Abstract
Introduction
Severe chronic obstructive pulmonary disease (COPD) is partly characterized by diminished skeletal muscle oxidative capacity and concurrent dyslipidemia. It is unknown whether such metabolic derangements increase the risk of cardiovascular disease. This study explored associations among physical activity (PA), muscle oxidative capacity, and coronary artery calcium (CAC) in COPDGene participants.
Methods
Data from current and former smokers with COPD (n = 75) and normal spirometry (n = 70) were retrospectively analyzed. Physical activity was measured for seven days using triaxial accelerometry (steps/day and vector magnitude units [VMU]) along with the aggregate of self-reported PA amount and PA difficulty using the PROactive D-PPAC instrument. Muscle oxidative capacity (k) was assessed via near-infrared spectroscopy, and CAC was assessed via chest computerized tomography.
Results
Relative to controls, COPD patients exhibited higher CAC (median [IQR], 31 [0–431] vs 264 [40–799] HU; p = 0.003), lower k (mean ± SD = 1.66 ± 0.48 vs 1.25 ± 0.37 min−1; p < 0.001), and lower D-PPAC total score (65.2 ± 9.9 vs 58.8 ± 13.2; p = 0.003). Multivariate analysis—adjusting for age, sex, race, diabetes, disease severity, hyperlipidemia, smoking status, and hypertension—revealed a significant negative association between CAC and D-PPAC total score (β, −0.05; p = 0.013), driven primarily by D-PPAC difficulty score (β, −0.03; p = 0.026). A 1 unit increase in D-PPAC total score was associated with a 5% lower CAC (p = 0.013). There was no association between CAC and either k, steps/day, VMU, or D-PPAC amount.
Conclusion
Patients with COPD and concomitantly elevated CAC exhibit greater perceptions of difficulty when performing daily activities. This may have implications for exercise adherence and risk of overall physical decline.
Keywords: coronary artery calcium, COPD, muscle, oxidative capacity, physical activity, respiratory
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of hospitalization and mortality in the USA.1 Of all COPD-related comorbidities, vascular and heart diseases are among the most prevalent, with coronary artery disease affecting one-in-six COPD patients,2,3 and a risk of arterial stiffness that increases with COPD disease severity.4 There is also evidence that patients with both vascular disease and COPD are at a two-fold greater risk of morbidity and mortality relative to the general population.5 Indeed, a recent analysis of ~7000 patients revealed a three-fold greater risk of mortality in those with concurrent severe COPD and high coronary artery calcium (CAC) relative to severe COPD alone.6 Collectively, the data suggest that the greatest mortality risk in COPD may not be lung disease, per se, but rather its association with cardiovascular disease (CVD).5,6
The mechanisms underpinning CVD risk in COPD are not well understood. Both diseases share risk factors (e.g., cigarette smoking) and common pathologies (e.g., a persistent low-grade systemic inflammation) .7 However, COPD is additionally characterized by airway inflammation and remodeling (i.e., chronic bronchitis) and/or parenchymal damage (ie, emphysema)8 causing exertional dyspnea.9 The resulting exercise intolerance leads to physical inactivity, deconditioning, and a subsequent worsening of respiratory symptoms in a downward spiral of physical decline.10 Physical inactivity independently predicts coronary artery disease11 and is an important factor in CVD mortality risk in patients with COPD.12
Physical deconditioning is partly characterized by a loss of skeletal muscle oxidative fibers,13 which is also an independent risk factor for mortality in COPD.14 When compared to smokers with normal lung function, COPD patients exhibit lower muscle oxidative capacity15,16 and a smaller fraction of type I muscle fibers17 in the lower limbs, even when accounting for smoking history and differences in physical activity.18 Patients with severe COPD and low skeletal muscle oxidative capacity also exhibit greater serum di- and triglycerides.19 Although these metabolic derangements would be expected to confer an increased risk of CVD (e.g., CAC burden), the associations among physical activity (PA), muscle oxidative capacity, and CAC have not been directly explored in COPD. A better understanding of these relationships will inform our understanding of COPD pathophysiology and potentially highlight whether reduced PA and/or physical deconditioning are associated with a greater cardiovascular risk burden. Accordingly, the aim of this study was to assess PA, muscle oxidative capacity, and coronary artery calcium in current and former smokers, with and without COPD, recruited from the COPDGene cohort. It was hypothesized that CAC burden would be associated negatively with both muscle oxidative capacity and markers of PA.
Methods
Participants
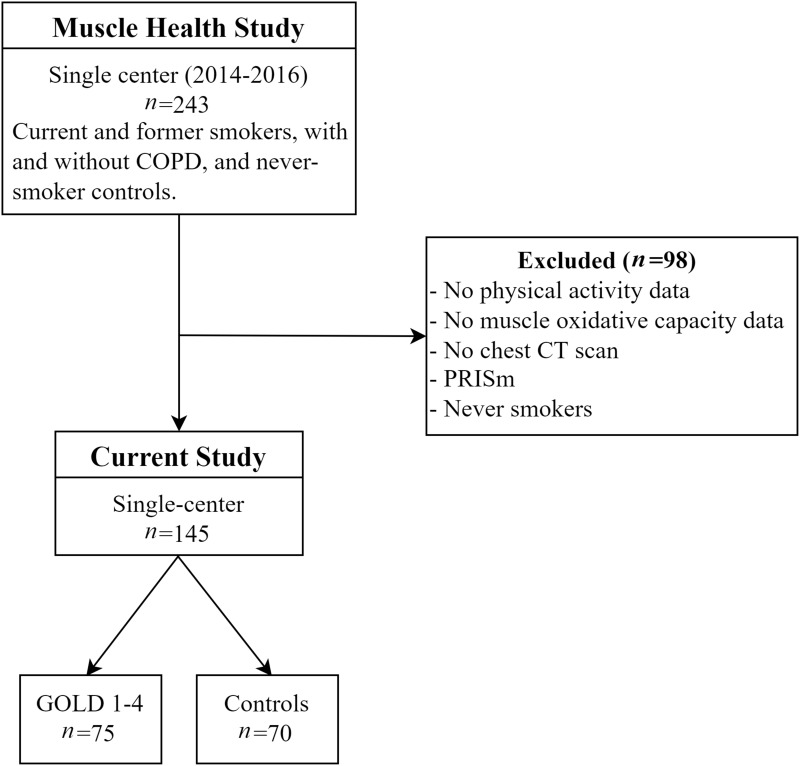
The study population was drawn from the single-center Muscle Health Study, an ancillary study of COPDGene, which enrolled a total of 243 participants at The Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center between 2014 and 2016. The present study is a retrospective analysis of 145 current and former smokers, with and without COPD, in whom the relevant assessments were available (Figure 1). The study was approved by the Institutional Review Board at The Lundquist Institute (#12756-03) and conducted in accordance with the Declaration of Helsinki except for principle 35 (public trial registration). All participants provided written, informed consent prior to the start of data collection.
Figure 1.
Consort diagram of participants recruited into the Muscle Health Study and allocated to the current analysis.
Abbreviation: PRISm, preserved ratio with impaired spirometry.
Study Overview
Each participant underwent the following assessments: demographics (age, sex, race, mass, and stature); resting vital signs (heart rate, blood pressure, oxygen saturation, and hemoglobin concentration); current medications; medical and smoking history; symptoms (Modified Medical Research Council Dyspnea Scale, mMRC;20 and COPD Assessment Test, CAT21); pulmonary function via spirometry;22 functional exercise performance via distance accomplished in the 6-minute Walk Test (6MWT); objective and subjective measures of PA; skeletal muscle oxidative capacity; and CAC.
Physical Activity
Objective measures of PA were assessed via a triaxial accelerometer (DynaPort MoveMonitor; McRoberts BV, The Hague, The Netherlands) which participants were asked to wear for 1 week. The device returned a count of the number of daily steps and the daily vector magnitude units (VMU; count/min) which provided data on the combined amount and intensity of accelerometer movement. Data were included in the analysis if the accelerometer was worn for >8 hours per day,23 between 08:00 and 23:00, for at least 4 of 7 days of the week.18
Measures of PA experience were assessed via the Daily-PROactive Physical Activity instrument in COPD (D-PPAC version) questionnaire relating to PA performed on the days in which the accelerometer was worn.24 The D-PPAC questionnaire consisted of seven questions: two relating to PA amount (viz., “How much walking did you do outside today? How many chores did you do outside the house today?) and five relating to the difficulty of, and perceived limitation in, completing activities of daily living (viz., How much difficulty did you have getting dressed today? How often did you avoid doing activities because of your lung problems today? How breathless were you in general during your activities today? How tired were you in general during your activities today? How often did you have to take breaks during your physical activities today?).24 Output from the instrument was subcategorized into two domains in which raw ratings were converted into Rassh scores on a 0 (worst) to 100 (best) scale: i) D-PPAC amount comprised the sum result of the two questions on PA amount (each rated on a 0–4 scale) combined with VMU and step data from the accelerometer; ii) D-PPAC difficulty comprised the sum result of the five questions on PA difficulty (each rated on a 0–4 scale). The D-PPAC total score, which represented the patient experience with PA, was then calculated as the mean of both domains. The D-PPAC instrument is a reliable, valid, and responsive measure in diverse COPD patients,25 with strong test–retest reliability in smokers with and without COPD (ICC ≥0.88).24
Muscle Oxidative Capacity
In a single visit to the laboratory, muscle oxidative capacity was assessed in duplicate from the medial gastrocnemius as muscle O2 consumption recovery rate constant (k, min−1) using near-infrared spectroscopy, as previously described.15,26 The technique is well validated and provides values for k that are directly proportional to muscle oxidative capacity measured in muscle biopsy samples27 and single muscle fibers of various biochemical phenotypes.28
Coronary Artery Calcium
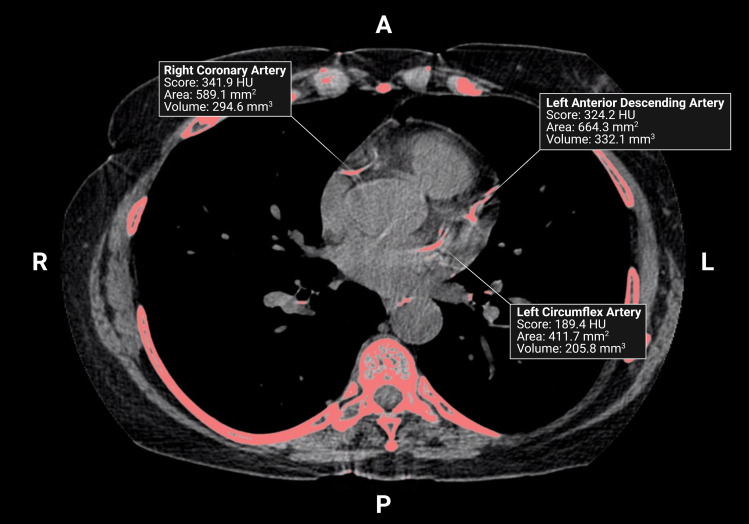
Each participant underwent two consecutive, non-contrast, ungated cardiac CT scans using parameters previously described.29,30 Scans were performed from carina to below the apex of the heart and images were acquired during breath hold to reduce motion artifact and improve image quality.31 Total CAC was reported as Agatston score following the aggregation of density and area of summed calcium deposits from the left main, left anterior descending, left circumflex, and right coronary arteries (Figure 2).
Figure 2.
A non-contrast, ungated chest CT scan from a 69-year-old male with COPD (FEV1, 30%Pred). Image was acquired during inspiratory breath-hold and shows calcium in the right coronary, left anterior descending, and left circumflex arteries. Created with Biorender.com.
Abbreviations: HU, Hounsfield Units; A, anterior; P, posterior; L, left; R, right.
Statistics
Analyses were performed using SAS for Windows version 9.4 (SAS Institute, Cary, NC). Participant characteristics (demographics, vital signs, medical and smoking history, pulmonary function, symptoms, 6MWT distance, PA, skeletal muscle oxidative capacity, and CAC) were compared between COPD patients (stratified by Global Obstructive Lung Disease [GOLD] stage32) and non-COPD controls using the chi-square statistic, Student’s t-test, and Kruskal–Wallis test, as appropriate. Effect size (Cohen’s d) was used to quantify the magnitude of the difference between group means (0.2, small; 0.5, medium; 0.8, large).33 Linear regression was used to assess associations between CAC (>0) and muscle oxidative capacity (k); CAC (>0) and D-PPAC total, amount, and difficulty scores; CAC (>0) and steps/day; and CAC (>0) and VMU from the accelerometer. Three multivariable linear models were used: model 1 adjusted for age, sex, and race; model 2 adjusted for age, sex, race, diabetes, GOLD stage, and hyperlipidemia; and model 3 adjusted for age, sex, race, diabetes, GOLD stage, hyperlipidemia, smoking status, and hypertension. To satisfy the assumptions of linear regression, values for CAC were log transformed and zero inflated. Discrete variables are presented as counts and percentages, and continuous variables as either mean (± standard deviation) or median (± interquartile range). Alpha level was set as 0.05.
Results
Demographics and Clinical Characteristics
Participant characteristics are reported in Table 1. Of 145 participants, 71 (49%) were female and 68 (47%) were African American. Based on the spirometric indices, the 75 patients with COPD were categorized as GOLD spirometry stage 1 (n = 20), stage 2 (n = 34), stage 3 (n = 14), and stage 4 (n = 7). Relative to controls, COPD patients were significantly older (p < 0.001), had a greater Non-Hispanic White representation (p < 0.001), and were more likely to be former smokers (p = 0.001). There were no significant between-group differences in BMI, sex, resting heart rate, diabetes, hyperlipidemia, hypertension, 6MWT, hemoglobin concentration, or steps/day (p > 0.05). Per definition, COPD patients exhibited lower FEV1 and FEV1%predicted (p < 0.001). The COPD patients also exhibited lower resting SpO2 (p < 0.001), VMU (p = 0.008), D-PPAC total score (p = 0.002), D-PPAC amount score (p = 0.034), D-PPAC difficulty score (p = 0.020), muscle oxidative capacity (p < 0.001), higher systolic blood pressure (p = 0.011), CAT score (p = 0.027), and CAC (p = 0.003) (Table 1). In total, 29/145 patients (20%) exhibited one-or-more pre-existing cardiovascular comorbidities that included myocardial infarction (n = 9), blood clots (n = 8), congestive heart failure (n = 7), coronary artery disease (n = 7), atrial fibrillation (n = 4), angina pectoris (n = 3), stroke (n = 2), transient ischemia (n = 2), and peripheral vascular disease (n = 1). Thirteen (45%) of those with comorbidities were controls.
Table 1.
Participant Characteristics
| Total (n = 145) | Controls (n = 70) | GOLD 1–4 (n = 75) | p | d | ||||
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Age (years)a | 63.6 | (9.3) | 59.9 | (8.9) | 67.0 | (8.5) | <0.001 | 0.82 |
| BMI (kg/m2)a | 28.1 | (6.2) | 28.8 | (6.7) | 27.4 | (5.6) | 0.153 | 0.23 |
| Male (n (%)) | 74 | (51) | 34 | (49) | 40 | (53) | 0.567 | – |
| Race, non-Hispanic White (n (%)) | 77 | (53) | 22 | (31) | 55 | (73) | <0.001 | - |
| Resting vitals | ||||||||
| SBP (mmHg)a | 134 | (18) | 130 | (14) | 137 | (21) | 0.011 | 0.39 |
| DBP (mmHg)a | 78 | (9) | 78 | (8) | 78 | (11) | 0.948 | 0.00 |
| HR (bt/min)a | 73 | (12) | 72 | (11) | 74 | (13) | 0.230 | 0.17 |
| SpO2 (%)b | 98 | (97–99) | 99 | (98–99) | 98 | (97–99) | <0.001 | - |
| Hemoglobin (g/dL)a | 13.8 | (1.4) | 13.7 | (1.3) | 13.9 | (1.6) | 0.480 | 0.14 |
| Medical history | ||||||||
| O2 therapy (n (%)) | 14 | (10) | 0 | (0) | 14 | (19) | - | - |
| Severe exacerbations (n (%)) | 20 | (14) | 4 | (6) | 16 | (21) | 0.006 | - |
| Diabetes (n (%)) | 19 | (13) | 12 | (17) | 7 | (9) | 0.164 | - |
| Hyperlipidemia (n (%)) | 54 | (37) | 23 | (33) | 31 | (41) | 0.291 | - |
| Hypertension (n (%)) | 73 | (50) | 33 | (47) | 40 | (53) | 0.456 | - |
| Pulmonary function (post-bronch) | ||||||||
| FVC (L)a | 3.23 | (0.86) | 3.37 | (0.77) | 3.10 | (0.91) | 0.044 | 0.32 |
| FEV1 (L)a | 2.18 | (0.82) | 2.68 | (0.59) | 1.72 | (0.72) | <0.001 | 1.46 |
| FEV1/FVC a | 0.67 | (0.16) | 0.80 | (0.05) | 0.54 | (0.14) | <0.001 | 2.47 |
| FEV1%Pred a | 82.0 | (27.3) | 101.4 | (12.8) | 63.7 | (24.6) | <0.001 | 1.92 |
| CT air trapping (HU)a | 18.5 | (14) | 10.3 | (7.8) | 27.2 | (14) | <0.001 | 1.49 |
| Smoking history | ||||||||
| Current smoker (n (%)) | 64 | (44) | 41 | (59) | 23 | (31) | - | - |
| Former smoker (n (%)) | 81 | (56) | 29 | (41) | 52 | (69) | 0.001 | - |
| Symptoms | ||||||||
| mMRC = 0 (n (%)) | 76 | (52) | 49 | (70) | 27 | (36) | 0.001 | - |
| CAT scoreb | 14 | (6–20) | 12 | (6–20) | 15 | (9–23) | 0.027 | - |
| Functional exercise performance | ||||||||
| 6MWT (ft)a | 1279 | (293) | 1311 | (252) | 1248 | (325) | 0.193 | 0.22 |
| Muscle oxidative capacity | ||||||||
| k (min−1)a | 1.45 | (0.47) | 1.66 | (0.48) | 1.25 | (0.37) | <0.001 | 0.97 |
| Physical activity | ||||||||
| Steps/day (n/d)a | 5532 | (3836) | 6101 | (3188) | 5028 | (4291) | 0.101 | 0.28 |
| Daily VMU (counts/min−1)a | 391 | (228) | 444 | (181) | 343 | (255) | 0.008 | 0.46 |
| D-PPAC amount score (0–100)a | 48.8 | (13.7) | 51.7 | (12.3) | 46.3 | (14.3) | 0.034 | 0.40 |
| D-PPAC difficulty score (0–100)a | 75.0 | (17.6) | 78.8 | (16.1) | 71.3 | (18.1) | 0.020 | 0.44 |
| D-PPAC total score (0–100)a | 61.7 | (12.2) | 65.2 | (9.9) | 58.8 | (13.2) | 0.003 | 0.55 |
| Coronary artery calcium | ||||||||
| Total score (HU)b | 115.4 | (2–628) | 31.5 | (0–431) | 264.4 | (40–799) | 0.003 | - |
| Total area (mm2)b | 209.1 | (8–866) | 68.3 | (0–534) | 543.3 | (80–1287) | 0.001 | - |
| Total density (HU)b | 0.5 | (0.2–0.6) | 0.4 | (0–0.6) | 0.6 | (0.4–0.6) | 0.225 | - |
| Total volume (mm3)b | 104.5 | (4–433) | 34.1 | (0–267) | 271.7 | (40–643) | 0.001 | - |
| Total score of 0 (n (%))a | 32 | (22) | 20 | (29) | 12 | (16) | 0.068 | 0.34 |
Notes: Data are aMean (SD); bMedian (IQR).
Abbreviations: Controls, smokers with normal spirometry; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; SpO2, oxygen saturation of hemoglobin; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; mMRC, modified Medical Research Council dyspnea scale; CAT, COPD assessment test; 6MWT, 6-minute walk test [distance]; k, muscle oxidative capacity; VMU, vector magnitude units; D-PPAC, Daily-PROactive Physical Activity instrument in COPD; HU, Hounsfield units; p, comparison of GOLD 1–4 and controls using chi-square statistic, Student’s t-test, or Kruskal–Wallis test; d, Cohen’s d effect size (for mean ± SD only).
Coronary Artery Calcium, Physical Activity, and Muscle Oxidative Capacity
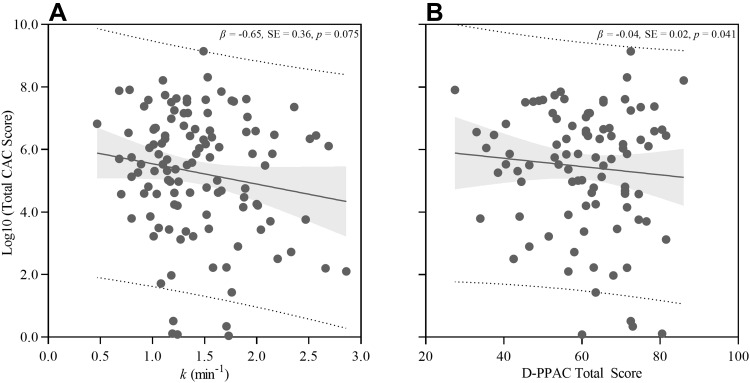
Univariable and multivariable linear regressions were used to assess the relationships between CAC (total score) and muscle oxidative capacity (k); CAC and D-PPAC total, amount, and difficulty scores; and CAC and both steps/day and VMU. The univariable analysis revealed a trending negative association between CAC and k (β, −0.65; p = 0.075; Figure 3A), but any association was lost after adjustments for age, sex, race, diabetes, GOLD stage, hyperlipidemia, smoking status, and hypertension (Model 3: β, −0.02; p = 0.953) (Table 2). When assessing the association between CAC and D-PPAC total score, the univariable model revealed a significant negative association (β, −0.04; p = 0.041; Figure 3B) which was strengthened after multivariable adjustment (β, −0.05; p = 0.013) (Table 2). Specifically, a 1 unit increase in D-PPAC total score was associated with 5% lower total CAC score (p = 0.013). The association between CAC and D-PACC was driven by the D-PPAC difficulty sub-domain (β, −0.03; p = 0.026), such that lower D-PACC difficulty score (i.e., higher self-reported difficulty experience with PA) was associated with higher CAC. There was no association between CAC and D-PPAC amount score (β, −0.03; p = 0.124) or CAC and the independent number of daily steps (β, −0.03; p = 0.124) or VMU (β, 0.00; p = 0.203) from the accelerometer.
Figure 3.
Univariable (unadjusted) analysis of total coronary artery calcium (CAC) versus muscle oxidative capacity (k) (A), and CAC versus D-PPAC total score (B). Regression (solid line); 95% confidence limits (grey shading); 95% prediction limits (dashed line).
Table 2.
Adjusted Regression Models for Associations Between CAC and (i) Muscle Oxidative Capacity (k), (ii) D-PPAC Total Score, (iii) D-PPAC Amount Score, and (iv) D-PPAC Difficulty Score
| CAC vs k | CAC vs D-PPAC Total Score | CAC vs D-PPAC Amount Score | CAC vs D-PPAC Difficulty Score | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | β | SE | p | β | SE | p | |
| Unadjusted | −0.65 | 0.36 | 0.075 | −0.04 | 0.02 | 0.041 | −0.04 | 0.02 | 0.035 | −0.02 | 0.02 | 0.232 |
| Model 1 | −0.22 | 0.33 | 0.506 | −0.06 | 0.02 | 0.004 | −0.04 | 0.02 | 0.026 | −0.03 | 0.01 | 0.021 |
| Model 2 | −0.09 | 0.36 | 0.806 | −0.05 | 0.02 | 0.008 | −0.03 | 0.02 | 0.069 | −0.03 | 0.01 | 0.026 |
| Model 3 | −0.02 | 0.35 | 0.953 | −0.05 | 0.02 | 0.013 | −0.03 | 0.02 | 0.124 | −0.03 | 0.01 | 0.026 |
Notes: Model 1 adjusted for age, sex, race. Model 2 adjusted for age, sex, race, diabetes, GOLD status, hyperlipidemia. Model 3 adjusted for age, sex, race, diabetes, GOLD status, hyperlipidemia, smoking status, and hypertension.
Abbreviations: CAC, coronary artery calcium; k, muscle oxidative capacity; D-PPAC, Daily-PROactive Physical Activity instrument; β, beta coefficient; SE, standard error.
Discussion
The aims of this study were to assess relationships among subjective and objective measures of physical activity, muscle oxidative capacity, and coronary artery calcium in current and former smokers, with and without COPD. The main finding was a significant association between CAC and ratings of PA difficulty derived from the D-PPAC sub-domain, such that individuals with greater perceived difficulty in PA exhibited greater CAC. Ratings of PA difficulty were also the basis for an association between D-PPAC total score and CAC. Contrary to our hypothesis, there was no association between CAC and D-PPAC amount, CAC and objective measures of PA (steps/day or VMU), or CAC and skeletal muscle oxidative capacity.
Our multivariable analysis revealed that a 1 unit increase in D-PPAC total score was associated with a 5% lower CAC (Table 2). Thus, a 4 unit increase in D-PPAC total score (the minimum important difference25 would be associated with a 20% lower CAC. The association, which was driven by the perceived difficulty ratings rather than the amount of activity (D-PPAC amount score or accelerometry measurement), remained after multivariable adjustment.
The mechanistic basis for this relationship is unclear. It is possible that CAC (and/or arterial stiffness) directly contributes to increased perceptions of PA difficulty in this group, but such a hypothesis has not been tested. It could also be that PA difficulty is higher in individuals with lower cardiorespiratory fitness and/or higher respiratory symptoms. Indeed, good cardiorespiratory fitness appears to be protective against plaque burden34 and slows the progression of early atherosclerosis.35 Moreover, low cardiorespiratory fitness mediates exercise-related symptoms, specifically increasing ratings of dyspnea in COPD.36
Previous research has shown that physical inactivity is an independent predictor of both CAC37 and coronary artery diseases.11 Yet, after adjustment for hypertension, smoking status, diabetes mellitus, and hypercholesterolemia, we found no association of current PA levels with CAC. There are several possible explanations for this lack of association. First, our cohort of COPD patients and controls had very low levels of activity, averaging ~5500 steps per day. This is only minimally above the step-defined “sedentary” threshold,23,38 and such low levels of PA may have attenuated the association with CAC. Second, this was a cross-sectional, observational study that was not designed to capture PA history. As such, longitudinal data are needed to better explore the relationship between chronic (lifelong) physical inactivity and CAC development in COPD. Lastly, tobacco use is a known predictor of CAC formation in young adults, exhibiting an odds ratio of 1.67 in individuals with CAC >100 HU.39 Although long-term PA may partially attenuate the increased CVD risk associated with smoking,40 the dynamics of this relationship in COPD are unknown. We speculate that the levels of PA exhibited by our cohort are insufficient to offset the negative effects of long-term smoking on CAC development.
Prior data from our group showed that patients with severe COPD exhibited lower k relative to smokers without COPD (p < 0.001) and that k was inversely associated with di- and triglyceride concentrations.19 Furthermore, in a large cohort of asymptomatic subjects, Bittencourt et al showed that triglyceride-rich lipoprotein cholesterol associated positively with CAC.41 These observations led to our hypothesis that COPD patients with low k may be at greater risk of CAC development, and thus CVD, second to metabolic inflexibility and hyperlipidemia. Despite our COPD patients exhibiting significantly lower values for k than smoking controls (1.25 versus 1.66 min−1), the trending negative association between k and CAC in the univariable analysis was obviated after adjustment for demographics and common CAC covariates. This suggests that k is not a predominating factor in the pathophysiology of CAC formation in current and former smokers, with or without COPD. Not only does age associate negatively with muscle oxidative capacity in otherwise healthy adults,42 it was also a significant confounder for CAC burden in our cohort. In addition, there is a well-documented association between smoking status/cumulative smoking exposure and inflammation leading to subclinical-atherosclerosis.43 Accordingly, both age and smoking history may be predictive of CAC formation in current and former smokers.
Study Implications
Breathlessness during physical exertion and general exercise intolerance are characteristic of COPD.44 Our data show that, regardless of GOLD stage, COPD patients with concomitantly elevated CAC exhibit greater perceptions of difficulty when performing daily activities (due to symptoms of dyspnea or fatigue). This heightened perceptual response places these patients at greater risk of deconditioning and overall physical decline. Moreover, a principal factor underpinning exercise adherence in patients with chronic diseases is “enjoyment and absence of unpleasant experiences”.45 As such, COPD patients with elevated CAC may be less likely to adhere to regular exercise. Not only will this negatively affect quality of life, it will also reduce the efficacy of pulmonary rehabilitation—a primary component of which is exercise training.46
Study Limitations
First, it is pertinent that CAC as an endpoint for CVD risk is limited in that it does not account for uncalcified plaque which is less stable and potentially associated with greater incidence of cardiovascular events.47 In fact, analysis of plaque morphology reveals higher CAC but lower mixed plaque in athletes relative to controls.48 Several studies also show a J-shaped relationship between self-reported PA and CAC49–51 such that moderate levels of activity appear most protective against CAC formation. Thus, while lower k congruent with higher di- and triglycerides19 might increase CVD risk in severe COPD patients, our retrospective analysis was unable to establish a subsequent link to CAC formation. A longitudinal assessment of CAC and k progression in COPD patients may be required to further explore this phenomenon.
Second, when interpreting the present findings, the perceptual assessments of the D-PPAC instrument also warrant brief consideration. The D-PPAC tool requires patients to answer a series of questions using daily recall, e.g., “How much difficulty did you have [getting dressed today]?”. The instrument does not allow a qualitative interpretation of the various factors that constitute “difficulty”, nor does it distinguish between difficulty responses attributable to cardiorespiratory fitness and those attributable to respiratory disease. In addition, COPD patients exhibit greater affective responses and sensations of respiratory distress (specifically air hunger) during PA relative to controls.52 As such, a more comprehensive assessment of the qualitative aspects of PA experience would offer greater insight into the mechanisms underpinning PA “difficulty” and its association with CAC burden in COPD.
Lastly, our model accounted for age, sex, race, diabetes, GOLD status, hyperlipidemia, smoking status, and hypertension, but we did not collect data on fat mass index or distribution, both of which are considered prominent risk factors for most cardiovascular diseases.53
Conclusions
Patients with COPD and concomitantly elevated CAC exhibit greater perceptions of difficulty when performing daily activities. Not only are these patients potentially at greater cardiovascular risk due to increased CAC burden, but their experience of difficulty during activity places them at greater risk of deconditioning and overall physical decline. The heightened perceptual response to physical activity may also have implications for exercise adherence and the success of the exercise component of pulmonary rehabilitation.
Disclosure
Nicholas B. Tiller is funded by a fellowship from the Tobacco-Related Disease Research Program (TRDRP; award no. T31FT1692). Asghar Abbasi is funded by a fellowship from the Tobacco-Related Disease Research Program (TRDRP; award no. 28FT-0017). Harry B. Rossiter is supported by grants from NIH (R01HL151452, R01HL153460, P50HD098593, R01DK122767, and P2CHD086851) and the Tobacco Related Disease Research Program (T31IP1666). He reports consulting fees from Omniox Inc. and is involved in contracted clinical research with Boehringer Ingelheim, GlaxoSmithKline, Novartis, AstraZeneca, Astellas, United Therapeutics, Genentech and Regeneron. He is a visiting professor at the University of Leeds, UK. Alessandra Adami is funded by an NIH grant (R01HL151452) and by grants from Swiss National Science Foundation (P300P3_151705 and P300PB_167767). The authors declare no other conflicts of interest in this work. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
References
- 1.Products - national vital statistics reports - homepage; 2021. Available from. https://www.cdc.gov/nchs/products/nvsr.htm. Accessed July 4, 2021.
- 2.Karch A, Vogelmeier C, Welte T, et al. The German COPD cohort COSYCONET: aims, methods and descriptive analysis of the study population at baseline. Respir Med. 2016;114:27–37. doi: 10.1016/j.rmed.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 3.Putcha N, Han MK, Martinez CH, et al. Comorbidities of COPD have a major impact on clinical outcomes, particularly in African Americans. Chronic Obstr Pulm Dis. 2014;1(1):105–114. doi: 10.15326/jcopdf.1.1.2014.0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAllister DA, Maclay JD, Mills NL, et al. Arterial stiffness is independently associated with emphysema severity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(12):1208–1214. doi: 10.1164/rccm.200707-1080OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest. 2005;128(4):2640–2646. doi: 10.1378/chest.128.4.2640 [DOI] [PubMed] [Google Scholar]
- 6.Budoff MJ, Lutz SM, Kinney GL, et al. Coronary artery calcium on noncontrast thoracic computerized tomography scans and all-cause mortality. Circulation. 2018;138(21):2437–2438. doi: 10.1161/CIRCULATIONAHA.118.036835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakanishi R, Berman DS, Budoff MJ, et al. Current but not past smoking increases the risk of cardiac events: insights from coronary computed tomographic angiography. Eur Heart J. 2015;36(17):1031–1040. doi: 10.1093/eurheartj/ehv013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corlateanu A, Mendez Y, Wang Y, Garnica R de JA, Botnaru V, Siafakas N. Chronic obstructive pulmonary disease and phenotypes: a state-of-the-art. Pulmonology. 2020;26(2):95–100. doi: 10.1016/j.pulmoe.2019.10.006 [DOI] [PubMed] [Google Scholar]
- 9.Mahut B, Caumont-Prim A, Plantier L, et al. Relationships between respiratory and airway resistances and activity-related dyspnea in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2012;7:165–171. doi: 10.2147/COPD.S29745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decramer M. Tiotropium as essential maintenance therapy in COPD. Eur Respir Rev. 2006;15(99):51–57. doi: 10.1183/09059180.00009906 [DOI] [Google Scholar]
- 11.Sattelmair J, Pertman J, Ding EL, Kohl HW, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease. Circulation. 2011;124(7):789–795. doi: 10.1161/CIRCULATIONAHA.110.010710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng SWM, McKeough Z, Alison J, Dennis S, Hamer M, Stamatakis E. Associations of total and type-specific physical activity with mortality in chronic obstructive pulmonary disease: a population-based cohort study. BMC Public Health. 2018;18(1):268. doi: 10.1186/s12889-018-5167-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel MS, Natanek SA, Stratakos G, et al. Vastus lateralis fiber shift is an independent predictor of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190(3):350–352. doi: 10.1164/rccm.201404-0713LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watz H, Pitta F, Rochester CL, et al. An official European Respiratory Society statement on physical activity in COPD. Eur Respir J. 2014;44(6):1521–1537. doi: 10.1183/09031936.00046814 [DOI] [PubMed] [Google Scholar]
- 15.Adami A, Cao R, Porszasz J, Casaburi R, Rossiter HB. Reproducibility of NIRS assessment of muscle oxidative capacity in smokers with and without COPD. Respir Physiol Neurobiol. 2017;235:18–26. doi: 10.1016/j.resp.2016.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maltais F, Simard AA, Simard C, Jobin J, Desgagnés P, LeBlanc P. Oxidative capacity of the skeletal muscle and lactic acid kinetics during exercise in normal subjects and in patients with COPD. Am J Respir Crit Care Med. 1996;153(1):288–293. doi: 10.1164/ajrccm.153.1.8542131 [DOI] [PubMed] [Google Scholar]
- 17.Natanek SA, Gosker HR, Slot IGM, et al. Heterogeneity of quadriceps muscle phenotype in chronic obstructive pulmonary disease (COPD); implications for stratified medicine? Muscle Nerve. 2013;48(4):488–497. doi: 10.1002/mus.23784 [DOI] [PubMed] [Google Scholar]
- 18.Adami A, Corvino RB, Calmelat RA, Porszasz J, Casaburi R, Rossiter HB. Muscle oxidative capacity is reduced in both upper and lower limbs in COPD. Med Sci Sports Exerc. 2020;52(10):2061–2068. doi: 10.1249/MSS.0000000000002364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li R, Adami A, Chang CC, Tseng CH, Hsiai TK, Rossiter HB. Serum acylglycerols inversely associate with muscle oxidative capacity in severe COPD. Med Sci Sports Exerc. 2021;53(1):10–18. doi: 10.1249/MSS.0000000000002441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hajiro T, Nishimura K, Tsukino M, Ikeda A, Koyama H, Izumi T. Analysis of clinical methods used to evaluate dyspnea in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158(4):1185–1189. doi: 10.1164/ajrccm.158.4.9802091 [DOI] [PubMed] [Google Scholar]
- 21.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 22.Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. an official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. doi: 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demeyer H, Mohan D, Burtin C, et al. Objectively measured physical activity in patients with COPD: recommendations from an international task force on physical activity. Chronic Obstr Pulm Dis. 2021;8(4):528–550. doi: 10.15326/jcopdf.2021.0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gimeno-Santos E, Raste Y, Demeyer H, et al. The PROactive instruments to measure physical activity in patients with chronic obstructive pulmonary disease. Eur Respir J. 2015;46(4):988–1000. doi: 10.1183/09031936.00183014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Aymerich J, Puhan MA, Corriol-Rohou S, et al. Validity and responsiveness of the Daily- and Clinical visit-PROactive physical activity in COPD (D-PPAC and C-PPAC) instruments. Thorax. 2021;76(3):228–238. doi: 10.1136/thoraxjnl-2020-214554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adami A, Rossiter HB. Principles, insights, and potential pitfalls of the noninvasive determination of muscle oxidative capacity by near-infrared spectroscopy. J Appl Physiol. 2018;124(1):245–248. doi: 10.1152/japplphysiol.00445.2017 [DOI] [PubMed] [Google Scholar]
- 27.Ryan TE, Brophy P, Lin CT, Hickner RC, Neufer PD. Assessment of in vivo skeletal muscle mitochondrial respiratory capacity in humans by near-infrared spectroscopy: a comparison with in situ measurements. J Physiol. 2014;592(15):3231–3241. doi: 10.1113/jphysiol.2014.274456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.RCI Wüst, van der Laarse WJ, Rossiter HB. On-off asymmetries in oxygen consumption kinetics of single Xenopus laevis skeletal muscle fibres suggest higher-order control. J Physiol. 2013;591(3):731–744. doi: 10.1113/jphysiol.2012.241992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of multi-ethnic study of atherosclerosis (Mesa) and coronary artery risk development in young adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439 [DOI] [PubMed] [Google Scholar]
- 30.Budoff MJ, Nasir K, Kinney GL, et al. Coronary artery and thoracic calcium on noncontrast thoracic CT scans: comparison of ungated and gated examinations in patients from the COPD Gene cohort. J Cardiovasc Comput Tomogr. 2011;5(2):113–118. doi: 10.1016/j.jcct.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi: 10.3109/15412550903499522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 33.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Routledge; 1988. doi: 10.4324/9780203771587 [DOI] [Google Scholar]
- 34.Kermott CA, Schroeder DR, Kopecky SL, Behrenbeck TR. Cardiorespiratory fitness and coronary artery calcification in a primary prevention population. Mayo Clin Proc Innov Qual Outcomes. 2019;3(2):122–130. doi: 10.1016/j.mayocpiqo.2019.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lakka TA, Laukkanen JA, Rauramaa R, et al. Cardiorespiratory fitness and the progression of carotid atherosclerosis in middle-aged men. Ann Intern Med. 2001;134(1):12–20. doi: 10.7326/0003-4819-134-1-200101020-00008 [DOI] [PubMed] [Google Scholar]
- 36.Crisafulli E, Aiello M, Tzani P, et al. A high degree of dyspnea is associated with poor maximum exercise capacity in subjects with COPD with the same severity of air-flow obstruction. Respir Care. 2019;64(4):390–397. doi: 10.4187/respcare.06336 [DOI] [PubMed] [Google Scholar]
- 37.Rozanski A, Arnson Y, Gransar H, et al. Associations among self-reported physical activity, coronary artery calcium scores, and mortality risk in older adults. Mayo Clin Proc. 2020;4(3):229–237. doi: 10.1016/j.mayocpiqo.2020.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tudor-Locke C, Craig CL, Thyfault JP, Spence JC. A step-defined sedentary lifestyle index: <5000 steps/day. Appl Physiol Nutr Metab. 2013;38(2):100–114. doi: 10.1139/apnm-2012-0235 [DOI] [PubMed] [Google Scholar]
- 39.Javaid A, Mitchell JD, Villines TC. Predictors of coronary artery calcium and long‐term risks of death, myocardial infarction, and stroke in young adults. J Am Heart Assoc. 2021;10(22):e022513. doi: 10.1161/JAHA.121.022513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teramoto M, Moonie S, Cross CL, Chino M, Alpert PT. Association of leisure-time physical activity to cardiovascular disease prevalence in relation to smoking among adult nevadans. PLoS One. 2015;10(5):e0128424. doi: 10.1371/journal.pone.0128424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bittencourt MS, Santos RD, Staniak H, et al. Relation of fasting triglyceride-rich lipoprotein cholesterol to coronary artery calcium score (from the ELSA-Brasil Study). Am J Cardiol. 2017;119(9):1352–1358. doi: 10.1016/j.amjcard.2017.01.033 [DOI] [PubMed] [Google Scholar]
- 42.Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526(Pt 1):203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McEvoy JW, Nasir K, DeFilippis AP, et al. The relationship of cigarette smoking with inflammation and subclinical vascular disease: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35(4):1002–1010. doi: 10.1161/ATVBAHA.114.304960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Donnell DE. Impacting patient-centred outcomes in COPD: breathlessness and exercise tolerance. Eur Respir Rev. 2006;15(99):37–41. doi: 10.1183/09059180.00009903 [DOI] [Google Scholar]
- 45.Collado-Mateo D, Lavín-Pérez AM, Peñacoba C, et al. Key factors associated with adherence to physical exercise in patients with chronic diseases and older adults: an umbrella review. Int J Environ Res Public Health. 2021;18(4):2023. doi: 10.3390/ijerph18042023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Troosters T, Casaburi R, Gosselink R, Decramer M. Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(1):19–38. doi: 10.1164/rccm.200408-1109SO [DOI] [PubMed] [Google Scholar]
- 47.Thomas IC, Forbang NI, Criqui MH. The evolving view of coronary artery calcium and cardiovascular disease risk. Clin Cardiol. 2018;41(1):144–150. doi: 10.1002/clc.22842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aengevaeren VL, Mosterd A, Sharma S, et al. Exercise and coronary atherosclerosis. Circulation. 2020;141(16):1338–1350. doi: 10.1161/CIRCULATIONAHA.119.044467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Imran TF, Patel Y, Ellison RC, et al. Walking and calcified atherosclerotic plaque in the coronary arteries: the national heart, lung, and blood institute family heart study. Arterioscler Thromb Vasc Biol. 2016;36(6):1272–1277. doi: 10.1161/ATVBAHA.116.307284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwaśniewska M, Kostka T, Jegier A, et al. Regular physical activity and cardiovascular biomarkers in prevention of atherosclerosis in men: a 25-year prospective cohort study. BMC Cardiovasc Disord. 2016;16:65. doi: 10.1186/s12872-016-0239-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwaśniewska M, Jegier A, Kostka T, et al. Long-term effect of different physical activity levels on subclinical atherosclerosis in middle-aged men: a 25-year prospective study. PLoS One. 2014;9(1):e85209. doi: 10.1371/journal.pone.0085209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith J, Albert P, Bertella E, Lester J, Jack S, Calverley P. Qualitative aspects of breathlessness in health and disease. Thorax. 2009;64(8):713–718. doi: 10.1136/thx.2008.104869 [DOI] [PubMed] [Google Scholar]
- 53.Larsson SC, Bäck M, Rees JMB, Mason AM, Burgess S. Body mass index and body composition in relation to 14 cardiovascular conditions in UK biobank: a Mendelian randomization study. Eur Heart J. 2020;41(2):221–226. doi: 10.1093/eurheartj/ehz388 [DOI] [PMC free article] [PubMed] [Google Scholar]