Abstract
Pulmonary arterial hypertension (PAH) is a progressive vasculopathy that is advanced by the time symptoms develop. As symptoms are nonspecific and the condition uncommon, continued progression toward end-stage disease occurs for an average of 2 years between symptom onset and diagnosis. There is need for earlier diagnosis and treatment, as most patients are severely symptomatic when diagnosed and their mortality is high despite therapy. Screening can help; however, it is not straightforward due to the diversity of patient profiles and lack of sufficiently accurate tools. Echocardiography, currently the best available screening tool, lacks both sensitivity and specificity. The low prevalence of PAH renders many screening tools unfit for purpose. However, this may be overcome, in some instances, by using enrichment tools to preselect screening populations. The majority of data are available for systemic sclerosis. A recent study has demonstrated how lung function can be used to enrich PAH prevalence in a systemic sclerosis population. A screening bundle then selects patients for right heart catheterisation with improved rates of sensitivity compared to current guidelines.
Introduction
Screening is defined as the systematic testing of asymptomatic individuals to search for preclinical disease and mildly symptomatic patients to prevent progression and/or development of the disease [1]. Whether screening is worthwhile depends on many factors. Screening is only beneficial when early detection leads to therapy that modifies the natural history of the condition. If a disease is curable at any stage or there is no available therapy then screening is not recommended. The challenges involved in screening are exemplified by the recent demonstration that mammography-based screening for breast cancer may be futile [2].
Furthermore, it is critical that screening involves easily accessible and real world tools, and that the methodology has been tested in a cross-sectional study to determine the performance of the screening tool(s) compared with gold standard investigation [1].
If low-cost, noninvasive, perfect (sensitivity 100% and specificity 100%) tests are available and we have disease modifying therapies, then screening is worthwhile independent of disease prevalence. When available screening tools are less perfect, and invasive confirmatory tests are required, then Bayesian probability comes into play and screening becomes ineffective where the pre-test probability is low. If one screens a population with a disease prevalence of 0.5%, even a screening test with 99% sensitivity and 99% specificity produces twice as many false positives as true positives due to the large number of disease-free individuals in the population.
Thus when constructing a screening programme many factors come into play. 1) Is early diagnosis worthwhile? 2) Do we have validated cost-effective and acceptable screening tools? 3) Where an invasive gold standard diagnosis is required, is the positive predictive value of screening test results sufficiently high that one can reasonably recommend intervention?
Pulmonary hypertension meets the classical requirements of a disease where screening should be beneficial. The long-term prognosis of patients diagnosed on a routine clinical basis with pulmonary arterial hypertension (PAH) remains poor, even with optimal use of all currently available therapies [3]. Despite extensive education programmes most patients are severely symptomatic and have advanced haemodynamic impairment at diagnosis. However, we know that currently available therapies delay symptom progression and are effective in mildly symptomatic patients [4].
After a decade of educational effort the delay between symptom onset and diagnosis remains approximately 2 years, and most patients have haemodynamically advanced disease and are in functional class III or IV at the time of diagnosis [5]. PAH patients presenting with clinically advanced disease (functional class III/IV) have a much higher mortality than those identified earlier in the course of their disease [3, 6, 7].
However, screening is not straightforward in PAH due to the diversity of the diseases associated with PAH. The prevalence (pre-test probability) of PAH varies in at-risk populations as screening tests exhibit differing efficacy in each subgroup. Finally, one has to “calibrate” screening tools as increased specificity is linked to reduced sensitivity.
Systemic sclerosis (SSc)-associated PAH is a good example of how screening can work despite a relatively low incidence of PAH and a background population prevalence of ∼5–12% [8–10]. In the follow-up of the ItinerAir study, it was shown that patients with scleroderma-associated PAH identified early through an active screening programme had far better survival when compared to a parallel group of patients identified through usual clinical care [11, 12]. This study was limited by lead-in bias and small numbers but provides preliminary data to support the concept that screening may be useful. In addition, a US registry of early scleroderma PAH showed a better 3-year survival compared to other cohorts within the past 3 years [13].
We know that a diffusing capacity of the lung for carbon monoxide (DLCO) <60% predicted enriches the population prevalence of PAH by approximately five-fold [10]. In the recently published DETECT study, the prevalence of pulmonary hypertension was increased to 19% in a selected at-risk population (DLCO <60% pred and SSc for >3 years). This allowed the development of a practical screening tool [14], which will be discussed later.
The current European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines recommend annual echocardiography screening in symptomatic patients with scleroderma spectrum disease and state that annual echocardiography screening may be considered in asymptomatic patients. This is largely based on expert opinion [15]. table 1 shows the risk factors for PAH and associated guidelines for substrate recognition [16].
Table 1. Approach following substrate recognition.
| Substrate | Further assessment | Rationale |
| BMPR2 mutation | Yearly echocardiogram; RHC if echocardiogram demonstrates evidence of PAH# | Early detection of PAH; 20% chance of developing PAH |
| First degree relative of patient with BMPR2 mutation or within pedigree of two or more patients with a diagnosis of PAH | Genetic counselling and recommendation for BMPR2 genotyping; proceed as above if positive | Autosomal dominant transmission |
| Systemic sclerosis | Yearly echocardiogram; RHC if echocardiogram demonstrates evidence of PAH# | Approximately 8–12% (by RHC) prevalence of PAH in systemic sclerosis |
| HIV infection | Echocardiogram if symptoms or signs suggestive of PAH; RHC if echocardiogram demonstrates evidence of PAH# | 0.5% prevalence of PAH |
| Portal hypertension | Echocardiogram if OLT considered; RHC if echocardiogram demonstrates evidence of PAH# | 4% prevalence of PAH in candidates for OLT; PAH is predictive of poor OLT outcome |
| Prior appetite suppressant use (fenfluramine) | Echocardiogram only if symptomatic | Incidence of PAH is ∼0.005% if agent used for >3 months |
| Congenital heart disease with shunt | Echocardiogram and RHC at time of diagnosis; consider repair of defect if significant left-to-right shunt present | High probability of PAH developing in unrepaired shunt (Eisenmenger syndrome) |
| Recent acute pulmonary embolism | Ventilation/perfusion scintigraphy 3 months after event if symptomatic; pulmonary angiogram if positive | 3% risk of chronic thromboembolic PH; negative ventilation/perfusion scan excludes chronic thromboembolism |
| Sickle cell disease | Yearly echocardiogram; RHC if echocardiogram demonstrates evidence of PAH# | Increased mortality if PH present, early detection of PH, 30% develop PH or ∼10% develop PAH |
BMPR2: bone morphogenetic protein receptor 2; PAH: pulmonary arterial hypertension; RHC: right heart catheterisation; OLT: orthotopic liver transplantation; PH: pulmonary hypertension. #: high right ventricular systolic pressure or right heart chamber enlargement. Reproduced from [16] with permission from the publisher.
Echocardiography
Echocardiography is currently the most effective screening tool for PAH. It is virtually universally available and is noninvasive. Despite these strengths it has significant limitations.
Echocardiography experience is crucial; failure to obtain an estimate of projected systolic pulmonary artery pressure (PAP) has been reported in between 20 and 39% of patients [17, 18]. Doppler-derived pressure estimations, while correlating with invasively measured systolic PAP in populations, show poor reliability in individual patients with both under- and overestimation of actual systolic PAP [19–22]. It is believed that the prevalence of PAH in a population with tricuspid regurgitation jet <2.8 m·s−1 is very low. However, from the DETECT study we now know that over one-third of all SSc-PAH in a screening population is found in patients with a tricuspid regurgitation jet <2.8 m·s−1; thus, the performance of this threshold is dependent on the severity of the haemodynamic perturbation in the study population rather than the accuracy of echocardiographic assessment of the estimated systolic PAP in isolation [14].
We may consider whether other echocardiographic variables can help. For example, would right ventricular dysfunction (e.g. reduced tricuspid annular plane systolic excursion) help identify those patients missed by relying on pressure estimates [23]? The recent DETECT study also sheds some light on this question. 28 echocardiographic variables were included in the analysis (including tricuspid annular plane systolic excursion); however, only tricuspid regurgitation velocity, right ventricular area and right atrial area made it into the final model due to their discriminatory property in PAH versus non-pulmonary hypertension groups. In addition, in some countries, the cost of echocardiography as an upfront screening tool may be prohibitive.
Therefore, echocardiography cannot always be relied on, particularly in associated PAH where pressures are less elevated at diagnosis. Furthermore, the right ventricle does not react uniformly to pressure overload in different morbidities [24]. Rest echocardiography may be more reliable in patients presenting with advanced haemodynamics, such as Eisenmenger syndrome [25], idiopathic PAH [26] or bone morphogenetic protein receptor 2 (BMPR2), than those presenting with less haemodynamic perturbation, such as connective tissue disease (CTD)-PAH [14]. In SSc, for example, echocardiographic parameters of right ventricular function are usually well preserved at diagnosis [27].
Exercise echocardiography
Exercise echocardiography is considerably more complex and expensive as a screening procedure, and the potential for confounders and unreliability increase as complexity increases; for example, if one tries to estimate cardiac output at peak exercise. Unfortunately, at present, we do not know the prognostic relevance of exercise-induced pulmonary hypertension and exercise echocardiography is currently not recommended in pulmonary hypertension screening.
That said, the logic behind exercise testing is impeccable, as >70% of the vascular bed is lost before pressures reach pathological levels at rest [28]. Currently there is limited evidence in respect of the future outcome in patients with exercise pulmonary hypertension. The precise threshold at which one should diagnose exercise pulmonary hypertension is unresolved, since exercise mean PAP can frequently exceed 30 mmHg, particularly among the elderly [29]. Furthermore, it is not known if different PAH subgroups react differently in response to exercise.
Data from the UK national registry of CTD-PAH showed that ∼20% of scleroderma patients with exercise pulmonary hypertension at catheter (mean PAP >30 mmHg) progressed to PAH requiring advanced therapy within 3 years [6]. Steen et al. [30] showed that exercise echocardiography unmasked pulmonary hypertension in a scleroderma population at increased risk for PAH (DLCO <60% pred, forced vital capacity (FVC)/DLCO >1.6 and estimated systolic PAP 30–50 mmHg). In this study, 44% of patients had an increase of >20 mmHg in estimated right ventricular systolic pressure (RVSP) on echocardiography during exercise. These patients then underwent right heart catheterisation (RHC) and 62% were found to have elevated pressures [30].
In contrast, a study by Codullo et al. [31] using exercise echocardiography and follow-up in a low-risk scleroderma population (asymptomatic without overt pulmonary hypertension; tricuspid regurgitation velocity <3 m·s−1) found that only one in 19 patients developed PAH requiring advanced therapy when using an increase in systolic PAP of ≥18 mmHg after exercise as the criterion [32]. The utility of exercise echocardiography in this population is unresolved; it may contribute to the identification of patients with pulmonary hypertension but normal resting echocardiography or may identify a population with a relatively low rate of progression to PAH, depending on the precise subpopulation studied.
Lung function
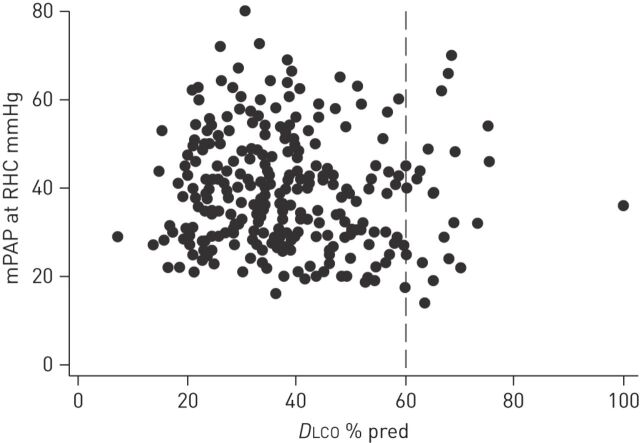
Steen et al. [33] were the first to show that patients with PAH and limited SSc have significantly lower mean DLCO several years before diagnosis. Mukerjee et al. [20] demonstrated in 85 SSc patients that a threshold of DLCO of 50% pred was associated with a high specificity (90%), however, at the price of low sensitivity (39%) for SSc-PAH. From the ItinerAir study [10] we know that a DLCO of >60% pred has a high specificity in excluding PAH, thus focusing on those with a DLCO <60% pred renders screening much more effective as an enrichment strategy in SSc. figure 1 shows the distribution of DLCO among 243 SSc-pulmonary hypertension patients at the Royal Free Hospital (London, UK) in whom full lung function was available. This demonstrates that <10% have a DLCO >60% pred and only one patient had a DLCO >80% pred. Unfortunately, this cannot be applied to other causes of PAH, in idiopathic PAH, for example, only a modest reduction in DLCO has been reported [5, 34]. Lower values of DLCO may have other implications, Gunther et al. [35] have shown that pulmonary veno-occlusive disease (PVOD) is present more frequently in SSc populations. Patients with more than two signs of PVOD on computed tomography had lower values of DLCO than those with at least one sign. Further support for this observation comes from a pathology study where pulmonary vein obstruction was found more often in tissue samples from CTD patients than from other PAH groups [36]. However, it should be noted that the studies suggesting a high prevalence of PVOD in SSc-PAH are outliers, since the original epoprostenol study in SSc-PAH did not identify the occurrence of pulmonary oedema in the population [37, 38], even during long-term follow-up, and the response to therapy in the recent SERAPHIN (Study with an Endothelial Receptor Antagonist in Pulmonary Arterial Hypertension to Improve Clinical Outcomes) study was identical in the idiopathic PAH and CTD-PAH subpopulations [39].
Figure 1.
Distribution of diffusing capacity of the lung for carbon monoxide (DLCO) among 243 scleroderma patients who underwent right heart catheterisation (RHC) at the Royal Free Hospital (London, UK) in whom full lung function at the time of diagnosis was available. The results demonstrate that <10% had a DLCO >60% pred and only one patient had a DLCO >80% pred. mPAP: mean pulmonary artery pressure; % pred: % predicted.
N-terminal pro-brain natriuretic peptide
Biomarkers such as N-terminal pro-brain natriuretic peptide (NT-proBNP) may offer additional value in screening. However, NT-proBNP levels might not perform uniformly in different forms of PAH. It has been reported that in SSc NT-proBNP levels are higher than in idiopathic PAH, despite less haemodynamic impairment [40].
NT-proBNP release typically occurs with volume overload and ventricular contractile dysfunction [41, 42]. Williams et al. [42] showed a cut-off value of 395 pg·mL−1 has a sensitivity of 55.9% and specificity of 95.1% for identification of SSc-PAH, suggesting that a low NT-proBNP may be helpful in ruling out SSc-PAH. Of note, however, this study included relatively few control patients, not all of whom were catheterised.
Screening algorithms incorporating echocardiography results
Mukerjee et al. [20] were the first to report on an active screening programme in SSc. Cardiac catheterisation was performed in all patients where the tricuspid regurgitation velocity was >3 m·s−1 and/or the corrected DLCO was <50% pred without advanced pulmonary fibrosis (or a fall in DLCO by >20% within the previous 2 years). The authors found both tests to be adequate in advanced PAH; however, neither exhibited reliability to exclude PAH where pre-test probability was high [20].
In the first prospective multicentre study, Hachulla et al. [10] applied a screening algorithm in a large scale population of SSc based on symptom (dyspnoea) and Doppler echocardiography. Doppler echocardiography was performed in a SSc population without severe pulmonary function abnormalities. A tricuspid regurgitation velocity >3 m·s−1 directly led to RHC. Patients with a tricuspid regurgitation velocity >2.5–3 m·s−1 were only referred for RHC if unexplained dyspnoea was also present. Patients with a tricuspid regurgitation velocity <2.5 m·s−1 were assumed not to have pulmonary hypertension irrespective of the presence of unexplained breathlessness. In this study, Hachulla et al. [10] were able to detect PAH at a mild stage; however, due to the design this study was not able to detect false negatives and only 33 out of 599 patients underwent RHC.
Screening algorithms incorporating lung function results
Given the limitations of echocardiography several groups used lung function test results among other data to investigate screening algorithms. Fortunately, we know that a DLCO of <60% pred enriches the population prevalence of PAH five-fold [10].
Allanore et al. [43] found that NT-proBNP levels at >97% of manufacturer levels, particularly when combined with a DLCO/alveolar volume <70%, could predict the development of PAH in eight out of 101 patients in an SSc cohort over a 36-month period of follow-up. A similar approach was used by Thakkar et al. [44], who derived a screening algorithm incorporating NT-proBNP and pulmonary function test results, but this score has not yet been validated.
Meune et al. [45] developed a simple score (Cochin risk score) to predict the risk of pulmonary hypertension SSc constituted by simple clinical observations (age, FVC and DLCO). The overall predictive value of the Cochin risk score was 0.87 (95% CI 0.79–0.95) and it showed a 89.5% sensitivity and a 74.1% specificity in the detection of patients developing pulmonary hypertension during follow-up.
Schreiber et al. [46] used a large cohort of SSc patients who underwent RHC to derive a simple formula using DLCO and peripheral arterial oxygen saturation to predict mean PAP. Using a predicted threshold of 30 mmHg sensitivity was 59.3% and specificity was 70.8% in a split-sample cohort.
Screening algorithms combining echocardiography and lung function
Gladue et al. [47] reported on combining the results of transthoracic echocardiography and lung function to predict the presence of PAH in two cohorts of SSc enriched for PAH. They developed a matrix of transthoracic echocardiogram and pulmonary function test results to assess if this combination would improve the negative predictive value, as relying on a RVSP of >50 mmHg alone 36% of PAH cases would have been missed. The combination of RVSP >50 mmHg and DLCO <60% pred captured 94% of patients. Controls were not catheterised [47].
The questions we must pose in all these studies are whether all patients allocated to the control group were free of pulmonary hypertension. Because the gold standard was not included in all patients, estimates of sensitivity are positively biased. The majority of these studies have used echocardiography amongst other tests to select their baseline populations and we therefore do not know how many patients have been missed (false negatives). Furthermore, most studies included a high proportion of patients with advanced symptomatic disease, thus they are biased toward easier discrimination between disease and controls.
DETECT
In a high-risk population (SSc >3 years and DLCO <60% pred), the recent DETECT study (multicentre, international, cross-sectional) demonstrated that a simple algorithm is a sensitive and noninvasive tool for screening of PAH [14]. It is the first study that addresses the false-negative rate by mandating RHC in all patients. In a population of 466 patients with SSc at increased risk for PAH (DLCO <60% pred and >3 year history of scleroderma spectrum), 19% of patients had RHC-confirmed PAH. Almost two-thirds of patients with PAH were in functional class I or II. From this data set a two-step algorithm has been developed to identify which patients should be referred for echocardiography and subsequently catheterisation. Six simple clinical variables were identified on regression analysis that increased the likelihood of finding PAH, namely: FVC/DLCO; current or past teleangiectasias; anti-centromere antibody; NT-proBNP; serum urate; and right axis deviation on ECG. A combination of these parameters led to an echocardiogram referral. After echocardiography the prediction score from step one plus two echocardiographic variables determines referral to RHC. Sensitivity is very high (96%) and specificity is 48%, so the rate of referral to RHC required using the DETECT algorithm is still substantial (62%). In summary, the algorithm out-performs the current ESC/ERS recommendation because of the high sensitivity despite referring a similar number for catheterisation.
Strikingly, in the DETECT study one in five patients with PAH was asymptomatic (functional class 1 despite SSc) and one-third of patients had a tricuspid regurgitation velocity of <2.8 m·s−1 which renders symptoms or echocardiography in isolation insufficient to screen for increased pulmonary pressures. In addition, it was found that not only tricuspid regurgitation velocity, but also right atrial area, right ventricular area and right ventricular and left ventricular parameters did not perform well in isolation.
Limitations of screening
The available data on screening in SSc-PAH sheds light on other important issues as well. Not all identified pulmonary hypertension is PAH, other forms of pulmonary hypertension can occur as well, lung disease [48], scleroderma-related left heart dysfunction leading to post-capillary pulmonary hypertension, chronic thrombembolic disease and PVOD [35], have all been recorded [49]. One must also consider the consequences of screening tools that may dominantly identify patients at risk for conditions for which there is no specific therapy. More studies are needed to help discriminate between the different forms of pulmonary hypertension as we may be underestimating the importance of other forms of pulmonary hypertension, which are less treatable or not treatable per se. In scleroderma, by 10 years after onset 50% of patients with diffuse SSc will have significant pulmonary fibrosis, and left ventricular diastolic dysfunction due to myocardial involvement is common. As both lung disease on high-resolution computed tomography and left ventricular dysfunction via wedge resection are arbitrarily defined, it is very likely that a subgroup of patients is presently being ineffectively treated with potentially toxic therapies.
Recommendations in CTD-PAH
Despite increasing knowledge that combination screening tools perform better than individual tests, recommendations in CTD-PAH have focused on transthoracic echocardiography and do not provide detailed recommendations for patients. There is increasing evidence-based data (presented in previous sections) of the characteristics of different noninvasive screening tools in SSc. Recently, a multidisciplinary group of cardiologists, pulmonologists and rheumatologists developed the first such recommendations [50]. Using a well-defined consensus methodology (RAND/University of California at Los Angeles (CA, USA) methodology), the authors incorporated a systematic review of the literature and consensus to develop a set of recommendations with a goal to facilitate screening and early detection of CTD-PAH. Tables 2–4 provide key highlights of these recommendations.
Table 2. General recommendations for screening and early detection of connective tissue disease (CTD)-pulmonary arterial hypertension (PAH).
| All patients with SSc should be screened for PAH |
| Patients with mixed CTD or other CTD with scleroderma features (referred to as scleroderma spectrum disorders) should be screened in a similar way to patients with SSc |
| Screening of asymptomatic patients is not recommended for mixed CTD or other CTD patients without features of scleroderma (including systemic lupus erythematosus, rheumatoid arthritis, inflammatory myositis and Sjögrens syndrome) |
| All SSc and scleroderma spectrum patients with a positive, noninvasive screening (as presented in these recommendations) should be referred for RHC |
| RHC is mandatory for diagnosis of PAH |
| Acute vasodilator testing is not required as part of the evaluation of PAH in patients with SSc, scleroderma spectrum disorders or other CTD |
SSc: systemic sclerosis; RHC: right heart catheterisation. Reproduced with modification from [50] with permission from the publisher.
Table 4. Recommendations for right heart catheterisation (RHC) for systemic scleroderma and scleroderma spectrum disorder.
| Signs or symptoms# required for RHC | |
| Transthoracic echocardiogram | |
| TR velocity 2.5–2.8 m·s−1 | Yes |
| TR velocity >2.8 m·s−1 | No |
| Right atrial (major dimension >53 mm) or right ventricular enlargement (mid-cavity dimension >35 mm), irrespective of TR velocity | No |
| Pulmonary function test | |
| FVC/DLCO >1.6 and/or DLCO <60% pred¶ | Yes |
| FVC/DLCO >1.6 and/or DLCO <60% pred and NT-proBNP more than twice upper limit of normal¶ | No |
| Composite measure | |
| Meets DETECT algorithm in patients with DLCO <60% pred and disease duration >3 years | No |
TR: tricuspid regurgitation; FVC: forced vital capacity; DLCO: diffusing capacity of the lung for carbon monoxide; % pred: % predicted; NT-proBNP: N-terminal pro-brain natriuretic peptide. #: symptoms include dyspnoea on rest or exercise, fatigue, pre-syncope/syncope, chest pain, palpitations, dizziness and light-headedness; signs include loud pulmonic sound and peripheral oedema. ¶: transthoracic echocardiogram without overt systolic dysfunction, greater than grade I diastolic dysfunction or greater than mild mitral or aortic valve disease or evidence of pulmonary hypertension. Reproduced with modification from [50] with permission from the publisher.
Table 3. Initial screening evaluation in patients with systemic sclerosis and scleroderma spectrum disorders.
| PFT with DLCO |
| Transthoracic echocardiogram |
| NT-proBNP |
| DETECT algorithm if DLCO <60% pred and >3 years disease duration |
| Frequency of noninvasive tests: |
| Transthoracic echocardiogram on annual basis as a screening test |
| Transthoracic echocardiogram if new signs or symptoms develop |
| PFT with DLCO on annual basis as a screening test |
| PFT with DLCO if new signs or symptoms develop |
| NT-proBNP if new signs of symptoms develop |
PFT: pulmonary function test; DLCO: diffusing capacity of the lung for carbon monoxide; NT-proBNP: N-terminal pro-brain natriuretic peptide; % pred: % predicted. Reproduced with modification from [50] with permission from the publisher.
Specific conditions
BMPR2 positive patients and relatives of patients with familial PAH
Current guidelines recommend yearly echocardiograms in asymptomatic carriers of the BMPR2 mutation and RHC if the echocardiogram is abnormal. BMPR2-positive patients have a lifetime risk of PAH which is probably ∼20% [51]. This has recently been challenged [52], but as yet we have no feel for the age by which >10% of carriers might have PAH. Gas transfer abnormalities are typically modest in idiopathic PAH so lung function testing is unlikely to be helpful as an enrichment strategy in this population [5]. Whether further investigation would lead to the identification of particular mutations associated with a higher prevalence of PAH is presently unknown. It remains unclear whether following current guidelines will accurately discriminate between those with and without PAH.
Gruenig et al. [53] have shown that exercise elevation of pulmonary pressures is more common among first degree relatives of individuals with familial PAH, and in some progression to overt PAH is observed. However, considerably more work is required to determine whether exercise pulmonary hypertension is a reliable predictor of PAH and whether such screening is cost-effective.
Haemolytic anaemia and sickle cell
High-quality data about the prevalence of PAH and screening is available for sickle cell anaemia from the study by Parent et al. [21]. The background prevalence was found to be 6%. In this study a low tricuspid regurgitation velocity of 2.5 m·s−1 was used as a threshold, irrespective of symptoms. The authors found echocardiography to perform poorly in retrospect and proposed an algorithm that includes NT-proBNP and 6-min walk data in addition to echocardiography; the predictive value was 62% and with a false negative rate of least 7% [21].
CTEPH
The current European registry data highlight the importance of previous venous thromboembolism events as an aetiological factor for the development of chronic thromboembolic pulmonary hypertension (CTEPH), along with a significant role for associated medical risk factors as co-existing mechanisms in the disease process [54]. As there is evidence that CTEPH may also develop in patients without previous pulmonary embolism it is difficult to determine the exact prevalence. However, two Italian studies have been published in the setting of pulmonary embolism: one found a cumulative incidence of symptomatic CTEPH of 1% 6 months after venous thromboembolism increasing to 3.8% after 2 years, the other reported an incidence of ∼1% in a follow-up of 4 years [55, 56].
In addition, there are known risk factors for CTEPH such as splenectomy, cancer, ventriculoatrial shunt, chronic inflammatory disease, antiphospholipid antibodies and hypothyroidism [57]. Given these common risk factors screening methods would be useful, but due to our limited knowledge of the prevalence of CTEPH in these populations at present, screening (apart from regular assessments for dyspnoea) is not known to be cost-effective and worthwhile.
The guidance of the American Heart Association/American College of Cardiology guidance is to perform ventilation/perfusion (V′/Q′) scanning in patients in whom dyspnoea remains a problem 3 months after a pulmonary embolism and in all patients with an unknown cause of pulmonary hypertension in order to exclude CTEPH. V′/Q′scanning is preferred to a computed tomography pulmonary angiogram, because of the higher sensitivity [58], but it can miss distal disease. Although sensitive, V′/Q′ scanning lacks specificity and could lead to unnecessary investigation of patients with previous pulmonary embolism. A prospective evaluation of the discriminant value of V′/Q′in patients with previous pulmonary embolism would help clarify the precise role of this investigation in screening for CTEPH in symptomatic and asymptomatic patients.
Portopulmonary hypertension
The prevalence of portopulmonary hypertension may reach 5–8% among patients with advanced liver disease evaluated for liver transplantation [59]. Echocardiographic screening is advised by guidelines as mortality increases with attempted liver transplantation.
Krowka et al. [60] reported on a screening algorithm among transplantation candidates in a prospective fashion. They used Doppler echocardiography to determine PAP in 1235 patients. They found 101 (8%) patients to have an estimated PAP of >50 mmHg and they subsequently underwent catheterisation. 90% of patients were found to have a mean PAP >25 mmHg, 65% of those were diagnosed with portopulmonary hypertension (mean PAP >25 mmHg and/or pulmonary vascular resistance >240 dyn·s·cm−5). There was discordance between echocardiogram and RHC, and in 22% of patients tricuspid regurgitation velocity could not be recorded. Overall correlation between PAP and systolic PAP measured during catheterisation was reasonable [60].
Thus, in portopulmonary hypertension the prevalence appears to be less than the required 10% threshold for screening with current tools. However, this study could easily have missed false negatives and in portopulmonary hypertension high output elevation of pulmonary pressures is not necessarily associated with any vasculopathy.
HIV
As in other forms of PAH, most patients with HIV-related PAH are in functional class III and IV at diagnosis. In HIV the estimated prevalence of PAH has been reported as 0.5% [61], and has not changed in recent years [62]. Currently, there is no recommendation on how to screen for PAH in asymptomatic HIV patients; echocardiography is recommended in the presence of unexplained dyspnoea.
Two studies demonstrated the problems with inaccuracy and false positives by selecting patients for RHC with Doppler echocardiography. In the study of Sitbon et al. [62], a tricuspid regurgitation velocity of 2.5 m·s−1 and 2.8 m·s−1 was associated with a false-positive rate of 72% and 29%, respectively. In their cohort, Selby et al. [22] showed that 20% of patients echocardiography measurements were found to deviate by >10 mmHg from the invasively derived figure.
Congenital heart disease
Eisenmenger syndrome is a complex clinical syndrome including cyanosis and erythrocytosis associated with a prolonged, usually very substantial, elevation of pulmonary pressures, and right ventricular hypertrophy is readily diagnosed using clinical observation and echocardiography. Presently we only have evidence for symptomatic management of those with significant exercise limitation [63] and modest observational evidence for prognostic benefit [64]. Presently there is no need for screening, simply advice that all patients with unrepaired shunts should be followed by expert centres.
However, if we were ever to show that prevention of transition from flow and pressure overload to pulmonary vasculopathy were possible a very different set of questions would arise, given that measurements of pulmonary pressures alone would provide no insight, and measuring pulmonary vascular resistance in this population is much more complex than in other cohorts. Assessment of pulmonary vascular compliance is one possible tool to consider in the future for this population [65].
One particularly difficult population is the Fontan population, where pulmonary vasculopathy can only lead to elevation of resistance and reduced cardiac output, without elevation of pulmonary pressures. Presently we have no evidence base for treating these patients and it is probable that “screening”, should it ever be applied, would have to be invasive.
Conclusion
Developing screening programmes for PAH is worthwhile because it is an uncommon progressive disorder, with nonspecific symptoms and disease modifying therapies are available. Currently available screening tools lack specificity and sensitivity and the required diagnostic test is invasive, thus population enrichment remains pivotal in developing any adequate screening programme. For SSc-PAH we now know the critical elements of such a screening programme, unfortunately this data is not generalisable to other forms of PAH. We have, however, learnt the importance of considering the false negative rates in studies where the gold standard test is not applied in all subjects, and this should inform the development of screening programmes for other cohorts.
Supplementary Material
Footnotes
Support statement: The authors wrote the manuscript and no support was provided regarding the content and no author received any payment for this work.
Conflict of interest: Disclosures can be found alongside the online version of this article at err.ersjournals.com
Provenance: Publication of this peer-reviewed article was supported by Actelion Pharmaceuticals Ltd, Switzerland (principal sponsor, European Respiratory Review issue 130).
References
- 1.Black WC, Welch HG. Screening for disease. AJR Am J Roentgenol 1997; 168: 3–11. [DOI] [PubMed] [Google Scholar]
- 2.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med 2012; 367: 1998–2005. [DOI] [PubMed] [Google Scholar]
- 3.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010; 122: 156–163. [DOI] [PubMed] [Google Scholar]
- 4.Galiè N, Rubin LJ, Hoeper M, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet 2008; 371: 2093–2100. [DOI] [PubMed] [Google Scholar]
- 5.Hurdman J, Condliffe R, Elliot CA, et al. ASPIRE registry: Assessing the Spectrum of Pulmonary hypertension Identified at a REferral Centre. Eur Respir J 2012; 39: 945–955. [DOI] [PubMed] [Google Scholar]
- 6.Condliffe R, Kiely DG, Peacock AJ, et al. Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am J Respir Crit Care Med 2009; 179: 151–157. [DOI] [PubMed] [Google Scholar]
- 7.Launay D, Sitbon O, Hachulla E, et al. Survival in systemic sclerosis-associated pulmonary arterial hypertension in the modern management era. Ann Rheum Dis 2012. [in press DOI: 10.1136/annrheumdis-2012-202489]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avouac J, Airò P, Meune C, et al. Prevalence of pulmonary hypertension in systemic sclerosis in European Caucasians and metaanalysis of 5 studies. J Rheumatol 2010; 37: 2290–2298. [DOI] [PubMed] [Google Scholar]
- 9.Mukerjee D, St George D, Coleiro B, et al. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approach. Ann Rheum Dis 2003; 62: 1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005; 52: 3792–3800. [DOI] [PubMed] [Google Scholar]
- 11.Hachulla E, Carpentier P, Gressin V, et al. Risk factors for death and the 3-year survival of patients with systemic sclerosis: the French ItinerAIR-Sclerodermie study. Rheumatology (Oxford) 2009; 48: 304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humbert M, Yaici A, de Groote P, et al. Screening for pulmonary arterial hypertension in patients with systemic sclerosis: clinical characteristics at diagnosis and long-term survival. Arthritis Rheum 2011; 63: 3522–3530. [DOI] [PubMed] [Google Scholar]
- 13.Chung L, Domsic RT, Lingala B, et al. Survival and predictors of mortality in systemic sclerosis associated pulmonary arterial hypertension: outcomes from the PHAROS registry. Arthritis Care Res (Hoboken) 2013. [in press DOI: 10.1002/acr.22121]. [DOI] [PubMed] [Google Scholar]
- 14.Coghlan JG, Denton CP, Grünig E, et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2013. [in press DOI: 10.1136/annrheumdis-2013-203301]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30: 2493–2537. [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53: 1573–1619. [DOI] [PubMed] [Google Scholar]
- 17.Kowal-Bielecka O, Avouac J, Pittrow D, et al. Echocardiography as an outcome measure in scleroderma-related pulmonary arterial hypertension: a systematic literature analysis by the EPOSS group. J Rheumatol 2010; 37: 105–115. [DOI] [PubMed] [Google Scholar]
- 18.Denton CP, Cailes JB, Phillips GD, et al. Comparison of Doppler echocardiography and right heart catheterization to assess pulmonary hypertension in systemic sclerosis. Br J Rheumatol 1997; 36: 239–243. [DOI] [PubMed] [Google Scholar]
- 19.Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009; 179: 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukerjee D, St George D, Knight C, et al. Echocardiography and pulmonary function as screening tests for pulmonary arterial hypertension in systemic sclerosis. Rheumatology (Oxford) 2004; 43: 461–466. [DOI] [PubMed] [Google Scholar]
- 21.Parent F, Bachir D, Inamo J, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med 2011; 365: 44–53. [DOI] [PubMed] [Google Scholar]
- 22.Selby VN, Scherzer R, Barnett CF, et al. Doppler echocardiography does not accurately estimate pulmonary artery systolic pressure in HIV-infected patients. AIDS 2012; 26: 1967–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forfia PR, Fisher MR, Mathai SC, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med 2006; 174: 1034–1041. [DOI] [PubMed] [Google Scholar]
- 24.Jurcut R, Giusca S, Ticulescu R, et al. Different patterns of adaptation of the right ventricle to pressure overload: a comparison between pulmonary hypertension and pulmonary stenosis. J Am Soc Echocardiogr 2011; 24: 1109–1117. [DOI] [PubMed] [Google Scholar]
- 25.Moceri P, Dimopoulos K, Liodakis E, et al. Echocardiographic predictors of outcome in eisenmenger syndrome. Circulation 2012; 126: 1461–1468. [DOI] [PubMed] [Google Scholar]
- 26.Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 2010; 137: 376–387. [DOI] [PubMed] [Google Scholar]
- 27.de Groote P, Gressin V, Hachulla E, et al. Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis 2008; 67: 31–36. [DOI] [PubMed] [Google Scholar]
- 28.Reid LM. Structure and function in pulmonary hypertension. New perceptions. Chest 1986; 89: 279–288. [DOI] [PubMed] [Google Scholar]
- 29.Kovacs G, Berghold A, Scheidl S, et al. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J 2009; 34: 888–894. [DOI] [PubMed] [Google Scholar]
- 30.Steen V, Chou M, Shanmugam V, et al. Exercise-induced pulmonary arterial hypertension in patients with systemic sclerosis. Chest 2008; 134: 146–151. [DOI] [PubMed] [Google Scholar]
- 31.Codullo V, Caporali R, Cuomo G, et al. Stress Doppler echocardiography in systemic sclerosis: evidence for a role in the prediction of pulmonary hypertension. Arthritis Rheum 2013; 65: 2403–2411. [DOI] [PubMed] [Google Scholar]
- 32.D'Alto M, Ghio S, D'Andrea A, et al. Inappropriate exercise-induced increase in pulmonary artery pressure in patients with systemic sclerosis. Heart 2011; 97: 112–117. [DOI] [PubMed] [Google Scholar]
- 33.Steen V, Medsger TA, Jr. Predictors of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Arthritis Rheum 2003; 48: 516–522. [DOI] [PubMed] [Google Scholar]
- 34.Burke CM, Glanville AR, Morris AJ, et al. Pulmonary function in advanced pulmonary hypertension. Thorax 1987; 42: 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Günther S, Jaïs X, Maitre S, et al. Computed tomography findings of pulmonary venoocclusive disease in scleroderma patients presenting with precapillary pulmonary hypertension. Arthritis Rheum 2012; 64: 2995–3005. [DOI] [PubMed] [Google Scholar]
- 36.Dorfmuller P, Humbert M, Perros F, et al. Fibrous remodeling of the pulmonary venous system in pulmonary arterial hypertension associated with connective tissue diseases. Hum Pathol 2007; 38: 893–902. [DOI] [PubMed] [Google Scholar]
- 37.Badesch DB, McGoon MD, Barst RJ, et al. Long term survival among patients with scleroderma-associated pulmonary arterial hypertension treated with intravenous epoprostenol. J Rheumatol 2009; 36: 2244–2249. [DOI] [PubMed] [Google Scholar]
- 38.Badesch DB, Tapson VF, McGoon MD, et al. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trial. Ann Intern Med 2000; 132: 425–434. [DOI] [PubMed] [Google Scholar]
- 39.Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013; 369: 809–818. [DOI] [PubMed] [Google Scholar]
- 40.Chung L, Liu J, Parsons L, et al. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest 2010; 138: 1383–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dimitroulas T, Giannakoulas G, Karvounis H, et al. Natriuretic peptides in systemic sclerosis-related pulmonary arterial hypertension. Semin Arthritis Rheum 2010; 39: 278–284. [DOI] [PubMed] [Google Scholar]
- 42.Williams MH, Handler CE, Akram R, et al. Role of N-terminal brain natriuretic peptide (N-TproBNP) in scleroderma-associated pulmonary arterial hypertension. Eur Heart J 2006; 27: 1485–1494. [DOI] [PubMed] [Google Scholar]
- 43.Allanore Y, Borderie D, Avouac J, et al. High N-terminal pro-brain natriuretic peptide levels and low diffusing capacity for carbon monoxide as independent predictors of the occurrence of precapillary pulmonary arterial hypertension in patients with systemic sclerosis. Arthritis Rheum 2008; 58: 284–291. [DOI] [PubMed] [Google Scholar]
- 44.Thakkar V, Stevens WM, Prior D, et al. N-terminal pro-brain natriuretic peptide in a novel screening algorithm for pulmonary arterial hypertension in systemic sclerosis: a case-control study. Arthritis Res Ther 2012; 14: R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meune C, Avouac J, Airò P, et al. Prediction of pulmonary hypertension related to systemic sclerosis by an index based on simple clinical observations. Arthritis Rheum 2011; 63: 2790–2796. [DOI] [PubMed] [Google Scholar]
- 46.Schreiber BE, Valerio CJ, Keir GJ, et al. Improving the detection of pulmonary hypertension in systemic sclerosis using pulmonary function tests. Arthritis Rheum 2011; 63: 3531–3539. [DOI] [PubMed] [Google Scholar]
- 47.Gladue H, Steen V, Allanore Y, et al. Combination of echocardiographic and pulmonary function test measures improves sensitivity for diagnosis of systemic sclerosis-associated pulmonary arterial hypertension: analysis of 2 cohorts. J Rheumatol 2013; 40: 1706–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cottin V, Nunes H, Mouthon L, et al. Combined pulmonary fibrosis and emphysema syndrome in connective tissue disease. Arthritis Rheum 2011; 63: 295–304. [DOI] [PubMed] [Google Scholar]
- 49.Chatterjee S Pulmonary hypertension in systemic sclerosis, Semin Arthritis Rheum 2011; 41: 19–37. [DOI] [PubMed] [Google Scholar]
- 50.Khanna D, Gladue H, Channick R, et al. Recommendations for screening and detection of connective-tissue disease associated pulmonary arterial hypertension. Arthritis Rheum 2013. [in press DOI: 10.1002/art.38172]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Newman JH, Trembath RC, Morse JA, et al. Genetic basis of pulmonary arterial hypertension: current understanding and future directions. J Am Coll Cardiol 2004; 43: Suppl. 12, 33S 39S. [DOI] [PubMed] [Google Scholar]
- 52.Larkin EK, Newman JH, Austin ED, et al. Longitudinal analysis casts doubt on the presence of genetic anticipation in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 186: 892–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grünig E, Weissmann S, Ehlken N, et al. Stress Doppler echocardiography in relatives of patients with idiopathic and familial pulmonary arterial hypertension: results of a multicenter European analysis of pulmonary artery pressure response to exercise and hypoxia. Circulation 2009; 119: 1747–1757. [DOI] [PubMed] [Google Scholar]
- 54.Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011; 124: 1973–1981. [DOI] [PubMed] [Google Scholar]
- 55.Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350: 2257–2264. [DOI] [PubMed] [Google Scholar]
- 56.Becattini C, Agnelli G, Pesavento R, et al. Incidence of chronic thromboembolic pulmonary hypertension after a first episode of pulmonary embolism. Chest 2006; 130: 172–175. [DOI] [PubMed] [Google Scholar]
- 57.Kim NH, Lang IM. Risk factors for chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2012; 21: 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tunariu N, Gibbs SJ, Win Z, et al. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J Nucl Med 2007; 48: 680–684. [DOI] [PubMed] [Google Scholar]
- 59.Krowka MJ, Miller DP, Barst RJ, et al. Portopulmonary hypertension: a report from the US-based REVEAL Registry. Chest 2012; 141: 906–915. [DOI] [PubMed] [Google Scholar]
- 60.Krowka MJ, Swanson KL, Frantz RP, et al. Portopulmonary hypertension: results from a 10-year screening algorithm. Hepatology 2006; 44: 1502–1510. [DOI] [PubMed] [Google Scholar]
- 61.Speich R, Jenni R, Opravil M, et al. Primary pulmonary hypertension in HIV infection. Chest 1991; 100: 1268–1271. [DOI] [PubMed] [Google Scholar]
- 62.Sitbon O, Lascoux-Combe C, Delfraissy JF, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med 2008; 177: 108–113. [DOI] [PubMed] [Google Scholar]
- 63.Galièe N, Beghetti M, Gatzoulis MA, et al. Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation 2006; 114: 48–54. [DOI] [PubMed] [Google Scholar]
- 64.Dimopoulos K, Inuzuka R, Goletto S, et al. Improved survival among patients with Eisenmenger syndrome receiving advanced therapy for pulmonary arterial hypertension. Circulation 2010; 121: 20–25. [DOI] [PubMed] [Google Scholar]
- 65.Ben-Yehuda O, Barnett C. Magnetic resonance assessment of pulmonary artery compliance: a promising diagnostic and prognostic tool in pulmonary hypertension? JACC Cardiovasc Imaging 2009; 2: 296–298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.