Abstract
Objectives
There are limited data on factors influencing antibiotic prescription among insured patients. We assessed for correlates of an antibiotic prescription among insured patients.
Design
A cross-sectional study.
Setting
The study was conducted at the National Health Insurance Fund offices, Dar es Salaam, Tanzania.
Data source
We captured data from the claim forms, containing inpatient and outpatient treatment information for insured patients, for the month of September 2019.
Outcome variable
Receipt of an antibiotic prescription.
Exposure variables
Age, sex, diagnosis, prescriber qualification, health facility level, ownership and department were exposure variables. Predictors of receipt of an antibiotic prescription were determined by Poisson regression analysis.
Results
Of 993 analysed patients, the mean (±SD) age was 36.3 (±23.2) years, 581 (58.5%) were females and 535 (53.9%) were adults. The prevalence of antibiotic prescription was 46.4% (95% CI 42.8% to 50.0%). Strong predictors of an antibiotic prescription were being a child (1.7, 95% CI 1.3 to 2.2); acute upper respiratory tract infection (URTI) of multiple and unspecified sites (1.6, 95% CI 1.3 to 1.4); chronic rhinitis, nasopharyngitis and pharyngitis (4.0, 95% CI 2.4 to 6.4); being attended by a clinical officer (1.9, 95% CI 1.2 to 3.0); attending a health centre (1.5, 95% CI 1.1 to 2.0); attending a public facility (1.2, 95% CI 1.0 to 1.4) and visiting an inpatient department (2.0, 95% CI 1.2 to 3.4).
Conclusions
Among insured patients, being a child, acute URTI, being attended by a clinical officer or dental therapist, being attended by an assistant medical/dental officer, attending a health centre or a district hospital, attending a public health facility and visiting an inpatient department predicted an antibiotic prescription. Incorporation of these findings in revisions or establishment of targeted antimicrobial stewardship programmes may lead to better antibiotic prescribing practices that are critical for combating antibiotic resistance.
Keywords: Public health, Clinical audit, THERAPEUTICS, CLINICAL PHARMACOLOGY
Strengths and Limitations of this study.
To our knowledge, this is the first study in Tanzania to address predictors of receipt of an antibiotic prescription among insured patients.
Insured patients being an increasing patient population in recent times and its anticipated risk of polypharmacy, studying antibiotic utilization in this group is important.
Being a cross-sectional design, our study, does not account for seasonal variations in antibiotic use, it lacks robustness in establishing causality, and is less generalizable.
Our data does not account for rejected claim forms, thereby making the results less generalizable.
We did not adjust for specific confounders, all variables with p < 0.2 were entered in the multivariable regression to model the receipt of an antibiotic prescription. We, therefore interpret our findings with caution.
Introduction
Curtailing antibiotic consumption is important to global health. Antibiotic use and misuse may predispose to development of resistant bacteria.1–4 Furthermore, it is estimated that half of the prescribed antimicrobials are inappropriate.5 We should strive to preserve antibiotics at all costs by providing a balance between access and excess as both have detrimental consequences. Delayed access may promote mortality from bacterial infections while excessive use increases selection pressure thereby favouring the development of resistant strains.6 Increased antibiotic exposure in healthcare settings is among the key modifiable drivers of antibiotic resistance.7 8
Emergence and spread of antibiotic-resistant bacteria far outweigh the speed with which newer antibiotics receive market approval.9 Humans, animals, as well as the surroundings, face the catastrophic consequences of antibiotic resistance.10 11 The consequences of which are associated with higher morbidity, longer duration of hospital stay, higher mortality rates and increased healthcare cost.12 13 These consequences are more pronounced in low/middle-income countries (LMICs) due to burden of infections, limited resources, poor health system, and weak regulatory enforcement to oversee antibiotic quality assurance, prescriptions and dispensing outlets.5
In Tanzania, resistance to commonly prescribed antibiotics was demonstrated in up to 60% of β-lactamase bacterial isolates from inpatients and outpatients attending a tertiary healthcare facility.14 In another study, 43.3% of Staphylococcus aureus nasal isolates from inpatients, which are resistant to methicillin, were also resistant to second-generation cephalosporin, cefoxitin.15 Some studies in children found bacterial pathogens resistant to multiple antibiotics.16 17 Therefore, the need for curbing antibiotic prescriptions so as to contribute in the fight against antibiotic resistance is warranted.
It has been argued that the more we procrastinate on taking urgent action to protect the current antibiotics we have, the more difficult and expensive it will be to tackle antibiotic resistance in the future.18 To combat the problem of increased use of antibiotics and its consequence, building capacity in areas of antimicrobial stewardship programmes (ASPs) and infection control is important.19–21 Globally, ASPs in hospitals have shown promise in reducing irrational antibiotic prescriptions. However, implementation challenges and heterogeneity in structures for antimicrobial stewardship in LMICs emphasise the need for tailored stewardship programmes.22 23
It is known that factors from healthcare providers, patients and the health system may influence the antibiotic prescription rate. Moreover, there are limited data regarding local factors influencing receipt of an antibiotic prescription among insured patients in Tanzania. This poses a key barrier in developing and implementing targeted ASPs. We conducted a study to identify factors that influence receipt of an antibiotic prescription among insured patients. ASPs in LMICs are often not comprehensively implemented and this may be partly because of a lack of resources and awareness of local important factors that influence antibiotic prescription.24 25
Materials and methods
We did a cross-sectional study of antibiotic prescription to patients insured by the National Health Insurance Fund (NHIF) involving claim forms submitted to the fund by health facilities in Dar es Salaam City Council (formerly Ilala municipal council) in Dar es Salaam. We chose insured patients because of having a high antibiotic prescription prevalence.26 Moreover, there are limited data on factors influencing receipt of an antibiotic prescription among this group. Part of the methodology has previously been published.27 Briefly, data collection from the claim forms was accomplished using a specially designed form. All forms submitted for claims, containing inpatient and outpatient information, in the period of 1 month of September 2019, were included in the study. Each claim form submitted to the insurance fund represented a request for payment or reimbursement for a single patient visit after receiving a service by a provider. A decade average of reimbursement rate is about 98.0%.28 Prescribers and designated healthcare workers at the respective health facility could access the claim forms and prepare them before submitting to the insurance fund. We accessed only the claim forms processed by the fund for paying the healthcare facilities for the services they have offered in the respective month of September. We could not access rejected claim forms, so they were not part of our sampling frame. We excluded forms for patients attended by physiotherapists or occupational therapists as they were not prescribers.
Claim forms for 378 patients were our initial sample size and were obtained by assuming 67.7% as prevalence of receiving an antibiotic prescription,29 a margin of error of 5% and a 10% chance of incomplete forms.30 However, in view of readily available patient claim forms, absence of additional risk to patients and affordability of data collection process, the planned sample size was increased to claim forms for 1100 patients. This was done in order to obtain precise estimates and to have enough data for subgroup analysis with adequate statistical power. Claim forms included in the study were selected randomly31 from the eligible forms (2A and B) for the month of September 2019 submitted to NHIF headquarters.
The dependent variable was receipt of an antibiotic prescription. It was a no/yes dichotomous variable. A no/yes question was recorded whether the client received an antibiotic prescription during the health facility visit. The independent variables were sociodemographic (sex (male, female), age (child <18 years, adult (18 years and above but <60 years), elderly (≥60 years)), level of health facility (dispensary, health centre, district hospital, regional referral hospital, national referral hospital), ownership of health facility (public vs private), final International Classification of Diseases, Tenth Revision (ICD-10) diagnosis code, department visited (inpatient vs outpatient), surgical procedure, polypharmacy (optimal number of drugs per encounter ≤3), generic name prescribing (optimal 100%), safe injection prescribing (encounter with an injection prescribed, optimal ≤10%), Essential Drug List prescribing (optimal 100%), and prescriber qualification such as clinical officer or dental therapist, assistant medical/dental officer, medical/dental officer, specialist, consultant. The patient, prescriber and health facility factors that may influence receipt of an antibiotic prescription were derived from the NHIF claim forms 2A and B and were selected on theoretical basis of similar studies.
There were no missing data in our study as our data source was the patient claim forms submitted to the insurance fund for payment claims by health facilities. Health facilities ensure the completeness of the claim forms so as to avoid any delay in the payment process. We used IBM SPSS Statistics software V.23 to analyse our data. Descriptive statistics summarised categorical variables, whereas numerical data were summarised by using mean and median. Χ2 test determined the associations between dependent variable (receipt of an antibiotic prescription) and independent variables (factors that influence receipt of an antibiotic prescription) and Fisher’s exact test was used when cell count is less than five. To identify predictors of receipt of an antibiotic prescription, we performed a Poisson regression with robust variance analysis. To control for confounding, first univariable analysis was done and then factors with a p value cut-off point of <0.2 were entered into the multivariable model. We did not adjust for specific confounders.
Patient and public involvement
It was not possible to involve patients and the public in the design, conduct and reporting of the study; however, dissemination plans of the findings to relevant authorities exist.
Results
Patient characteristics
Sociodemographic characteristics of patients of this study have been published elsewhere.27 In summary, out of 993 patients who met the analysis criteria, most were adults (n=535, 54%) and of female sex (n=581, 59%). The average age (±SD) was 36.3 (±23.2) years. Most patients visited the outpatient department (n=975, 98%) and private healthcare facilities (n=525, 53%). Majority of patients (n=548, 55.2%) attended a national referral hospital facility and most (n=437, 44.0%) received a specialist consultation (table 1). The complete list of patient characteristics is found in the online supplemental file 1. The outcome of interest, receipt of an antibiotic prescription, was found in 46.4% (n=357) of patients.
Table 1.
Sociodemographic and other patient characteristics
| Characteristic (N=993) | n (%) |
| Age in years | |
| Mean (SD)=36.3 (23.2), median=37.0 | |
| Children (<18) | 264 (26.6) |
| Adults (18–59) | 535 (53.9) |
| Elderly (≥60) | 194 (19.5) |
| Sex | |
| Male | 412 (41.5) |
| Female | 581 (58.5) |
| Level of health facility | |
| Dispensary | 102 (10.3) |
| Health centre | 119 (12.0) |
| District hospital | 101 (10.2) |
| Regional referral hospital | 123 (12.4) |
| National referral hospital | 548 (55.2) |
| Ownership of health facility | |
| Public | 468 (47.1) |
| Private | 525 (52.9) |
| Department visited | |
| Outpatient | 975 (98.2) |
| Inpatient | 18 (1.8) |
| Any procedure/surgery done | |
| No | 940 (94.7) |
| Yes | 53 (5.3) |
| Prescriber qualification | |
| Clinical officer/dental therapist | 132 (13.3) |
| Assistant medical/dental officer | 18 (1.8) |
| Medical/dental officer | 320 (32.2) |
| Specialist | 437 (44.0) |
| Consultant | 86 (8.7) |
bmjopen-2022-062147supp001.pdf (364.9KB, pdf)
Diagnoses were reported using ICD-10 diagnostic criteria. Among patients, ‘other disorders of urinary system’ (n=102, 10.3%) was the most common. ‘Other disorders of urinary system’, ICD-10—N39 diagnostic code, encompasses diagnoses such as: urinary tract infection, site not specified; persistent proteinuria, unspecified; stress incontinence; other specified urinary incontinence; other specified disorders of urinary system and disorders of urinary system, unspecified. The prevalence of acute upper respiratory tract infection (URTI) of multiple and unspecified sites was 6.5% (n=65), whereas that of acute tonsillitis was 2.4% (n=24). A complete list of prevalence of diagnoses among the study participants is found in online supplemental file 1.
Patient characteristics by receipt of an antibiotic prescription
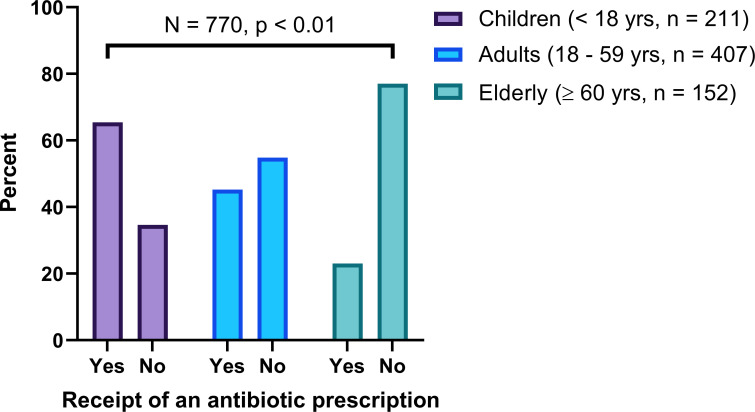
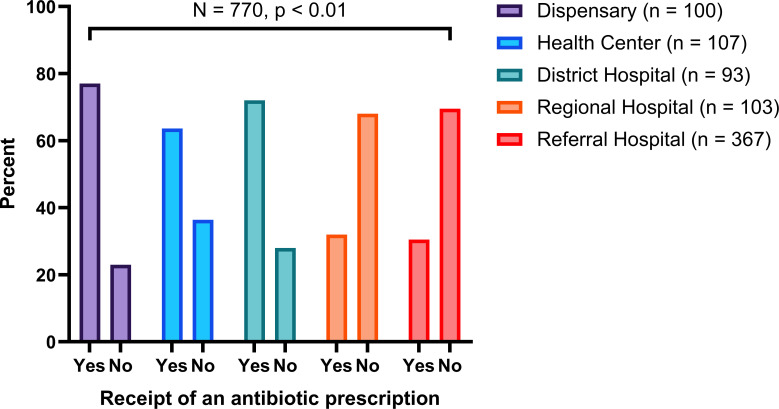
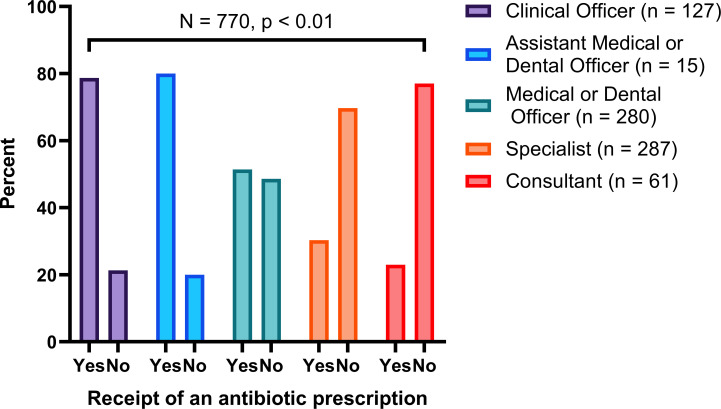
About two-thirds of children (65.4%) received an antibiotic prescription when compared with adults (45.2%) and the elderly (23.0%) (figure 1). Over three-quarters of patients (77.0%) who attended lower-level health facilities such as dispensaries received an antibiotic prescription compared with those who attended health facilities at the level of a national referral hospital (30.5%) (figure 2). A higher proportion (80.0%) of patients who were attended by either assistant medical or dental officers received an antibiotic prescription when compared with those attended by medical or dental officers (51.4%), specialists (30.3%) and consultants (23.0%) (figure 3).
Figure 1.
Receipt of an antibiotic prescription by age group.
Figure 2.
Receipt of an antibiotic prescription by the level of health facility.
Figure 3.
Receipt of an antibiotic prescription by prescriber qualification.
More than two-thirds of patients (70.6%) who visited the inpatient department received an antibiotic prescription compared with those who visited the outpatient department (45.8%). Most patients with acute tonsillitis (95.8%) and those with other disorders of urinary system (93.7%) were prescribed an antibiotic. A complete list of distribution of study characteristics by receipt of an antibiotic prescription is presented in online supplemental file 2.
bmjopen-2022-062147supp002.pdf (84.5KB, pdf)
Factors associated with receipt of an antibiotic prescription
Evidence of an association between the following factors and receipt of an antibiotic prescription was observed. The prevalence of receipt of an antibiotic prescription was highest among patients with chronic rhinitis, nasopharyngitis and pharyngitis, and was about four times higher compared with those who have no such a diagnosis. This was followed by a diagnosis of other disorders of the bladder in which the prevalence of receipt of an antibiotic prescription was about 3.5 times higher compared with those without such a diagnosis (adjusted prevalence ratio (aPR)=3.5, 95% CI 2.5 to 4.8, p<0.001). Moreover, having a diagnosis of acute URTI of multiple and unspecified sites was associated with receipt of an antibiotic prescription at a prevalence of about 1.6 times higher than those who were not (aPR=1.6, 95% CI 1.3 to 1.4, p<0.001).
The prevalence of receipt of an antibiotic prescription was about 1.7 times higher in children compared with that in the elderly. Attending a health centre was associated with about 1.5 times higher prevalence of receipt of an antibiotic prescription compared with those who attended the national referral hospital (aPR=1.5, 95% CI 1.1 to 2.0, p<0.009). Similarly, attending a district hospital predicted receipt of an antibiotic prescription (aPR=1.5, 95% CI 1.1 to 1.9, p<0.004) when compared with those who attended the national referral hospital. Furthermore, the prevalence of receipt of an antibiotic prescription was about 1.9 times higher in patients attended by a clinical officer or a dental therapist compared with those attended by a consultant. In addition, being attended by an assistant medical or dental officer was associated with an antibiotic prevalence of about two times higher than those being attended by a consultant (table 2). Patients with non-ideal generic prescriptions had an antibiotic prescription prevalence of 1.3 times higher than that of patients with ideal generic prescriptions (aPR=1.3, 95% CI 1.1 to 1.5, p<0.002).
Table 2.
Poisson regression analysis of factors influencing receipt of an antibiotic prescription
| Characteristic (N=770) | Univariate regression | Multivariate regression | ||
| cPR (95% CI) | P value | aPR (95% CI) | P value | |
| Age in years | ||||
| Children (<18) | 2.8 (2.1 to 3.9) | <0.001 | 1.7 (1.3 to 2.2) | <0.001 |
| Adults (18–59) | 2.0 (1.4 to 2.7) | <0.001 | 1.5 (1.1 to 1.9) | 0.004 |
| Elderly (≥60) | 1 (ref) | 1 (ref) | ||
| Any medical procedure/surgery done | ||||
| Yes | 1.4 (1.1 to 1.9) | 0.01 | 1.3 (0.8 to 2.0) | 0.34 |
| No | 1 (ref) | 1 (ref) | ||
| Chronic rhinitis, nasopharyngitis and pharyngitis—J31 | ||||
| Yes | 2.2 (2.0 to 2.3) | <0.001 | 4.0 (2.4 to 6.4) | <0.001 |
| No | 1 (ref) | 1 (ref) | ||
| Other disorders of bladder—N32 | ||||
| Yes | 2.2 (2.0 to 2.3) | <0.001 | 3.5 (2.5 to 4.8) | <0.001 |
| No | 1 (ref) | 1 (ref) | ||
| Disease of the pulp and periapical tissues—K04 | ||||
| Yes | 2.2 (2.0 to 2.4) | <0.001 | 3.4 (2.3 to 4.8) | <0.001 |
| No | 1 (ref) | 1 (ref) | ||
| Infections of genitourinary tract in pregnancy—O23 | ||||
| Yes | 2.2 (2.0 to 2.3) | <0.001 | 2.9 (2.1 to 4.0) | <0.001 |
| No | 1 (ref) | 1 (ref) | ||
| Cutaneous abscess, furuncle and carbuncle—L02 | ||||
| Yes | 2.2 (2.0 to 2.3) | <0.001 | 3.0 (1.9 to 4.9) | <0.001 |
| No | 1 (ref) | 1 (ref) | ||
| Acute pharyngitis—J02 | ||||
| Yes | 1.8 (1.3 to 2.6) | 0.002 | 2.7 (1.1 to 6.3) | 0.03 |
| No | 1 (ref) | 1 (ref) | ||
| Acute tonsillitis—J03 | ||||
| Yes | 2.1 (1.9 to 2.4) | <0.001 | 2.3 (1.8 to 3.0) | <0.001 |
| No | 1 (ref) | 1 (ref) | ||
| Acute URTI of multiple and unspecified sites—J06 | ||||
| Yes | 1.8 (1.6 to 2.1) | <0.001 | 1.6 (1.3 to 1.9) | <0.001 |
| No | 1 (ref) | 1 (ref) | ||
| Candidiasis—B37 | ||||
| Yes | 1.9 (1.5 to 2.4) | <0.001 | 1.6 (1.2 to 2.1) | 0.002 |
| No | 1 (ref) | 1 (ref) | ||
| Prescriber qualification | ||||
| Clinical officer/dental therapist | 3.4 (2.1 to 5.5) | <0.001 | 1.9 (1.2 to 3.0) | 0.005 |
| Assistant medical/dental officer | 3.5 (2.1 to 5.9) | <0.001 | 2.0 (1.1 to 3.4) | 0.02 |
| Medical/dental officer | 2.2 (1.4 to 3.6) | 0.001 | 1.6 (1.1 to 2.5) | 0.03 |
| Specialist | 1.3 (0.8 to 2.1) | 0.27 | 1.3 (0.8 to 1.9) | 0.25 |
| Consultant | 1 (ref) | 1 (ref) | ||
| All medications prescribed using their generic names | ||||
| No | 1.2 (1.0 to 1.4) | 0.02 | 1.3 (1.1 to 1.5) | 0.002 |
| Yes | 1 (ref) | 1 (ref) | ||
| Presence of injectable formulation in the prescription | ||||
| Yes | 1.4 (1.1 to 1.7) | 0.003 | 1.4 (1.1 to 1.8) | 0.004 |
| No | 1 (ref) | 1 (ref) | ||
| Level of health facility | ||||
| Dispensary | 2.5 (2.1 to 3.0) | <0.001 | 1.3 (0.9 to 1.8) | 0.14 |
| Health centre | 2.1 (1.7 to 2.6) | <0.001 | 1.5 (1.1 to 2.0) | 0.009 |
| District hospital | 2.4 (1.9 to 2.9) | <0.001 | 1.5 (1.1 to 1.9) | 0.004 |
| Regional referral hospital | 1.1 (0.8 to 1.4) | 0.77 | 1.0 (0.7 to 1.4) | 0.97 |
| National referral hospital | 1 (ref) | 1 (ref) | ||
| Ownership of health facility | ||||
| Public | 0.7 (0.6 to 0.8) | <0.001 | 1.2 (1.0 to 1.4) | 0.03 |
| Private | 1 (ref) | 1 (ref) | ||
| Department visited | ||||
| Inpatient | 1.5 (1.1 to 2.1) | 0.007 | 2.0 (1.2 to 3.4) | 0.01 |
| Outpatient | 1 (ref) | 1 (ref) | ||
aPR, adjusted prevalence ratio; cPR, crude prevalence ratio; ref, reference category; URTI, upper respiratory tract infection.
Moreover, patients who attended a public hospital had an antibiotic prescription prevalence of about 1.2 times higher compared with those who attended a private hospital, whereas attending an inpatient department predicted receipt of an antibiotic prescription compared with attending an outpatient department (aPR=2.0, 95% CI 1.2 to 3.4, p<0.01). Similar prevalence of receipt of an antibiotic prescription was seen in patients having diagnoses of candidiasis, and acute URTI of multiple and unspecified sites. The complete list of variables subjected to univariate and multivariate analyses is found in online supplemental file 3.
bmjopen-2022-062147supp003.pdf (70.1KB, pdf)
Discussion
We conducted a cross-sectional study among insured patients to determine factors influencing receipt of an antibiotic prescription. We assessed factors related to patient, prescriber and the health facility. Factors related to patient, with strong evidence of association with receipt of an antibiotic prescription, included being a child and having a diagnosis of URTI. The prescriber-related factor influencing receipt of antibiotic prescription was being attended by a clinical officer or dental therapist and assistant medical/dental officer. Furthermore, absence of ideal generic prescribing and presence of injectable formulation in the prescription both independently predicted receipt of an antibiotic prescription. Factors related to the health facility that were associated with receipt of an antibiotic prescription included attending either a health centre or a district hospital, attending a public health facility and visiting an inpatient department.
Antimicrobial stewardship is the most promising strategy to stop misuse and excessive use of antibiotics. However, implementation of such programmes is challenging and thus, research looking into ways of strengthening ASPs is critical for ensuring optimal clinical outcomes, minimal unintended consequences of antibiotics use, improved susceptibility rates to targeted antibiotics, optimal resource utilisation and hence, control of bacterial infections. The thrust of our study was to define factors that are strong predictors of an antibiotic prescription so that ASPs may see where to put emphasis.
We have identified diagnosis of URTI, both acute and chronic, as the strong predictors of an antibiotic prescription in our study population. This means that the microbiology laboratory aspect of antimicrobial stewardship such as provision of culture and sensitivity results on a regular basis or preparation of annual antibiotic susceptibility pattern needs to be established and strengthened. There are criteria, WHO or Integrated Management of Childhood Illness (IMCI), for prescribing an antibiotic for URTI. However, when clinicians are unwilling to go through the procedures or when procedures are not available, prescription of an antibiotic will be the easy way out and without taking risk for possibility of untreated or delayed treatment of a bacterial infection. Although most URTIs have a viral aetiology and have a self-limiting course, antibiotics are commonly prescribed.32 This observation is in line with other previous pieces of published literature that have demonstrated this association.33–36 The patient and the public should be informed that most of the acute URTIs are viral in origin and they require supportive therapy and not antibiotics. This will decrease patient antibiotic expectation. Although some studies show no evidence,37 facility-specific guidelines and algorithms, adapted from national standard treatment guidelines, should be established with respect to properly diagnosing and treating URTIs.38–40
Our data show that the prevalence of receipt of an antibiotic prescription was high among children and with a decreasing trend towards the elderly. URTIs and non-bloody diarrhoea being prevalent in children and mostly treated with antibiotics despite being viral in origin and contrary to treatment guidelines may explain this finding.41 42 This observation is comparable with other published results.26 33 43–45 Moreover, immune senescence in the elderly causes atypical presentations of infectious disease symptoms such as fever and cough, whereas in children they are more pronounced.46 ASPs should be strengthened in paediatrics so as to decrease antibiotic prescriptions as there is strong evidence supporting such an approach.47 48 Despite the challenges of implementing ASPs in paediatrics, clinical education, caregiver education, updated facility-specific guidelines and prospective audit and feedback are stewardship interventions shown to decrease antibiotic utilisation.49–51
Being attended by a clinical officer or dental therapist is another factor which appears to influence prescription of an antibiotic. The prevalence of receipt of an antibiotic prescription was about 1.7 times higher in patients who were attended by either a clinical officer or dental therapists when compared with those seen by consultants. Similarly, the prevalence of antibiotic prescription in patients attended by an assistant medical/dental officer was twice higher than that of patients seen by a consultant. Clinical officers, dental therapists, and assistant medical/dental officers being less experienced and less trained to prescribe probably explains this observation. Moreover, clinical officers, dental therapists, assistant medical/dental officers usually work in primary healthcare facilities in which there is a high volume of patients and fewer resources, which increase the likelihood of irrational medication prescriptions including antibiotics.52 This antibiotic prescribing disparity between prescribers with different qualifications was also demonstrated in previous studies.53 Another study in Hubei, China similarly found that prescribers with higher qualifications were less likely to prescribe antibiotics.52 This finding emphasises the need for antibiotic stewardship interventions to target clinical officers, dental therapists and assistant medical/dental officers through clinical education. Opportunities and protected time for clinicians to address knowledge gap through continuing medical education have been found to improve antibiotic utilisation.54–56 Therefore, it is important for hospital policies and administrators to provide clinicians with such opportunities.
Ideally, all medications in a prescription should be written in their generic names as per WHO/International Network of Rational Use of Drugs (INRUD) prescribing indicators. We observed strong evidence of an association between non-ideal generic prescribing and receipt of an antibiotic prescription. This observation may be explained by the fact that both suboptimal generic prescribing and overprescribing antibiotics are indicators of poor prescribing practice.33 In addition, presence of an injectable formulation in the prescription was associated with receipt of an antibiotic. The presence of an injectable formulation in the prescription may be indicative of the severity of the illness or infection and this may explain why the prevalence of receiving an antibiotic prescription is higher. It is essential that ASPs enable prescribers to adhere to generic prescribing and other good prescribing practices.
Studies have shown that patients’ likelihood of receiving an antibiotic prescription is influenced by the type of health facility they have attended to. A study in Ghana showed that attending a health centre or a clinic is associated with receipt of an antibiotic prescription.33 Similarly, we have revealed that there is strong evidence of an association between patient attending a health centre and receiving an antibiotic prescription when compared with those attending a national referral hospital. This observation may be attributed to limited resources in terms of medications and diagnostic capabilities resulting in empirical prescribing of antibiotics. Indeed, targeting lower-level health facilities with antimicrobial stewardship interventions such clinical education, facility-specific guidelines for common infections, and antibiotic oversight through prospective audit and feedback may decrease antibiotic prescriptions.48 50
Surprisingly, our study showed that attending a public health facility was associated with a higher prevalence of receipt of an antibiotic prescription. This was a surprising finding as private health facilities are driven by profit, so we did expect them to prescribe more medications including antibiotics to patients when compared with public health facilities. We speculate that insured patients are more likely to attend private health facilities where prescribers better adhere to insurance guidelines than those in public facilities. This was in line with a South African study by Mohlala et al.57 Similarly, an Australian study also showed a higher prevalence of antibiotic prescriptions for treatment and not for prophylaxis in public hospitals when compared with private hospitals.58 This is a worrisome finding as, in general, majority of patients are likely to be seen in public health facilities thus antibiotic prescriptions might be higher than what we have observed. Clinical education, facility-specific guidelines and antibiotic oversight should be established or strengthened in public health facilities.
Attending the inpatient department was also associated with a prevalence of receipt of an antibiotic prescription two times higher than that of patients who visited the outpatient department. This high antibiotic prevalence could be explained by the fact that inpatients tend to have a more severe illness when compared with those treated at the outpatient department. A similar observation of high antibiotic prescriptions among inpatients was found in a study by Bediako-Bowan et al in Ghana.59 Facility-specific guidelines for inpatient management should be established or strengthened to minimise antibiotic prescriptions.
To our knowledge, this is the first study in Tanzania to address predictors of receipt of an antibiotic prescription among insured patients. For insured patients, being an increasing patient population in recent times and its anticipated risk of polypharmacy, studying antibiotic utilisation in this group is important. Our data do not account for rejected claim forms, thereby making the results less generalisable. Using patient claim forms submitted to the insurance fund as our data source ensured no missing data as incomplete forms are not processed for payment and usually returned to the healthcare provider. However, limitations of this study include inherent weakness of cross-sectional studies as they lack robustness in establishing causality, lack of generalisability of the study findings as our study population was only insured patients and inability to account for seasonal variations in antibiotic use. Moreover, the overly large sample size used may cause small differences in observations to be statistically significant without any clinical significance. Furthermore, we did not adjust for specific confounders; all variables with p<0.2 were entered in the multivariable regression to model the main effect. We therefore interpret our findings with caution.
Conclusions
Factors influencing antibiotic prescription in Tanzania are similar to the factors reported in literature. Being a child, having a diagnosis of URTIs, being attended by a clinical officer, dental therapist and assistant medical/dental officer, attending a health centre or district hospital, and attending a public health facility appear to be the most important factors that when targeted through antimicrobial stewardship activities may have an important impact on antibiotic misuse and excessive use.
Supplementary Material
Acknowledgments
We thank Mr Gilbert Kubenea and Sr Rehema Hassan who helped us to retrieve the files of potentially eligible participants from the archives. We appreciate the help of Dr Ngalela Kateule and Sr Neema Manga, for assisting us to obtain the sampling frame list. Research assistant, Mr Roman Mathias, assisted with the data collection. We acknowledge that details of the methods have been published elsewhere. Finally, we thank the management of NHIF for permission and cooperation during the conduct of the study.
Footnotes
Contributors: MAK, PS and SM conceptualised and designed the study, and collected, analysed, and interpreted the data. MAK drafted the initial manuscript and acts as a guarantor. MAK, PS and SM critically revised the manuscript and approved the final version to be submitted.
Funding: This work was supported by the Ministry of Education, Science and Technology, Tanzania.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplemental information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Ethical approval from the research and publication committee of MUHAS was sought and was granted (reference no. DA.287/298/01A). We requested further permission from the Director of National Health Insurance Fund (NHIF) to proceed with the study using NHIF database after informing him of the purpose of the study and possible benefits to NHIF as well as to the society at large. Utmost confidentiality was maintained as no personal identifiers were collected by our data capture tool.
References
- 1.Gelband H, Miller-petrie M, Pant S. The state of the world ’ S antibiotics 2015. Wound Healing Southern Africa 2015;8:30–4. [Google Scholar]
- 2.Roca I, Akova M, Baquero F, et al. The global threat of antimicrobial resistance: science for intervention. New Microbes New Infect 2015;6:22–9. 10.1016/j.nmni.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei X, Zhang Z, Walley J. Reducing antibiotic for child upper respiratory infections in rural China: an RCT, process evaluation and cost-effectiveness analysis. American journal of respiratory and critical care medicine Conference: american thoracic society international conference, ATS 2018 United states 2018;197. [Google Scholar]
- 4.Yates TD, Davis ME, Taylor YJ, et al. Not a magic pill: a qualitative exploration of provider perspectives on antibiotic prescribing in the outpatient setting. BMC Fam Pract 2018;19:96. 10.1186/s12875-018-0788-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharland M, Saroey P, Berezin EN. The global threat of antimicrobial resistance--The need for standardized surveillance tools to define burden and develop interventions. J Pediatr 2015;91:410–2. 10.1016/j.jped.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 6.Das P, Horton R. Antibiotics: achieving the balance between access and excess. Lancet 2016;387:102–4. 10.1016/S0140-6736(15)00729-1 [DOI] [PubMed] [Google Scholar]
- 7.Holmes AH, Moore LSP, Sundsfjord A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016;387:176–87. 10.1016/S0140-6736(15)00473-0 [DOI] [PubMed] [Google Scholar]
- 8.Karam G, Chastre J, Wilcox MH, et al. Antibiotic strategies in the era of multidrug resistance. Crit Care 2016;20:136. 10.1186/s13054-016-1320-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz J, Castro I, Calabuig E, et al. Non-Antibiotic treatment for infectious diseases. Rev Esp Quimioter 2017;30 Suppl 1:66–71. [PubMed] [Google Scholar]
- 10.Williams-nguyen J, Sallach JB, Bartelt-hunt S. State of the science. Journal of Environmental Quality 2016;45:394–406. [DOI] [PubMed] [Google Scholar]
- 11.Khabbaz R, Cars O, Kumar S. Implementation of the global action plan on antimicrobial resistance. WHO GAP AMR Newsletter N° 2017;32:1–4. [Google Scholar]
- 12.Manyi-Loh C, Mamphweli S, Meyer E, et al. Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications. Molecules 2018;23:795. 10.3390/molecules23040795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman ND, Temkin E, Carmeli Y. The negative impact of antibiotic resistance. Clin Microbiol Infect 2016;22:416–22. 10.1016/j.cmi.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 14.Kajeguka DC, Nambunga PP, Kabissi F, et al. Antimicrobial resistance patterns of phenotype extended spectrum beta-lactamase producing bacterial isolates in a referral hospital in northern Tanzania. Tanzan J Health Res 2015;17:1–8. 10.4314/thrb.v17i3.2 [DOI] [Google Scholar]
- 15.Kumburu HH, Sonda T, Leekitcharoenphon P, et al. Hospital Epidemiology of Methicillin-Resistant Staphylococcus aureus in a Tertiary Care Hospital in Moshi, Tanzania, as Determined by Whole Genome Sequencing. Biomed Res Int 2018;2018:1–12. 10.1155/2018/2087693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christopher A, Mshana SE, Kidenya BR, et al. Bacteremia and resistant gram-negative pathogens among under-fives in Tanzania. Ital J Pediatr 2013;39:27. 10.1186/1824-7288-39-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed M, Mirambo MM, Mushi MF, et al. Bacteremia caused by multidrug-resistant bacteria among hospitalized malnourished children in Mwanza, Tanzania: a cross sectional study. BMC Res Notes 2017;10:62. 10.1186/s13104-017-2389-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tibrewal R. a review on combating antibiotic resistance. International Journal of Medical and Biomedical Studies 2018;1. [Google Scholar]
- 19.Abu Sin M, Nahrgang S, Ziegelmann A, et al. [Global and national strategies against antibiotic resistance]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2018;61:507–14. 10.1007/s00103-018-2722-2 [DOI] [PubMed] [Google Scholar]
- 20.Prentiss T, Weisberg K, Zervos J. Building capacity in infection prevention and antimicrobial stewardship in low- and middle-income countries: the role of partnerships Inter-countries. Curr Treat Options Infect Dis 2018;10:7–16. 10.1007/s40506-018-0140-5 [DOI] [Google Scholar]
- 21.Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis 2013;13:1057–98. 10.1016/S1473-3099(13)70318-9 [DOI] [PubMed] [Google Scholar]
- 22.Smith I, Lescure X, Singh S. The implementation of antimicrobial stewardship in low, middle and high income countries. International Journal of Infectious Diseases 2018;73:140. [Google Scholar]
- 23.World Health Organization . Antimicrobial stewardship interventions: a practical guide. Copenhagen: WHO Regional Office for Europe, 2021. [Google Scholar]
- 24.Cox JA, Vlieghe E, Mendelson M, et al. Antibiotic stewardship in low- and middle-income countries: the same but different? Clinical Microbiology and Infection 2017;23:812–8. 10.1016/j.cmi.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 25.Sangeda RZ, Kibona J, Munishi C, et al. Assessment of implementation of antimicrobial resistance surveillance and antimicrobial stewardship programs in Tanzanian health facilities a year after Launch of the National action plan. Front Public Health 2020;8:454. 10.3389/fpubh.2020.00454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okoro RN, Nmeka C, Erah PO. Antibiotics prescription pattern and determinants of utilization in the National health insurance scheme at a tertiary hospital in Nigeria. Afr Health Sci 2019;19:2356–64. 10.4314/ahs.v19i3.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khalfan MA, Sasi PG, Mugusi SF. The prevalence and pattern of antibiotic prescription among insured patients in Dar ES Salaam Tanzania. Pan Afr Med J 2021;40:140. 10.11604/pamj.2021.40.140.29584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Embrey M, Mbwasi R, Shekalaghe E. National Health Insurance Fund’s relationship to retail drug outlets: a Tanzania case study. Journal of Pharmaceutical Policy and Practice 2021;14:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irunde H, Minzi O, Moshiro C. Assessment of rational medicines prescribing in healthcare facilities in four regions of Tanzania. JPPCM 2017;3:225–31. 10.5530/jppcm.2017.4.64 [DOI] [Google Scholar]
- 30.Kirkwood B, Sterne J. Calculation of required sample size. In: Essentials of medical statistics, 2003: 413–24. [Google Scholar]
- 31.OpenEpi - Toolkit Shell for Developing New Applications.. Available: http://www.openepi.com/Random/Random.htm [Accessed 16 Nov 2021].
- 32.Kilipamwambu A, Bwire GM, Myemba DT, et al. WHO/INRUD core prescribing indicators and antibiotic utilization patterns among primary health care facilities in Ilala district, Tanzania. JAC Antimicrob Resist 2021;3:dlab049. 10.1093/jacamr/dlab049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahiabu M-A, Tersbøl BP, Biritwum R, et al. A retrospective audit of antibiotic prescriptions in primary health-care facilities in eastern region, Ghana. Health Policy Plan 2016;31:250–8. 10.1093/heapol/czv048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shamsuddin S, Akkawi ME, Zaidi STR, et al. Antimicrobial drug use in primary healthcare clinics: a retrospective evaluation. Int J Infect Dis 2016;52:16–22. 10.1016/j.ijid.2016.09.013 [DOI] [PubMed] [Google Scholar]
- 35.Ahmad A, Khan MU, Malik S, et al. Prescription patterns and appropriateness of antibiotics in the management of cough/cold and diarrhea in a rural tertiary care teaching hospital. J Pharm Bioallied Sci 2016;8:335. 10.4103/0975-7406.199340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogawski ET, Platts-Mills JA, Seidman JC, et al. Early antibiotic exposure in low-resource settings is associated with increased weight in the first two years of life. J Pediatr Gastroenterol Nutr 2017;65:350–6. 10.1097/MPG.0000000000001640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato D, Goto T, Uda K, et al. Impact of national guidelines for antimicrobial stewardship to reduce antibiotic use in upper respiratory tract infection and gastroenteritis. Infect Control Hosp Epidemiol 2021;42:280–6. 10.1017/ice.2020.427 [DOI] [PubMed] [Google Scholar]
- 38.Neuman MI, Hall M, Hersh AL, et al. Influence of hospital guidelines on management of children hospitalized with pneumonia. Pediatrics 2012;130:e823–30. 10.1542/peds.2012-1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenkins TC, Irwin A, Coombs L, et al. Effects of clinical pathways for common outpatient infections on antibiotic prescribing. Am J Med 2013;126:327–35. 10.1016/j.amjmed.2012.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foolad F, Nagel JL, Eschenauer G, et al. Disease-based antimicrobial stewardship: a review of active and passive approaches to patient management. J Antimicrob Chemother 2017;72:3232–44. 10.1093/jac/dkx266 [DOI] [PubMed] [Google Scholar]
- 41.Integrated Management of Childhood Illness. Geneva: World Health Organization; 2005.. [Google Scholar]
- 42.The Treatment of diarrhea: a manual for physicians and other senior health workers. 4th Rev. Geneva: World Health Organization; 2005. [Google Scholar]
- 43.Luisa M, Amore CD, Ceradini J. Prevalence of antibiotic use in a tertiary care hospital in Italy, 2008 – 2016. Italian Journal of Pediatrics 2019;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Novan Y. I. P, Primadi A, Mahfudz M, Suharjono . Comparison of antibiotic prescriptions in adults and children with upper respiratory tract infections in Bangka Tengah primary health care centers. J Basic Clin Physiol Pharmacol 2020;30:1–4. 10.1515/jbcpp-2019-0248 [DOI] [PubMed] [Google Scholar]
- 45.Seni J, Mapunjo SG, Wittenauer R, et al. Antimicrobial use across six referral hospitals in Tanzania: a point prevalence survey. BMJ Open 2020;10:e042819. 10.1136/bmjopen-2020-042819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beckett CL, Harbarth S, Huttner B. Special considerations of antibiotic prescription in the geriatric population. Clin Microbiol Infect 2015;21:3–9. 10.1016/j.cmi.2014.08.018 [DOI] [PubMed] [Google Scholar]
- 47.Don D, Barbieri E, Daverio M. Implementation and impact of pediatric antimicrobial stewardship programs: a systematic scoping review. Antimicrobial Resistance and Infection Control 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Probst V, Islamovic F, Mirza A. Antimicrobial stewardship program in pediatric medicine. Pediatr Investig 2021;5:229–38. 10.1002/ped4.12292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kinoshita N, Komura M, Tsuzuki S, et al. The effect of preauthorization and prospective audit and feedback system on oral antimicrobial prescription for outpatients at a children's hospital in Japan. J Infect Chemother 2020;26:582–7. 10.1016/j.jiac.2020.01.013 [DOI] [PubMed] [Google Scholar]
- 50.Bagga B, Stultz JS, Arnold S, et al. A culture change: impact of a pediatric antimicrobial stewardship program based on guideline implementation and prospective audit with feedback. Antibiotics 2021;10. doi: 10.3390/antibiotics10111307. [Epub ahead of print: 27 10 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Branstetter JW, Barker L, Yarbrough A, et al. Challenges of antibiotic stewardship in the pediatric and neonatal intensive care units. J Pediatr Pharmacol Ther 2021;26:659–68. 10.5863/1551-6776-26.7.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu C, Liu C, Wang D, et al. Intrinsic and external determinants of antibiotic prescribing: a multi-level path analysis of primary care prescriptions in Hubei, China. Antimicrob Resist Infect Control 2019;8:132. 10.1186/s13756-019-0592-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roumie CL, Halasa NB, Edwards KM, et al. Differences in antibiotic prescribing among physicians, residents, and nonphysician clinicians. Am J Med 2005;118:641–8. 10.1016/j.amjmed.2005.02.013 [DOI] [PubMed] [Google Scholar]
- 54.Doron S, Davidson LE. Antimicrobial stewardship. Mayo Clin Proc 2011;86:1113–23. 10.4065/mcp.2011.0358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Regev-Yochay G, Raz M, Dagan R, et al. Reduction in antibiotic use following a cluster randomized controlled multifaceted intervention: the Israeli judicious antibiotic prescription study. Clin Infect Dis 2011;53:33–41. 10.1093/cid/cir272 [DOI] [PubMed] [Google Scholar]
- 56.Weiss K, Blais R, Fortin A, et al. Impact of a multipronged education strategy on antibiotic prescribing in Quebec, Canada. Clin Infect Dis 2011;53:433–9. 10.1093/cid/cir409 [DOI] [PubMed] [Google Scholar]
- 57.Mohlala G, Peltzer K, Phaswana-Mafuya N, et al. Drug prescription habits in public and private health facilities in 2 provinces in South Africa. East Mediterr Health J 2010;16:324–8. 10.26719/2010.16.3.324 [DOI] [PubMed] [Google Scholar]
- 58.Cotta MO, Chen C, Tacey M, et al. What are the similarities and differences in antimicrobial prescribing between Australian public and private hospitals? Intern Med J 2016;46:1182–8. 10.1111/imj.13209 [DOI] [PubMed] [Google Scholar]
- 59.Bediako-Bowan AAA, Owusu E, Labi A-K, et al. Antibiotic use in surgical units of selected hospitals in Ghana: a multi-centre point prevalence survey. BMC Public Health 2019;19:1–10. 10.1186/s12889-019-7162-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-062147supp001.pdf (364.9KB, pdf)
bmjopen-2022-062147supp002.pdf (84.5KB, pdf)
bmjopen-2022-062147supp003.pdf (70.1KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplemental information.