Abstract
Purpose
Frailty is a prevalent condition in older adults. Identification of frailty using an electronic Frailty Index (eFI) has been successfully implemented across general practices in the United Kingdom. However, in Australia, the eFI remains understudied. Therefore, we aimed to (i) examine the feasibility of deriving an eFI from Australian general practice records and (ii) describe the prevalence of frailty as measured by the eFI and the prevalence with socioeconomic status and geographic remoteness.
Participants and Methods
This retrospective analysis included patients (≥70 years) attending any one of >700 general practices utilizing the Australian MedicineInsight data platform, 2017–2018. A 36-item eFI was derived using standard methodology, with frailty classified as mild (scores 0.13–0.24); moderate (0.25–0.36) or severe (≥0.37). Socioeconomic status (Socio-Economic Indexes for Areas (SEIFA) index)) and geographic remoteness (Australian Statistical Geography Standard (ASGC) remoteness areas) were also examined.
Results
In total, 79,251 patients (56% female) were included, mean age 80.0 years (SD 6.5); 37.4% (95% CI 37.0–37.7) were mildly frail, 16.7% (95% CI 16.4–16.9) moderately frail, 4.8% (95% CI 4.7–5.0) severely frail. Median eFI score was 0.14 (IQR 0.08 to 0.22); maximum eFI score was 0.69. Across all age groups, moderate and severe frailty was significantly more prevalent in females (P < 0.001). Frailty severity increased with increasing age (P < 0.001) and was strongly associated with socioeconomic disadvantage (P < 0.001) but not with geographic remoteness.
Conclusion
Frailty was identifiable from routinely collected general practice data. Frailty was more prevalent in socioeconomically disadvantaged groups, women and older patients and existed in all levels of remoteness. Routine implementation of an eFI could inform interventions to prevent or reduce frailty in all older adults, regardless of location.
Keywords: primary health care, family practice, frailty, electronic health records, geriatric assessment, aged
Introduction
Across the world, people are living longer, but with more chronic conditions, disability and frailty.1,2 Older people with frailty are high users of emergency services and hospitals.3 This growing demand for health care resources is concerning as it might exceed capacity to meet the needs of patients in the future, even beyond the end of the pandemic. Frailty is a challenging public health priority2 and is characterized by an increased vulnerability resulting from age-related decline in reserve and function across multiple physiological systems.1 Frailty is consistently associated with adverse health outcomes including functional dependency, hospitalizations, residential aged-care admission and death.2,4,5
Promotion of healthy aging and disease prevention within general practice and community settings may assist in tackling frailty trajectories.6 In particular, evidence suggests that nutritional supplements, strength training, management of chronic disease and addressing contributors to frailty (such as polypharmacy, sarcopenia, weight loss) can delay and potentially reverse frailty and improve quality of life (QOL).7–9 But first, frailty needs to be routinely identified.
General practice is well placed to screen for frailty10 and well positioned to manage the frail patient, addressing the multifaceted issues that arise during geriatric assessment, managing chronic disease, minimizing polypharmacy, promoting exercise and employing nutritional interventions.11 This is important, with recent Australian government-funded reablement and restorative care programs to increase the availability of multidisciplinary services for older people with frailty in the community.12 Recognizing frailty and referring patients with frailty in need of these services may potentially offer cost-effective strategies to manage aging members in the community and reduce the pressure on acute care services.13
Despite calls for routine frailty screening for all older adults,14 there is no consensus on an operational definition of frailty.15 Screening for frailty needs reliable and accurate tools that are easily administered by the clinician in their clinical environment. Currently, there is no single recommended frailty screening tool for general practice, and despite the large number of frailty scales available,16 they are time-consuming and complex to administer in a real-life clinical setting and their application in general practice has been limited. Frailty screening tools such as frailty indexes that are automated from Electronic Medical Records (EMRs) may overcome some of those hurdles in general practice11 and be welcomed by busy clinicians.
One such tool that has been developed and validated in the United Kingdom primary care setting is the electronic Frailty Index (eFI), developed by Clegg et al using routine general practice care databases in England.17 The eFI is based on Rockwood’s cumulative deficit model of frailty,18 identifies 36 deficits and classifies individuals into either fit, mild, moderate or severe frailty, and has demonstrated robust predictive validity for outcomes of hospitalization, residential aged-care admission and mortality.17
Therefore, in this context, we aimed to investigate whether it would be possible to apply a similar approach to an Australian healthcare environment using an existing electronic national primary care data platform. The primary objective of this study was to investigate the practical feasibility of calculating the eFI from general practice routinely collected data. Specifically, our goal was to determine if an Australian National primary care data collection platform has sufficient data available to define at enough eFI deficits and if it produces an eFI that fits with the expected parameters of a frailty index. A secondary objective was to describe the prevalence of frailty (as measured by the eFI) and its distribution across socioeconomic status and geographic remoteness.
Methods
Design and Setting
This is a retrospective exploratory study of a de-identified cohort of older patients, aged ≥70 years, attending Australian general practices participating in the MedicineInsight data platform.
Data Source
MedicineInsight is a large-scale, national primary care data platform, managed by NPS MedicineWise. It supports quality improvement in Australian primary care and post-market surveillance of medicines in >700 consenting general practices who provide ongoing data, covering a population of >3 million patients.19 MedicineInsight patients are broadly similar to patients who visited a general practitioner in the Medical Benefits Schedule (MBS) data as measured by age, gender and socioeconomic status.20,21 Details of the data collection process used by MedicineInsight and database characteristics are published elsewhere.20 In summary, the data contain several years’ worth of anonymized patient demographic and clinical data extracted directly from the clinical information systems of participating general practices from all Australian states and territories. Items extracted from patient encounters include medical history (diagnoses/conditions), prescriptions, investigations, pathology test results, observations, allergies, and immunization. The authors had access to a 25% random sample of patients (n=3,473,336) provided by MedicineInsight (an independent external group) under contract to the University of New South Wales for research.
Study Population
Patients
A 25% random sample of patients from Australian general practices, with complete data from 1 January 2016 to 31 December 2018, who participated in the MedicineInsight data platform was available for this study. The target group for the analysis comprised patients aged ≥70 years at 1 January 2017 who had a recorded attendance at a participating practice for at least three (non-administrative) encounters on different days with a general practitioner (GP) between 1 January 2016 and 31 December 2018. Patients were excluded if they had incomplete data on age and sex, had died, or had been made inactive (ie, no longer attending the practice) prior to 1 January 2017.
Electronic Frailty Index (eFI) Deficits
We developed a set of rules to define the different deficits (eg, health conditions, medical history, symptoms, clinical signs, pathology test values, medications, and psychosocial circumstances) based on Read codes, a coded thesaurus of clinical terms used in general practice software in the United Kingdom, provided by Andrew Clegg, who designed the eFI (personal correspondence). This list of Read codes defined deficit synonyms and where relevant medicines and pathology test result values related to the condition. Supplementary Table 1 summarizes the definitions used. Additional input was provided by clinicians including general practitioners (LPL, MC), geriatrician (DN), pharmacist (MW), general practice nurses and aged care clinical nurse specialist (ETL, MT) to ensure that definitions reflected the Australian clinical environment. These rules were then applied to the diagnoses, reason for encounter, reason for prescription, prescription, pathology, and observational data fields extracted from the MedicineInsight data. Deficits were limited to any mention of the deficit recorded in the data any time prior to the censure date for the patient. The censure date was the date of the last non-administrative encounter for each patient with a GP between 1 January 2017 and 31 December 2018. Information on deficits related to prescription or pathology results was restricted to deficits recorded in the data in the 12 months prior to the censure date for each patient. While there is no international agreement on the cut-off for polypharmacy,22 in this study it was defined as ≥5 current prescriptions funded by the Australian Pharmaceutical Benefits Scheme, for comparability with the eFI developers’ cut-off.17 Cut-points for pathology results were defined by reported laboratory reference ranges. Deficits were coded “1” if present in the MedicineInsight data for the eligible patient, or 0 if absent from the database. The eFI score was calculated by dividing the total number of deficits present (maximum 36) by 36, giving a score between 0 and 1.17
Other Variables
Socioeconomic status was determined according to the Socio-Economic Indexes for Areas (SEIFA) (1–5, 5 being highest, 1 being lowest) developed by the Australian Bureau of Statistics and summarizes information about the economic and social conditions of people and households within an area based on data items from the five-yearly Census covering education, income, employment, occupation and housing.23 Geographic remoteness was defined by the Australian Standard Geographic Classification (ASGC) remoteness areas which divides Australia into broad geographic regions (major cities, inner regional, outer regional, remote and very remote) for statistical purposes.24 Age (years) and sex (male and female) were also recorded for analysis.
Feasibility Measures
Feasibility data or the derivation of the eFI was included if sufficient data were available to define and extract ≥90% of eFI deficits, produces an eFI that is consistent with the expected frailty index submaximal limit at about two-thirds of the deficits tested,25 extracted data quality is suitable to analyze the aggregated eFI and potential for future operationalization of the eFI calculation in the current context in which it is tested.
Analysis
The prevalence of no frailty, mild frailty, moderate frailty and severe frailty was calculated for the study population based on eFI cut-offs used by Clegg et al: ≤0.12 represents no frailty; 0.13 to 0.24 mild frailty; 0.25 to 0.36 moderate frailty and ≥0.37 severe frailty.17
Descriptive analysis was used to characterize the deficit prevalence, patient population and frailty severity. For frailty severity analysis, we combined moderate and severe frailty groups. We analyzed five-year age groups for comparability purposes as patients aged 75+ years in Australia are eligible for the annual in-depth Health Assessment in general practice.26 We report counts and proportions for discrete variables. Mantel–Haenszel chi-squared tests were used to compare socio-demographic characteristics (including remoteness and socio-economic status) according to frailty. All analyses were conducted in SAS 9.4 (Cary NC).
Ethics
Approval to conduct this study was granted by the University of New South Wales Human Research Ethics Committee (#HC190384) and NPS MedicineWise Data Governance Committee (DG 2018–015).
All MedicineInsight data requests are approved by an independent Data Governance Committee that includes GPs, researchers, consumer representatives and data security experts to mitigate risks to participants. Data is collected, used, and stored in accordance with Australian privacy laws. In addition, patient’s identifiable information, for example, name, date of birth and street address, is not collected. Patients have the option not to participate by informing the consenting practice.20
Results
We identified 79,251 patients eligible for inclusion in the study, ie, where ≥90% of variables were available to calculate the eFI. Participants had a mean age of 80 years (SD 6.5) with a larger representation of females (56%).
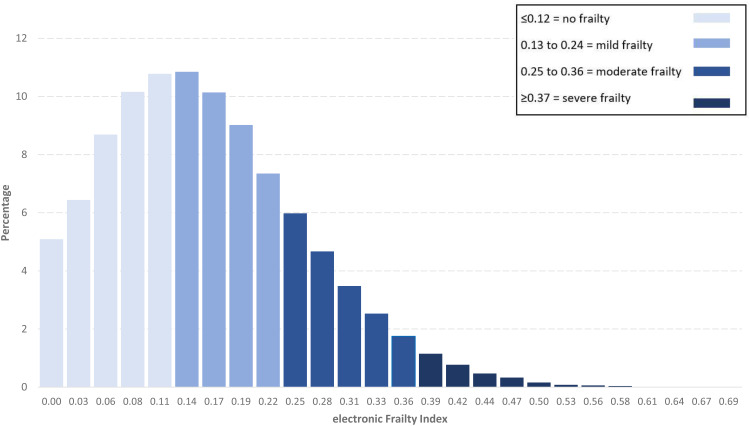
In total, 32,625 (41.2%, 95% CI 40.8–41.5) of patients were classified as not frail according to the eFI score, 29,608 (37.4%, 95% CI 37.0–37.7) were mildly frail, 13,197 (16.7%, 95% CI 16.4–16.9) moderately frail and 3821 (4.8%, 95% CI 4.7–5.0) were severely frail. The frequencies of individual eFI scores for all patients ≥70 years showed a skewed distribution (Figure 1). The median eFI score was 0.14 (IQR 0.08 to 0.22) with the maximum observed eFI score 0.69.
Figure 1.
Overall distribution of electronic Frailty Index scores in the Australian MedicineInsight sample (n = 79,251).
Frailty severity increased with age (test for trend P < 0.001). The prevalence of frailty levels was significantly higher among females compared to males (P < 0.001), and while absolute proportions at each level of frailty decreased with increasing remoteness, the difference was not significant. The most socio-economically advantaged (SEIFA index 5) were more likely to be classified as no frailty (column 2, Table 1) and increased proportions of mildly frail and moderately-severely frail were seen among the most disadvantaged (SEIFA index 1) (Table 1) (P < 0.001).
Table 1.
Socio-Demographic Characteristics by Frailty Severity Levels in the Australian MedicineInsight Sample
| All Participants n (%) | eFI No Frailty n (%) | eFI Mild Frailty n (%) | eFI Moderate-Severe Frailty n (%) | p-value | |
|---|---|---|---|---|---|
| 79,251 (100) | 32,625 (41.2) | 29,608 (37.4) | 17,018 (21.5) | ||
| Gender | |||||
| Male | 35,228 (44.5) | 15,965 (45.3) | 12,882 (36.6) | 6381 (18.1) | <0.001 |
| Female | 44,023 (55.5) | 16,660 (37.8) | 16,726 (37.9) | 10,637 (24.2) | |
| Age, median (IQR) | 79 (75–84) | 76 (73–81) | 79 (75–84) | 83 (78–88) | |
| Age groups, years | |||||
| 70–74 | 19,309 (24.4) | 11,291 (58.5) | 6333 (32.8) | 1685 (8.7) | <0.001 |
| 75–79 | 23,395 (29.5) | 10,806 (46.2) | 8943 (38.2) | 3646 (15.6) | |
| 80–84 | 17,152 (21.6) | 5813 (33.9) | 7005 (40.8) | 4334 (25.3) | |
| 85–89 | 11,319 (14.3) | 2989 (26.4) | 4381 (38.7) | 3949 (34.9) | |
| ≥90 | 8076 (10.2) | 1726 (21.4) | 2946 (36.5) | 3404 (42.2) | |
| Age groups (male) | |||||
| 70–74 | 9311 (26.4) | 5655 (60.7) | 2939 (31.6) | 717 (7.7) | <0.001 |
| 75–79 | 10,817 (30.7) | 5418 (50.1) | 3932 (36.4) | 1467 (13.6) | |
| 80–84 | 7603 (21.6) | 2896 (38.1) | 3024 (39.8) | 1683 (22.1) | |
| 85–89 | 4702 (13.4) | 1370 (29.1) | 1882 (40.0) | 1450 (30.8) | |
| ≥90 | 2795 (7.9) | 626 (22.4) | 1105 (39.5) | 1064 (38.1) | |
| Age groups (female) | |||||
| 70–74 | 9998 (22.7) | 5636 (56.4) | 3394 (33.9) | 968 (9.7) | <0.001 |
| 75–79 | 12,578 (28.6) | 5388 (42.8) | 5011 (39.8) | 2179 (17.3) | |
| 80–84 | 9549 (21.7) | 2917 (30.6) | 3981 (41.7) | 2651 (27.8) | |
| 85–89 | 6617 (15.0) | 1619 (24.5) | 2499 (37.8) | 2499 (37.8) | |
| ≥90 | 5281 (12.0) | 1100 (20.8) | 1841 (34.9) | 2340 (44.3) | |
| Remotenessa | |||||
| Major city | 47,580 (60.1) | 19,805 (41.6) | 17,625 (37.0) | 10,150 (21.3) | 0.585 |
| Inner regional | 22,044 (27.8) | 8713 (39.5) | 8375 (37.9) | 4956 (22.5) | |
| Outer regional | 8934 (11.3) | 3784 (42.4) | 3353 (37.5) | 1797 (20.1) | |
| Remote/very remote | 693 (0.9) | 323 (46.6) | 255 (36.8) | 115 (16.6) | |
| Index of disadvantageb | |||||
| Score 1 (most disadvantaged) | 13,415 (16.9) | 5220 (38.9) | 5121 (38.2) | 3074 (22.9) | <0.001 |
| Score 2 | 13,889 (17.6) | 5272 (37.9) | 5417 (39.0) | 3200 (23.0) | |
| Score 3 | 20,134 (25.5) | 8672 (43.1) | 7348 (36.5) | 4114 (20.3) | |
| Score 4 | 13,039 (16.5) | 5340 (40.9) | 4851 (37.2) | 2848 (21.8) | |
| Score 5 (most advantaged) | 18,501 (23.4) | 7994 (43.2) | 6773 (36.6) | 3734 (20.2) |
Notes: aASGC Remoteness Areas can be found at https://www.abs.gov.au/websitedbs/d3310114.nsf/home/remoteness+structure. bSocio-economic Indexes for Areas (SEIFA) can be found at https://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa.
Abbreviation: eFI, electronic Frailty Index.
As seen in Table 2, polypharmacy was the most commonly identified deficit for the eFI (61.7%), followed by arthritis (55.3%), hypertension (52.1%), urinary system disease (36.1%), visual impairment (31.3%), respiratory disease (29.5%) and osteoporosis (22.5%). The deficits with the lowest prevalence were housebound (0.03%), social vulnerability (1.2%), weight loss/anorexia (2.4%) and foot problems (3.1%).
Table 2.
Prevalence of Individual Deficits Contributing to the eFI Score in the Australian MedicineInsight Sample
| Deficits | n | % |
|---|---|---|
| Activity limitation | 3066 | 3.9 |
| Anaemia and haematinic deficiency | 15,034 | 18.8 |
| Arthritis | 43,791 | 55.3 |
| Atrial fibrillation | 12,878 | 16.3 |
| Cerebrovascular disease | 8923 | 11.3 |
| Chronic kidney disease | 15,037 | 18.9 |
| Diabetes | 15,808 | 19.9 |
| Dizziness | 15,869 | 20.0 |
| Dyspnoea | 7626 | 9.6 |
| Falls | 10,893 | 13.7 |
| Foot problems | 2451 | 3.1 |
| Fragility fracture | 9288 | 11.7 |
| Hearing impairment | 7799 | 9.8 |
| Heart Failure | 8060 | 10.2 |
| Heart valve disease | 5966 | 7.5 |
| Housebound | 20 | 0.03 |
| Hypertension | 41,315 | 52.1 |
| Hypotension/syncope | 6405 | 8.1 |
| Ischaemic heart disease | 14,823 | 18.7 |
| Memory and cognitive impairment | 8183 | 10.3 |
| Mobility and transfer problems | 2646 | 3.3 |
| Osteoporosis | 17,823 | 22.5 |
| Parkinsonism and tremor | 3093 | 3.9 |
| Peptic ulcer | 3824 | 4.8 |
| Peripheral vascular disease | 3568 | 4.5 |
| Polypharmacy | 48,875 | 61.7 |
| Requirement for care | 6697 | 8.5 |
| Respiratory disease | 23,353 | 29.5 |
| Skin ulcer | 4933 | 6.2 |
| Sleep disturbance | 9423 | 11.9 |
| Social vulnerability | 948 | 1.2 |
| Thyroid disease | 13,307 | 16.8 |
| Urinary incontinence | 7923 | 10.0 |
| Urinary system disease | 28,641 | 36.1 |
| Visual impairment | 24,762 | 31.3 |
| Weight loss and anorexia | 1878 | 2.4 |
Notes: Prevalence % estimates are out of the total eligible sample of 79,251.
Discussion
To our knowledge, this research is the first large-scale Australian study to derive an eFI based on routinely collected general practice data. Results demonstrated that it was indeed feasible to derive an eFI from Australian general practice records, including the identification of all (100%) 36 individual components of the eFI, and fits with the expected parameters of a frailty index maximum observed score of ≤0.70.27 Our study also showed that the eFI developed by Clegg et al’17 - which is contractual for general practices across the United Kingdom,28 was translatable to Australian primary care clinical systems.
Prevalence of frailty was high in our study: 17,018 patients (21.5%) were moderately or severely frail and a further 29,608 patients (37.4%) were mildly frail. This prevalence is comparable to that found by Clegg et al,17 who reported that frailty prevalence (moderate plus severe frailty) in English general practice was 15% and 20% in their internal and external validation cohorts, respectively, and prevalence of mild frailty was 35% and 37% for internal and external cohorts, respectively. More details on gender and age distribution and severity estimates can be found in Supplementary Table 2. Similarly, a further validation of the eFI in Welsh general practice reported frailty prevalence as 15% (again, combining moderate and severe frailty categories), and mild frailty as between 33% and 37% depending on which cohort of patients was studied.4 Furthermore, the present study showed a significant age-associated increase in frailty prevalence, as well as a higher prevalence of frailty in females than males (24.2% vs 18.1%); these findings are both consistent with the literature.1,2,17,18,29,30
Frailty was more prevalent in those with socioeconomic disadvantage in our study. This is consistent with multiple international studies, including in both low- to middle-30 and high-income countries.31–34 While this study was cross-sectional in nature, it is expected that determinants such as socioeconomic status might be predictive of frailty, given that socioeconomic inequalities are known drivers of adverse health outcomes.35 While not within the scope of this study or available in our database, the prevalence of frailty between racial and ethnic groups would also be worth examining in multicultural countries such as Australia. A pilot study using the eFI in an older population in London found the prevalence of frailty to differ between ethnic groups,36 and a recent scoping review finding a higher prevalence of frailty in Indigenous adults from settler-colonies when compared to their non-Indigenous counterparts.37 The literature also shows an inconsistent link between frailty and remoteness, with frailty prevalence higher in rural areas in some studies,38 but not others.39 The present study found that frailty existed in all levels of remoteness (major cities, regional and remote areas). Therefore, routine implementation of an eFI could inform interventions to prevent or reduce frailty in all older adults, regardless of location.
Frailty is associated with poorer outcomes2,5 but may be amenable to treatment that reduces frailty progression and poor outcomes.16 Treatments with evidence of effectiveness include nutritional supplementation, physical training, cognitive therapy and health education.7,8,16 Early detection may facilitate targeted intervention at specific points along the frailty spectrum9,16 and better inform discussions regarding trajectories of health and goals of care.15 Recent international clinical practice guidelines recommend that all older adults be offered screening for frailty using a rapid and validated instrument which is suitable to the clinical setting.9 The value of using an eFI to identify frailty from EMRs in primary care is the time efficiency it confers to busy clinicians. For instance, an automated eFI can be applied in lieu of clinical frailty measurement, given that it has been found to show convergent validity with several frailty measurements internationally, including the Clinical Frailty Scale (CFS), Edmonton Frailty Scale (EFS), standard FI,40 and FI based on comprehensive geriatric assessment (FI-CGA).41 An Australian study in general practice using the eFI against Fried’s physical frailty phenotype scale found the eFI to have a high level of accuracy in identifying frailty (area under the Receiver Operating Characteristic curve = 0.9).42 The utility of periodic monitoring of eFI estimates and frailty trajectories among older patients in general practice cannot be overemphasized. It is useful for profiling of high-risk groups, assisting in clinical decision-making and informing public health policy on modifiable risk factors, as confirmed in a recent systematic review.43 Indeed, research from England has reported that the eFI in general practice can inform healthcare service use, including the need for community services in the forthcoming six months.29 Similarly, in Wales, the National Health Service (NHS) eFI has shown good predictive ability for outcomes such as 5-year mortality, hospitalization, care home admission and 1-year mortality following hospitalisation.4
Strengths and Limitations
We have shown that a frailty index can be calculated from routinely collected data from multiple general practices across several states and territories. We were also able to map its distribution by socioeconomic areas and geographic location, giving further insights into the prevalence of frailty across Australia. The use of this existing and ongoing large-scale dataset is a key advantage of our study, without the need for additional significant fiscal investment or time from busy clinicians, as is using the coding from the NHS eFI. Despite these advantages, our study has some disadvantages. The cross-sectional nature of the analysis precludes confirmation of causal inferences, but the associations are still present even if not aetiologically relevant or the directionality is uncertain or reversed44,45 and are worth examining for future service planning. This sample includes >700 practices across the country, and there may be some differentials in patient casemix and frailty prevalence. Some general practice data are not well recorded in Australia,20 eg, social vulnerability, physical activity and nutrition, and therefore, we suspect that some information could be under-reported. This issue has also been flagged internationally. For example, a 2019 study of the eFI in the UK (n = 42,593) indicated that although the correlation between the eFI and coded level of frailty [mild, moderate or severe] was high (85.3%), there was much variability between general practices and the software used. The authors of this study concluded that software improvements and staff training were urgently needed to adequately record frailty status.46
Implications for Practice
Frailty identification should preferably take place before a “crisis point” that leads patients to the emergency department, and general practice is an ideal setting for identification to occur. This study has provided new understandings of frailty from the information already available in data routinely collected by GPs, which may be used to identify and “flag” frailty without the need to use additional frailty instruments or invest in additional software or other tools. The development of a validated automated data extraction tool would have the potential to overcome the known barriers to frailty screening including awareness of frailty:47 frailty assessment measures which are often impractical in the GP setting,48 frailty questionnaires which patients find difficult to complete and the time-consuming nature of screening.49 Screening of frailty and institution of, and support for, tailored interventions have the potential to improve health outcomes in older patients such as quality of life, physical and psychosocial functioning.7,50
Frailty identification can also provide a framework to discuss and elicit goals of care with older patients and caregivers and empower shared decision-making by clinician and patient/caregivers regarding “frailty-aware care”51 and use of health interventions that can reduce non-beneficial and/or harmful treatments at the end of life.15 Together, these may facilitate timely interventions to slow the progression of frailty and/or improve QOL.
To enhance the identification of frailty in the future, an automated process may be the way forward. While the eFI could potentially be built into a field already extracted from EMRs,42 to be comparable with the UK, there are differences that need to be considered. For example, in Australia, there remains no standardization for EMR software, therefore data quality could be influenced by the formatting capabilities and clinical coding of the software. There is also much diversity of software available to general practices; therefore, achieving efficient healthcare interoperability is a potential barrier to the implementation of eFIs in Australia.42,52 To enhance future related research, engaging both health care consumers and general practice teams in the study design is necessary.
Conclusion
Our study contributes to the first large-scale application of an eFI to routinely collected Australian primary care data. It demonstrates that the United Kingdom’s eFI is translatable to Australian primary care clinical systems, as all eFI variables were extractable. Furthermore, using the Australian eFI, we found frailty to be present across all levels of remoteness (major cities, regional and remote areas) but more prevalent in those with socioeconomic disadvantage, an association worth exploring further. Therefore, routine implementation of an automated eFI could inform strategies to identify at-risk patients and manage frailty in older Australian adults regardless of location.
Acknowledgments
We would like to thank Dr Robert Menzies, Professor Teng Liaw and Professor Roslyn Poulos for initial assistance with the study protocol. We are grateful to the general practices and general practitioners who participate in MedicineInsight and the patients whose de-identified data make this work possible.
Funding Statement
This study was funded by a grant from the School of Population Health at the University of New South Wales. The funder had no role in the study design, conduct, reporting or decision to publish.
Ethics Statement
Ethics approval was granted by the University of New South Wales Human Research Ethics Committee (#HC190384) and NPS MedicineWise Data Governance Committee (DG 2018-015).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365–1375. doi: 10.1016/S0140-6736(19)31786-6 [DOI] [PubMed] [Google Scholar]
- 3.Lowthian JA, Jolley DJ, Curtis AJ, et al. The challenges of population ageing: accelerating demand for emergency ambulance services by older patients, 1995–2015. Med J Aust. 2011;194(11):574. doi: 10.5694/j.1326-5377.2011.tb03107.x [DOI] [PubMed] [Google Scholar]
- 4.Hollinghurst J, Fry R, Akbari A, et al. External validation of the electronic frailty index using the population of wales within the secure anonymised information linkage databank. Age Ageing. 2019;48(6):922–926. doi: 10.1093/ageing/afz110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis ET, Dent E, Alkhouri H, et al. Which frailty scale for patients admitted via emergency department? A cohort study. Arch Gerontol Geriatr. 2019;80:104–114. doi: 10.1016/j.archger.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 6.Pond CD, Regan C. Improving the delivery of primary care for older people. Med J Aust. 2019;211(2):60–62.e61. doi: 10.5694/mja2.50236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apóstolo J, Cooke R, Bobrowicz-Campos E, et al. Effectiveness of interventions to prevent pre-frailty and frailty progression in older adults: a systematic review. JBI Database Syst Rev Implement Rep. 2018;16(1):140–232. doi: 10.11124/JBISRIR-2017-003382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Travers J, Romero-Ortuno R, Bailey J, Cooney MT. Delaying and reversing frailty: a systematic review of primary care interventions. Br J Gen Pract. 2019;69(678):e61–e69. doi: 10.3399/bjgp18X700241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dent E, Morley JE, Cruz-Jentoft AJ, et al. Physical frailty: ICFSR international clinical practice guidelines for identification and management. J Nutr Health Aging. 2019;23(9):771–787. doi: 10.1007/s12603-019-1273-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callaghan S, Smith SM. Frailty in family practice. Fam Pract. 2017;34(5):508–510. doi: 10.1093/fampra/cmx029 [DOI] [PubMed] [Google Scholar]
- 11.Abbasi M, Rolfson D, Khera AS, Dabravolskaj J, Dent E, Xia L. Identification and management of frailty in the primary care setting. CMAJ. 2018;190(38):E1134–E1140. doi: 10.1503/cmaj.171509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Department of Health. About the Commonwealth Home Support Programme (CHSP). Australian Government; 2020. Available from: https://www.health.gov.au/initiatives-and-programs/commonwealth-home-support-programme-chsp/about-The-commonwealth-home-support-programme-chsp. Accessed October 27, 2022. [Google Scholar]
- 13.Lewin G, Allan J, Patterson C, Knuiman M, Boldy D, Hendrie D. A comparison of the home-care and healthcare service use and costs of older Australians randomised to receive a restorative or a conventional home‐care service. Health Soc Care Community. 2014;22(3):328–336. doi: 10.1111/hsc.12092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morley JE, Vellas B, Abellan van Kan G. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–397. doi: 10.1016/j.jamda.2013.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardona-Morrell M, Lewis E, Suman S, et al. Recognising older frail patients near the end of life: what next? Eur J Intern Med. 2017;17(S0953–6205):30376–X. [DOI] [PubMed] [Google Scholar]
- 16.Walston J, Buta B, Xue Q-L. Frailty screening and interventions: considerations for clinical practice. Clin Geriatr Med. 2018;34(1):25–38. doi: 10.1016/j.cger.2017.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45(3):353–360. doi: 10.1093/ageing/afw039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World. 2001;1:323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NPS MedicineWise. MedicineInsight Data Book Version 3.0. NPS MedicineWise; 2020. [Google Scholar]
- 20.Busingye D, Gianacas C, Pollack A, et al. Data resource profile: medicineinsight, an Australian national primary health care database. Int J Epidemiol. 2019;48(6):1741–1741h. doi: 10.1093/ije/dyz147 [DOI] [PubMed] [Google Scholar]
- 21.NPS MedicineWise. General Practice Insights Report July 2018–June 2019. Sydney: NPS MedicineWise; 2020. [Google Scholar]
- 22.Nwadiugwu MC. Frailty and the risk of polypharmacy in the older person: enabling and preventative approaches. J Aging Res. 2020;2020:6759521. doi: 10.1155/2020/6759521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Australian Bureau of Statistics. Census of Population and Housing: socio-Economic Indexes for Areas (SEIFA), Australia. ABS; 2011. Available from: https://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa2011?opendocument&navpos=260. Accessed October 27, 2022.
- 24.Australian Bureau of Statistics. Australian Statistical Geography Standard (ASGS): volume 5 - remoteness structure. ABS; 2018. Available from: https://www.abs.gov.au/ausstats/abs@.nsf/mf/1270.0.55.005. Accessed October 27, 2022. [Google Scholar]
- 25.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8. doi: 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Department of Health. Health assessment for people aged 75 years and older. Australian Government; 2014. Available from: https://www1.health.gov.au/internet/main/publishing.nsf/Content/mbsprimarycare_mbsitem_75andolder. Accessed October 27, 2022. [Google Scholar]
- 27.Drubbel I, Numans ME, Kranenburg G, Bleijenberg N, de Wit NJ, Schuurmans MJ. Screening for frailty in primary care: a systematic review of the psychometric properties of the frailty index in community-dwelling older people. BMC Geriatr. 2014;14(1):27. doi: 10.1186/1471-2318-14-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Health Service. Supporting routine frailty identification and frailty through the GP Contract 2017/2018. NHS England; 2019. Available from: https://www.england.nhs.uk/wp-content/uploads/2017/04/gms-contract-batch-coding-statement-v1.pdf. Accessed October 27, 2022. [Google Scholar]
- 29.Boyd PJ, Nevard M, Ford JA, Khondoker M, Cross JL, Fox C. The electronic frailty index as an indicator of community healthcare service utilisation in the older population. Age Ageing. 2018;48(2):273–277. doi: 10.1093/ageing/afy181 [DOI] [PubMed] [Google Scholar]
- 30.Hoogendijk EO, Rijnhart JJM, Kowal P, et al. Socioeconomic inequalities in frailty among older adults in six low- and middle-income countries: results from the WHO Study on global AGEing and adult health (SAGE). Maturitas. 2018;115:56–63. doi: 10.1016/j.maturitas.2018.06.011 [DOI] [PubMed] [Google Scholar]
- 31.Hoogendijk EO, Heymans MW, Deeg DJH, Huisman M. Socioeconomic inequalities in frailty among older adults: results from a 10-year longitudinal study in the Netherlands. Gerontology. 2018;64(2):157–164. doi: 10.1159/000481943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunner EJ, Shipley MJ, Ahmadi-Abhari S, et al. Midlife contributors to socioeconomic differences in frailty during later life: a prospective cohort study. Lancet Public Health. 2018;3(7):e313–e322. doi: 10.1016/S2468-2667(18)30079-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendonça N, Kingston A, Yadegarfar M, et al. Transitions between frailty states in the very old: the influence of socioeconomic status and multi-morbidity in the Newcastle 85+ cohort study. Age Ageing. 2020;49(6):974–981. doi: 10.1093/ageing/afaa054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franse CB, van Grieken A, Qin L, Melis RJF, Rietjens JAC, Raat H. Socioeconomic inequalities in frailty and frailty components among community-dwelling older citizens. PLoS One. 2017;12(11):e0187946. doi: 10.1371/journal.pone.0187946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steptoe A, Zaninotto P. Lower socioeconomic status and the acceleration of aging: an outcome-wide analysis. Proc Natl Acad Sci. 2020;117(26):14911–14917. doi: 10.1073/pnas.1915741117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pradhananga S, Regmi K, Razzaq N, Ettefaghian A, Dey AB, Hewson D. Ethnic differences in the prevalence of frailty in the United Kingdom assessed using the electronic Frailty Index. Aging Medicine. 2019;2(3):168–173. doi: 10.1002/agm2.12083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis ET, Howard L, Cardona M, et al. Frailty in indigenous populations: a scoping review. Public Health Front. 2021;9:785460. doi: 10.3389/fpubh.2021.785460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dent E, Dal Grande E, Price K, Taylor AW. Frailty and usage of health care systems: results from the South Australian Monitoring and Surveillance System (SAMSS). Maturitas. 2017;104:36–43. doi: 10.1016/j.maturitas.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 39.Yu P, Song X, Shi J, et al. Frailty and survival of older Chinese adults in urban and rural areas: results from the Beijing Longitudinal Study of Aging. Arch Gerontol Geriatr. 2012;54(1):3–8. doi: 10.1016/j.archger.2011.04.020 [DOI] [PubMed] [Google Scholar]
- 40.Brundle C, Heaven A, Brown L, et al. Convergent validity of the electronic frailty index. Age Ageing. 2018;48(1):152–156. doi: 10.1093/ageing/afy162 [DOI] [PubMed] [Google Scholar]
- 41.Abbasi M, Khera S, Dabravolskaj J, et al. A cross-sectional study examining convergent validity of a frailty index based on electronic medical records in a Canadian primary care program. BMC Geriatr. 2019;19(1):109. doi: 10.1186/s12877-019-1119-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ambagtsheer RC, Beilby J, Dabravolskaj J, Abbasi M, Archibald MM, Dent E. Application of an electronic Frailty Index in Australian primary care: data quality and feasibility assessment. Aging Clin Exp Res. 2018;31. doi: 10.1007/s40520-018-1023-9 [DOI] [PubMed] [Google Scholar]
- 43.Welstead M, Jenkins ND, Russ TC, Luciano M, Muniz-Terrera G, Systematic A. Review of frailty trajectories: their shape and influencing factors. Gerontologist. 2020;61(8):e463–e475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savitz DA, Wellenius GA. Can cross-sectional studies contribute to causal inference? It Depends. Am J Epidemiol. 2022. doi: 10.1093/aje/kwac037 [DOI] [PubMed] [Google Scholar]
- 45.Shahar E, Shahar DJ. Causal diagrams and the cross-sectional study. Clin Epidemiol. 2013;5:57–65. doi: 10.2147/CLEP.S42843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Millares-Martin P. Large retrospective analysis on frailty assessment in primary care: electronic Frailty Index versus frailty coding. BMJ Health Care Inform. 2019;26(1):1. doi: 10.1136/bmjhci-2019-000024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gwyther H, Shaw R, Jaime Dauden EA, et al. Understanding frailty: a qualitative study of European healthcare policy-makers’ approaches to frailty screening and management. BMJ Open. 2018;8(1):e018653. doi: 10.1136/bmjopen-2017-018653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Kempen JA, Schers HJ, Jacobs A, et al. Development of an instrument for the identification of frail older people as a target population for integrated care. Br J Gen Pract. 2013;63(608):e225–e231. doi: 10.3399/bjgp13X664289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruiz JG, Priyadarshni S, Rahaman Z, et al. Validation of an automatically generated screening score for frailty: the care assessment need (CAN) score. BMC Geriatr. 2018;18(1):106. doi: 10.1186/s12877-018-0802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee PH, Lee YS, Chan DC. Interventions targeting geriatric frailty: a systemic review. J Clin Gerontol Geriatr. 2012;3(2):47–52. doi: 10.1016/j.jcgg.2012.04.001 [DOI] [Google Scholar]
- 51.Boreskie KF, Hay JL, Boreskie PE, Arora RC, Duhamel TA. Frailty-aware care: giving value to frailty assessment across different healthcare settings. BMC Geriatr. 2022;22(1):13. doi: 10.1186/s12877-021-02722-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Canaway R, Boyle DI, Manski-Nankervis JAE, et al. Gathering data for decisions: best practice use of primary care electronic records for research. Med J Aust. 2019;210(S6):S12–S16. doi: 10.5694/mja2.50026 [DOI] [PMC free article] [PubMed] [Google Scholar]