Abstract
Glucosyltransferase (GTF) enzymes of mutans streptococci are considered virulence factors due to their ability to synthesize adhesive glucans, which facilitate cell-to-cell adherence and accumulation. In this study we report the cloning, expression, and characterization of the catalytic (CAT) and glucan-binding (GLU) domains of S. mutans GTF-I encoded by gtfB. The CAT and GLU polypeptides represent amino acid residues 253 to 628 and 1183 to 1473, respectively, of S. mutans GTF-I. Antibodies to recombinant CAT and GLU were generated in rabbits and purified by affinity chromatography. Purified anti-CAT antibodies significantly inhibited water-insoluble glucan synthesis by S. mutans and S. sobrinus GTFs (P < 0.0001 and P < 0.05, respectively). The purified anti-GLU antibodies significantly inhibited both water-insoluble and water-soluble glucan synthesis by S. mutans GTFs (P < 0.0001 and P < 0.05, respectively). These results demonstrate that anti-CAT and anti-GLU antibodies are capable of inhibiting a variety of GTF activities. Since antibodies to S. mutans in saliva are implicated in protection against disease, we next assessed the ability of CAT and GLU polypeptides to induce mucosal antibody responses in mice. Intranasal (i.n.) immunization of mice with CAT showed significantly (P < 0.005) elevated levels of specific immunoglobulin G (IgG) antibody activity in serum and specific IgA antibody activity in serum, saliva, vaginal washes, and fecal samples. GLU immunized animals showed significantly (P < 0.005) elevated levels of specific IgA antibody activity in serum and vaginal secretions. Taken together, these results demonstrate that the recombinant CAT and GLU polypeptides are effective in inducing both mucosal and systemic immune responses. The ability of these polypeptides to induce a mucosal IgA immune response in mice after i.n. immunization supports their use as subunit vaccine candidates in the development of an anticaries vaccine.
Glucosyltransferase (GTF) enzymes of Streptococcus mutans are important for the cariogenicity of this organism due to their synthesis of water-soluble and water-insoluble glucans from sucrose (13, 15). Three different genes encoding distinct GTFs have been characterized and named gtfB, gtfC, and gtfD (1, 10, 22, 31). The gtfB gene product, GTF-I, synthesizes a water-insoluble glucan polymer, whereas the gtfD gene product, GTF-S, synthesizes a water-soluble glucan polymer. The gtfC gene encodes an enzyme, GTF-SI, which is able to synthesize both water-soluble and water-insoluble glucans. These glucans play an important role in dental plaque formation of S. mutans by facilitating the accumulation of bacteria on the tooth surfaces. The special in vivo significance of insoluble glucan synthesis in caries formation on smooth tooth surfaces has been confirmed in two separate rat models (20, 32). Specifically, S. mutans mutants defective in insoluble glucan synthesis display reduced cariogenicity.
The GTFs have been shown to contain two distinct domains, i.e., the N-terminal catalytic site which binds and hydrolyzes sucrose (18) and the C-terminal repetitive domain involved in binding of glucans and presumably the chain extension of growing glucan polymers (11, 19). Based on sequence similarities between GTFs and a superfamily of related amylolytic enzymes with a (β/α)8-barrel domain, it has been suggested that the catalytic domain in GTFs displays the (β/α)8-barrel structure properties (5, 16). Even though the catalytic Asp-451 residue involved in the attachment of sucrose to the GTF enzyme has been identified, in addition to other functionally important amino acids (e.g., Asp-413, Trp-491, and His-561) (12, 18, 30), the contribution of these amino acids to the precise mechanism of enzymatic activity is still unknown.
Due to the importance of GTFs in the cariogenicity of S. mutans, these proteins are of interest as immunogens in vaccine development against S. mutans-induced dental caries. Recent studies involving immunizations with synthetic peptides consisting of a lysine backbone and peptides from the catalytic or glucan-binding region (representing amino acids 448 to 457 and 1303 to 1324 in Streptococcus downei GTF-I, respectively) have shown a reduction in the level of smooth surface and sulcal caries of immunized rats after infection with Streptococcus sobrinus (28). In the same study, a reduction was also seen in the level of sulcal dental caries of immunized rats after infection with S. mutans. Other peptides representing overlapping areas of the catalytic domain have been synthesized as eight-branched constructs on a lysine core (23). Rats immunized with these constructs showed significant reductions in dental caries after infection with S. mutans compared to sham-immunized controls.
Here we describe the construction of two recombinant polypeptides derived from segments of the S. mutans GTF-I catalytic (CAT) or glucan-binding (GLU) regions representing amino acid residues 253 to 628 and 1183 to 1473, respectively. The CAT and GLU polypeptides both included the sequences previously implicated in inducing caries immunity in rats, as well as all other functionally important amino acids (12, 18, 23, 28, 30). The immunogenic properties of the CAT and GLU polypeptides were determined after immunization of rabbits and mice. The ability of the rabbit antibodies to CAT and GLU to inhibit water-insoluble and water-soluble glucan synthesis by GTFs from S. mutans and S. sobrinus was evaluated in an in vitro glucan synthesis system. Furthermore, we assessed the ability of CAT and GLU to induce mucosal immune responses in mice immunized via the intranasal (i.n.) route.
MATERIALS AND METHODS
Genetic construction.
DNA fragments encoding the catalytic and glucan-binding domains in gtfB from S. mutans were PCR amplified from plasmid pYNB13 (30) (provided by H. K. Kuramitsu, Buffalo, N.Y.). PCR primers were chosen according to the published nucleotide sequence (22), and appropriate restriction sites were introduced for subcloning (NcoI at the 5′ end of the upper primer and XhoI at the 5′ end of the lower primer). CAT and GLU fragments, representing bp 759 to 1887 and bp 3551 to 4422, respectively, were cloned into plasmid pGEM-T (Promega, Madison, Wis.) and transformed into Escherichia coli JM109. Transformed colonies were screened by blue-white selection on Luria-Bertani agar plates (1% tryptone, 0.5% yeast extract, 1% NaCl) containing isopropylthio-β-d-galactoside, 5-bromo-4-chloro-3-indolyl-β-d-galactoside, and 50 μg of carbenicillin per ml (selection for pGEM-T). Plasmid preparations were made from selected white colonies by using the Wizard Minipreps DNA Purification Systems (Promega), and the presence of an insert was confirmed by XhoI and NcoI digestions followed by gel electrophoresis. The 1.1-kb CAT and 0.9-kb GLU inserts were separated from vector sequences by restriction enzyme digestion with NcoI and XhoI, followed by gel electrophoresis and purification with the QIAEX gel extraction kit (Qiagen, Chatsworth, Calif.). The purified fragments were subcloned into the expression vector pET20b(+) (Novagen, Madison, Wis.), and the plasmids, named pET20b(+)-CAT and pET20b(+)-GLU, were electroporated into Salmonella typhimurium BRD509 containing pGP1-2 (7). Transformed colonies were selected on L agar plates (1% tryptone, 0.5% yeast extract, 0.5% NaCl, 0.1% dextrose, 1.8% agar) containing 50 μg of carbenicillin per ml [selection for pET20b(+)-CAT or pET20b(+)-GLU] and 50 μg of kanamycin per ml (selection for pGP1-2). The transformants were examined for the presence of plasmids with the sizes 7.1 kb (pGP1-2) and either 4.8 kb [pET20b(+)-CAT] or 4.6 kb [pET20b(+)-GLU].
Recombinant protein expression and purification.
S. typhimurium BRD509(pGP1-2) containing either pET20b(+)-CAT or pET20b(+)-GLU was grown to mid log phase at 30°C before the cells were induced by a temperature shift from 30 to 42°C for 30 min (27). The cells were grown an additional 2 h at 30°C and then harvested by centrifugation. The pelleted cells were solubilized in TTE buffer (0.05 M Tris-HCl, pH 8.0; 0.1% Triton X-100; 2 mM EDTA) and sonicated on ice, and insoluble proteins were recovered by centrifugation. The pellet was washed twice in wash buffer (0.05 M Tris, pH 8.0; 0.1 M NaCl; 0.5% Triton X-100; 0.01 M EDTA) with sonication between washes. The CAT or GLU polypeptides present in inclusion bodies were solubilized in a urea buffer (8 M urea, 50 mM Tris-HCl [pH 7.9], 0.5 M NaCl, 1 mM EDTA, 30 mM 2-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride).
The recombinant polypeptides used for rabbit immunizations were refolded by dialyzing them against 50 mM Tris-HCl (pH 7.9)–0.5 M NaCl–10% glycerol (8), and the purity was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The polypeptides used for mouse immunizations were solubilized as described above but refolded by dialyzing them against water. These preparations were then extracted with 1 volume of a 9:1 phenol-water solution containing 0.2% triethylamine and 0.5% sodium deoxycholate to eliminate any possible contaminating lipopolysaccharide (LPS) (17). After separation of the two phases by centrifugation (14,000 × g), the proteins in the phenol phase were reextracted with 0.2% triethylamine and 0.5% sodium deoxycholate. When phenol was removed by dialyzing into 10 mM Tris-HCl (pH 7.9) and 0.15 M NaCl, the polypeptides formed aggregates. The LPS content was determined by a Limulus assay as recommended by the manufacturer (Biowhittaker, Walkersville, Md.).
Rabbit immunizations.
Antibodies against CAT or GLU recombinant polypeptides were raised in rabbits by immunization via the subcutaneous route on day 0 with 100 μg of protein in complete Freund adjuvant, and on days 14 and 28 with 100 μg of protein in incomplete Freund adjuvant. Blood was collected by cardiac puncture on day 42, and serum was obtained by centrifugation. All animal studies were performed according to National Institutes of Health guidelines and protocols approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee. The antibodies were purified from the serum by affinity chromatography on cyanogen bromide-activated Sepharose columns with immobilized CAT or GLU polypeptides (4).
Western blot analysis.
The ability of the affinity-purified antibodies to react with native GTFs was tested by Western blot analysis. A crude cell lysate from S. mutans GS-5 was made as previously described (29). Briefly, 5 ml of chilled ethanol was added to a 5-ml poststationary-phase culture and left for 30 min. The precipitate was recovered by centrifugation (10,000 × g) and solubilized in 250 μl of 8 M urea in 10 mM potassium phosphate buffer (pH 7.2). The GTFs were solubilized by shaking for 1 h at 25°C, and the insoluble proteins were recovered by centrifugation. The solubilizing procedure was repeated three times, and the supernatants were pooled. Then 10 μl of the crude GTF lysate was used for Western blot analysis along with 0.24 μg of GLU and 0.18 μg of CAT.
GTFs.
GTFs from S. mutans SJ32 were obtained as previously described for S. mutans JF (26). Briefly, after bacterial growth in glucose-containing defined medium, enzymes were isolated by affinity chromatography on Sephadex G-100 (Pharmacia, Piscataway, N.J.) by using 3 M guanidine HCl as the eluting solvent. This GTF-rich pool was then subjected to fast protein liquid chromatography (FPLC) on Superose 6 (Pharmacia) with 6 M guanidine for elution. The gel filtration step removes non-GTF and other glucan-binding proteins from GTF preparations of S. mutans, as demonstrated by SDS-PAGE, after which only components with enzyme activity were observed. The S. mutans GTF preparation taken to this level of enrichment synthesized 51 to 81% water-soluble glucan by filter assay and likely contained a mixture analogous to the gene products of S. mutans GS5 gtfB, gtfC, and gtfD (1, 10, 22, 31). This preparation was used in the assays for the inhibition of GTF activity. GTFs from S. sobrinus 6715 were obtained in a similar manner (affinity chromatography on Sephadex G-150, followed by FPLC gel filtration on Superose 6). The FPLC GTF preparation taken to this level of enrichment contained a mixture of GTF isozymes, including GTF-I and GTF-S, but was essentially free of other proteins. Approximately 90% of the glucan synthesized by this GTF preparation was found to be water insoluble under the conditions of the assay described below. This preparation was also used for GTF-inhibition assays.
Antibody inhibition of glucan synthesis.
Rabbit antibody was evaluated for its ability to inhibit glucan synthesis catalyzed by S. mutans and S. sobrinus GTFs, as prepared above. In the assay for S. mutans GTF inhibition, glass test tubes were precoated with 70 μl of 0.05% bovine serum albumin in sodium phosphate-buffered saline and 0.2% sodium azide (PBSA) (pH 6.5). Next, 10-μl volumes of rabbit IgG (760 μg/ml) in PBSA (1:5 dilution) were added to the tube. Normal rabbit IgG (Sigma, St. Louis, Mo.) was used as the negative control. Then, 20 μl of S. mutans GTF, containing 5 μg of GTF per ml of BSA-PBSA was added. This 100-μl mixture was preincubated for 2 h at 37°C. Then 0.85 mg of sucrose, 19 nCi of [14C-glucose]-sucrose (approximately 40,000 cpm) was added in 0.1 ml of PBSA in the absence of primer dextran. Incubation proceeded for 3 h at 37°C. Water-insoluble glucan was collected on Whatman GF/F glass fiber filters and washed with 1 ml of PBSA, and the radioactivity determined as previously described (26). Synthesis of water-soluble glucan was collected from the filtrate by precipitation with 3.2 volumes (4 ml) of 95% ethanol with 4 mg of dextran T-10 (Pharmacia) as the carrier, followed by centrifugation as previously described (25). The assay for inhibition of S. sobrinus GTF activity followed essentially the same protocol except that 0.07 μg of GTF was added to each tube and the final incubation was for 2 h. The amount of water-soluble glucan synthesized under these conditions was less than 3% of the total glucan synthesized. The percent inhibition of S. mutans and S. sobrinus GTFs was calculated as follows: [(glucan production in the presence of rabbit IgG lacking specific antibody activity − glucan production in the presence of IgG anti-CAT or anti-GLU)/glucan production in the presence of rabbit IgG lacking specific antibody activity] × 100.
Mouse immunizations.
Groups of BALB/c mice (five per group), 10 weeks of age, were used for i.n. immunization with 50 μg of purified recombinant CAT or GLU. Each dose was applied slowly into the nares, and each application did not exceed a volume of 20 μl. The vaccine was delivered equally to both nares. The immunizations were given on days 0, 10, and 20, and saliva, blood, fecal, and vaginal samples were obtained prior to immunization and on day 27. Saliva samples were collected after stimulation of the salivary flow by intraperitoneal injection of 5 μg of carbachol (Sigma) (9). Blood samples were obtained with heparinized capillary pipettes from the retroorbital plexus, and serum was collected after centrifugation and stored at −70°C until assayed for antibody activity by enzyme-linked immunosorbent assay (ELISA). Fecal samples were derived by vortexing three fecal pellets in 600 μl of borate-buffered saline containing 0.02% azide, 1 mM phenylmethylsulfonyl fluoride, 1 mM EDTA, and 2% fetal calf serum. Insoluble material was removed by centrifugation. Vaginal samples were obtained by washing the vagina twice with 50 μl of phosphate-buffered saline. All secretion samples were stored at −70°C until analyzed by ELISA for the presence of specific IgA antibodies and for the total concentration of IgA.
ELISA.
The levels of specific antibodies in samples were determined on Maxisorp microtiter plates (Nunc, Roskilde, Denmark) coated with CAT or GLU (3 μg/ml). The total levels of IgA in secretions were detected by coating them with an optimal concentration of antibodies to mouse IgA. Peroxidase-labeled antibodies to mouse IgA or IgG were used as detection reagents, followed by o-phenylenediamine substrate with H2O2. The antibody concentrations in individual samples were determined as previously described (9). The detecting and coating antibodies used in this study were purchased from Southern Biotechnology Associates, Inc., Birmingham, Ala. The levels of antibody in the samples were logarithmically transformed, and statistical analyses (Student’s t test) of differences between groups were performed by using the InStat program (GraphPad Software, San Diego, Calif.).
RESULTS
Recombinant protein expression.
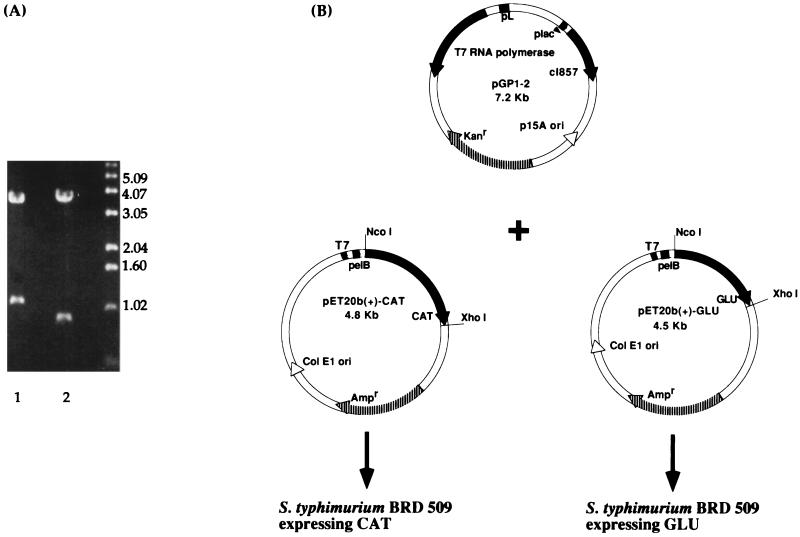
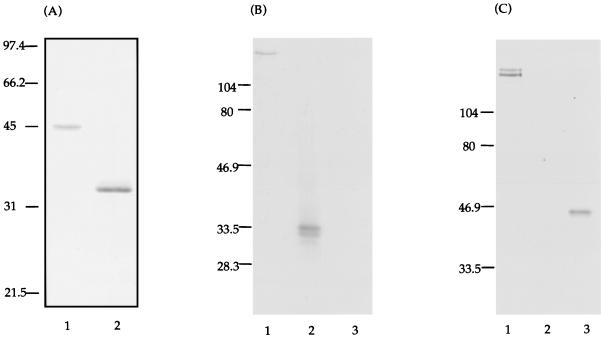
Based on previous predictions of functionally important domains in GTFs (18, 19, 23, 30), two regions of the S. mutans gtfB gene were each PCR amplified and cloned into pET20b(+). Restriction enzyme digestion of either pET20b(+)-CAT or pET20b(+)-GLU with XhoI and NcoI revealed fragments of the predicted sizes 1.14 and 0.87 kb, respectively (Fig. 1A). The two constructs were each electroporated into S. typhimurium BRD509 along with pGP1-2 (Fig. 1B), which provides a source of T7 RNA polymerase. The expression of T7 RNA polymerase is under the control of the λPL promoter, which is regulated by a temperature-inducible λ repressor. The expressed polypeptides, i.e., CAT and GLU, were found in inclusion bodies when the cultures were induced at 42°C (Fig. 2A), 37°C, or 30°C (data not shown). The actual sizes of CAT (∼45 kDa) and GLU (∼33.5 kDa) matched the predicted sizes of 42.5 and 33.4 kDa, respectively. The CAT and GLU polypeptides were solubilized as described in Materials and Methods and used for systemic immunizations in rabbits or mucosal immunizations in mice. The LPS contents in the GLU and CAT preparations used for mucosal immunizations were 0.001 and 0.09%, respectively.
FIG. 1.
(A) Restriction enzyme digestion with XhoI and NcoI of the constructs pET20b(+)-CAT (lane 1) and pET20b(+)-GLU (lane 2). Molecular size standards in kilobases are indicated on the right. (B) Maps of the plasmids used for transformation of S. typhimurium BRD509 expressing the recombinant proteins CAT and GLU. The salmonella host was cotransformed with the plasmid pGP1-2, thus providing the temperature-inducible T7 RNA polymerase.
FIG. 2.
(A) Coomassie blue stain of SDS-PAGE of the recombinant CAT (lane 1) and GLU (lane 2) polypeptides. The CAT and GLU polypeptides were purified and resolubilized from inclusion bodies. Molecular weight markers in kilodaltons are indicated on the left. (B) Western blot of a crude extract of S. mutans GTFs (lane 1), purified GLU (lane 2), and purified CAT (lane 3). The blot was probed with biotinylated anti-GLU antibodies and developed with alkaline phosphatase-conjugated streptavidin. (C) Western blot identical to that shown in panel B but probed with biotinylated anti-CAT antibodies and developed with alkaline phosphatase-conjugated streptavidin.
Western blot analysis.
Affinity-purified antibodies, derived by immunizing rabbits with recombinant CAT or GLU polypeptides, were tested for their ability to recognize native S. mutans GTFs, as well as the CAT and GLU polypeptides. Antibodies raised against GLU showed reactivity with a high-molecular-weight protein in the S. mutans cell lysate (∼168 kDa) and the GLU polypeptide used for immunization (Fig. 2B). Antibodies raised against CAT showed reactivity with two distinct proteins (∼155 and 168 kDa) in the native S. mutans GTF preparation, in addition to the CAT polypeptide used for immunization (Fig. 2C). Neither anti-CAT nor anti-GLU antibodies cross-reacted with each other.
Inhibition of GTF activity.
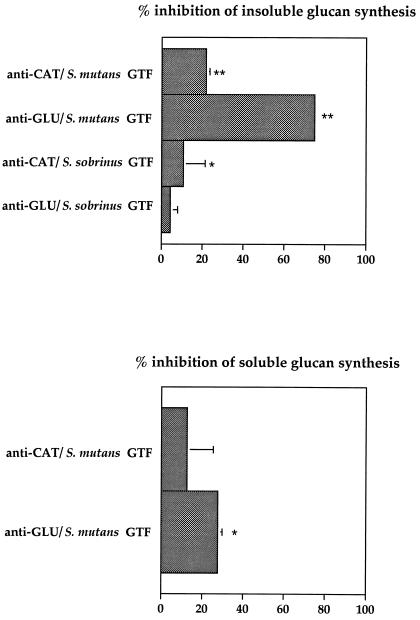
The affinity-purified rabbit antibodies against CAT or GLU polypeptides were tested for their ability to inhibit glucan synthesis by GTFs purified from S. mutans or S. sobrinus (Fig. 3). The anti-CAT and anti-GLU antibodies reduced significantly (P < 0.0001) the insoluble-glucan synthesis by S. mutans GTF by 22 and 75%, respectively. The anti-CAT but not the anti-GLU antibodies significantly inhibited S. sobrinus insoluble-glucan synthesis (P < 0.03). The level of inhibition of soluble-glucan synthesis by S. mutans GTFs was significant (P < 0.04) for anti-GLU antibodies. The synthesis of water-soluble glucan by our S. sobrinus GTF preparation is quite low, and neither CAT- nor GLU-specific antibodies showed any significant inhibition of this particular glucan production (data not shown).
FIG. 3.
Inhibition of insoluble- and soluble-glucan production by rabbit antibodies raised against CAT or GLU. The differences in glucan production with specific antibody compared to that seen with rabbit IgG lacking specific antibody activity were considered statistically significant at a level of P < 0.05 (∗) or P < 0.0001 (∗∗). Bars show the mean ± the standard deviation (SD) (n = 4).
Immune responses in mice after i.n. immunization with GLU or CAT.
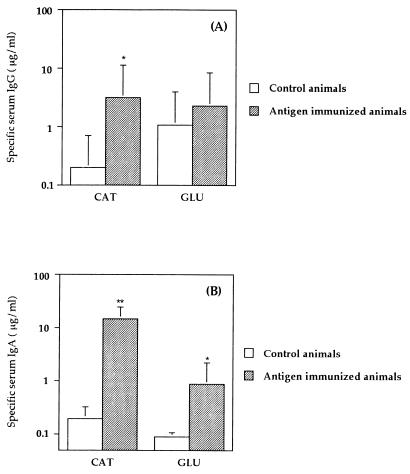
The serum antibody responses in mice immunized by the i.n. route with the CAT polypeptide showed enhanced levels of specific IgG in serum (P = 0.0023) compared to nonimmunized controls (Fig. 4A). The GLU-immunized animals did not show significantly elevated levels of specific antibody due to the level of cross-reacting antibodies in the serum of nonimmunized control animals. Mice immunized with CAT or GLU recombinant polypeptides showed significantly enhanced levels of specific serum IgA antibody activity (P < 0.0001 and P = 0.0006, respectively) compared to nonimmunized controls (Fig. 4B).
FIG. 4.
Serum IgG (A) or IgA (B) responses to CAT or GLU 1 week after the third i.n. immunization of BALB/c mice. Results are shown as geometric means ×/÷ the SD for five mice immunized with CAT or GLU or for nonimmunized controls. The antibody activities were significantly different from nonimmunized mice at a level of P < 0.005 (∗) or P < 0.0001 (∗∗).
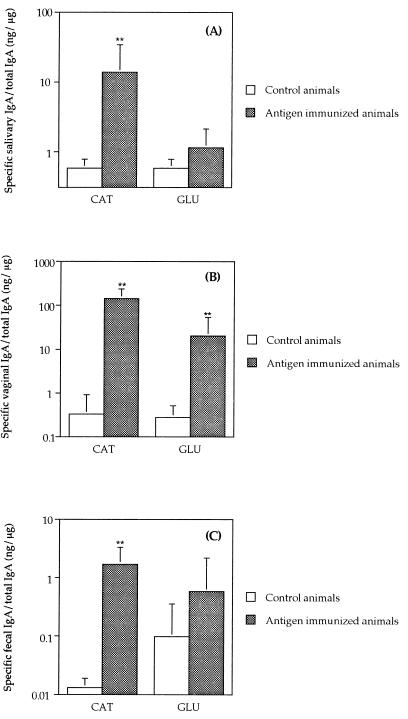
Antibody responses in mucosal secretions in animals immunized by the i.n. route with CAT recombinant polypeptide showed significantly enhanced levels of specific salivary IgA (P < 0.0001) (Fig. 5A). Both CAT and GLU induced vaginal IgA antibody responses (P < 0.0001) compared to the nonimmunized control group (Fig. 5B). Interestingly, the level of specific antibodies in animals immunized with CAT constitutes 15% of the total level of IgA found in the vaginal washes. A high specific intestinal IgA response was induced in mice immunized with CAT (P < 0.0001) (Fig. 5C). The GLU-immunized group showed a specific IgA response in the intestine, although it was not statistically different from that of the nonimmunized controls.
FIG. 5.
Salivary IgA (A), vaginal IgA (B), or intestinal IgA (C) responses to CAT or GLU 1 week after the third i.n. immunization. Results are shown as the geometric means of the specific IgA response/total IgA (×/÷ the SD) for five mice immunized with CAT or GLU or for nonimmunized controls. The antibody activities were significantly different from nonimmunized mice at a level of P < 0.0001 (∗∗).
DISCUSSION
The characterization of the genes encoding GTFs of mutans streptococci is providing valuable information on the functional domains of these enzymes, which contribute to the virulence of this organism. In the present study, we have cloned the two proposed functional regions of S. mutans GTF-I, CAT and GLU. Antibodies were generated in rabbits against CAT and GLU and shown to significantly inhibit glucan synthesis by GTFs from both S. mutans and S. sobrinus. Other investigators have constructed fusion proteins consisting of the saliva-binding alanine-rich repeat region of antigen I/II (also known as PAc), a cell surface adhesin of S. mutans, and the glucan-binding domain of GTF-I or the catalytic domain of GTF-I. Antibodies generated to the fusion protein containing the glucan-binding domain inhibit water-insoluble glucan synthesis by S. mutans GTFs, whereas water-soluble glucan synthesis by S. mutans GTFs is only weakly inhibited (32). We have shown that both soluble- and insoluble-glucan synthesis by S. mutans GTFs was significantly inhibited with antibodies raised against GLU. Furthermore, we demonstrated an inhibitory effect of antibodies raised against catalytic on insoluble-glucan synthesis by both S. mutans and S. sobrinus GTFs. The inhibitory effect of antibodies against the CAT domain was not seen with antibodies against the previously described fusion protein containing the catalytic site (32). A possible explanation for these findings could reflect the difference in the polypeptides used for generating the antibodies. Our CAT has 12 additional amino acids of the GTF-I N-terminal end and an additional 100 amino acids of the C-terminal end compared to the fusion protein containing the catalytic site.
Antibodies against GLU inhibited water-insoluble-glucan synthesis by S. mutans GTFs better than antibodies against CAT. This could be due to the repetitive nature of the GLU domain, which increases the probability of antibody binding, or to the poor accessibility of antibodies to the CAT domain. On the other hand, antibodies to CAT inhibit insoluble-glucan synthesis of GTFs from S. sobrinus better than antibodies to GLU. Since the antibodies were generated to polypeptides derived from gtfB from S. mutans GS-5, the stronger inhibition of anti-CAT antibodies of S. sobrinus GTF activity could be explained by the higher degree of homology between these two species in the catalytic region than in the glucan-binding region (21). The same interspecies inhibition pattern was seen with an antibody against a 21-mer peptide representing the catalytic domain of GTFs but not with an antibody against a peptide representing the glucan-binding domain of GTFs (26). The higher degree of homology in the catalytic region than the glucan-binding domain between different GTFs was also reflected by our Western blot analysis. A single band from the crude GTF extract, presumably GTF-I based on the molecular weight, reacted with anti-GLU antibodies, whereas the anti-CAT antibodies reacted with two distinct bands, i.e., GTF-I and GTF-SI, based on the molecular weights (10, 22, 31). Neither anti-CAT nor anti-GLU antiserum could recognize GTF-S, which supports previous findings that the highest similarity exists between the GTF-I and GTF-SI proteins (10). The anti-GLU antisera did not recognize the S. mutans cell-surface glucan-binding protein despite the amino acid sequence homology between the glucan-binding domain of GTF-I and glucan-binding protein (2).
Several studies have shown that antibodies raised against peptides representing the functional domains of GTFs are capable of inhibiting glucan synthesis (3, 14, 23, 25, 26). To our knowledge, this is the first time that the domains representing all amino acids predicted to be important to sucrase or glucan-binding activity have been cloned and expressed as recombinant polypeptides and the first time that antibodies raised against these larger recombinant polypeptides were shown to inhibit GTF activity. The inhibition of S. mutans GTF activity by polyclonal rabbit antibodies raised against the recombinant GLU or CAT polypeptides was stronger than the inhibition demonstrated by rat sera from animals immunized with the GLU or CAT peptides consisting of four 22-mer or 21-mer peptides, respectively, attached to a lysine core (28).
The ultimate goal for studying virulence factors from S. mutans would be to understand the pathogenesis of mutans streptococci and to develop a mucosal vaccine which inhibits these factors and reduces the disease process. It has previously been shown that salivary IgA from humans who had been naturally immunized with GTF is capable of inhibiting GTF activity (24). We therefore tested the recombinantly expressed CAT and GLU polypeptides for their ability to induce a mucosal antibody response in mice when administered i.n. An advantage of immunizing with large compared to small polypeptides in future human immunization studies would be that genetic factors might restrict the ability of different individuals to respond to the small peptides. The CAT polypeptide induced a significant specific IgG response in serum and significant specific IgA responses in serum and saliva when given by the i.n. route. CAT was also able to induce a generalized mucosal IgA response, as evidenced by the induction of IgA antibodies in vaginal secretions and fecal samples. The GLU polypeptide induced significant specific IgA responses in serum and vaginal samples. The moderate immune response to GLU may be caused by the repeating nature of this domain. It has previously been shown by Gravekamp et al. (6) that an inverse relationship exists between the number of repeats and the immunogenicity of the alpha C protein from Streptococcus agalactiae, and this may be a mechanism whereby repeat elements contribute to the evasion of host immunity. The GLU polypeptide has poor immunogenicity compared to the CAT polypeptide, but we have demonstrated a very strong GTF inhibitory effect by anti-GLU antibodies when present in low amounts (0.038 mg/ml) in the in vitro glucan synthesis assay. This suggests that lower anti-GLU salivary IgA antibody levels could be sufficient in protection against dental caries.
The previously described CAT peptide consisting of four 21-mer peptides on a lysine core matrix (28) did not induce significant elevated IgA antibody activity in rats injected with peptide in the salivary gland vicinity (23), but it did induce protective immune responses to either S. mutans or S. sobrinus infection in an experimental rat model for dental caries (28). It is unknown what antibody level is sufficient in the induction of protective immunity against dental caries, and only future protection studies will answer this question. The findings that antibodies to both CAT and GLU polypeptides inhibit GTF activity and that these recombinant polypeptides were capable of inducing significant specific antibody responses when delivered as a mucosal vaccine in the absence of adjuvant is very promising for their future use in development of a human vaccine against dental caries.
ACKNOWLEDGMENTS
We thank Cecily Harmon for excellent technical assistance.
This work was supported by USPSH grants DE09081, DE06746, DE04733, and DE06153.
This work was done by Christina Jespersgaard in partial fulfillment of the requirements for a Ph.D. from The University of Aarhus.
REFERENCES
- 1.Aoki H, Shiroza T, Hayakawa M, Sato S, Kuramitsu H K. Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect Immun. 1986;53:587–594. doi: 10.1128/iai.53.3.587-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banas J A, Russell R R B, Feretti J J. Sequence analysis of the gene for the glucan-binding protein of Streptococcus mutans Ingbritt. Infect Immun. 1990;58:667–673. doi: 10.1128/iai.58.3.667-673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chia J-S, Lin R-H, Lin S-W, Chen J-Y, Yang C-S. Inhibition of glucosyltransferase activities of Streptococcus mutans by a monoclonal antibody to a subsequence peptide. Infect Immun. 1993;61:4689–4695. doi: 10.1128/iai.61.11.4689-4695.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Stroeber W. Current protocols in immunology. Vol. 2. New York, N.Y: Greene Publishing Associates, Inc./John Wiley & Sons, Inc.; 1991. [Google Scholar]
- 5.Devulapalle K S, Goodman S D, Gao Q, Hemsley A, Mooser G. Knowledge-based model of a glucosyltransferase from the oral bacterial group of mutans streptococci. Protein Sci. 1997;6:2489–2493. doi: 10.1002/pro.5560061201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gravekamp C, Kasper D L, Michel J L, Kling D E, Carey V, Madoff L C. Immunogenicity and protective efficacy of the alpha C protein of group B streptococci are inversely related to the number of repeats. Infect Immun. 1997;65:5216–5221. doi: 10.1128/iai.65.12.5216-5221.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hajishengallis G, Harokopakis E, Hollingshead S K, Russell M W, Michalek S M. Construction and oral immunogenicity of a Salmonella typhimurium strain expressing a streptococcal adhesin linked to the A2/B subunits of cholera toxin. Vaccine. 1996;14:1545–1548. doi: 10.1016/s0264-410x(96)00093-x. [DOI] [PubMed] [Google Scholar]
- 8.Hajishengallis G, Koga T, Russell M W. Affinity and specificity of the interactions between Streptococcus mutans antigen I/II and salivary components. J Dent Res. 1994;73:1493–1502. doi: 10.1177/00220345940730090301. [DOI] [PubMed] [Google Scholar]
- 9.Harokopakis E, Hajishengallis G, Greenway T E, Russell M W, Michalek S M. Mucosal immunogenicity of a recombinant Salmonella typhimurium-cloned heterologous antigen in the absence or presence of coexpressed cholera toxin A2 and B subunits. Infect Immun. 1997;65:1445–1454. doi: 10.1128/iai.65.4.1445-1454.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honda O, Kato C, Kuramitsu H K. Nucleotide sequence of the Streptococcus mutans gtfD gene encoding the glucosyltransferase-S enzyme. J Gen Microbiol. 1990;136:2099–2105. doi: 10.1099/00221287-136-10-2099. [DOI] [PubMed] [Google Scholar]
- 11.Kato C, Kuramitsu H K. Carboxyl-terminal deletion analysis of the Streptococcus mutans glucosyltransferase-I enzyme. FEMS Microbiol Lett. 1990;60:299–302. doi: 10.1016/0378-1097(90)90321-g. [DOI] [PubMed] [Google Scholar]
- 12.Kato C, Nakano Y, Lis M, Kuramitsu H K. Molecular genetic analysis of the catalytic site of Streptococcus mutans glucosyltransferases. Biochem Biophys Res Commun. 1992;189:1184–1188. doi: 10.1016/0006-291x(92)92329-v. [DOI] [PubMed] [Google Scholar]
- 13.Kuramitsu H K. Virulence factors of mutans streptococci: role of molecular genetics. Crit Rev Oral Biol Med. 1993;4:159–176. doi: 10.1177/10454411930040020201. [DOI] [PubMed] [Google Scholar]
- 14.Laloi P, Munro C L, Jones K R, Macrina F L. Immunologic characteristics of a Streptococcus mutans glucosyltransferase B sucrose-binding site peptide-cholera toxin B-subunit chimeric protein. Infect Immun. 1996;64:28–36. doi: 10.1128/iai.64.1.28-36.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loesche W J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacGregor E A, Jespersen H M, Svensson B. A circularly permuted α-amylase-type α/β-barrel structure in glucan-synthesizing glucosyltransferases. FEBS Lett. 1996;378:263–266. doi: 10.1016/0014-5793(95)01428-4. [DOI] [PubMed] [Google Scholar]
- 17.Manthey C L, Vogel S N. Elimination of trace endotoxin protein from rough chemotype LPS. J Endotoxin Res. 1994;1:84–91. [Google Scholar]
- 18.Mooser G, Hefta S A, Paxton R J, Shively J E, Lee T D. Isolation and sequence of an active-site peptide containing a catalytic aspartic acid from two Streptococcus sobrinus alpha-glucosyltransferases. J Biol Chem. 1991;266:8916–8922. [PubMed] [Google Scholar]
- 19.Mooser G, Wong C. Isolation of a glucan-binding domain of glucosyltransferase (1,6-α-glucan synthase) from Streptococcus sobrinus. Infect Immun. 1988;56:880–884. doi: 10.1128/iai.56.4.880-884.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munro C, Michalek S M, Macrina F L. Cariogenicity of Streptococcus mutans V403 glucosyltransferase and fructosyltransferase mutants constructed by allelic exchange. Infect Immun. 1991;59:2316–2323. doi: 10.1128/iai.59.7.2316-2323.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell R R, Shiroza T, Kuramitsu H K, Ferretti J J. Homology of glucosyltransferase gene and protein sequences from Streptococcus sobrinus and Streptococcus mutans. J Dent Res. 1988;67:543–547. doi: 10.1177/00220345880670030401. [DOI] [PubMed] [Google Scholar]
- 22.Shiroza T, Ueda S, Kuramitsu H K. Sequence analysis of the gtfB gene from Streptococcus mutans. J Bacteriol. 1987;169:4263–4270. doi: 10.1128/jb.169.9.4263-4270.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith D J, Shoushtari B, Heschel R L, King W F, Taubman M A. Immunogenicity and protective immunity induced by synthetic peptides associated with a catalytic subdomain of mutans group streptococcal glucosyltransferase. Infect Immun. 1997;65:4424–4430. doi: 10.1128/iai.65.11.4424-4430.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith D J, Taubman M A, Ebersole J L. Salivary IgA antibody to glucosyltransferase in man. Clin Exp Immunol. 1985;61:416–424. [PMC free article] [PubMed] [Google Scholar]
- 25.Smith D J, Taubman M A, Holmberg C F, Eastcott J, King W F, Ali-Salaam P. Antigenicity and immunogenicity of a synthetic peptide derived from a glucan-binding domain of mutans streptococcal glucosyltransferase. Infect Immun. 1993;61:2899–2905. doi: 10.1128/iai.61.7.2899-2905.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith D J, Taubman M A, King W F, Eida S, Powell J R, Eastcott J. Immunological characteristics of a synthetic peptide associated with a catalytic domain of mutans streptococcal glucosyltransferase. Infect Immun. 1994;62:5470–5476. doi: 10.1128/iai.62.12.5470-5476.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabor S, Richardson C C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taubman M A, Holmberg C J, Smith D J. Immunization of rats with synthetic peptide constructs from the glucan-binding or catalytic region of mutans streptococcal glucosyltransferase protects against dental caries. Infect Immun. 1995;63:3088–3093. doi: 10.1128/iai.63.8.3088-3093.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomita Y, Zhu X, Ochiai K, Namiki Y, Okada T, Ikemi T, Fukushima K. Evaluation of three individual glucosyltransferases produced by Streptococcus mutans using monoclonal antibodies. FEMS Microbiol Lett. 1996;145:427–432. doi: 10.1111/j.1574-6968.1996.tb08611.x. [DOI] [PubMed] [Google Scholar]
- 30.Tsumori H, Minami T, Kuramitsu H K. Identification of essential amino acids in the Streptococcus mutans glucosyltransferases. J Bacteriol. 1997;179:3391–3396. doi: 10.1128/jb.179.11.3391-3396.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueda S, Shiroza T, Kuramitsu H K. Sequence analysis of the gtfC gene from Streptococcus mutans GS-5. Gene. 1988;69:101–109. doi: 10.1016/0378-1119(88)90382-4. [DOI] [PubMed] [Google Scholar]
- 32.Yamashita Y, Bowen W H, Burne R A, Kuramitsu H K. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun. 1993;61:3811–3817. doi: 10.1128/iai.61.9.3811-3817.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu H, Nakano Y, Yamashita Y, Ohio T, Koga T. Effects of antibodies against cell surface protein antigen PAc-glucosyltransferase fusion proteins on glucan synthesis and cell adhesion of Streptococcus mutans. Infect Immun. 1997;65:2292–2298. doi: 10.1128/iai.65.6.2292-2298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]