Abstract
Incubation of human epithelial cells with nanomolar concentrations of chromatographically purified Serratia marcescens hemolysin (ShlA) caused irreversible vacuolation and subsequent lysis of the cells. Vacuolation differed from vacuole formation by Helicobacter pylori VacA. Sublytic doses of ShlA led to a reversible depletion of intracellular ATP. Restoration to the initial ATP level was presumably due to the repair of the toxin damage and was inhibited by cycloheximide. Pores formed in epithelial cells and fibroblasts without disruption of the plasma membrane, and the pores appeared to be considerably smaller than those observed in artificial lipid membranes and in erythrocytes and did not allow the influx of propidium iodide or trypan blue. All cytotoxic effects induced by isolated recombinant ShlA were also obtained with exponentially growing S. marcescens cells. The previously suggested role of the hemolysin in the pathogenicity of S. marcescens is supported by these data.
Almost all strains of Serratia marcescens secrete a cytotoxin (7, 9–11, 34) that causes hemolysis of human and animal erythrocytes (35) and the release of the inflammatory mediators leukotrienes and histamine from leukocytes (23). Previous studies (27) have shown an increase in the pathogenicity of the pathogenic Escherichia coli strain 536/21 after transformation with the S. marcescens shlA and shlB genes. E. coli strains transformed with the S. marcescens hemolysin-determining genes, shlA and shlB, display the same hemolytic properties as the S. marcescens parent (10, 33, 36). Nontoxic ShlA protein (termed ShlA*) is secreted via a signal peptide mechanism (Sec system) into the periplasmic space. The ShlB protein is located in the outer membrane and mediates secretion of ShlA* across the outer membrane and conversion of ShlA* to an active hemolysin (termed ShlA) (33, 36). Membrane binding of ShlA and pore formation on human erythrocytes have been shown (35). The size of ShlA pores in human erythrocytes has been estimated with osmotic protection experiments (35). Erythrocytes can be protected from ShlA pore formation by the presence of protective oligosaccharides. Dilution of the protective oligosaccharides results in osmolysis. ShlA forms pores through which dextrins with a molecular mass of up to 1,400 Da diffuse (34). Pores ranging in size from 1 to 3 nm also form in planar artificial lipid membranes (36).
Since ShlA lyses all mammalian erythrocytes tested, there is no evidence for a high-affinity binding site. However, the cytoplasmic membranes of gram-negative prokaryotes are not affected by ShlA (40).
There are no published reports on the action of ShlA on cultured eukaryotic cells of various origins. Due to the uropathogenicity of S. marcescens, the cytotoxic effects on epithelial cells are of great interest. Here, we demonstrate that epithelial cells are depleted of ATP by low doses of ShlA. ATP depletion was reversed in a medium lacking ShlA. Restoration of the ATP level was inhibited by cycloheximide. At higher doses, ShlA induced an extended vacuolation, and the cells were lysed. The pores created by ShlA in nucleated cells appeared to be considerably smaller than those deduced from studies with human erythrocytes (38). Hemolytic S. marcescens strains showed the same effects on cell cultures as isolated recombinant ShlA.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Cells were grown in TY medium, consisting of 0.8% tryptone (Difco Laboratories), 0.5% yeast extract, and 0.5% NaCl, at pH 7.0.
TABLE 1.
Strains of E. coli and S. marcescens and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| E. coli | ||
| WM1576 | K38 HrfC pGP1-2 | 46 |
| BL21(DE3) | Lysogenic for phage λ carrying the phage T7 RNA polymerase gene under control of the lacUV5 promoter | 44 |
| S. marcescens | ||
| VA15854 | Human isolate | 34 |
| W1128 | Human isolate | 32 |
| CDC 04:H4 | Human isolate | 34 |
| Plasmids | ||
| pRO3 | pT7-5 shlA shlB | 39 |
| pES15 | pT7-5 shlA | 33 |
| pRO14 | pSUKS1 shlA shlB | 39 |
| pRO2 | pT7-6 shlB shlA(Δ256-1578) | 39 |
Hemolysis assay.
Hemolysis was determined as described previously (20). Serial dilutions of hemolytic samples were prepared with phosphate-buffered saline or HU buffer (6 M urea in 50 mM HEPES, pH 6.8), and 100-μl aliquots of each dilution were incubated with 1 ml of erythrocyte suspension for 15 min at 22°C and then centrifuged for 1 min in a microcentrifuge. The absorbance at 405 nm (A405) of released hemoglobin was measured spectroscopically. Hemolytic activities are presented either as the percentage of the total erythrocytes lysed (percent hemolysis) or as hemolytic units (HU) per milliliter, which were determined as described previously (3, 20). The HU (release of 50% of the total hemoglobin) is defined as [(A405 for the sample with hemolysin − A405 for the control without hemolysin) × 100]/(A405 for total lysis caused by sodium dodecyl sulfate − A405 for the control).
For the determination of hemolysis in an exponentially growing bacterial culture, 20 ml of a culture of S. marcescens or E. coli BL21 was incubated at 37°C until an optical density at 578 nm of 0.5 was reached. From the sediment of washed erythrocytes, 0.66 ml was added to the culture, and hemolysis was determined after a 15-min incubation at 37°C. The bacterial cells and unlysed erythrocytes were removed by centrifugation, the supernatant was diluted 1:100 in water, and the A405 of the released hemoglobin was measured. As a control, untransformed E. coli was examined in the same manner. Total lysis of added erythrocytes was determined with each culture after addition of 0.2 ml of 20% sodium dodecyl sulfate, followed by incubation at 37°C for 5 min.
Isolation of ShlA, ShlA255, and ShlA* from culture supernatants and cells.
Methods for the synthesis and purification of ShlA, ShlA255, and ShlA* (20) and E. coli hemolysin (HlyA) (4) have been described previously. ShlA, ShlA255, and HlyA were in precipitated spent media; ShlA* was isolated from the periplasm.
Activation of ShlA* by complementation with ShlA255.
Aliquots (20 μl) containing ShlA* were mixed with ShlA255 (5 μl) contained in the peak fractions of column chromatography and incubated for 2 min at 22°C, after which 200 μl of an 8% suspension of human erythrocytes in 0.9% NaCl was added. Hemolysis was determined spectroscopically at 405 nm after a 15-min incubation, as described previously (21, 29).
Cell cultures.
Cultures of keratinocytes (48), endothelial cells (48), monocytes (16), fibroblasts (49), and epithelial cells (HeLa [ATCC CCL 2], Hec1B [ATCC HTB 113], Chang [ATCC L20.2 clone 1-5c4], RT112 [bladder carcinoma cell line], and HEp-2 [ATCC CCL 23]) were used. Granulocytes and T cells were isolated from the blood of healthy volunteers (51).
Determination of K+.
Monolayers of epithelial cells cultured in 250-ml flasks were washed with Hanks’ balanced salt solution (HBSS) (Sigma, St. Louis, Mo.) and then incubated in 10 ml of HBSS with 20 HU of ShlA at 37°C under a 5% CO2 atmosphere. At the times given in Results, the K+ concentration in the supernatant was measured directly. Cells were washed with HBSS and lysed with 1 ml of 1% Triton X-100. For determination of the K+ concentration in the supernatant and cells, an Eppendorf IFOX 5053 flame photometer was used.
Determination of intracellular ATP.
Cellular ATP was measured by chemiluminescence as described previously (5).
Determination of cytotoxicity by the LDH release assay.
Release of lactate dehydrogenase (LDH) from eukaryotic cells indicates permeability of the membrane and cell death. The amount of LDH release was determined with a microplate assay according to the instructions of the manufacturer (Boehringer, Mannheim, Germany).
Incubation with inhibitors.
Epithelial cells were incubated with ShlA until the initial ATP level decreased to 30% or lower. The culture supernatant was replaced by medium containing 10% fetal bovine serum (FBS) (Sigma) and 2 μg of cycloheximide per ml, 500 μM ouabain, or 25 nM bafilomycin A1. Cells were incubated at 37°C under a 5% CO2 atmosphere, and the ATP concentration was determined at various time points. Vacuolated cell were incubated with 200 nM bafilomycin A1 as described previously (14).
Evaluation of vacuolation.
Epithelial cells were grown in a 24-well plate until confluent. After treatment with ShlA, cells were photographed with a phase-contrast invertoscope with or without neutral red staining.
Neutral red or trypan blue staining and determination of influx of propidium iodide.
Cells were incubated for 5 min at 37°C with complete culture medium containing 5 mM neutral red. After being washed with phosphate-buffered saline, the cells were fixed with 70% ethanol and 0.37% HCl. The absorbance was measured at 534 nm. Cells were incubated for 1 min at 37°C with 0.5% trypan blue solution (Sigma). The supernatant was removed, and the cells were examined in the invertoscope. T cells or HEp-2 cells suspended at 105 cells per ml in HBSS with propidium iodide were measured for bright red fluorescence (4, 48) in a FACScan flow cytometer (Becton Dickinson).
Osmotic protection with oligosaccharides.
The monosaccharide sorbitol (82 Da) and the oligosaccharides sucrose (342 Da), maltotriose (504 Da), maltopentaose (828 Da), dextrin 20 (900 Da), maltoheptaose (1,152 Da), dextran 15 (1,400 Da), and dextran 4 (4,000 Da) were solubilized in culture medium at a concentration of 30 mM. HEp-2 cells were treated with a sublytic dose of ShlA (3 HU/ml) in medium containing the oligosaccharides as described above. Cytotoxicity was determined with the LDH release assay.
Protein analytical procedures.
Proteins were separated by polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate, as described previously (21). Protein concentrations were determined as described previously (6, 41).
RESULTS
Vacuolation of human epithelial cells.
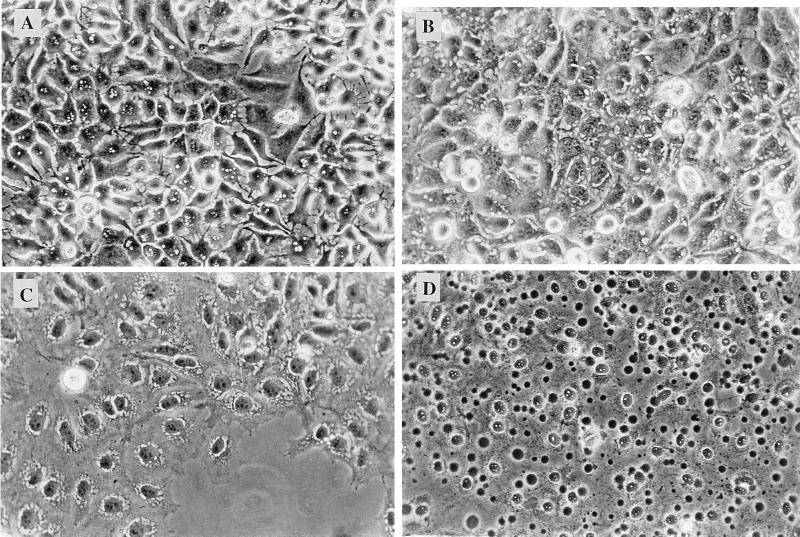
Adherent HEp-2, RT112, HeLa, Chang, and Hec1B epithelial cells were treated with a lytic dose (30 HU/ml) of a bacterial culture supernatant containing ShlA, with 30 HU of purified ShlA/ml, or with exponentially growing S. marcescens strains at a multiplicity of infection (MOI) ranging from 1 to 20. As observed by phase-contrast microscopy, vacuoles were formed with all epithelial cell lines regardless of which form of active ShlA was used. As a typical example, vacuolation of HEp-2 cells treated with 30 HU of purified recombinant ShlA/ml was observed within 15 min, followed by lysis after 40 min (Fig. 1). The same results were obtained with a culture supernatant of the ShlA-secreting recombinant E. coli strain that contained 30 HU of ShlA/ml. In contrast, Chang cells completely lysed after 15 min. Cells were treated with neutral red after vacuolation became visible. Vacuoles induced by ShlA (or ShlA-containing spent medium) did not stain with neutral red (not shown) or with trypan blue (not shown). As a control, the same cell cultures were treated with VacA-containing spent medium of Helicobacter pylori. Vacuoles were formed and were stained by neutral red; however, no lysis was detected. The beginning of vacuolation with VacA took at least 2 h, as was observed previously (13, 26), whereas the first vacuoles of ShlA-treated cells were seen after 15 min. The effects of VacA and ShlA on HeLa, RT112, Hec1B, and HEp-2 cells are compared in Table 2; the results obtained with the various epithelial cell lines were similar. Unexpectedly, vacuoles were not induced by ShlA in fibroblasts; VacA was not tested. Similar results were obtained with epithelial cell lines (HeLa, HEp-2, and Hec1B) inoculated with the exponentially growing S. marcescens strains VA15854 and CDC 04:H4 at an MOI of 20, but vacuolation was visible only after 1 h. S. marcescens W1128 did not induce vacuoles within 1 h and was less hemolytic in the standard liquid hemolysis assay (52% lysis) than CDC 04:H4 (87% lysis). Inoculation of the HeLa, HEp-2, and Hec1B epithelial cell lines with an MOI of greater than 100 led to complete lysis within 30 min.
FIG. 1.
Phase-contrast photomicrographs of ShlA-induced vacuolation of HEp-2 cells after 10 (A), 20 (B) 30 (C), and 45 (D) min. Cells were cultured in a 24-well plate until they reached subconfluence. The medium was supplemented with 100 μl of a bacterial culture supernatant containing ShlA (30 HU/ml). After incubation at 37°C under an atmosphere of 5% CO2, cells were examined by phase-contrast microscopy. Magnification, ×230.
TABLE 2.
Effects of ShlA and VacA on epithelial cells
| Effect of toxin action | Characteristic for:
|
|
|---|---|---|
| ShlA | VacA | |
| Toxin aggregation | Yes | Yes |
| Activity half-life at room temperature | 3 min | Stable |
| Induction of vacuolation reversible by bafilomycin A1 | No | Yes |
| Reversion of formed vacuoles by bafilomycin A1 | No | Yes |
| Neutral red-stainable vacuoles | No | Yes |
| Lysis of target cells | Yes | No |
ATP depletion and K+ efflux from various eukaryotic cells induced by low doses of ShlA.
The ATP concentrations in primary cell cultures of keratinocytes, endothelial cells, fibroblasts, monocytes, the epithelial cell lines HeLa, HEp-2, Chang, RT112, and Hec1B, and granulocytes and T cells freshly prepared from human blood were determined prior to and after treatment with various concentrations of ShlA (1.5 to 3 HU/ml). The results are summarized in Table 3. Keratinocytes and endothelial cells were resistant to exposure to ShlA (1 and 2 μg/ml; specific activity, 1.5 HU/μg) for 1 h at 37°C. Blood cells were slightly sensitive, but fibroblasts and all epithelial cells tested were very sensitive to ShlA. Chang cells were more sensitive and completely lysed when treated with 1 μg of ShlA/ml for 1 h. ATP depletion in epithelial cells was coupled to the efflux of potassium. ShlA-treated cells showed a decrease in cellular potassium concentrations with a corresponding increase in potassium concentrations in the supernatant. After treatment of HEp-2 cells with ShlA (2 HU/ml), the cellular potassium level, relative to 100% at 0 min, decreased to 25% after 30 min and to 15% after 60 min. ATP depletion and potassium efflux are also observed with ShlA* activated with ShlA255 (20), and the effects are similar to the effects of ShlA, whereas ShlA* alone and ShlA255 alone were completely inactive (data not shown).
TABLE 3.
ATP depletion in various cells induced by ShlA or viable S. marcescens strains
| Cell species | % of initial ATP level remaininga after incubation for 1 h at 37°C with:
|
||||
|---|---|---|---|---|---|
| ShlA
|
S. marcescensb
|
||||
| 1.5 HU/ml | 3 HU/ml | VA15854 | W1128 | CDC 04:H4 | |
| Granulocytes | 75 | 55 | NTc | NT | NT |
| Monocytes | 62 | 35 | NT | NT | NT |
| T cells | 85 | 80 | NT | NT | NT |
| Fibroblasts | 51 | 5 | NT | NT | NT |
| Endothelial cells | 100 | 100 | NT | NT | NT |
| Keratinocytes | 100 | 100 | NT | NT | NT |
| HeLa | 40 | 5 | 19 | 94 | 8 |
| HEp-2 | 50 | 14 | 32 | 100 | 28 |
| Hec1B | 12 | 0 | 22 | 100 | 15 |
| RT112 | 29 | 0 | NT | NT | NT |
| Chang | Lysed | Lysed | NT | NT | NT |
The values represent the means from three independent experiments performed in triplicate.
MOI, approximately 20; ShlA specific activity, 1.5 HU/μg.
NT, not tested.
ShlA-secreting, exponentially growing S. marcescens cells applied to epithelial cell lines at an MOI of approximately 10 also evoked ATP depletion.
ATP depletion and vacuolation.
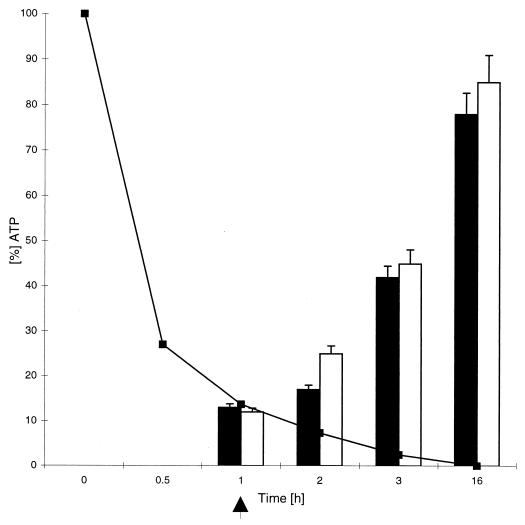
HEp-2 and HeLa cells were treated with sublytic doses of ShlA (2 HU/ml) as described above. After 3 h of incubation, the cells were completely depleted of ATP (Fig. 2). At this time point, many very small vacuoles were observed by phase-contrast microscopy (data not shown). After 3.5 h of incubation, typical spacious vacuoles (Fig. 1A) appeared; these vacuoles were identical (in lack of trypan blue- and neutral red staining and appearance under the phase-contrast microscope) to those induced by a 10-fold-higher hemolytic activity (30 HU) after 20 min.
FIG. 2.
Time course of ATP depletion induced by ShlA (2 HU/ml) in fibroblasts and HEp-2 cells (■). In a second experiment, the medium was changed after depletion of the initial ATP level to about 10% (indicated by the arrow), and the restoration of the ATP level in fibroblasts (open bars) and HEp-2 cells (filled bars) was measured. Similar results were obtained with HeLa cells. Values shown are the means and standard deviations from experiments carried out three times in triplicate.
Fibroblasts and epithelial cells can recover from ShlA-induced ATP depletion.
Cells were treated with 1.5 HU of ShlA/ml in HBSS. The initial drop in the intracellular ATP level depended on the cell species (Table 3). Under these conditions, epithelial cells were as sensitive to ShlA as fibroblasts, and were more sensitive than the other cell species tested (Table 3). After the intracellular ATP level had decreased to about 10%, the culture supernatant containing the toxin was replaced by complete medium containing FBS (Sigma). Similar to results of previous studies with staphylococcal α-toxin (49), in fibroblasts and all epithelial cells depleted of ATP by ShlA, the initial ATP level was restored after 16 h at 37°C (Fig. 2). In another experiment the efficiency of this ATP restoration was shown to be dependent on the initial drop of the ATP level (Fig. 3, cells without cycloheximide) and was presumably due to cell lysis. Depletion of the initial ATP level to lower than 20% decreased the recuperation capacity of HEp-2 cells significantly (Fig. 3), and cells began to lyse (indicated by trypan blue staining [data not shown]). Prolonged toxin treatment led to an increased ATP depletion to less than 5% of the initial ATP level and to vacuolation with subsequent cell lysis. The time course of ATP restoration in epithelial cells differed slightly from that in fibroblasts. Fibroblasts recuperated completely from an ATP depletion to 10% within 16 h (Fig. 2), which indicated integrity of the cells and resistance to ShlA, whereas epithelial cells appeared to be more sensitive to ShlA.
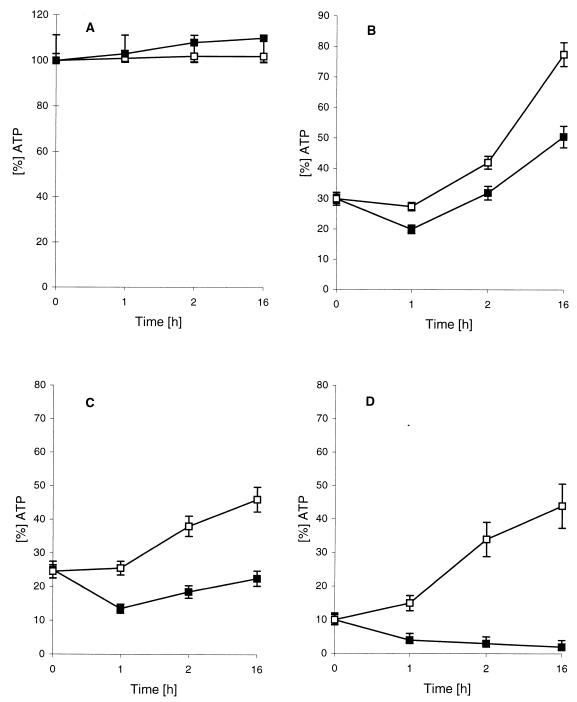
FIG. 3.
Influence of cycloheximide on restoration of the initial ATP level in HEp-2 cells. (A) Control experiment. Cells were not treated with ShlA. (B to D) Initial ATP level depleted by ShlA to 30% (B) 25% (C), and 10% (D), followed by a change in the medium (to one with 5% FBS). □, no cycloheximide; ■, 2 μg of cycloheximide/ml. Similar results were obtained with HeLa cells. Values are means and standard deviations from experiments carried out three times in triplicate.
Influence of inhibitors on restoration of the ATP level and on vacuolation of toxin-treated fibroblasts and epithelial cells.
Cycloheximide, an inhibitor of protein biosynthesis in nucleated cells, was used to investigate the influence of protein biosynthesis on the restoration of the ATP level after ShlA treatment. Fibroblasts and HEp-2, HeLa, and Hec1B cells were incubated with ShlA until the initial ATP level had decreased to 30, 25, or 10%. The ShlA-containing supernatants were replaced by complete medium (with FBS) with or without cycloheximide. In fibroblasts, recovery of the ATP level was completely inhibited by cycloheximide. In HEp-2 or HeLa cell cultures treated with ShlA, the initial ATP level was restored up to 80% within 16 h, but cycloheximide inhibited the restoration of ATP level (Fig. 3B to D [HEp-2 cells]). In cells not treated with ShlA and cycloheximide, the ATP levels stayed constant during 16 h of incubation (Fig. 3A). Depending on the initial decrease in ATP, the inhibitory effect of cycloheximide increased (Fig. 3B to D).
Bafilomycin A1, an inhibitor of vacuolar-type ATPases, inhibits the induction of vacuole formation by VacA and causes the disappearance of vacuoles previously formed by VacA (14). Bafilomycin A1 caused no inhibition of ShlA-induced vacuolation on epithelial cells and no disappearance of ShlA-induced vacuoles (Table 2). In addition, bafilomycin A1 had no significant inhibitory effect on ATP depletion after it was used to treat HEp-2 or HeLa cells treated with ShlA.
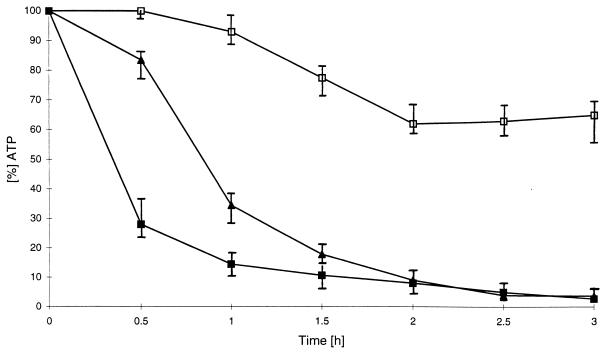
Ouabain, another ATPase inhibitor, inhibits the K+/Na+ transporter in the plasma membrane. After treatment of HEp-2 and HeLa cells with ShlA and ouabain, the decrease of the initial ATP level was clearly retarded until 30 min and then decreased to zero (Fig. 4). This decrease in the ATP level was caused by ShlA-induced lysis, as indicated by trypan blue staining (data not shown). Vacuolation was not altered or inhibited by ouabain.
FIG. 4.
Influence of 500 μM ouabain on ATP depletion by ShlA in HEp-2 cells. ■, 2 HU of ShlA per ml; □, 500 μM ouabain; ▴ 2 HU of ShlA per ml plus 500 μM ouabain. Values shown are the means and standard deviations from experiments carried out three times in triplicate.
Pores in endothelial cells, fibroblasts, T cells, and epithelial cells formed at sublytic ShlA concentrations exclude marker molecules smaller than 700 Da.
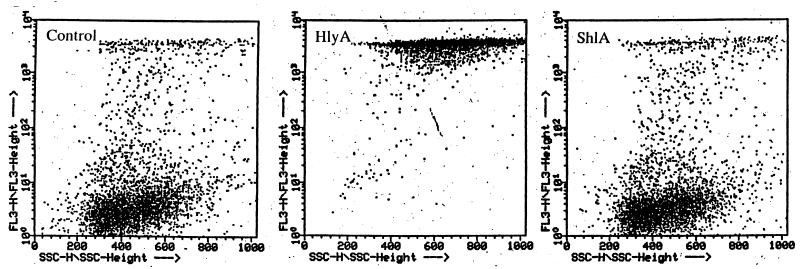
Uptake of Vital dyes (trypan blue and neutral red) by cells treated with low doses of ShlA (up to 2 μg/ml [2 HU/ml]) was measured photometrically. Depending on the cell line, ShlA concentrations were adjusted to yield an ATP level of approximately 10% (Table 3). ATP-depleted endothelial cells, fibroblasts, and epithelial cells were stained with trypan blue. ATP-depleted cells showed no increased trypan blue uptake in comparison with untreated cells (data not shown). Epithelial cells were treated with 30 HU of purified ShlA/ml or with an ShlA-containing supernatant of recombinant E. coli(pRO3) (30 HU/ml) for 15 to 20 min. Significant vacuolation was observed, but no trypan blue uptake was detected. Enhanced vacuolation over 30 min yielded increased trypan blue uptake, indicating lysis, as observed by phase-contrast microscopy (Fig. 1). Propidium iodide (Mr, 668) uptake by ShlA-treated T cells was measured by flow cytometry. Cells were incubated with various concentrations of purified ShlA for 1 h at 37°C. T cells, with the ATP level depleted to 10% were used for fluorescence-activated cell sorter analysis. A FACScan plot of propidium iodide influx in ATP-depleted T cells is shown in Fig. 5. ShlA-treated cells showed no propidium iodide influx, in contrast to HlyA-treated cells, which took up the dye as expected, since HlyA is known to create larger pores in eukaryotic membranes.
FIG. 5.
Demonstration that HlyA and ShlA produce pores in T cells that are either permeable (HlyA) or nonpermeable (ShlA) to propidium iodide. Cells were treated with 1 μg of HlyA/ml and 4 μg of ShlA/ml in HBSS buffered with 10 mM HEPES, pH 7.4. ShlA evoked an ATP depletion to 10% of the original ATP level after 60 min of incubation at 37°C. The medium was then replaced with HBSS supplemented with 10 μg of propidium iodide/ml. After 30 min at 37°C, samples were subjected to flow cytometric analysis. Experiments were carried out in duplicate, and similar results were obtained.
Protection of HEp-2 cells against osmotic lysis by oligosaccharides.
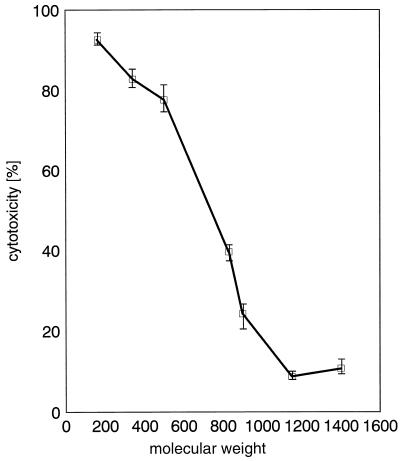
Incubation of HEp-2 cells with ShlA and a number of oligosaccharides at a concentration of 30 mM reduced the cytotoxicity of ShlA significantly. The protective effect of the oligosaccharides depended on their molecular weights. Sucrose (Mr, 342) and maltotriose (Mr, 504) had no effect on the cytotoxicity of ShlA (determined by LDH release), whereas maltopentaose (Mr, 828) reduced cytotoxicity to about 40% and maltoheptaose (Mr, 1,152) and dextran 15 (Mr, 1,400) nearly completely inhibited the cytotoxicity of ShlA (to below 10%) (Fig. 6). When the medium of ShlA-treated cells was changed to a medium free of ShlA and protective oligosaccharides, the cytotoxicity of cell-bound ShlA was markedly increased, indicating cell lysis. Interestingly, ATP depletion was not affected by the presence of any oligosaccharide under the conditions described above; the ATP level decreased like for the cases shown in Table 3 and Fig. 2. Hemolysis-negative ShlA* (inactive form) was not cytotoxic, but cytotoxicity and osmoprotective effects similar to those with ShlA were observed when ShlA* was complemented with ShlA255 (data not shown).
FIG. 6.
Influence of oligosaccharides on the cytotoxicity of ShlA to HEp-2 cells. Sorbitol (Mr, 182), sucrose (342), maltotriose (504), maltopentaose (828), dextrin 20 (900), maltoheptaose (1,152), dextran 15 (1,400), or dextran 4 (4,000) was added to a final concentration of 30 mM in complete culture medium. Cells were coincubated with 3 HU of ShlA/ml for 1 h at 37°C under a 5% CO2 atmosphere. Values shown are the means and standard deviations from experiments carried out three times in triplicate.
DISCUSSION
Vacuolation was observed with recombinant-ShlA-containing supernatants of E. coli and with chromatographically purified ShlA. Since recombinant ShlA is the only protein secreted by the E. coli BL21 shlB shlA transformant, ShlA was the main protein in the culture supernatant; only minor amounts of contaminating cytoplasmic proteins of E. coli were present. For purification, ShlA must be precipitated from the supernatant and chromatographed. During these procedures, ShlA remains native and active, since crude extracts and purified ShlA were indistinguishable in their hemolytic or cytolytic effects.
ShlA-induced vacuolation in epithelial cells was quite different from vacuolation induced by VacA. VacA-induced vacuoles originate from massive swelling of membranous compartments of late stages of the endocytic pathway by acting in the cell cytosol (13, 15). The lumen of the spacious vacuoles is acidified by the activity of a membrane-bound vacuolar-type ATPase (14, 30), which is inhibited by bafilomycin A1. In contrast to that with VacA, vacuolation induced by ShlA was very fast and was not inhibited or reversed by bafilomycin A1. In addition, vacuolation induced by VacA initiates around the nucleus, whereas ShlA-induced vacuoles appeared simultaneously throughout the cytoplasm. The obviously different genesis of ShlA-induced vacuoles is supported by the observation that ShlA-induced vacuoles were not stained by neutral red, which indicates that they were not acidified. Apparently, vacuoles induced by ShlA are not derived from late endosomes, as has been shown for VacA (13). A phenotype similar to that of ShlA-induced vacuolation has been observed with aerolysin from Aeromonas hydrophila (1). Abrami et al. (1) have shown that pore formation by this toxin leads to permeabilization of the plasma membrane for small ions without membrane disruption and that the toxin causes dramatic vacuolation of the endoplasmic reticulum but not of other intracellular compartments. Since vacuolation with ShlA was observed only in epithelial cells, and not in fibroblasts, vacuolation by ShlA may be specific for epithelial cells. Explanations for this difference may be that fibroblasts affect the toxin activity or that they are more efficient than epithelial cells in repairing the lesions caused by ShlA. The mechanism of vacuolation is not known, and examination of this process may lead to a better understanding of the different behaviors of epithelial cells and fibroblasts.
Vacuolation obtained with purified ShlA was identical to vacuolation observed with exponentially growing hemolytic S. marcescens cells, indicating that vacuolation is caused mainly by ShlA. The supernatant of exponentially growing S. marcescens cells was only slightly hemolytic. In S. marcescens cultures, ShlA was cytotoxic mainly while adsorbed to the bacteria (cell-bound hemolytic activity [23]), since released ShlA forms aggregates and precipitates and is proteolytically degraded (the half-life of hemolytic activity in culture medium is 3 min at 37°C). Therefore, S. marcescens exerts hemolytic and vacuolating activities mainly in direct contact with the target cells, as has also been shown with erythrocytes (8). A similar kind of bacterium-mediated vacuolation has also been observed after infection with Vibrio parahaemolyticus (22) or with E. coli O157:H7 (17) but has not been studied with isolated toxins.
The key to understanding this vacuolation might be the observed ATP depletion in target cells. Prior to vacuolation, ShlA-treated cells were depleted of ATP. As with staphylococcal α-toxin, the observed ATP depletion induced by ShlA was caused by potassium efflux. It was clearly shown that ATP depletion was caused not by an ATP efflux but by an enhanced Na+/K+-ATPase activity, as indicated by ouabain inhibition of ATP depletion. An imbalance in the cellular potassium levels activates the ATP-driven Na+/K+-pump and decreases intracellular ATP. This imbalance in ions also occurs in osmoprotected cells, and therefore they become depleted of ATP. Potassium efflux and ATP depletion evoked by ShlA indicate that small pores were formed in the nucleated cell membranes. These pores were not permeable to trypan blue or propidium iodide. The pores formed by staphylococcal α-toxin are also not permeable to these dyes, in contrast to those formed by E. coli hemolysin HlyA, which are permeable to propidium iodide. The consequence of this pore formation in epithelial cells appeared to be the induction of vacuolation by ShlA. ShlA pores in the plasma membrane lead to the osmotic influx of water into the cells with swelling and subsequent lysis, as has been shown with erythrocytes, where osmotic protection by oligosaccharides with molecular masses of greater than 900 Da prevents osmolysis (38). On nucleated epithelial cells, the pore diameter appears to be smaller, because oligosaccharides larger than 900 Da protected HEp-2 cells from ShlA-mediated lysis but not from ATP depletion. It is obvious that the cells released potassium through the pores formed by ShlA (resulting in ATP depletion) and that the pores and ShlA were not affected directly by the oligosaccharides. The larger oligosaccharides prevented osmolysis because they are too large to enter the erythrocytes through the ShlA pores and thus counterbalance the internal osmotic pressure of the erythrocytes and nucleated eukaryotic cells. These data clearly show that ShlA forms pores not only in artificial bilayers and erythrocytes but also in epithelial cells.
ATP depletion in ShlA-treated cells was reversed by a change to a medium free of ShlA. This restoration of the initial ATP level depended on the time at which the medium was changed. After the change of medium, the ATP level decreased over the first 30 min and then increased continuously. This time course of ATP level restoration indicates that the fibroblasts and epithelial cells are able to inactivate the toxin pores by possibly closing the pores, by proteolytic degradation of ShlA, or by a complete shedding of the toxin from the membrane. However, staphylococcal α-toxin has been shown to remain inactive on the cell surface (49) without shedding or proteolytic degradation.
Unexpectedly, the repair of ShlA pores was inhibited by cycloheximide, whereas restoration of the ATP level in fibroblasts after treatment with staphylococcal α-toxin is unaffected by cycloheximide (47). The degree of inhibition by cycloheximide depended on the initial ATP depletion. HEp-2 cells with the ATP level depleted to 30% of the initial level were more viable and the ATP level was restored up to 80%, indicating a presumed cell death of about 20%. The extended destruction of HEp-2 cells by ShlA treatment led to minimal ATP level restoration (about 50%). Interestingly, the inhibitory effect of cycloheximide was higher with lower initial ATP levels. These data suggest that the repair of ShlA pores is dependent on protein synthesis and that extended ShlA treatment exhausts the capacity for ATP restoration.
Cytotoxicity of ShlA, as indicated by LDH release, was shown. ShlA itself has not been shown to have proteolytic or lipolytic activity to date. The hemolytic activity of S. marcescens did not originate from the exoprotease secreted by this bacterium (37). Also, no phospholipase activity, like that of the α-toxin of Clostridium perfringens (phospholipase D), was found. In contrast, ShlA needs phosphatidylethanolamine for activation by ShlB (20) and binds phosphatidylethanolamine strongly without degradation. ShlA is also a potent hemolysin even at 0°C (38), which excludes any enzymatic activity. The hemolytic activity of ShlA is due to its pore-forming capacity. This does not exclude a second cytotoxic activity, but no functional similarities with other toxins (e.g., the bifunctional protein adenylate cyclase of Bordetella pertussis [19]) have been observed.
ShlA showed target cell specificity different from that of other bacterial pore-forming toxins, such as staphylococcal α-toxin and E. coli hemolysin (48, 50). ShlA was capable of inducing ATP depletion and potassium efflux in epithelial cells and in fibroblasts. ShlA activity on epithelial cells differs from the specificity of staphylococcal α-toxin on keratinocytes and endothelial cells (45, 48) and monocytes (5), which are unaffected by ShlA. ShlA has no high-affinity binding site (38) (like that of α-toxin), and the pore structure is certainly not a heptamer (38) as has been shown for staphylococcal α-toxin (42). The different molecular organizations of the two toxins may contribute to the different target cell specificities.
S. marcescens as a pathogen has the capacity to damage tissue, and this depends on ShlA secretion (23, 27). Edwardsiella tarda synthesizes a hemolysin that is homologous to ShlA (43). Hemolysin production by E. tarda enhances HEp-2 cell invasion significantly. For Proteus mirabilis, which also contains a homologous hemolysin, fimbriae and properties such as swarming are more important than hemolysin production for host cell invasion (2). Adhesion to epithelial cells mediated by type 1 fimbriae of S. marcescens is known (24, 51). Fimbriae of S. marcescens also contribute to superoxide production (27, 28) and phagocytosis (28). It is not known whether ShlA is involved in adhesion or putative invasion of S. marcescens, but the cytolytic activity may enable S. marcescens to penetrate tissue layers and invade the host organism. Examination of putative invasion and the dependence of the repair of ShlA pores on protein biosynthesis may lead to a better insight into the behavior of this pore-forming toxin on eukaryotic cell membranes and into the bacterium-host cell interaction in the pathogenicity of S. marcescens.
ACKNOWLEDGMENTS
We thank V. Braun and S. Bhakdi for support and helpful discussions, W. Fischer for excellent photographs illustrating vacuolation, and Karen A. Brune for editing the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 323, project B1).
REFERENCES
- 1.Abrami L, Fivaz M, Glauser P E, Parton R G, van-der-Goot F G. A pore-forming toxin interacts with a GPI-anchored protein and causes vacuolation of the endoplasmic reticulum. J Cell Biol. 1998;140:525–540. doi: 10.1083/jcb.140.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison C, Coleman N, Jones P L, Hughes C. Ability of Proteus mirabilis to invade human urothelial cells is coupled to motility and swarming differentiation. Infect Immun. 1992;60:4740–4746. doi: 10.1128/iai.60.11.4740-4746.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernheimer A W. Assay of hemolytic toxins. Methods Enzymol. 1988;165:213–217. doi: 10.1016/s0076-6879(88)65033-6. [DOI] [PubMed] [Google Scholar]
- 4.Bhakdi S, Martin E. Superoxide generation by human neutrophils induced by low doses of Escherichia coli hemolysin. Infect Immun. 1991;59:2955–2962. doi: 10.1128/iai.59.9.2955-2962.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhakdi S, Muhly M, Korom S, Hugo F. Release of interleukin-1β associated with potent cytocidal action of staphylococcal alpha-toxin on human monocytes. Infect Immun. 1989;57:3512–3519. doi: 10.1128/iai.57.11.3512-3519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Braun V, Focareta T. Pore-forming bacterial protein hemolysins (cytolysins) Crit Rev Microbiol. 1991;18:115–158. doi: 10.3109/10408419109113511. [DOI] [PubMed] [Google Scholar]
- 8.Braun V, Günther H, Neuss B, Tautz C. Hemolytic activity of Serratia marcescens. Arch Microbiol. 1985;141:371–376. doi: 10.1007/BF00428852. [DOI] [PubMed] [Google Scholar]
- 9.Braun V, Hobbie S, Ondraczek R. Serratia marcescens forms a new type of cytolysin. FEMS Microbiol Lett. 1992;79:299–305. doi: 10.1111/j.1574-6968.1992.tb14056.x. [DOI] [PubMed] [Google Scholar]
- 10.Braun V, Neuss B, Ruan Y, Schiebel E, Schöffler H, Jander G. Identification of the Serratia marcescens hemolysin determinant by cloning into Escherichia coli. J Bacteriol. 1987;169:2113–2120. doi: 10.1128/jb.169.5.2113-2120.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun V, Schoenherr R, Hobbie S. Enterobacterial hemolysins: activation, secretion and pore formation. Trends Microbiol. 1993;1:211–216. doi: 10.1016/0966-842x(93)90134-d. [DOI] [PubMed] [Google Scholar]
- 12.Carbonell G V, Vidotto M C. Virulence factors in Serratia marcescens: cell-bound hemolysin and aerobactin. J Med Biol Res. 1992;25:1–8. [PubMed] [Google Scholar]
- 13.Catrenich C E, Chestnut M H. Character and origin of vacuoles induced in mammalian cells by the cytotoxin of Helicobacter pylori. J Med Microbiol. 1992;37:389–395. doi: 10.1099/00222615-37-6-389. [DOI] [PubMed] [Google Scholar]
- 14.Cover T L, Reddy L Y, Blaser M J. Effects of ATPase inhibitors on the response of HeLa cells to Helicobacter pylori vacuolating toxin. Infect Immun. 1993;61:1427–1431. doi: 10.1128/iai.61.4.1427-1431.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Bernard M, Arico B, Papini E, Rizzuto R, Grandi G, Rappuoli R, Montecucco C. Helicobacter pylori toxin VacA induces vacuole formation by acting in the cell cytosol. Mol Microbiol. 1997;26:665–674. doi: 10.1046/j.1365-2958.1997.5881952.x. [DOI] [PubMed] [Google Scholar]
- 16.Denholm E M, Wolber F M. A simple method for the purification of human peripheral blood monocytes. J Immunol Methods. 1991;144:247–251. doi: 10.1016/0022-1759(91)90092-t. [DOI] [PubMed] [Google Scholar]
- 17.Fratamico P M, Buchanan R L, Cooke P H. Virulence of an Escherichia coli O157:H7 sorbitol-positive mutant. Appl Environ Microbiol. 1993;59:4245–4252. doi: 10.1128/aem.59.12.4245-4252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gadeberg O V, Orskov I. In vitro cytotoxic effect of alpha-hemolytic Escherichia coli on human blood granulocytes. Infect Immun. 1984;45:255–260. doi: 10.1128/iai.45.1.255-260.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glaser P, Sakamoto H, Bellalou J, Ullmann A, Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988;7:3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hertle R, Brutsche S, Groeger W, Hobbie S, Koch W, Könninger U, Braun V. Specific phosphatidylethanolamine dependence of Serratia marcescens cytotoxin activity. Mol Microbiol. 1997;26:853–865. doi: 10.1046/j.1365-2958.1997.6031978.x. [DOI] [PubMed] [Google Scholar]
- 21.Hilger M, Braun V. Superlytic hemolysin mutants of Serratia marcescens. J Bacteriol. 1995;177:7202–7209. doi: 10.1128/jb.177.24.7202-7209.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoashi K, Ogata K, Taniguchi H, Yamashita H, Tsuji K, Mizuguchi Y, Ohtomo N. Pathogensis of Vibrio parahaemolyticus: intraperitoneal and orogastric challenge experiments in mice. Microbiol Immunol. 1990;34:355–366. doi: 10.1111/j.1348-0421.1990.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 23.König W, Faltin Y, Scheffer J, Schöffler H, Braun V. Role of cell-bound hemolysin as a pathogenicity factor for Serratia infections. Infect Immun. 1987;55:2554–2561. doi: 10.1128/iai.55.11.2554-2561.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreft B, Carstensen O, Straube E, Bohnet S, Hacker J, Marre R. Adherence to and cytotoxicity of Escherichia coli for eukaryotic cell lines quantified by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) Int J Med Microbiol Virol Parasitol Infect Dis. 1992;276:231–242. doi: 10.1016/s0934-8840(11)80010-1. [DOI] [PubMed] [Google Scholar]
- 25.Leranoz S, Orus P, Berlanga M, Dalet F, Vinas M. New fimbrial adhesins of Serratia marcescens isolated from urinary tract infections: description and properties. J Urol. 1997;157:694–698. [PubMed] [Google Scholar]
- 26.Leunk R D, Johnson P T, David B C, Kraft W G, Morgan D R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988;26:93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- 27.Marré R, Hacker J, Braun V. The cell-bound hemolysin of Serratia marcescens contributes to uropathogenicity. Microb Pathog. 1989;7:153–156. doi: 10.1016/0882-4010(89)90034-x. [DOI] [PubMed] [Google Scholar]
- 28.Mizunoe Y, Matsumoto T, Haraoka M, Sakumoto M, Kubo S, Kumazawa J. Effect of pili of Serratia marcescens on superoxide production and phagocytosis of human polymorphonuclear leukocytes. J Urol. 1995;154:1227–1230. [PubMed] [Google Scholar]
- 29.Ondraczek R, Hobbie S, Braun V. In vitro activation of the Serratia marcescens hemolysin through modification and complementation. J Bacteriol. 1992;174:5086–5094. doi: 10.1128/jb.174.15.5086-5094.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papini E, Satin B, Bucci C, de Bernard M, Telford J L, Manetti R, Rappuoli R, Zerial M, Montecucco C. The small GTP binding protein rab7 is essential for cellular vacuolation induced by Helicobacter pylori cytotoxin. EMBO J. 1997;16:15–24. doi: 10.1093/emboj/16.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pellett S, Welch R A. Escherichia coli hemolysin mutants with altered target cell specificity. Infect Immun. 1996;64:3081–3087. doi: 10.1128/iai.64.8.3081-3087.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poole K, Braun V. Influence of growth temperature and lipopolysaccharide on hemolytic activity of Serratia marcescens. J Bacteriol. 1988;170:5146–5152. doi: 10.1128/jb.170.11.5146-5152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poole K, Schiebel E, Braun V. Molecular characterization of the hemolysin determinant of Serratia marcescens. J Bacteriol. 1988;170:3177–3188. doi: 10.1128/jb.170.7.3177-3188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruan Y, Braun V. Hemolysin as a marker for Serratia. Arch Microbiol. 1990;154:221–225. doi: 10.1007/BF00248958. [DOI] [PubMed] [Google Scholar]
- 35.Schiebel E, Braun V. Integration of the Serratia marcescens haemolysin into human erythrocyte membranes. Mol Microbiol. 1989;3:445–453. doi: 10.1111/j.1365-2958.1989.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 36.Schiebel E, Schwarz H, Braun V. Subcellular location and unique secretion of the hemolysin of Serratia marcescens. J Biol Chem. 1989;264:16311–16320. [PubMed] [Google Scholar]
- 37.Schmitz G, Braun V. Cell-bound and secreted proteases of Serratia marcescens. J Bacteriol. 1985;161:1002–1009. doi: 10.1128/jb.161.3.1002-1009.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoenherr R, Hilger M, Broer S, Benz R, Braun V. Interaction of Serratia marcescens hemolysin (ShlA) with artificial and erythrocyte membranes: demonstration of the formation of aqueous multistate channels. Eur J Biochem. 1994;223:655–663. doi: 10.1111/j.1432-1033.1994.tb19038.x. [DOI] [PubMed] [Google Scholar]
- 39.Schoenherr R, Tsolis R, Focareta T, Braun V. Amino acid replacements in the Serratia marcescens haemolysin ShIA define sites involved in activation and secretion. Mol Microbiol. 1993;9:1229–1237. doi: 10.1111/j.1365-2958.1993.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 40.Sieben S, Hertle R, Gumpert J, Braun V. Expression and secretion of Serratia marcescens hemolysin in stable protoplast type L-forms of Proteus mirabilis. Arch Microbiol. 1998;170:236–242. doi: 10.1007/s002030050638. [DOI] [PubMed] [Google Scholar]
- 41.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. . (Erratum, 163:279, 1987.) [DOI] [PubMed] [Google Scholar]
- 42.Song L, Hobaugh M R, Shustak C, Cheley S, Bayley H, Gouaux J E. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science. 1996;274:1859–1866. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 43.Strauss E J, Ghori N, Falkow S. An Edwardsiella tarda strain containing a mutation in a gene with homology to shlB and hpmB is defective for entry into epithelial cells in culture. Infect Immun. 1997;65:3924–3932. doi: 10.1128/iai.65.9.3924-3932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Studier F W, Moffatt B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 45.Suttorp N, Hessz T, Seeger W, Wilke A, Koob R, Lutz F, Drenckhahn D. Bacterial exotoxins and endothelial permeability for water and albumin in vitro. Am J Physiol. 1988;255:C368–C376. doi: 10.1152/ajpcell.1988.255.3.C368. [DOI] [PubMed] [Google Scholar]
- 46.Tabor S, Richardson C C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valeva, A. 1997. Personal communication.
- 48.Walev I, Martin E, Jonas D, Mohamadzadeh M, Müller-Klieser W, Kunz L, Bhakdi S. Staphylococcal alpha-toxin kills human keratinocytes by permeabilizing the plasma membrane for monovalent ions. Infect Immun. 1993;61:4972–4979. doi: 10.1128/iai.61.12.4972-4979.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walev I, Palmer M, Martin E, Jonas D, Weller U, Höhn-Bentz H, Husmann M, Bhakdi S. Recovery of human fibroblasts from attack by the pore-forming α-toxin of Staphylococcus aureus. Microb Pathog. 1994;17:187–201. doi: 10.1006/mpat.1994.1065. [DOI] [PubMed] [Google Scholar]
- 50.Walev I, Reske K, Palmer M, Valeva A, Bhakdi S. Potassium-inhibited processing of IL-1β in human monocytes. EMBO J. 1995;14:1607–1614. doi: 10.1002/j.1460-2075.1995.tb07149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weir D M. Handbook of experimental immunology. Oxford, United Kingdom: Blackwell Scientific Publishers; 1986. [Google Scholar]
- 52.Yamamoto T, Ariyoshi A, Amako K. Fimbriae-mediated adherence of Serratia marcescens strain US5 to human urinary bladder surface. Microbiol Immunol. 1985;29:677–681. doi: 10.1111/j.1348-0421.1985.tb00871.x. [DOI] [PubMed] [Google Scholar]