the stimuli typically increase performance of a response paired with the same, but not a different, reward as the stimulus itself was paired with in training. Nonetheless, if a third stimulus is paired with a reward not earned by either of the instrumental responses, its effects are quite general, elevating performance of reward-seeking responses regardless of the identity of the reward with which they have previously been paired. Evidence that these processes are, in fact, distinct comes from motivational and neural manipulations that demonstrate this dissociation (Corbit & Balleine, 2005; Corbit et al., 2007; Corbit & Balleine, 2011).
With regard to alcohol, using the PIT paradigm we have previously confirmed that alcohol-predictive stimuli increase performance of an alcohol-seeking response. However, when the specificity of this effect was examined, in contrast to the outcome-specific effects seen with natural rewards such as sucrose, an alcohol-predictive stimulus elevated responding not only for alcohol, but also for sucrose (Corbit & Janak, 2007). This could be explained if the effects of alcohol-paired cues rely on non-specific behavioural arousal rather than on outcome-specific encoding and expectancy, however, alternative explanations such as detrimental effects of the pharmacological effects alcohol during training on discrimination are difficult to disentangle behaviourally.
Many studies have attributed a critical role to the nucleus accumbens (NAC) in reward processes including mediating the effects of conditioned stimuli on reward seeking including alcohol seeking (Katner & Weiss, 1999; Corbit et al., 2001; Nicola, 2007; Chaudhri et al., 2010). Importantly, outcome-specific and generally-arousing PIT effects differentially rely on the NAC shell and core, respectively (Corbit & Balleine, 2011). This allows us to formulate the hypothesis that if the non-specific PIT effects observed with alcohol-paired stimuli are due to a general activating effect of the stimuli, then these effects should rely on the NAC core but be unaffected by manipulations of the shell. Based on previous findings related to PIT effects observed with natural rewards, we make the opposite prediction that the specific effects of a sucrose-paired stimulus should rely on the NAC shell, but be relatively unaffected by manipulations of the core.
This experiment examined the role of the NAC core and shell subregions in mediating the impact of alcohol vs. sucrose predictive stimuli on alcohol- and sucrose-seeking responses within the PIT paradigm by reversibly inactivating the core or shell prior to testing and comparing to performance to that observed following a saline infusion.
Methods
Subjects and apparatus.
Thirty-two experimentally naïve male Long-Evans rats (Harlan, Indianapolis, IN) weighing approximately 350g at the beginning of the experiment served as subjects. The rats were singly housed in individually ventilated polycarbonate cages, in a temperature- (20 ± 1 °C) and humidity-controlled vivarium that was maintained on a 12/12-h light–dark cycle (lights on at 07:00 h; behavioural testing conducted during the light phase). Rats and had free access to laboratory chow and water in the home cage. All procedures were approved by the Institutional Animal Care and Use Committee of the Ernest Gallo Clinic and Research Center at the University of California, San Francisco in accordance with the Guide for the Care and Use of Laboratory Animals. Training and testing took place in 16 Med Associates (East Fairfield, VT) operant chambers housed within sound- and light-attenuating shells. Each chamber was equipped with two pumps, each of which was fitted with a syringe that delivered a specific volume of solution (0.1 ml) into a recessed magazine in the chamber when activated. The chambers contained retractable levers that could be inserted to the left and right of the magazine. The boxes also contained a white noise generator and a solanoid that, when activated, delivered a 5 Hz clicker stimulus. All stimuli were adjusted to 80 dB in the presence of background noise of 60 dB provided by a ventilation fan. A 3 W, 24 V houselight mounted on the top-center of the wall opposite the levers and magazine provided illumination. Computers equipped with MED-PC software controlled the equipment and recorded magazine entries and lever-press responses.
Ethanol Acclimation.
To familiarize the rats with the taste and pharmacological effects of ethanol and ensure consumption during training, they were given the opportunity to consume it in the homecage prior to training; the rats initially were given free access to 10% ethanol (10E; v/v) in tap water in the home cage. Since animals would ultimately receive training with sucrose reward as an alternative reward to 10E, sucrose was never added to the 10E solution. 10E was available 24 hrs a day for 14 days. For the next 14 days of acclimation, the 10E was made available for 1 hr per day at the same time that training would subsequently take place. The rats had free access to water throughout this phase in a separate bottle fixed to the home cage. Rats were weighed daily and 10E consumption was recorded in order to calculate g/kg alcohol levels. In an attempt to match the extensive pre-exposure to 10E, prior to training, animals were also pre-exposed to a 2% sucrose solution (2S; wt./v), which would serve as the other reward. Animals received two exposures to 100ml of the sucrose solution in a drinking bottle over night in the home cage which they readily consumed; water was also available. This amount was chosen to roughly match the total volume of EtOH consumed during pre-exposure.
Pavlovian Training.
The rats received 10 sessions of Pavlovian conditioning. Two auditory stimuli (white noise and clicker) served as conditional stimuli. One of these stimuli (E+) was paired with ethanol delivery while the other stimulus (S+) was paired with sucrose (counterbalanced). Six presentations of each stimulus were given in each session in random order interspersed with periods in which no stimuli were present. The average length of the variable intertrial interval was 4.5 min. The stimulus presentations were 2 min long. During each stimulus, 0.2 ml of the appropriate outcome (10E or 2S) was delivered on a random time (RT) 30 sec schedule. While the schedule of delivery was random and thus the number of outcomes varied across sessions, on average the animals received 4.8 ml of 10E across the 75 min session which should lead to significant blood alcohol levels. The number of magazine entries during each stimulus as well as in a pre-stimulus interval of equal length (2 min) was measured. The magazine was inspected at the end of the training sessions to ensure that the solutions had been consumed.
Instrumental training.
The animals were next trained to respond on the two levers to self-administer 10E or 2S. For half of the animals, responding on the left lever produced 0.1 ml of 10E whereas responding on the right lever delivered 0.1ml of 2S. The remaining animals received the opposite assignments. Training for the two levers occurred independently on alternating days and all sessions were 60 min in duration. Initially the animals received two days of training for each outcome in which responding was reinforced on a continuous reinforcement schedule and were then shifted to a random ratio (RR) 2 schedule for two sessions per outcome.
Surgery.
Rats were assigned to the core or shell group in an attempt to equate baseline instrumental response rates for the two groups. Animals were ranked based on their instrumental response rates and then allocated to core and shell groups to match response rates prior to surgery as closely as possible. Stereotaxic surgery was conducted under isoflurane anaesthesia to implant with 26 gauge guide cannulae (Plastics One, Roanoke, VA) targeted at either the core (AP: +1.2 mm, ML: +/− 1.8 mm, DV: −3.8 mm; coordinates relative to bregma, and dura for DV) or shell (AP: +1.6 mm, ML: +/− .75 mm, DV: −4.0 mm). The tips of the guide cannulae were positioned 3 mm dorsal to the intended infusion site thus the final DV coordinates were 3 mm more ventral than the position of the guides. Once in place, cannulae were anchored with machine screws and dental acrylic.
Retraining.
Ten days after surgery, rats received one session of training under the RR2 schedule for each outcome, and were then were shifted to a RR4 schedule for an additional three sessions per outcome. Rats had one additional session of Pavlovian training prior to testing.
Pavlovian-instrumental transfer tests.
Subjects received two pairs of extinction tests (ethanol and sucrose lever tested under both inactivation and control conditions). During each test, one lever was available and each stimulus was presented twice interspersed with intervals of no stimulus (Ø). The 22-minute test contained 8, 2 min bins (two white noise trials [N] and two clicker trials [C] alternated with four Ø trials in the following order: N,C,C,N). Each stimulus bin was separated from the subsequent baseline (Ø) bin by one minute and there was a two-minute extinction period prior to the first pre-CS bin.
Infusions.
For each pair of transfer tests half of the animals from each group received infusions of a combination of the GABA-B receptor agonist, baclofen, and the GABA-A receptor agonist, muscimol (B/M; 1.0/0.1 mM, Sigma, St Louis MO), or saline vehicle via an infusion cannulae (33 gauge; Plastics One) extending 3 mm below the guide cannula tip (0.3 μl per min/total volume of 0.3 μl delivered per hemisphere) 10 minutes prior to test. Infusions took place over one minute and the cannulae were left in place for an additional two minutes to allow for diffusion.
Histology.
Following the final test session, animals received an overdose of sodium pentobarbital prior to being perfused transcardially with saline followed by 10% formalin. Brains were extracted and stored in formalin overnight and then transferred to a 25% sucrose solution for 48 h. Coronal sections (50μm) of tissue were sliced, mounted, and stained with thionin, to allow verification of infusion track and placement and assessment of any extraneous damage.
Data Analysis.
Data were analysed using repeated measures analysis of variance (ANOVA). Significant main effects and interactions were analyzed with further ANOVA and significant simple effects were examined with pairwise comparisons. The threshold for significance was adjusted with a Bonferroni correction to account for the family-wise error for such comparisons. Where the statistical software (SPSS), which only reports values up to three decimal places, indicated a p-value of .000 we report this value, although the actual value will be some small number greater than zero. Preliminary analyses indicated no effect either of lever (left vs. right), stimulus (white noise vs. clicker) or test order [F’s<1]; therefore the data were collapsed across those factors.
Results
Ethanol Acclimation.
Three rats were excluded from all analyses for low consumption of alcohol (less than 1 ml per day) in the homecage, which in our experience, results in failure to acquire alcohol self-administration. The average consumption per rat during the 24 h access period was 11.2 ml (+/− SEM; 1.0 ml). The average consumption per rat during the 1 h access in the home cages was 3.5 (0.3) ml, which produced an average alcohol level of 0.70 (0.06) g/kg.
Histology.
Figure 1 displays the placement of cannula tips for rats included in the behavioural analysis. One and four animals were excluded from the core and shell groups, respectively because placements were outside the target area resulting in final group sizes of 13 and 10 for the core and shell groups.
Figure 1.
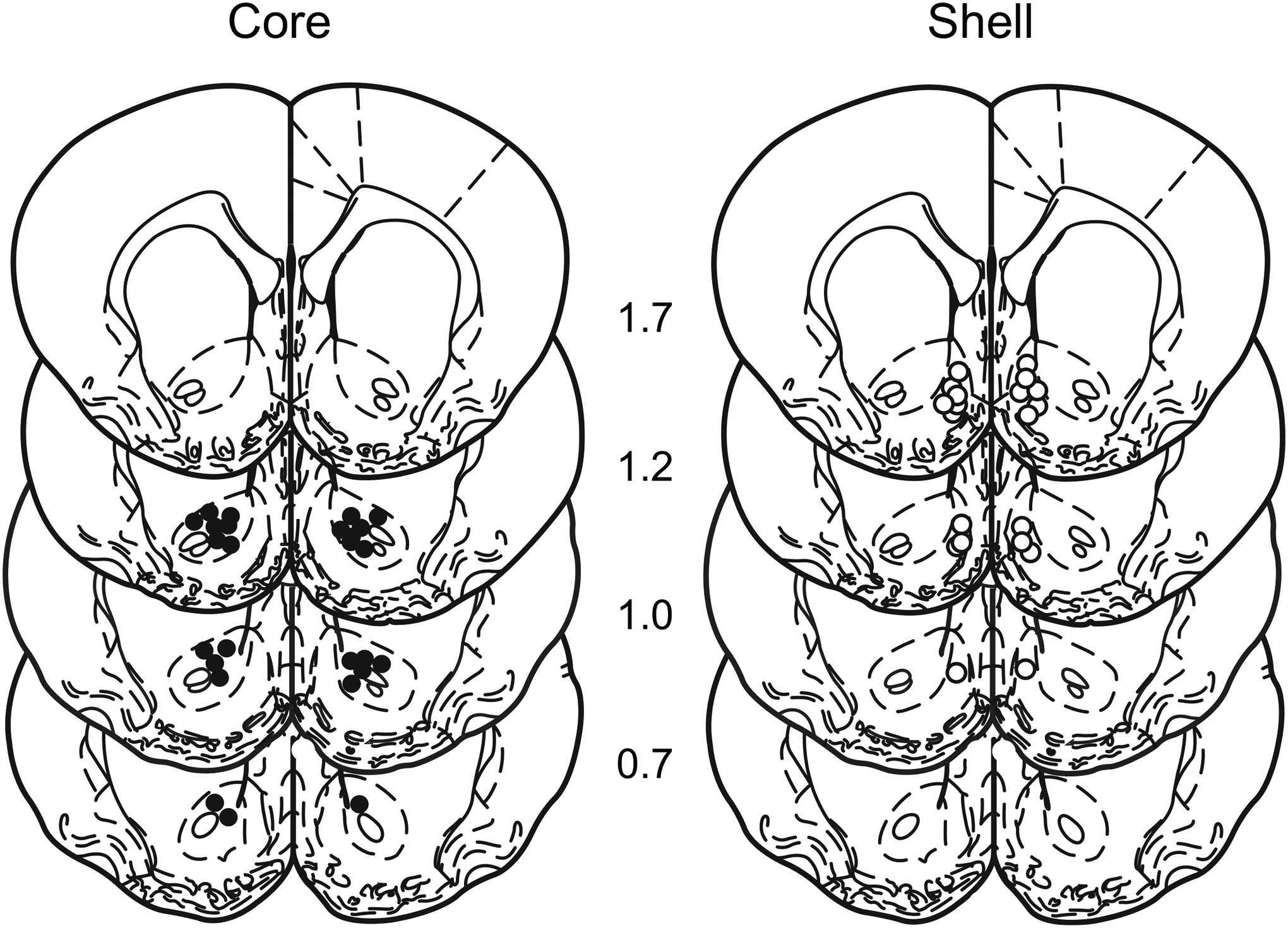
Schematic representation of the placement of cannula tips for the NAC core (left) and shell (right) groups. Numbers indicate distance anterior to bregma according to Paxinos & Watson, 1998.
Pavlovian Training.
The training data are presented in Figure 2A. Animals increased magazine entries during the stimuli across days of training. ANOVA indicated a significant effect of day [F(9,180)=9.4, p=0.000], and an effect of stimulus, indicating that animals entered the magazine more during the CSs than the pre-CS interval [F(2,40)=81.2, p=.000] and a stimulus by day interaction indicating that responding increased during the CSs, but not during the pre-CS period, across days [F(18, 360)=10.1, p=0.000]. There was no effect of group and no interactions with this factor [day; F(1,20)=0.72, p=0.691; stimulus; F(1,20)=0.1, p=0.748]. Direct comparison of the ethanol and sucrose stimuli demonstrated no difference in responding to the two stimuli [F(1,20)=2.2, p=0.147] and no interactions with this factor [day; F(1,20)=0.503, p=0.871; group; F(1,20)=0.002,p=0.966] indicating similar acquisition of a Pavlovian response to the two stimuli.
Figure 2. Pavlovian conditioning.
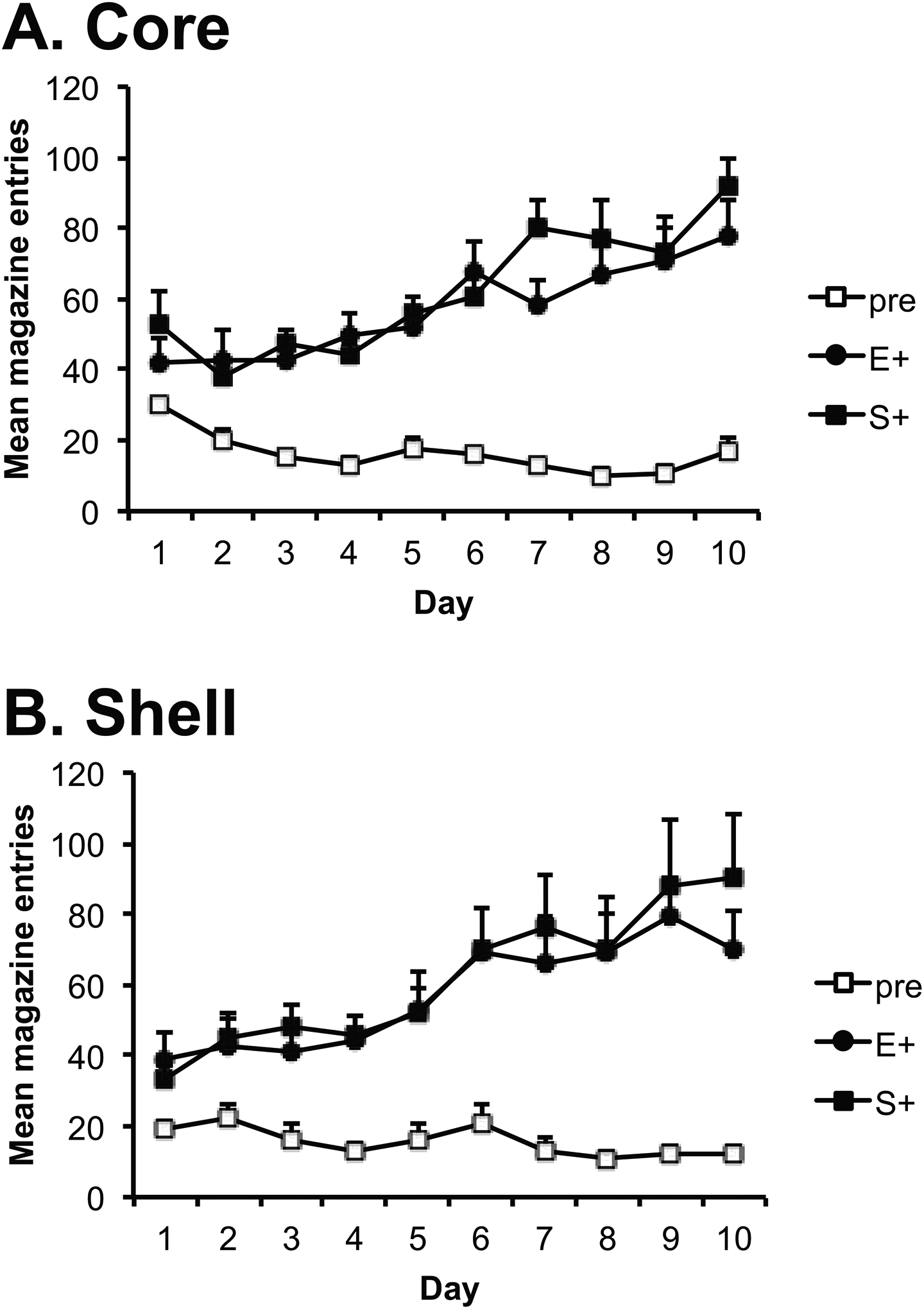
Mean number of magazine entries during presentations of the ethanol- (E+) or sucrose-paired (S+) stimuli and during the pre-CS intervals (+ SEM) across days of Pavlovian training for the core (A) and shell (B) groups. Rats in both groups learned to enter the magazine selectively during the stimulus periods.
Instrumental training.
The rats acquired the lever-press responses for both EtOH and sucrose and responding increased across days [Fig. 2B; F (7,147)=19.1, p= 0.000]. The rats responded more on the lever delivering sucrose, [F (1, 21)=24.0, p= 0.000] and there was an interaction between training day and lever [F (7, 147)=3.1, p= 0.002] indicating that the increase across days was greater for the sucrose lever. Importantly, there was no effect of group [F(1,20)=0.29, p=0.867] and no interactions with this factor [lever; F(1,20)=0.19,p=0.671; day; F(7,140)=1.94,p=0.181; group × day × lever; F(7,140)=1.01, p=0.425] indicating that core and shell animals responded similarly in training. The average volume of EtOH consumed for the final 3 days of instrumental training was 4.0 (0.8) and 4.2 (0.7) ml for rats in the core and shell groups, respectively, which corresponds to a mean EtOH intake of 0.7 (0.14) and 0.7 (0.13) g/kg for animals in the core and shell groups, respectively.
Pavlovian-instrumental transfer test.
For both groups, following saline infusion responding on the ethanol lever was elevated in the presence of the ethanol stimulus but to a far lesser extent in the presence of the sucrose stimulus. In contrast, as has been previously observed, responding on the sucrose lever was elevated equally by both the ethanol and sucrose-related stimuli (Corbit & Janak, 2007). This demonstrates that an ethanol-paired stimulus impacts performance of both the ethanol and sucrose responses, consistent with a general activating effect on reward-directed performance whereas the effects of the sucrose stimulus are specific to responses earning a common reward.
Inactivation of the core attenuated the impact of reward-predictive cues on responding although animals still showed some evidence of increased responding specifically in the presence of the stimulus that predicted the reward associated with the available lever. This suggests that core inactivation had an especially strong effect on the general form of PIT. Analysis of the test data for the core group including the factors of lever (ethanol vs. sucrose), infusion (saline vs. B/M) and stimulus (baseline, E+, S+) revealed a main effect of infusion, with animals responding less following B/M infusion [F(1,12)=10.54, p=0.007]. There was a significant effect of stimulus; overall, responding was greater during the stimuli than baseline intervals [F(2,24)=10.9, p=0.000]. There was no main effect of lever [F(1,12)= 2.09, p=0.174] but there was a lever by stimulus interaction [F(2,24)=4.53, p=0.021] indicating that the impact of the stimuli differed according to which lever was being tested. Neither the lever by infusion interaction, nor 3-way interaction were significant but there was an interaction between stimulus and infusion [F(2,24)=5.73, p=0.009]. To explore the interactions further, we examined the effects of the stimuli and inactivation on the ethanol and sucrose levers independently. For the ethanol lever, there was an effect of infusion indicating that rats responded less following B/M [F(1,12)=16.95, p=.001]. There was an effect of stimulus indicating that responding differed during the baseline, E+ and S+ intervals [F(2,24)=7.1, p=0.004]. Further, there was an interaction between these factors [F(2, 24)=6.45, p=0.006]. We next examined the effect of stimulus separately for saline and B/M conditions and, when an effect of stimulus was detected, used pairwise comparisons to examine evidence of the excitatory influence of each E+ and S+ relative to the baseline period. Following saline infusion, there was a significant effect of stimulus [F(2,24)=6.99, p=0.004] and responding during E+, but not S+ was significantly enhanced [p=0.032, p=0.181, respectively]. Following B/M infusion there was no effect of stimulus indicating that inactivation of the core attenuated PIT for the ethanol lever [F(2,24)=2.23, p=0.130]. For the sucrose lever, there was no effect of infusion [F(1,12)=0.29, p=0.559], or stimulus [F(2,24)=0.593, p=0.561], but there was an interaction between these factors [F(2,24)=10.08, p=0.001]. We next examined the stimulus effect following saline and B/M infusion. Following saline, there was a significant effect of stimulus [F(2,24)=5.41, p=0.012] and while responding during E+ was only marginally elevated, relative to baseline [p=0.052] S+ significantly enhanced responding [p=0.023]. Following B/M infusion there was a significant effect of stimulus [F(2,24)=3.58, p=0.044] and S+ but not E+ elevated responding relative to baseline [p=0.031, p=0.731, respectively].
Inactivation of the shell had a different pattern of effects. When the shell was inactivated, responding on the ethanol lever was similar in the presence of E+ and S+ whereas following saline infusion, the ethanol stimulus selectively elevated responding. For the sucrose lever, when the shell was inactivated, rats responded robustly and equally in the presence of both reward-predictive stimuli. This pattern did not differ from the pattern seen following saline infusion where both E+ and S+ are already observed to elevate responding. This suggests that inactivation of the shell interferes with the outcome-specificity of PIT effects, where specific PIT effects are otherwise present, but that the excitatory impact of the stimuli remains able to influence performance. ANOVA indicated no effect of lever [F(1,9)=2.19, p=0.173] or infusion [F(1,9)=0.076, p=0.789] but there was an effect of stimulus [F(2,18)=27.50, p=0.000]. There was no lever by stimulus interaction [F(2,18)=2.49, p=0.111], no lever by inactivation interaction [F(1,9)=0.07, p=0.834] and no three-way interaction [F(2,18)=2.79, p=0.088]. There was, however, a stimulus by inactivation interaction [F(2,18)=3.84, p=0.041]. We next examined the effect of stimulus and infusion on each lever. For the ethanol lever, there was an effect of stimulus [F(2,18)=14.7,p=0.000], no effect of inactivation [F(1,9)=0.17, p=692], but an interaction between these factors [F(2,18)=7.39, p=0.005]. Following saline, there was a significant stimulus effect [F(2,18)=15.93, p=0.000] with the E+ but not S+ elevating responding from baseline [p=0.005, p=0.214, respectively]. Following B/M there was an effect of stimulus [F(2,18)=5.02, p=0.019] however, neither the E+ nor S+ when examined alone significantly elevated responding from baseline [p=0.187, p=0.058, respectively] suggesting that either E+ and S+ jointly differed from baseline or that, perhaps our correction for multiple corrections diminished power to detect any effects.
For the sucrose lever, there was an effect of stimulus [F(2,18)=12.76, p=0.000], no effect of inactivation [F(1,9)=0.00, p=0.987] and no interaction between these factors [F(2,18)=0.14, p=0.873]. Following saline infusion, there as an effect of stimulus [F(2,18)=8.19, p=0.003] and both E+ and S+ elevated responding relative to baseline [p=0.031, p=0.001, respectively]. Following B/M infusion, there was an effect of stimulus [F(2,18)=6.73, p=0.007] and both E+ and S+ elevated responding relative to baseline [p=0.031, p=0.031, respectively]. Together these results suggest that the PIT effects observed on the sucrose lever were unaffected by inactivation of the shell.
Discussion
A major obstacle for the treatment of alcohol use disorders is that even following periods of abstinence, the risk of relapse remains high. Environmental stimuli that act as reminders of previous alcohol use can trigger subjective craving, or activate neural systems that control alcohol-seeking outside conscious awareness and thus may contribute importantly to relapse risk (Grusser et al., 2002; Loeber et al., 2006; Fox et al., 2007; Sinha et al. 2009). Previous studies in both animals and humans have described a role for alcohol predictive stimuli in the development, maintenance and relapse of alcohol seeking and consumption (Le & Shaham, 2002). Therefore understanding the behavioural and neural control of stimulus effects on alcohol seeking will be important for producing successful treatment outcomes.
The current study demonstrates that stimuli previously paired with alcohol consumption can promote performance of an independently trained response that procures alcohol. As previously described, the effects of the alcohol stimulus generalize to other reward related behaviours, in this instance to a sucrose-seeking response. This is in contrast to the effects of a stimulus paired with the natural reward sucrose, the effects of which were relatively specific to a sucrose, but not alcohol, seeking response. Such findings suggest that stimuli associated with drugs may be particularly powerful in provoking reward-directed behaviours. While the current findings are consistent with demonstrations from other paradigms, such as cue-induced reinstatement, which similarly show that alcohol associated cues can trigger alcohol-seeking responses (Katner et al., 1999; Nie & Janak, 2003; Zironi et al., 2006) the PIT paradigm is unique in that it isolates the incentive and cuing properties of stimuli without being confounded by potential opportunities for stimulus-response or conditioned reinforcement learning. The current findings demonstrate that alcohol stimuli can impact the initiation and vigor of responses with which they have never been directly paired.
The heterogeneous nature of the NAC has been well documented (see Zahm, 2000 for a review) and the core and shell regions have been dissociated in a variety of behavioural tasks. Utilization of stimulus-related information is one way in which specific actions can be selected from amongst competing alternatives. Stimuli carry information not only about the valence of a predicted outcome but also about the unique features of that outcome and it is clear that the NAC contributes importantly to the way that both of these processes influence performance with the core controlling the general motivational effects of such stimuli and the shell directing choice based on the specific features of unique outcomes. This experiment builds on previous reports that the NAC core and shell contribute to the general and outcome-specific forms effects of reward-predictive stimuli, respectively, and extend these results to alcohol reward (Corbit & Balleine, 2001; Hall et al., 2001; Corbit & Balleine, 2011). Inactivation of the core attenuated PIT overall with a particularly strong effect on the impact of the ethanol-predictive stimulus eliminating its effects on both the ethanol and sucrose lever. The effect of the sucrose stimulus on the sucrose lever remained. Together with previous demonstrations that the core is responsible for the generally arousing form of PIT this finding adds weight to the suggestion that alcohol-predictive stimuli generate this form of PIT. These findings add to other demonstrations that the NAC core is critical for control of alcohol seeking by discrete cues in reinstatement and renewal paradigms (Chaudhri et al., 2010). Inactivation of the shell resulted in rats responding equally in the presence of both the reward-predictive stimuli, demonstrating an inability to integrate specific stimulus-outcome and response-outcome associations in order to selectively direct responding, yet, some excitatory effects of the stimuli remained and the impact of the E+ was reduced from saline conditions. This finding is at odds to some degree with lesion results where lesions of the shell eliminate specific PIT including the excitatory effect of the “same” stimulus (Corbit et al., 2001). However, the design used here differed somewhat to previous studies, for example, training parameters and absolute response rates differed substantially and permanent lesions versus inactivation for the first time at test could affect baseline responding differently. Further, the use of alcohol as a reinforcer could contribute to these differences; alcohol as a reinforcer could produce learning that differs from that produced by natural reward even when the same training procedures are used. For example, it is possible that both outcome-specific and arousing components contribute to alcohol seeking and the influence of E+, perhaps accounting for effects on the alcohol and sucrose lever, respectively, and why both core and shell manipulation appear to impact the magnitude of the E+ effect. This finding is also consistent with the demonstration that manipulations of both core and shell can affect PIT following training involving a single stimulus and response, under some conditions (Pecina & Berridge, 2013). Nonetheless, these results are consistent with the view that the NAC shell is involved in inhibiting inappropriate behaviours (Ambroggi et al., 2011). For example, inactivation of the shell, but not core, increases responding in a context that signals that alcohol is unavailable (extinction context) as well as responding on an inactive lever in either a context paired with alcohol self-administration or extinction of that response (Chaudhri et al., 2008). Similarly, shell inactivation increases magazine entries during the interstimulus interval in a Pavlovian paradigm where alcohol was only delivered during stimulus presentations (Millan et al., 2015). Effective decision making involves not only choosing an action to perform, but often also inhibiting alternative or competing responses. This capacity appears to be lost following inactivation of the shell. It is worth noting that multiple brain regions and neurotransmitter systems have been implicated in PIT effects which is not surprising considering that both instrumental and Pavlovian contingencies must be encoded and integrated in order to generate specific PIT effects. The role of regions such as the amygdala, dorsal striatum, ventral pallidum and VTA are discussed in a recent review (Corbit & Balleine, 2015).
These results have implications for the understanding of stimulus control of relapse and the neural control of this process. Between 75–85% of detoxified alcoholics relapse after detoxification (Boothby & Doering, 2005). This is often despite a stated desire for abstinence and negative consequences of continued alcohol use. PIT effects have been shown to be resistant to changes in the value of rewards used in training (Rescorla, 1994) or even extinction of the Pavlovian cues (Delamater, 1996; Hogarth et al., 2014) indicating that stimulus influences on responding operate outside of desire or evaluation of the outcome which may make it a useful model for understanding cue-induced relapse following treatment and despite the desire to remain abstinent.
It has also been suggested that over the course of time drug-associated behaviours become habitual (Dickinson et al., 2002; Everitt 2014), mediated by automatic action schemas triggered by cues associated with previous drug use (Tiffany, 1990). Indeed, pre-clinical data has demonstrated that rats given extended alcohol self-administration or consumption in the home cage more readily form habits defined as a lack of sensitivity to outcome devaluation (Corbit et al., 2012; 2014, Barker et al., 2015). From this point of view, while drugs of abuse are initially sought for their rewarding properties, following extended use, drug seeking transitions to habitual control that is independent of the immediate value of the drug. Stimuli play a particularly important role in habitual behaviors since, as demonstrated by outcome devaluation procedures, the expression of habits does not rely on an expectation of the outcome a particular response produces but rather is triggered by environmental stimuli that over time have come to predict that responding will be reinforced under those environmental conditions. Indeed, the influence of stimuli has been shown to grow in parallel with the development of habitual control (Holland, 2004). Thus, while habit learning may only explain some aspects of addictive behaviour, or relate to a subset of the population with Alcohol Use Disorders, understanding diminished deliberate control over use as well as an exaggerated influence of stimuli, could provide considerable insight into relapse and the development of more effective treatments. This framework may go some way to explaining why drug-seeking behaviour often lapses despite negative consequences and individuals’ desire to remain abstinent.
While initial PIT findings come from animal studies, similar results have more recently been demonstrated in humans. For example, PIT effects are observed in both social drinkers (Martinovic et al., 2014) and detoxified alcoholics (Garbusow et al., 2014). In social drinkers, beer cues biased responding toward a response that earned beer thus providing some evidence of a selective PIT effect (Martinovic et al., 2014). However, the forced choice procedure used in that study does not allow evaluation of any potential general PIT effects. Garbusow and colleagues (2014) examined PIT in recently detoxified alcoholics and found that the patient population was more likely to show a PIT effect and that when observed, the effects was stronger than in healthy controls. Interestingly, in that study, subjects were trained to respond for monetary reward and so the observed results suggest that alcoholics may be more susceptible to the influence of Pavlovian stimuli thus demonstrating altered decision-making processes that are not limited to behaviours directed towards alcohol. Furthermore, alcohol and money-predictive stimuli each had excitatory effects on responding (for money) suggesting that alcohol predictive stimuli have effects that generalize to other reward-directed behaviours as seen in the current study. More direct evidence that PIT may in fact relate to addiction comes from the recent report that the strength of PIT effects may be an indicator of relapse risk (Garbusow et al., 2015). In this study, fMRI analyses were conducted during PIT and patients were followed up for three months after testing. Recently detoxified alcoholics showed a stronger behavioural PIT effect than healthy controls. Of interest, the activation of the NAC during PIT was greater in patients that went on to relapse, than in those that successfully abstained or in healthy controls. While the PIT design in this experiment also used monetary rather than alcohol reward and does not directly assess alcohol seeking, other work has implicated NAC activation in cue reactivity and relapse (Heinz et al., 2004; Beck et al., 2012) and approach to alcohol cues (Wiers et al., 2014). Thus, these results suggest that PIT, as an index of susceptibility to Pavlovian influences, may prospectively help identify patients at greater risk for relapse. The design of the study by Garbusow et al. (2015) does not fully explore general and specific PIT effects, though the previous demonstration that alcohol stimuli enhanced responding for monetary reward suggests the effects may be general. This, as well as direct effects on alcohol-seeking responses would be of significant interest for future study. Where possible within the limits of fMRI resolution, additional information about subregions of the NAC as well as the role of other neural structures would also be of interest and may help dissociate the type of PIT that is generated.
In summary, we show that an alcohol-predictive stimulus can invigorate performance of an independently trained alcohol-seeking response. This influence extends to a sucrose-seeking response, a pattern that is distinct from that seen with natural rewards where stimulus effects tend to be specific to responses earning a common reward. Inactivation of the NAC core attenuated expression of PIT and in particular, the general excitatory effects of the alcohol stimulus. In contrast, inactivation of the NAC shell reduced the specificity of the stimulus effects further confirming a role in specific PIT. Recent demonstrations that the PIT paradigm can be extended to humans and populations with substance use disorders in particular suggest it may be a useful tool for studying the behavioural and neural control of stimulus influences on behavior and may identify individuals that will be particularly vulnerable to relapse.
Figure 3. Instrumental conditioning.
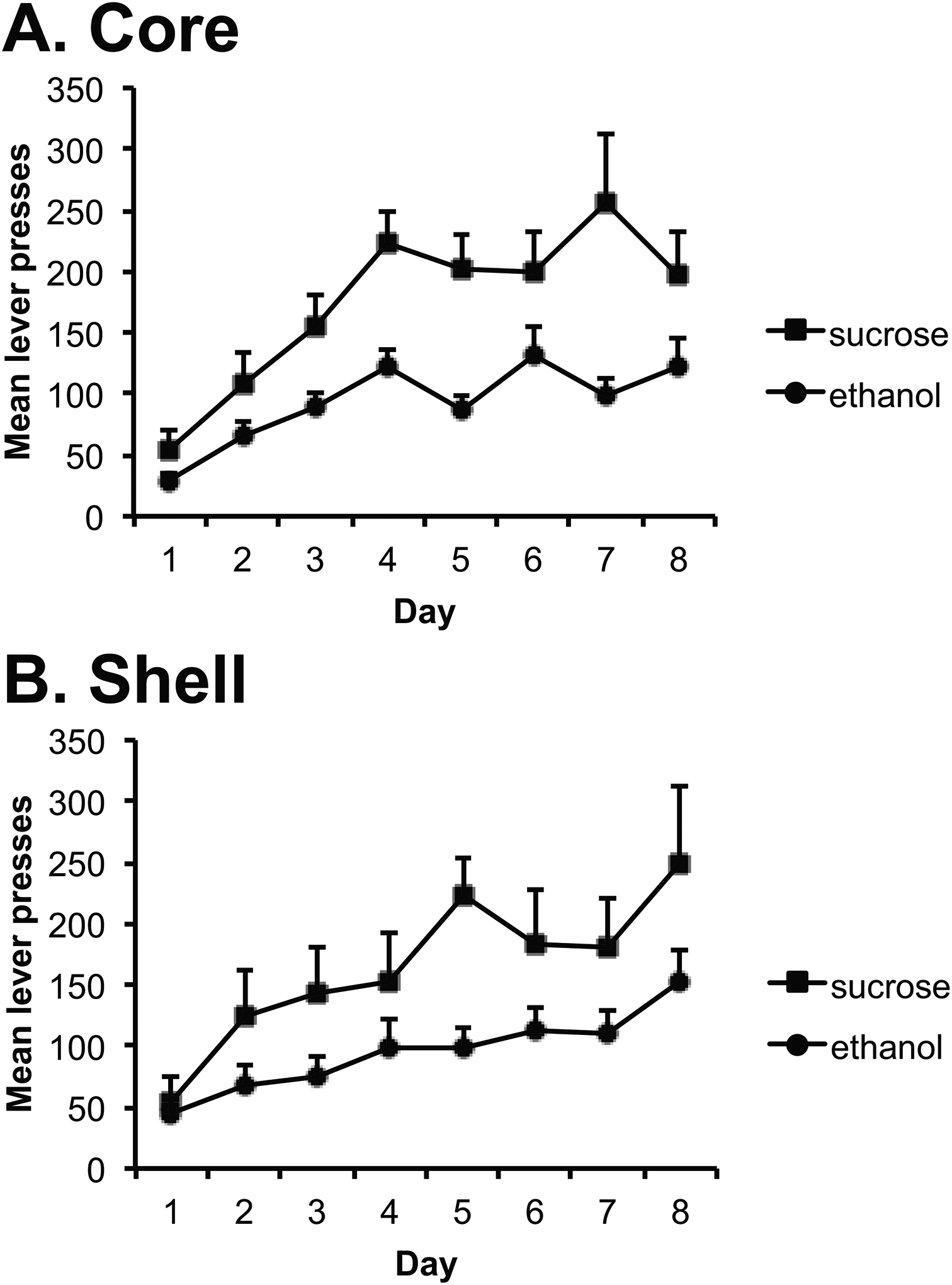
Mean lever-press responses earning ethanol and sucrose for the core (A) and shell (B) groups across days of training (+ SEM). For the first two days, each response was reinforced. Thereafter, responding was reinforced on an RR-2 schedule of reinforcement for days 3–5, and on an RR-4 schedule of reinforcement for days 6–8. Rats in both groups acquired the instrumental responses and increased response rates across days, but responding was higher for sucrose than for ethanol.
Figure 4.
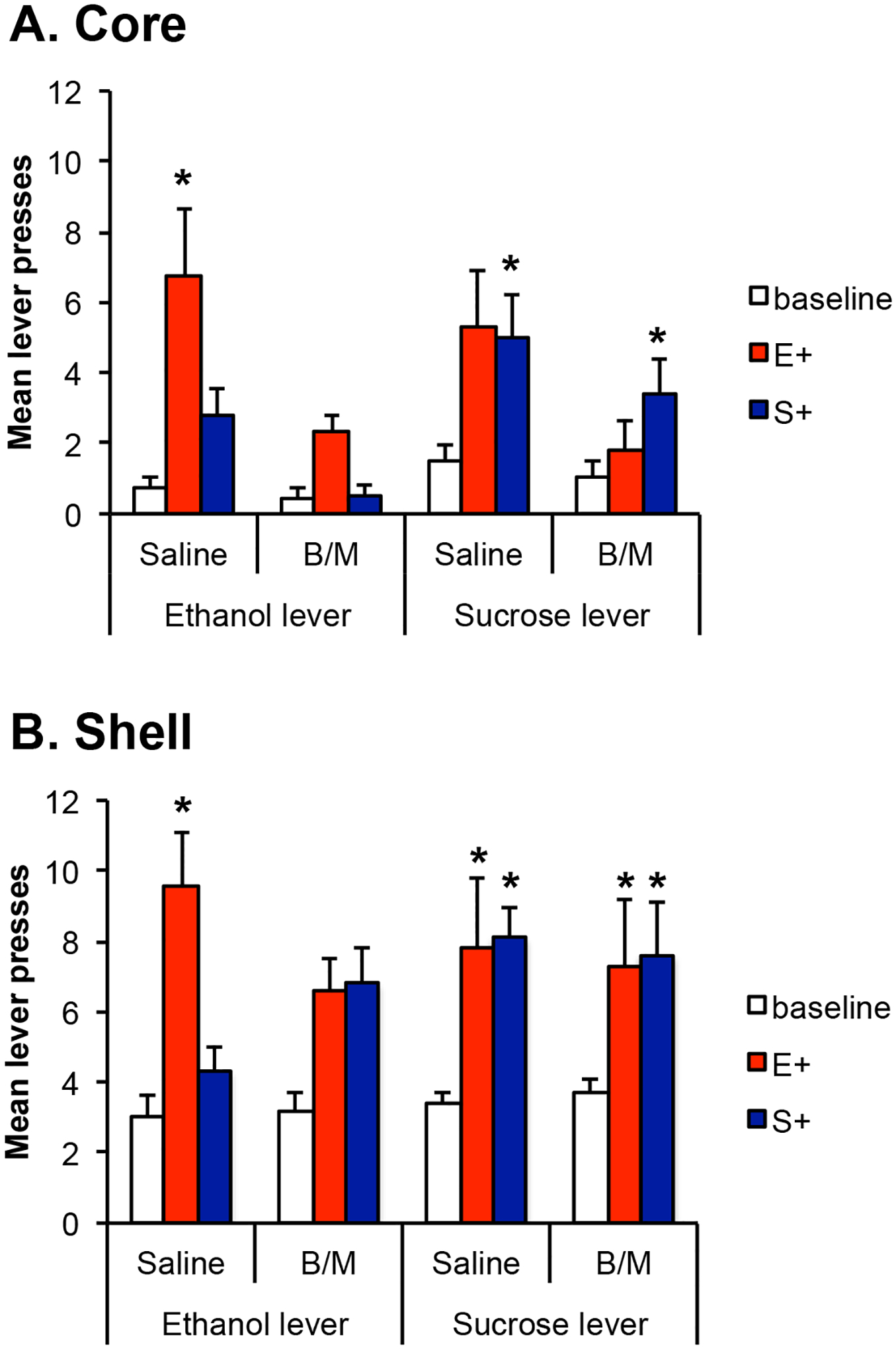
Effects of reversible inactivation of the core or shell on Pavlovian–instrumental transfer. (A) Mean lever presses (+SEM) on the ethanol- (left) and sucrose- (right) paired levers during baseline intervals and during presentations of the ethanol- (E+) and sucrose-paired (S+) stimuli following saline and baclofen/muscimol (B/M) infusions for rats in the core group. Presentation of E+ elevated responding on the ethanol lever relative to baseline and this effect was reduced by inactivation of the core. Both E+ and S+ elevated responding on the sucrose lever and the effect of E+ was reduced by inactivation. (B) Mean lever presses (+SEM) on the ethanol- (left) and sucrose- (right) paired levers during baseline intervals and during presentations of the ethanol- (E+) and sucrose-paired (S+) stimuli following saline and baclofen/muscimol (B/M) infusions for rats in the shell group. Presentation of E+ elevated responding on the ethanol lever relative to baseline. The specificity of this effect was lost following inactivation of the shell and excitatory effect of E+ attenuated. Presentation of both E+ and S+ elevated responding on the sucrose lever. There was no effect of inactivation of the shell on the sucrose response. * indicates a significant elevation in responding during the stimulus relative to baseline.
Acknowledgements
This research was supported by National Institutes of Health Grants AA014925 and AA018025 to PHJ and Australian National Health and Medical Research Council 1051037 and ABMRF to LHC. The authors declare no conflict of interest.
Abbreviations
- 10E
10% ethanol solution (wt./vol.)
- 2S
2% sucrose solution (wt./vol.)
- ANOVA
analysis of variance
- B/M
baclofen/muscimol
- CS
conditioned stimulus
- E+
ethanol-paired stimulus
- NAC
nucleus accumbens
- PIT
Pavlovian-instrumental transfer
- RR
random ratio
- S+
sucrose-paired stimulus
References
- Ambroggi F, Ghazizadeh A, Nicola SM, Fields HL. (2011). Roles of nucleus accumbens core and shell in incentive-cue responding and behavioral inhibition. J Neurosci., 31, 6820–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Corbit LH, Robinson DL, Gremel CM, Gonzales RA & Chandler LJ (2015). Corticostriatal circuitry and habitual ethanol seeking, Alcohol, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A Wustenberg T, Genauck A, Wrase J, Schlagenhauf F, Smolka et al. (2012). Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry, 69, 842–852. [DOI] [PubMed] [Google Scholar]
- Boothby LA, Doering PL (2005). Acamprosate for the treatment of alcohol dependence. Clin Ther, 27, 695–714. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Cone JJ, Janak PH. (2008). Reinstated ethanol-seeking in rats is modulated by environmental context and requires the nucleus accumbens core. Eur J Neurosci., 28, 2288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Schairer WW, Janak PH. (2010). Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology. 35, 783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW (2005) Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. J Neurosci 25, 962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH & Balleine BW (2011). The general and outcome-specific forms of Pavlovian-instrumental transfer are differentially mediated by the nucleus accumbens core and shell. The Journal of Neuroscience, 31, 11786–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, & Balleine BW (2015). Learning and Motivational Processes Contributing to Pavlovian–Instrumental Transfer and Their Neural Bases: Dopamine and Beyond. Current Topics in Behavioural Neuroscience. Epub ahead of print [DOI] [PubMed] [Google Scholar]
- Corbit LH & Janak PH (2007). Ethanol-associated cues produce general pavlovian-instrumental transfer. Alcoholism: Clinical and Experimental Research, 31, 766–774. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Janak PH, & Balleine BW (2007). General and outcome-specific forms of Pavlovian-instrumental transfer: the effect of shifts in motivational state and inactivation of the ventral tegmental area. European Journal of Neuroscience, 26, 3141–3149. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW (2001) The role of the nucleus accumbens in instrumental conditioning: evidence for a functional dissociation between core and shell. J Neurosci 21, 3251–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Nie H & Janak PH (2012). Habitual alcohol seeking: Time course and the contribution of subregions of the dorsal striatum. Biological Psychiatry, 72, 389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Nie H & Janak PH (2014). Habitual responding for alcohol depends upon both AMPA and D2 receptor signaling in the dorsolateral striatum. Frontiers in Behavioral Neuroscience, 8, 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamater AR (1996). Effects of several extinction treatments upon the integrity of Pavlovian stimulus-outcome associations. Animal Learning & Behavior, 24, 437–449. [Google Scholar]
- Dickinson A, Wood N, Smith JW (2002) Alcohol seeking by rats: action or habit? Quart J Exp Psychol 55B, 331–348. [DOI] [PubMed] [Google Scholar]
- Everitt BJ (2014) Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories - indications for novel treatments of addiction. Eur J Neurosci., 40, 2163–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R. (2007). Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clinical and Experimental Research, 31, 395–403. [DOI] [PubMed] [Google Scholar]
- Garbusow M, Schad DJ, Sebold M, Friedel E, Bernhardt N, Koch SP et al. (2015). Pavlovian-to-instrumental transfer effects in the nucleus accumbens relate to relapse in alcohol dependence. Addiction Biology, [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Garbusow M, Schad DJ, Sommer C, Jünger E, Sebold M, Friedel E, Wendt J, Kathmann N, Schlagenhauf F, Zimmermann US, Heinz A, Huys QJ, Rapp MA. (2014). Pavlovian-to-instrumental transfer in alcohol dependence: a pilot study. Neuropsychobiology, 70, 111–21. [DOI] [PubMed] [Google Scholar]
- Glasner SV, Overmier JB, Balleine BW (2005) The role of Pavlovian cues in alcohol seeking in dependent and nondependent rats. J Stud Alcohol 66, 53–61. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Heinz A, Raabe A, Wessa M, Podschus J, Flor H (2002). Stimulus-induced craving and startle potentiation in abstinent alcoholics and controls. Eur Psychiatry 17:188–193. [DOI] [PubMed] [Google Scholar]
- Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ (2001) Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behavior. Eur J Neurosci, 13, 1984–1992. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser S, Flor H, Braus D, Buchholz HG, Grunder G, Schreckenberger M, Smolka M, Rosch F, Mann K, Bartenstein P (2004) Correlation between dopamine D2 receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry 161, 1783–1789. [DOI] [PubMed] [Google Scholar]
- Hogarth L, Retzler C, Munafò MR, Tran DM, Troisi JR 2nd, Rose AK, Jones A, Field M (2014). Extinction of cue-evoked drug-seeking relies on degrading hierarchical instrumental expectancies. Behav Res Ther., 59, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC (2004). Relations between Pavlovian-instrumental transfer and reinforcer devaluation. Journal of Experimental Psychology: Animal Behavior Processes, 30, 104–117. [DOI] [PubMed] [Google Scholar]
- Katner SN, Magalong JG, Weiss F. (1999). Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology, 20, 471–479. [DOI] [PubMed] [Google Scholar]
- Katner SN, Weiss F. (1999). Ethanol-associated olfactory stimuli reinstate ethanol-seeking behavior after extinction and modify extracellular dopamine levels in the nucleus accumbens. Alcohol Clin Exp Res., 23,1751–60. [PubMed] [Google Scholar]
- Krank MD. (1989). Environmental Signals for Ethanol Enhance Free-Choice Ethanol-Consumption. Behavioral Neuroscience, 103, 365–372. [Google Scholar]
- Le A, Shaham Y. (2002). Neurobiology of relapse to alcohol in rats. Pharmacology & Therapeutics, 94, 137–156. [DOI] [PubMed] [Google Scholar]
- LeBlanc KH, Ostlund SB, Maidment NT. (2012). Pavlovian-to-instrumental transfer in cocaine seeking rats. Behav Neurosci., 126, 681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber S, Croissant B, Heinz A, Mann K, Flor H. (2006). Cue exposure in the treatment of alcohol dependence: effects on drinking outcome, craving and self-efficacy. The British journal of clinical psychology, 45, 515–529. [DOI] [PubMed] [Google Scholar]
- Mann K, Schäfer DR, Längle G, Ackermann K, Croissant B. (2005). The long-term course of alcoholism, 5, 10 and 16 years after treatment. Addiction, 100, 797–805. [DOI] [PubMed] [Google Scholar]
- Martinovic J, Jones A, Christiansen P, Rose AK, Hogarth L, Field M. (2014). Electrophysiological responses to alcohol cues are not associated with Pavlovian-to-instrumental transfer in social drinkers. PLoS One. 2014 Apr 14;9(4):e94605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, Reese RM, Grossman CD, Chaudhri N, & Janak PH (2015). Nucleus Accumbens and Posterior Amygdala Mediate Cue-Triggered Alcohol Seeking and Suppress Behavior During the Omission of Alcohol-Predictive Cues. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM (2007) The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berl) 191, 521–550. [DOI] [PubMed] [Google Scholar]
- Nie H, Janak PH (2003) Comparison of reinstatement of ethanol- and sucrose-seeking by conditioned stimuli and priming injections of allopregnanolone after extinction in rats. Psychopharmacology 168, 222–228. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan T, & Ehrman R (1990). Integrating systematic cue exposure with standard treatment in recovering drug dependent patients. Addictive Behaviors, 15, 355–365. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ (1998) Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol., 12, 15–22. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1998). The rat brain in stereotaxic coordinates, Ed4. San Diego: Academic. [DOI] [PubMed] [Google Scholar]
- Peciña S, & Berridge KC (2013). Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue‐triggered ‘wanting’for reward: entire core and medial shell mapped as substrates for PIT enhancement. European Journal of Neuroscience, 37(9), 1529–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA (1994). Transfer of instrumental control mediated by a devalued outcome. Animal Learning & Behavior, 22, 27–33. [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. (2009). Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology, 34, 1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST (1990) A cognitive model of drug urges ad drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev 97, 147–168. [DOI] [PubMed] [Google Scholar]
- Wiers CE, Stelzel C, Park SQ, Gawron CK, Ludwig VU, Gutwinski S, Heinz A, Lindenmeyer J, Wiers RW, Walter H, Bermpohl F. (2014). Neural correlates of alcohol-approach bias in alcohol addiction: the spirit is willing but the flesh is weak for spirits. Neuropsychopharmacology, 39, 688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS (2000) An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev 24, 85–105. [DOI] [PubMed] [Google Scholar]
- Zironi I, Burattini C, Aicardi G, Janak PH. (2006). Context is a trigger for relapse to alcohol. Behav Brain Res., 167, 150–5. [DOI] [PubMed] [Google Scholar]


