Abstract
The incidence of fungal infections caused by the opportunistic yeast Candida albicans has increased significantly in recent years. The ability to vaccinate selected patients against the organism would be advantageous. In this paper we describe a potential anti-C. albicans vaccine consisting of heat-killed C. albicans (HK-CA) in combination with the novel mucosal adjuvant LT(R192G), a genetically detoxified form of the heat-labile toxin of enterotoxigenic Escherichia coli. Groups of male CBA/J mice were immunized intranasally on three occasions at weekly intervals with 2 × 107 HK-CA per dose, alone or in conjunction with 10 μg of LT(R192G) per dose. Two weeks following the last application of antigen, some animals were challenged intravenously (i.v.) with 104, 105, or 106 viable C. albicans to assess protection as measured by survival and/or culture. Some groups of animals were footpad tested with C. albicans mannan to assess delayed-type hypersensitivity (DTH), and all the animals were bled for antibody assays. In two independent studies, all the animals immunized with HK-CA plus LT(R192G) were able to eradicate 104 C. albicans completely, as determined by kidney culture 4 weeks after challenge. Animals immunized with HK-CA only had reduced levels of C. albicans compared to the adjuvant or saline-only control. Greatly enhanced survival was observed when mice immunized with HK-CA plus LT(R192G) were challenged with 105 live C. albicans as well. Animals immunized with HK-CA plus LT(R192G) developed a significant DH response, while those given HK-CA alone developed only marginal DH responses. High immunoglobulin G (IgG) levels to cytoplasmic antigens developed in mice immunized with HK-CA plus LT(R192G), but they were found only after i.v. challenge. Addition of adjuvant shifted the antibody isotype production in i.v.-challenged animals to a response dominated by IgG2a. Clearly, intranasal immunization with killed C. albicans in conjunction with LT(R192G) afforded significant levels of protection. This novel approach offers new possibilities for the development of an effective vaccine against candidiasis for use in humans.
Candida albicans is a ubiquitous fungus, which, along with other species of Candida, may be found as part of the normal flora of humans (11). Although healthy individuals are regularly colonized with Candida, serious disease seldom occurs unless some precipitating factor alters the balance in favor of the fungus. Unfortunately, precipitating factors such as immunosuppressive therapies and diseases involving down-regulation of the immune system, including AIDS, are becoming more prevalent. Thus, serious forms of candidiasis are on the increase, and a vaccine capable of stimulating immunity in patients prior to the institution of immunosuppressive therapies could be of considerable value.
A rational approach to the development of a vaccine is problematic because the specific immune system mechanisms responsible for protective immunity have not been defined clearly. Moreover, candidiasis is a multifaceted disease which may manifest itself at multiple levels, including mucocutaneous tissue and internal organs. To complicate the issue further, the protective mechanisms associated with different tissues appear to be different. For example, cellular immunity appears to be critical for the defense of mucocutaneous tissue (1) but does not appear to be the sole critical factor in systemic defense (5). T lymphocytes appear to be important for the development of acquired immunity (7, 25, 44), but a specific role for T cells has not been established. It is not known, for example, whether T cells are critical for antibody production, for the development of cellular immune system phenomena, or for the production of cytokines that alter the activity of non-(antigen)-specific phagocytic cells. In fact, antibody, cellular immunity, and innate immunity as a composite may be responsible for the protection observed following immunization with the organism. Since life-threatening forms of candidiasis occur at the systemic level, and since the precise protective mechanisms at that level are ill defined, the development of a vaccine for the disease must be empirical.
In addition to our lack of knowledge about the component(s) of the immune system most responsible for acquired immunity at the systemic level, the nature of the immunogen(s) that stimulates a protective responses is also largely unknown. The best protective effects observed to date have been stimulated by immunization with viable cells from virulent (26, 40) or avirulent (3) strains of C. albicans. Killed cells or subcellular components have been moderately successful (2, 20, 35, 39, 49). Although some evidence has accumulated that antibody to specific immunogens, e.g., hsp90 (38), hsp75, or non-hsp96 (13), is protective systemically, much of the evidence is circumstantial.
With the advent of newer adjuvants and protocols for immunization, it seems timely to revisit the issue of immunization against C. albicans. An approach that has been explored recently involves mucosal immunization with inactivated organisms or purified immunogenic components derived from virulent organisms, delivered in combination with a mucosal adjuvant (17, 21).
Cholera toxin (CT) produced by various strains of Vibrio cholerae and heat-labile enterotoxin (LT) produced by some enterotoxigenic strains of Escherichia coli (10, 22, 36, 53, 54) are the two bacterial products with the greatest potential to function as mucosal adjuvants. Recent studies have examined the potential of CT and LT as mucosal adjuvants against a variety of bacterial and viral pathogens in vaccines containing whole killed organisms or purified subunits of relevant virulence determinants from these organisms. Representative examples include tetanus toxoid (52–54), inactivated influenza virus (28, 31), recombinant urease from Helicobacter spp. (34, 50), pneumococcal surface protein A from Streptococcus pneumoniae (51), Norwalk virus capsid protein (37), synthetic peptides from measles virus (29), and the human immunodeficiency virus type 1 C4/V3 peptide T1SP10 MN(A) (48). There are many other examples, and it is clear that both LT and CT have significant potential for use as adjuvants for mucosally administered antigens (see references 17 and 21 for recent reviews). However, the fact that these two proteins are toxic for humans and animals, even at low doses, precludes their practical use in vaccines.
A series of mutants of LT and CT have been developed in an effort to dissociate the adjuvant properties of these molecules from their toxic effects. One of these LT mutants, designated LT(R192G), was constructed by using site-directed mutagenesis to create a single amino acid substitution in the biologically active domain (A subunit). This mutation rendered the toxin insensitive to trypsin activation and consequently greatly diminished its toxicity without altering the intrinsic adjuvanticity characteristic of the native molecule (16). A number of reports published recently have evaluated the efficacy of LT(R192G) as an effective mucosal adjuvant (8, 32, 41).
In this paper, we report the effectiveness of a vaccine composed of heat-inactivated C. albicans and LT(R192G) to potentiate a protective immune response against intravenous (i.v.) challenge with live, virulent C. albicans.
MATERIALS AND METHODS
Mice.
Male CBA/J mice, 6 to 8 weeks of age, obtained from Jackson Laboratory (Bar Harbor, Maine) were housed in bioclean hoods and provided with food and water ad lib. All mice were allowed a 1-week acclimatization period before the experiments were begun.
Vaccine preparation.
C. albicans 20A, a serotype A isolate originally obtained from Errol Reiss at the Centers for Disease Control and Prevention, was used for all experiments. This strain was maintained at 4°C with monthly transfer on Sabouraud dextrose agar. Live C. albicans used for challenge and for intradermal (i.d.) inoculations was prepared as follows. Cultures of C. albicans were incubated for 24 h on slants of Sabouraud dextrose agar at 37°C, inoculated into Trypticase soy dialysate broth (43), and incubated at 37°C on a gyratory shaker operating at 165 rpm. The cells were harvested after 18 h and washed three times in nonpyrogenic saline (NPS). The final pellet was resuspended in NPS, and the cells were counted in a hemocytometer and diluted to the appropriate concentration in NPS. The viability of the culture was determined by plate count. C. albicans used for immunization was prepared as described above, except that the cell suspension was heated at 60°C for 2 h. The lack of viability of this preparation was confirmed by plating 109 cells on Sabouraud dextrose agar and incubating them at 37°C overnight. Heat-killed C. albicans is designated HK-CA below.
The mucosal adjuvant, LT(R192G), used in this study has been described previously and was purified in our laboratory by published procedures (9, 16). This adjuvant is a genetically detoxified mutant of LT, derived from enterotoxigenic E. coli, that is no longer sensitive to trypsin activation but retains the immunologic and adjuvant properties characteristic of the native LT molecule.
Immunization procedures and method.
Two types of experiments were performed. For intranasal (i.n.) immunizations, the mice were lightly anesthetized with Metofane (Pitman-Moore, Mundelein, Ill.) and the inoculum was delivered as two applications of 5 μl to each nostril for a total volume of 20 μl per dose.
The first set of experiments was carried out to determine whether vaccination as above protected immunized animals against C. albicans challenge. Three groups of 7 to 12 animals each were immunized i.n. on three occasions at weekly intervals with 2 × 107 HK-CA in conjunction with 10 μg of LT(R192G) per dose or with either 10 μg of LT(R192G) or NPS alone. Some experiments also included animals that were immunized twice i.d. with 2 × 106 viable C. albicans or once intragastrically (i.g.) with 2 × 107 viable C. albicans followed by a single i.d. inoculation with 2 × 106 viable organisms as described previously (18, 25). In one experiment, a group of animals was immunized twice with 2 × 107 HK-CA administered intragastrically. Two weeks after the last immunization, all the animals were challenged i.v. via the lateral tail vein with 104, 105, or 106 viable C. albicans. Studies to determine protection following i.v. challenge were conducted three times. In the first two experiments, protection was assessed by culturing the kidneys and brains of surviving animals for the presence of viable C. albicans 4 weeks after challenge with 104 live C. albicans. In the third experiment, protection was assessed by monitoring survival for 100 days after challenge with 105 or 106 live C. albicans. At the end of this period, all the surviving animals were sacrificed and blood was collected for serological analysis.
A second set of experiments was performed to analyze the levels of delayed-type hypersensitivity (DTH) and antibody responses 2 weeks following the completion of the immunization protocol, i.e., at the time when i.v. challenge was performed in the experiments described above. In these experiments, groups of five or six mice were immunized with HK-CA plus adjuvant or with HK-CA, adjuvant, or NPS alone, as described above. A control group was inoculated intradermally twice with 106 live C. albicans. These animals were then footpad tested to detect DTH and subsequently bled to determine antibody responses.
DTH.
The development of DTH in response to each immunization regimen was determined by the footpad assay as described previously (12, 14). Footpad thickness was measured 24 and 48 h after the antigen (mannan) was injected, and the mean thickness was calculated by subtracting preinjection measurements from postinjection measurements. Each mouse was inoculated in the footpad with 5 μg of C. albicans mannan as described previously (24).
Serological analysis.
All the animals were sacrificed by CO2 inhalation, and blood was immediately collected by cardiac puncture. Serum samples were separated by centrifugation in Microtainer tubes (Becton Dickinson & Co., Franklin Lakes, N.J.) and stored at −20°C until analyzed. Each serum sample was individually analyzed by enzyme-linked immunosorbent assays (ELISA). For all ELISAs, 96-well plates were coated with 5 μg of soluble cytoplasmic substances (SCS) per well prepared from viable C. albicans as described previously (19) or with a dithiothreitol extract (DTTE) from cell walls (42) and incubated overnight at 4°C. All subsequent steps were carried out at room temperature. After blocking nonspecific sites with 1% bovine serum albumin, twofold serial dilutions of serum from the experimental animals were added. Alkaline phosphatase-conjugated rabbit anti-mouse immunoglobulin G (IgG) or anti-mouse IgA (Sigma Chemical Co., St. Louis, Mo.) was used for determination of the total concentration of IgG or IgA in serum. Biotinylated anti-mouse IgG1, IgG2a, IgG2b, or IgG3 (PharMingen, San Diego, Calif.) followed by alkaline phosphatase-conjugated streptavidin (Southern Biotechnology Associates, Birmingham, Ala.) was used to quantify antibody isotypes. The optical density at 405 nm was determined with an ELISA reader (Bio-Tek Instruments, Inc., Winooski, Vt.) after addition of 100 μl of a 1-mg/ml solution of p-nitrophenyl phosphate (Sigma).
Western blot analyses were carried out essentially as described previously (23) by sodium dodecyl sulfate-gel electrophoresis on a 10% NuPage Bis-Tris gel run on an XCELL II mini-cell and blot module (Novex Experimental Technologies, San Diego, Calif.). The antigens for electrophoresis were prepared as follows. A 5-mg aliquot of SCS was dissolved in 1 ml of NPS, and 20 μl of this solution was added to 5 μl of sample buffer containing reducing agent. The molecular mass markers used were MultiMarkTM multi-colored standard (Novex Experimental Technologies), which ranged from 3 to 185 kDa. The samples were electrophoresed for 1.75 h at 100 V. Following protein transfer to nitrocellulose sheets, the membranes were blocked with 3% skim milk, and the strips of nitrocellulose were incubated with individual mouse sera diluted 1:100. The antigen-antibody reactions were detected with anti-mouse IgG (whole molecule) conjugated to alkaline phosphatase (Sigma) diluted 1:500, followed by a brief incubation in 5-bromo-4-chloro-3-indolyl phosphate–nitroblue tetrazolium substrate (Sigma).
Statistical analysis.
The total colony-forming units per organ were determined by standard dilution calculations and expressed as logarithmic numbers. Thereafter, logarithmic numbers for the respective groups were averaged to obtain geometric means. Since there is no logarithmic value for zero, it was assumed that a single organism could have been missed in the manipulations of the homogenates. Consequently, we used 0.4, the logarithmic value for 1, for the animals that were negative by culture. The Mann-Whitney test was used to determine statistical significance for culture and footpad data. P ≤ 0.05 were considered significant.
RESULTS
Protective immunity following immunization.
The first set of experiments was designed to determine whether i.n. administration of nonviable C. albicans in conjunction with LT(R192G) would stimulate protective immunity. Animals immunized i.n. with HK-CA in conjunction with LT(R192G), as well as those immunized with HK-CA, LT(R192G), or NPS alone, were challenged i.v. with 104 viable C. albicans 2 weeks following the last application of immunogen. Survival was monitored over a 28-day period, and then the animals were sacrificed and their kidneys and brains were cultured quantitatively. Animals immunized by previously proven methods of immunization, i.e., by i.g. and/or i.d. inoculations of viable C. albicans (18, 26), were included as controls.
As seen in Fig. 1, the results of two independent experiments demonstrate clearly that animals vaccinated i.n. with HK-CA plus LT(R192G) were able to eradicate C. albicans completely from their kidneys. Some of the animals immunized i.n. with HK-CA alone displayed reduced numbers of C. albicans in their kidneys the first time the experiment was performed; however, this observation was not a reproducible phenomenon in subsequent experiments. Animals immunized by i.g. followed by i.d. inoculation of viable C. albicans also had significantly (P ≤ 0.05) reduced levels of the yeast in their kidneys, but the reductions were not nearly as dramatic as those observed in animals immunized i.n. with HK-CA in conjunction with LT(R192G). The levels in the i.g.-i.d. group of animals were consistent with those previously demonstrated for this type of immunization (18, 26). As expected, control animals immunized i.n. with LT(R192G) alone or NPS alone had disseminated candidiasis, as indicated by the presence of large numbers of C. albicans in their kidneys. Moreover, as demonstrated in the experiment Fig. 1A, animals immunized i.g. with nonviable C. albicans did not develop protective responses. The inability of inactivated Candida to induce protection when administered i.g. has been previously observed in our laboratory. Consequently, i.g. immunization was not included the second experiment. The brains of all immunized and challenged animals were also cultured. Tissues from animals immunized i.n. with HK-CA plus adjuvant were negative, whereas the average levels of C. albicans found in the brains of animals in all the other groups were not significantly different (P ≤ 0.05) from those in the NPS controls (data not shown).
FIG. 1.
Demonstration of protective immunity by culture of kidneys 4 weeks following i.v. challenge with 104 viable C. albicans. Animals were immunized i.n. with HK-CA (2 × 107 organisms) with or without 10 μg of the adjuvant LT(R192G), adjuvant alone, or NPS on the same schedule. Other control groups included animals immunized by i.d. exposure to 2 × 106 viable C. albicans, animals vaccinated i.g. with 2 × 107 HK-CA, and animals given an i.g. immunization followed by an i.d. inoculation with live C. albicans as described in Materials and Methods. An i.v. challenge was performed 2 weeks following the last exposure to antigen. There were seven or eight animals per group.
To determine if the protection against a challenge with 104 viable C. albicans could be demonstrated with higher and more rapidly lethal doses of C. albicans, animals were immunized with either HK-CA plus LT(R192G) or LT(R192G) alone or were mock vaccinated with NPS. All the animals were subsequently challenged i.v. with 105 or 106 viable C. albicans 2 weeks following the last i.n. dose. Survival was monitored for 100 days. From preliminary experiments (data not shown), it was known that challenge of unimmunized animals with 105 or 106 organisms resulted in disease that was rapidly fatal; i.e., all animals receiving 106 organisms died within 48 h of inoculation and those given 105 organisms died within 3 weeks. Figure 2 shows the mortality pattern observed after i.v. challenge. At 18 days after the challenge with 105 C. albicans, there were no surviving mice in the LT(R192G) and the NPS control groups. In contrast, 90% of the animals in the group that received HK-CA plus LT(R192G) survived for the entire length of the experiment (100 days postchallenge) and had no detectable numbers of C. albicans in their kidneys at the time of sacrifice.
FIG. 2.
Survival of mice immunized with HK-CA plus LT(R192G), LT(R192G) alone, or NPS and challenged i.v. with 105 or 106 viable C. albicans 2 weeks following the last exposure to antigen. There were 7 to 12 animals per group.
A less dramatic but no less significant effect was observed when mice were challenged with 106 viable C. albicans. All the animals in the groups that were vaccinated with either LT(R192G) or NPS died within 72 h postchallenge. A greater survival rate was initially observed for animals immunized with HK-CA plus LT(R192G) (60% survival after 5 days); however, by day 15, only 20% of the animals were alive. Clearly, i.n. immunization with killed whole C. albicans in conjunction with the mucosal adjuvant LT(R192G) stimulated significant levels of protective immunity, even when the animals were challenged with doses of C. albicans which were rapidly fatal for unimmunized mice.
Cellular immunity in immunized animals.
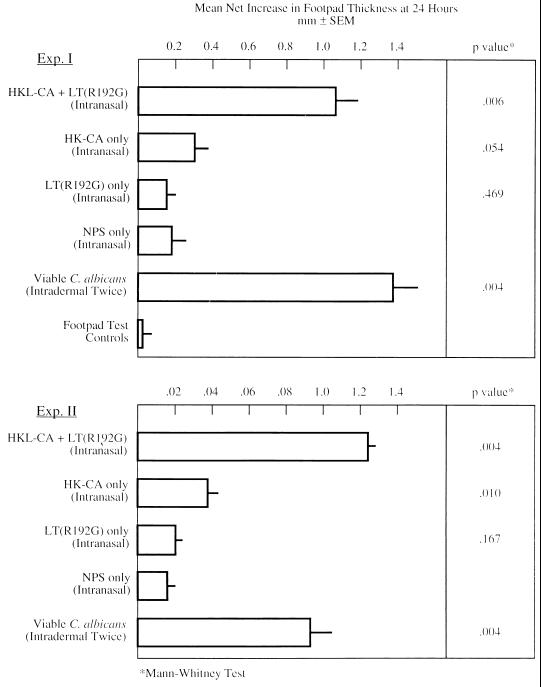
As an initial step to examining the nature of the protective immune response in vaccinated animals, groups of five or six mice were studied for the antibody and cellular immune responses developed after vaccination but prior to challenge. DTH was assessed by the footpad assay 2 weeks following the last of three immunizations with the fungus-adjuvant mixture. These mice were not challenged with live Candida at any point. The results depicted in Fig. 3 indicate that mice immunized with adjuvant alone developed no DTH response whereas those immunized with HK-CA alone developed minimal responses. Those responses were not statistically significant. In contrast, animals immunized with HK-CA plus LT(R192G) developed substantial and highly significant DTH reactions. The responses of the latter animals were comparable to those detected in animals inoculated i.d. or i.g.-i.d. with live C. albicans (18, 26). The immunological reactivity observed in the group immunized with HK-CA plus LT(R192G) indicates the development of a vigorous antigen-specific T-cell response, which correlated with protection and was elicited marginally by inactivated C. albicans in the absence of the mucosal adjuvant.
FIG. 3.
DTH to C. albicans mannan in mice immunized i.n. with HK-CA plus LT(R192G), HK-CA alone, NPS, LT(R192G) alone, or 106 viable C. albicans administered i.d. Footpad testing was performed on animals 2 weeks following the last exposure to HK-CA plus LT(R192G), HK-CA, LT(R192G), or NPS or 1 week following the last i.d. exposure to viable C. albicans. There were five or six animals per group. SEM, standard error of the mean.
Humoral immunity in immunized animals.
The levels of specific anti-Candida antibodies in serum were determined following i.n. immunization with the LT(R192G)-containing vaccine in animals treated in two different ways. First, footpad-tested animals from the previous experiment were sacrificed and bled 3 days after footpad testing. Footpad testing as described above was performed 2 weeks after the last of three applications of the vaccine. Thus, this first group of animals had not been challenged i.v. No antibody was detected in these animals, and when their responses were compared to those of unchallenged animals immunized i.g.-i.d., only two of eight animals in the latter group had demonstrable serum anti-Candida IgG 2 weeks following the second exposure to viable C. albicans (data not shown).
The second group of animals analyzed was that containing the mice that had been challenged i.v. with 104 viable C. albicans 2 weeks after the last application of vaccine and then sacrificed 4 weeks after the i.v. challenge. A control group, consisting of animals immunized i.g.-i.d. with viable Candida and challenged i.v. with viable C. albicans, as indicated above, was included in these assays. C. albicans-specific antibodies were measured by ELISA with anti-IgG whole molecule or sera specific for IgG1, IgG2a, IgG2b, IgG3, or IgA. Antibody detection was assessed with either the cytoplasmic extract, SCS, or the cell wall extract, DTTE.
As shown in Fig. 4A, the highest levels of IgG in serum against SCS were found in animals that had been immunized with HK-CA in conjunction with LT(R192G) and then challenged i.v. with viable C. albicans. These animals had the highest level of protection of any of the vaccine groups examined. There were detectable levels of IgG2a and IgG2b (data not shown) but no detectable IgG3 antibodies to SCS in these mice. Animals immunized with HK-CA alone and then challenged i.v. with viable C. albicans also developed antibodies to SCS, but the levels were much lower than those in animals that received HK-CA plus LT(R192G), and two of the animals had low levels of IgG1 antibodies specific for the SCS. No antibodies were detected in the negative control animals, i.e., animals given only adjuvant or NPS as the immunogen and then challenged i.v. with viable C. albicans. No IgG antibodies were detected against the cell wall antigen, DTTE, in any of the groups of mice, and none of the immunized and challenged animals had detectable IgA antibodies in serum specific for SCS (data not shown). Interestingly, the positive control group, i.e., the animals immunized by the i.g. and i.d. routes with viable C. albicans and then challenged i.v. with viable C. albicans, had lower total IgG levels but higher IgG1 levels compared to the group that received HK-CA plus LT(R192G).
FIG. 4.
ELISA analyses for total IgG (A) or IgG subclasses (B) specific for cytoplasmic antigens (SCS) of C. albicans in sera from animals immunized i.n. with HK-CA alone, HK-CA plus LT(R192G), LT(R192G) alone, or NPS or with viable C. albicans administered i.g.-i.d. Sera were obtained at the time of sacrifice for culture, i.e., 4 weeks following i.v. challenge of immunized animals with 104 viable C. albicans (solid circles) or 100 days following i.v. challenge of immunized animals with 105 viable C. albicans (open circles). There were 7 to 12 animals per group.
In the above experiment, animals immunized with HK-CA without LT(R192G) had approximately the same level of anti-SCS IgG2a as did animals immunized with HK-CA in conjunction with LT(R192G) and both groups of animals survived challenge with 104 viable C. albicans. To further explore the role of IgG1 and IgG2a in protection against lethal challenge, isotype levels were analyzed in animals immunized with HK-CA plus LT(R192G) that survived challenge with a 10-fold-higher dose (105) of viable C. albicans. It should be noted that in the 105 challenge group, control groups immunized with adjuvant alone or NPS succumbed to infection (Fig. 2), and consequently their sera were not available for analysis. As seen in Fig. 4, animals immunized with HK-CA plus LT(R192G) and challenged with 105 viable C. albicans had significant increases in anti-SCS total IgG levels. This increase was mostly due to a dramatic shift in the levels of anti-SCS IgG2a (Fig. 4B).
Since several investigators have suggested that antibody to specific antigens is protective against systemic candidiasis (27, 38), preliminary Western blot experiments were done to examine the total IgG response to cytoplasmic (SCS) and cell wall (DTTE) extracts in animals immunized in various ways. The Western blot analysis of a representative sample of the sera tested against SCS is shown in Fig. 5. The most interesting observation was that all of the animals immunized with HK-CA plus adjuvant (lanes 2 to 9) developed an antibody response to a number of SCS antigens. As seen in Fig. 5, a strong antibody response in these animals was directed against an antigen with a molecular mass of approximately 29 kDa. These were the animals that were completely protected against colonization of the kidneys and lethal i.v. challenge. Animals immunized with HK-CA alone (lanes 10 to 15), LT(R192G) alone (data not shown), or NPS (data not shown) prior to i.v. challenge developed no antibody responses to the cytoplasmic extract, as detected by Western blotting (Fig. 5), and were not protected against colonization following i.v. challenge. None of the animals immunized with HK-CA, with or without LT(R192G), exhibited an antibody response to the DTTE on Western blot analysis, although sera taken from mice immunized i.d. with viable C. albicans prior to i.v. challenge did have antibodies demonstrable to components of DTTE (not shown).
FIG. 5.
Western blot analyses of sera from animals immunized i.n. with HK-CA plus LT(R192G) or HK-CA alone. Lanes: 1, molecular size markers; 2 to 9, sera from mice immunized with HK-CA plus LT(R192G) and challenged with 105 viable C. albicans (sera were obtained 100 days postchallenge); 10 to 15, sera from mice immunized with HK-CA alone.
DISCUSSION
We have shown clearly that i.n. immunization with whole killed C. albicans mixed with the novel mucosal adjuvant LT(R192G) stimulated high levels of protection against systemic challenge with a highly virulent strain of C. albicans. Protective immunity was associated with substantial levels of DTH and with high levels of anti-SCS IgG in serum. A predominantly IgG2a response to soluble cytoplasmic antigens was observed in animals that survived a challenge with 105 C. albicans, and there were low levels of IgG1 and IgG2b in most of these mice as well. Antibody was not detectable to DTTE of the cell wall of the same strain of C. albicans used to immunize and infect animals.
The level of protection observed in the animals immunized with inactivated cells and adjuvant and then challenged with 105 viable C. albicans was remarkable in that 100% of the adjuvant- or NPS-only animals had died by 18 days whereas only 1 of 10 mice immunized with the organism plus adjuvant died over a course of 100 days. Similarly, although 80% of the immunized animals challenged with 106 organisms died over a period of 12 days, animals treated with adjuvant or NPS only were all dead within 2 to 3 days. When the kidneys and brains of animals immunized with HK-CA plus LT(R192G) were cultured 28 days following i.v. challenge, none of the animals examined in either of two independent experiments had any organisms detectable in the tissue homogenates. In the past, nonviable C. albicans has not been highly successful as an immunogen (25, 26, 40, 47). Considerably more success was attained when viable virulent C. albicans (26, 40) or an avirulent strain (3) was used, and there have been three reports of significant levels of protective immunity stimulated by C. albicans ribosomes (45), a mannoprotein (39), and antibody to an extracellular candidal protein, p43 (49).
The development of immunization protocols for candidiasis is complicated further by the fact that the mechanisms responsible for protection at the mucosal level appear to be different from those operative at the systemic level. In addition, when exploring protective mechanisms against candidiasis, it is imperative to define clearly whether the assessment of protection to a first exposure to viable C. albicans is being investigated or whether protection following immunization (acquired immunity) is under consideration. Most people have been exposed to C. albicans, usually in the gastrointestinal tract, since a high percentage of the population is colonized with the organism (11). Mucosal exposure stimulates protective systemic immunity, as demonstrated in an animal model (18) and as evidenced by the fact that most humans, despite lifelong colonization with the organism, never develop systemic disease. T lymphocytes are clearly involved in protective mechanisms, at both the mucosal (5) and systemic (7, 26, 46) levels, but the precise role of these T cells has not been defined.
The role of antibody in protection is controversial (6) and may depend upon whether one is evaluating the response to a first systemic exposure to viable C. albicans (27), a mucosal infection such as that in the vagina (15), or development of antibody to antigens that may actually enhance the infection (4). In our experiments with HK-CA and LT(R192G), the animals produced antibody against a number of SCS antigens, including a small antigen, i.e., approximately 29 kDa. A strong response to the 29-kDa antigen, in comparison to the other antigenic components of the SCS, was noted in the animals immunized with HK-CA plus LT(R192G) that were sacrificed 28 days following i.v. challenge with 104 live C. albicans (data not shown). Others (30, 33) have noted that patients with superficial or vaginal candidiasis have antibody responses to antigens that migrate in this region as well. While the evidence for involvement of antibodies to this antigen in any kind of protective mechanism is purely circumstantial, it may be profitable to examine this particular antigen in more detail for a role in the protective response.
We have shown that LT(R192G) can induce protective immunity when coadministered with whole inactivated bacteria (8) or viruses (32) or subunits of relevant virulence determinants from these pathogens (41). This adjuvant promotes the development of both humoral (antibody) and cell-mediated immune responses against the pathogen in both the systemic and mucosal compartments.
In a recent study by Chong et al. (8), the function of LT(R192G) in protection against typhoid-like disease was to upregulate or enhance the Th1 arm of the immune response against killed organisms. Specifically, mice immunized orally with killed Salmonella dublin in conjunction with LT(R192G) were protected against lethal challenge and had higher gamma interferon (IFN-γ) interleukin-2, and IgG responses than did mice immunized orally with killed S. dublin alone, which were not protected. Conclusive evidence for an association of IFN-γ with adjuvant-induced protection was provided in the studies in which neutralization of endogenous IFN-γ with anti-IFN-γ (monoclonal antibody R4-6A2) resulted in loss of protection against lethal oral challenge.
In the present study, we examined the ability of LT(R192G) to enhance the humoral and cellular immune responses against C. albicans and to induce protection against colonization and lethal i.v. challenge with wild-type C. albicans. Solid protection against colonization and lethal i.v. challenge was achieved following i.n. immunization with heat-killed whole organisms in conjunction with this adjuvant. Both the humoral and cellular immune responses against C. albicans were enhanced. A strong DTH response to mannan was observed in animals vaccinated with the mixture of HK-CA and LT(R192G). Moreover, isotype analysis of anti-Candida antibodies in protected animals revealed a predominance of antibodies of the IgG2a isotype, suggesting a strong Th1-type cytokine response. Studies to characterize the cytokine pattern observed in protected animals, using antigen restimulation studies and reverse transcriptase PCR analyses, are under way.
Currently, there are no vaccines available for human mycoses, and there is an urgent need to develop measures of prophylactic immunointervention against fungal pathogens. This is especially important if one considers a mass prophylactic treatment for immunocompromised human hosts. On the one hand, vaccines containing a live, replicating organism are associated with an inherent risk of infection, even when the vaccine strain is attenuated or exhibits low virulence. On the other hand, although subunit vaccines are safer, the cost and labor involved in the purification process of proteins or immunogenic carbohydrates represent a hindrance to the development of such vaccines. The concept of using inactivated C. albicans as a component of a potential anti-Candida vaccine is attractive because of its safety and the ease and low cost associated with the preparation of large numbers of cells of the inactivated yeast.
In future studies, we intend to determine if the protection conferred by this vaccine can be passively transferred to naive and immunocompromised mice and whether immunological protection supersedes the induction of immunosuppression in experimental animals. Concomitantly, our studies will determine if protection can be achieved in animals that are colonized with Candida prior to vaccination and if cross-protection against other Candida species can be achieved by using this mucosal immunization strategy. With the information obtained in the proposed studies, future vaccine strategies can be designed by using similar vaccination procedures for a variety of fungal pathogens. These are important issues because they take us beyond the phenomenological observations of “enhanced immunity” to a clearer understanding of the mechanisms of protection against Candida and the practical implications of the development of antifungal vaccines.
ACKNOWLEDGMENTS
This investigation was supported by Public Health Service grants AI12806 and AI42777 from the National Institutes of Health and by a grant from SmithKline Beecham Biologics.
We are grateful to Robert Johnson for assistance with statistical analyses.
REFERENCES
- 1.Balish E, Jensen J, Warner T, Brekke J, Leonard B. Mucosal and disseminated candidiasis in gnotobiotic SCID mice. J Med Vet Mycol. 1993;31:143–154. doi: 10.1080/02681219380000161. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee U, Mohapatra L N, Kumar R. Effect of immunization with formalin killed cells in complete Freund’s adjuvant in experimental candidosis. Indian J Med Res. 1985;81:454–458. [PubMed] [Google Scholar]
- 3.Bistoni F, Vecchiarelli A, Cenci E, Puccetti P, Marconi P, Cassone A. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect Immun. 1986;51:668–674. doi: 10.1128/iai.51.2.668-674.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bromuro C, La Valle R, Sandini S, Urbani F, Ausiello C M, Morelli L, Fe d’Ostiani C, Romani L, Cassone A. A 70-kilodalton recombinant heat shock protein of Candida albicans is highly immunogenic and enhances systemic murine candidiasis. Infect Immun. 1998;66:2154–2162. doi: 10.1128/iai.66.5.2154-2162.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantorna M T, Balish E. Role of CD4+ lymphocytes in resistance to mucosal candidiasis. Infect Immun. 1991;59:2447–2455. doi: 10.1128/iai.59.7.2447-2455.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadevall A. Antibody immunity and invasive fungal infections. Infect Immun. 1995;63:4211–4218. doi: 10.1128/iai.63.11.4211-4218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cenci E, Romani L, Vecchiarelli A, Puccetti P, Bistoni F. Role of L3T4+ lymphocytes in protective immunity to systemic Candida albicans infection in mice. Infect Immun. 1989;57:3581–3587. doi: 10.1128/iai.57.11.3581-3587.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong C, Frieberg M, Clements J D. LT(R192G), a non-toxic mutant of the heat-labile enterotoxin of Escherichia coli elicits enhanced humoral and cellular immune responses associated with protection against lethal oral challenge with Salmonella spp. Vaccine. 1998;16:732–740. doi: 10.1016/s0264-410x(97)00255-7. [DOI] [PubMed] [Google Scholar]
- 9.Clements J D, Finkelstein R A. Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect Immun. 1979;24:760–769. doi: 10.1128/iai.24.3.760-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clements J D, Hartzog N M, Lyon F L. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine. 1988;6:269–277. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- 11.Cohen R, Roth F J, Delgado E, Ahearn D G, Kalser M H. Fungal flora of the normal human small and large intestine. N Engl J Med. 1969;280:638–641. doi: 10.1056/NEJM196903202801204. [DOI] [PubMed] [Google Scholar]
- 12.Cooper M G. Delayed-type hypersensitivity in the mouse. I. Induction and elicitation by Salmonella adelaide flagellin and its derivatives. Scand J Immunol. 1972;1:167–178. doi: 10.1111/j.1365-3083.1972.tb00596.x. [DOI] [PubMed] [Google Scholar]
- 13.Costantino P J, Franklyn K M, Gare N F, Warmington J R. Production of antibodies to antigens of Candida albicans in CBA/H mice. Infect Immun. 1994;62:1400–1405. doi: 10.1128/iai.62.4.1400-1405.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowle A J. Delayed hypersensitivity in the mouse. Adv Immunol. 1975;20:197–264. doi: 10.1016/s0065-2776(08)60209-6. [DOI] [PubMed] [Google Scholar]
- 15.De Bernardis F, Boccanera M, Adriani D, Spreghini E, Santoni G, Cassone A. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect Immun. 1997;65:3399–3405. doi: 10.1128/iai.65.8.3399-3405.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickinson B L, Clements J D. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickinson B L, Clements J D. Use of Escherichia coli heat-labile enterotoxin as an oral adjuvant. In: Kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines. San Diego, Calif: Academic Press, Inc.; 1996. pp. 73–87. [Google Scholar]
- 18.Domer J E, Hector R F. Enhanced immune responses in mice treated with penicillin-tetracycline or trimethoprim-sulfamethoxazole when colonized intragastrically with Candida albicans. Antimicrob Agents Chemother. 1987;31:691–697. doi: 10.1128/aac.31.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domer J E, Moser S A. Experimental murine candidiasis: cell-mediated immunity after cutaneous challenge. Infect Immun. 1978;20:88–98. doi: 10.1128/iai.20.1.88-98.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckstein M, Barenholz Y, Bar L K, Segal E. Liposomes containing Candida albicans ribosomes as a prophylactic vaccine against disseminated candidiasis in mice. Vaccine. 1997;15:220–224. doi: 10.1016/s0264-410x(96)00137-5. [DOI] [PubMed] [Google Scholar]
- 21.Elson C. Cholera toxin as a mucosal adjuvant. In: Kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines. San Diego, Calif: Academic Press, Inc.; 1996. pp. 59–72. [Google Scholar]
- 22.Elson C O. Cholera toxin and its subunits as potential oral adjuvants. Immunol Today. 1989;146:29–33. doi: 10.1007/978-3-642-74529-4_3. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher S, Winston S E, Fullerr S A, Hurrell J G R. Immunoblotting and immunodetection. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1997. p. 10.8. [Google Scholar]
- 24.Garner R E, Kuruganti U, Czarniecki C W, Chiu H H, Domer J E. In vivo immune responses to Candida albicans modified by treatment with recombinant murine gamma interferon. Infect Immun. 1989;57:1800–1808. doi: 10.1128/iai.57.6.1800-1808.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giger D K, Domer J E, McQuitty J T., Jr Experimental murine candidiasis: pathological and immune responses to cutaneous inoculation with Candida albicans. Infect Immun. 1978;19:496–509. doi: 10.1128/iai.19.2.499-509.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giger D K, Domer J E, Moser S A, McQuitty J T., Jr Experimental murine candidiasis: pathological and immune responses in T-lymphocyte-depleted mice. Infect Immun. 1978;21:729–737. doi: 10.1128/iai.21.3.729-737.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han Y, Cutler J E. Antibody response that protects against disseminated candidiasis. Infect Immun. 1995;63:2714–2719. doi: 10.1128/iai.63.7.2714-2719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashigucci K, Ogawa H, Ishidate T, Yamashita R, Kamiya H, Watanabe K, Hattori N, Sato T, Suzuki Y, Nagamine T, Aizawa C, Tamura S, Kurata T, Oya A. Antibody responses in volunteers induced by nasal influenza vaccine combined with Escherichia coli heat-labile enterotoxin B subunit containing a trace amount of the holotoxin. Vaccine. 1996;14:113–119. doi: 10.1016/0264-410x(95)00174-y. [DOI] [PubMed] [Google Scholar]
- 29.Hathaway L J, Partidos C D, Vohra P, Steward M W. Induction of systemic immune responses to measles virus synthetic peptides administered intranasally. Vaccine. 1995;13:1495–1500. doi: 10.1016/0264-410x(95)00111-d. [DOI] [PubMed] [Google Scholar]
- 30.Ishiguro A, Homma M, Sukai T, Higashide K, Torii S, Tanaka K. Immunoblotting analysis of sera from patients with candidal vaginitis and healthy females. J Med Vet Mycol. 1992;30:281–292. doi: 10.1080/02681219280000371. [DOI] [PubMed] [Google Scholar]
- 31.Katz J M, Lu X, Young S A, Galphin J C. Adjuvant activity of the heat-labile enterotoxin from enterotoxigenic Escherichia coli for oral administration of inactivated influenza virus vaccine. J Infect Dis. 1997;175:352–363. doi: 10.1093/infdis/175.2.352. [DOI] [PubMed] [Google Scholar]
- 32.Katz J M, Lu X, Galphin J C, Clements J D. Heat-labile enterotoxin from E. coli as an adjuvant for oral influenza vaccination. In: Brown L E, Hampson A W, Webster R G, editors. Options for the control of influenza. III. New York, N.Y: Elsevier Science Publishing, Inc.; 1996. pp. 292–297. [Google Scholar]
- 33.Keller B I, Simmons P D, Ivanyl L. Identification of immunodominant antigens of Candida albicans in patients with superficial candidosis. Clin Immunol Immunopathol. 1990;54:347–353. doi: 10.1016/0090-1229(90)90048-u. [DOI] [PubMed] [Google Scholar]
- 34.Lee C K, Weltzin R, Thomas W D, Jr, Kleanthous H, Ermak T H, Soman G, Hill J E, Ackerman S K, Monath T P. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J Infect Dis. 1995;172:161–172. doi: 10.1093/infdis/172.1.161. [DOI] [PubMed] [Google Scholar]
- 35.Levy R, Segal E, Barr-Nea L. Systemic candidiasis in mice immunized with Candida albicans ribosomes. Mycopathologia. 1985;91:17–22. doi: 10.1007/BF00437281. [DOI] [PubMed] [Google Scholar]
- 36.Lycke N, Tsuji T, Holmgren J. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur J Immunol. 1992;22:2277–2281. doi: 10.1002/eji.1830220915. [DOI] [PubMed] [Google Scholar]
- 37.Mason H S, Ball J M, Shi J J, Jiang X, Estes M K, Arntzen C J. Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc Natl Acad Sci USA. 1996;93:5335–5340. doi: 10.1073/pnas.93.11.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matthews R C, Burnie J P, Howat D, Rowland T, Walton F. Autoantibody to heat-shock protein 90 can mediate protection against systemic candidosis. Immunology. 1991;74:20–24. [PMC free article] [PubMed] [Google Scholar]
- 39.Mencacci A, Torosantucci A, Spaccapelo R, Romani L, Bistoni F, Cassone A. A mannoprotein constituent of Candida albicans that elicits different levels of delayed-type hypersensitivity, cytokine production, and anticandidal protection in mice. Infect Immun. 1994;62:5353–5360. doi: 10.1128/iai.62.12.5353-5360.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mourad S, Friedman L. Active immunization of mice against Candida albicans. Proc Soc Exp Biol Med. 1961;106:570–572. doi: 10.3181/00379727-106-26405. [DOI] [PubMed] [Google Scholar]
- 41.O’Neal C M, Clements J D, Estes M K, Conner M E. Rotavirus 2/6 viruslike particles administered intranasally with cholera toxin, Escherichia coli heat-labile toxin (LT), and LT-R192G induce protection from rotavirus challenge. J Virol. 1998;72:3390–3393. doi: 10.1128/jvi.72.4.3390-3393.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reiss E, Stone S H, Hasenclever H F. Serological and cellular immune activity of peptidoglucomannan fractions of Candida albicans cell walls. Infect Immun. 1974;9:881–890. doi: 10.1128/iai.9.5.881-890.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Restrepo-Moreno A, Schneidau J D., Jr Nature of the skin-reactive principle in culture filtrates prepared from Paracoccidioides brasiliensis. J Bacteriol. 1967;93:1741–1748. doi: 10.1128/jb.93.6.1741-1748.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romani L, Mencacci A, Cenci E, Mosci P, Vitellozzi G, Grohmann U, Puccetti P, Bistoni F. Course of primary candidiasis in T cell-depleted mice infected with attenuated variant cells. J Infect Dis. 1992;166:1384–1392. doi: 10.1093/infdis/166.6.1384. [DOI] [PubMed] [Google Scholar]
- 45.Segal E, Nussbaum S, Barr-Nea L. Protection against systemic infections with various Candida species elicited by vaccination with Candida albicans ribosomes. Sabouraudia. 1985;23:275–285. [PubMed] [Google Scholar]
- 46.Sieck T G, Moors M A, Buckley H R, Blank K J. Protection against murine disseminated candidiasis mediated by a Candida albicans-specific T-cell line. Infect Immun. 1993;61:3540–3543. doi: 10.1128/iai.61.8.3540-3543.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soles P, Lim L Y, Louria D B. Active immunity in experimental candidiasis in mice. Sabouraudia. 1967;5:315–322. doi: 10.1080/00362176785190581. [DOI] [PubMed] [Google Scholar]
- 48.Staats H F, Nichols W G, Palker T J. Mucosal immunity to HIV-1: systemic and vaginal antibody responses after intranasal immunization with the HIV-1 C4/V3 peptide T1SP10 MN(A) J Immunol. 1996;157:462–472. [PubMed] [Google Scholar]
- 49.Tavares D, Ferreira P, Vilanova M, Videira A, Arala-Chaves M. Immunoprotection against systemic candidiasis in mice. Int Immunol. 1995;7:785–796. doi: 10.1093/intimm/7.5.785. [DOI] [PubMed] [Google Scholar]
- 50.Weltzin R, Kleanthous H, Guirakhoo F, Monath T P, Lee C K. Novel intranasal immunization techniques for antibody induction and protection of mice against gastric Helicobacter felis infection. Vaccine. 1997;15:370–376. doi: 10.1016/s0264-410x(97)00203-x. [DOI] [PubMed] [Google Scholar]
- 51.Wu H Y, Nahm M H, Guo Y, Russell M W, Briles D E. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J Infect Dis. 1997;175:839–846. doi: 10.1086/513980. [DOI] [PubMed] [Google Scholar]
- 52.Xu-Amano J, Jackson R J, Fujihashi K, Kiyono H, Staats H F, McGhee J R. Helper Th1 and Th2 cell responses following mucosal or systemic immunization with cholera toxin. Vaccine. 1994;12:903–911. doi: 10.1016/0264-410x(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 53.Xu-Amano J, Kiyono H, Jackson R J, Staats H F, Fujihashi K, Burrows P D, Elson C O, Pillai S, McGhee J R. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosal associated tissues. J Exp Med. 1993;178:1309–1320. doi: 10.1084/jem.178.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamamoto M, Vancott J L, Okahashi N, Marinaro M, Kiyono H, Fujihashi K, Jackson R J, Chatfield S N, Bluethmann H, McGhee J R. The role of Th1 and Th2 cells for mucosal IgA responses. Ann N Y Acad Sci. 1996;778:64–71. doi: 10.1111/j.1749-6632.1996.tb21115.x. [DOI] [PubMed] [Google Scholar]