Abstract
Unlike other type 4 pili, the neisserial pili consist of at least two distinct proteins, the highly variable major subunit PilE forming the pilus fiber and the tip-associated adhesin PilC. PilC protein purified either from gonococci or from Escherichia coli interacted with different human epithelial cell lines, primary epithelial and endothelial cells. The binding of PilC protein efficiently prevented the attachment of piliated Neisseria gonorrhoeae and Neisseria meningitidis to these cell types. Fluorescent beads coated with pili prepared from piliated wild-type N. gonorrhoeae also adhered to these cells, in contrast to beads coated with pili prepared from a piliated PilC-deficient mutant. In the latter case, the binding of fluorescent beads was restored after pretreatment of the pilus-loaded beads with purified PilC. Piliated wild-type N. gonorrhoeae, the piliated PilC-deficient mutant, and N. gonorrhoeae pili assembled in Pseudomonas aeruginosa agglutinated human erythrocytes, while nonpiliated gonococci did not. Consistently, purified PilC did not agglutinate or bind to human erythrocytes, suggesting that N. gonorrhoeae PilE is responsible for pilus-mediated hemagglutination.
Both Neisseria gonorrhoeae and Neisseria meningitidis primarily infect mucosal cell surfaces of humans, their exclusive host. N. meningitidis colonizes the nasopharyngeal epithelium and rarely disseminates via the vascular system to penetrate the blood-brain barrier. N. gonorrhoeae causes the sexually transmitted disease gonorrhoea, which has a low incidence of dissemination. Although the primary infection routes for N. gonorrhoeae and N. meningitidis are different, the binding specificities for human tissues are similar. For both pathogens, the initial colonization step is mediated by type 4 pili, and pilus-mediated binding also appears to be critical in defining host specificity. The type 4 pili function as adhesins for epithelial and endothelial cells.
Only recently has the nature of the pilus adhesin been elucidated. The adhesin critical for the binding of N. gonorrhoeae to human epithelial cells has been identified as PilC (37). The PilC protein is produced in small quantities and is encoded by two variant genes in N. gonorrhoeae MS11 (12). The expression of PilC is controlled by short variable G stretches affecting the translational reading frame and the expression of each pilC gene (12) and thus pilus-mediated adherence to epithelial cells (26, 28, 38). The PilC protein has been located at the tip of type 4 pili (37) as well as on the surface of N. gonorrhoeae (33, 36). Surface-bound PilC is involved in DNA uptake (36) and probably also in pilus transport (4). Purified PilC binds to human epithelial cells in vitro (37). Interestingly, the binding of PilC to epithelial cells prevents the binding of both N. gonorrhoeae and N. meningitidis, irrespective of the variant of PilC produced, indicating that both pathogens recognize related or identical receptors on human epithelial cells. Similar studies have been performed with an N. meningitidis strain which produces so-called class II type 4 pili, thus generalizing the function of PilC as a neisserial pilus adhesin (39).
Several previous researchers suggested a role for the major pilus subunit PilE in receptor recognition (28, 34, 38, 41, 46, 49). We recently proposed that N. gonorrhoeae pili comprise at least two distinct binding specificities, one for epithelial cells which is dependent on PilC and another for human erythrocytes (38). The ability of a piliated PilC-deficient mutant to agglutinate human erythrocytes and its lack of binding to epithelial cells already have suggested the possibility that PilE is a hemagglutinin (35); however, the question remains as to whether other factors contribute to hemagglutination. Only the use of purified components would identify the agglutinin for human erythrocytes. The concept of two different binding specificities located in two different components of the pilus is complicated by the fact that PilE undergoes antigenic variation (24), which influences epithelial cell-specific adherence (18, 38, 50) rather than erythrocyte binding (38).
The molecular basis of pilus-mediated binding of pathogenic Neisseria to human endothelial cells has not been elucidated so far. Virji et al. (48) demonstrated an efficient interaction of piliated variants of N. meningitidis and N. gonorrhoeae with human umbilical vein endothelial cells (HUVEC). In contrast to adherence to epithelial cells, the pilus-mediated binding of N. gonorrhoeae to HUVEC was not substantially influenced by antigenic variant PilE proteins (48). Also, N. meningitidis derivatives which expressed either class I or class II pili adhered similarly to HUVEC, suggesting that a common epitope of Neisseria pili was involved in the pilus-endothelial cell interaction.
In this study, we investigated the role of PilC purified either from N. gonorrhoeae or from Escherichia coli in the pilus-mediated binding of N. gonorrhoeae and N. meningitidis to epithelial and endothelial cells. We demonstrated the binding of purified PilC proteins to different cell types and performed adherence competition experiments by using purified PilC with N. gonorrhoeae and N. meningitidis strains. Recombinant Pseudomonas aeruginosa derivatives forming gonococcal pili were examined, demonstrating that PilE was the erythrocyte-specific adhesin of N. gonorrhoeae. Further evidence for the distinct adherence functions of PilE and PilC was provided by a novel in vitro binding assay with purified components.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All pathogenic bacterial strains used in this work are depicted in Table 1. E. coli strains were grown in Luria broth (LB) medium supplemented with ampicillin (100 μg ml−1) or erythromycin (250 μg ml−1) as needed. Gonococcal and meningococcal strains were grown on GC agar base with vitamin supplements (Becton Dickinson) at 37°C in 5% CO2. Recombinant gonococci were grown on GC agar base supplemented with tetracycline (10 μg ml−1), erythromycin (7 μg ml−1), chloramphenicol (12 μg ml−1), or isopropyl-β-d-thiogalactopyranoside (IPTG) (100 μg ml−1) as needed. P. aeruginosa K/2Pfs X90 was grown at 37°C on LB plates, and strain X91 was grown on LB plates containing carbenicillin at 500 μg ml−1. Plasmids pTR81, pHTR102, pIS25, and pHIS26 (Table 2) were maintained in E. coli K-12 strain DH5α grown at 37°C.
TABLE 1.
Strains used in this study
| Strain | Recipient strain, relevant genotype, and autonomous plasmid | Plasmid(s) used for gene replacement | Relevant phenotypea | Reference or source |
|---|---|---|---|---|
| N137 | N. gonorrhoeae MS11 variant E1; ΔpilE2 | P+ PilC+ PilEE1 Opa− | 6 | |
| N138 | N. gonorrhoeae MS11 variant F3; ΔpilE2 | P+ PilC+ PilEF3 Opa− | 6 | |
| N140 | N. gonorrhoeae MS11 variant C2; ΔpilE2 | P+ PilC+ PilEC2 Opa− | 8 | |
| N200 | N. gonorrhoeae MS11 variant A | P+ PilC+ PilEA Opa− | 22 | |
| N219 | N. gonorrhoeae MS11; ptetM25.2 | PS PilC+ PilEB1 Opa− | 38 | |
| N300 | N219; opaC::cat; pTH7 | pC20, Hermes-7 | PS PilC+ PilEB1 Opa− | 13 |
| N303 | N219; opaC::cat; ptetM25.2 (opa-50+) | pC20, pEMK55 | PS PilC+ PilEB1 Opa50+ | 17 |
| N556 | N. gonorrhoeae MS11; pilC1::cat-1 pilC2::catlowb | pTR36, pTR45 | P+ PilC− PilEN556 Opa− | 35 |
| N560 | N655; pHIS26 | pIS26 | P− PilC2His6+ PilE− Opa− | 37 |
| N621 | N. gonorrhoeae MS11; pilC1::cat-1 pilC2::catlow | pTR36, pTR45 | P− PilC− PilE+ Opa− | 35 |
| N655 | N. gonorrhoeae MS11; ΔpilE1 pilC1::ermC′ pilC2::catlow | pTR30, pTR32 | P− PilC− PilE− Opa− | This work |
| N862 | N. meningitidis A1493 | P+ PilC+ PilE+ Opa− | 39 | |
| N879 | N219; pHIS26 | pIS26 | PS PilC2His6+ PilE+ Opa− | This work |
| N881 | N621; pHIS26 | pIS26 | P+ PilC2His6+ PilE+ Opa− | This work |
| X90 | P. aeruginosa K/Pfs2 | P− PilC− | 11 | |
| X91 | X90; pRH117 | P+ PilC− PilEF3+ | 6a | |
| H2627 | E. coli DH5α; pIS25 | PilC2His6+ | This work | |
| H2628 | E. coli DH5α; pHIS26 | PilC2His6+ | This work |
P+, piliated; PS, S pilin; P−, nonpiliated.
catlow, marker for low-level resistance to chloramphenicol (≤8 μg/ml).
TABLE 2.
E. coli plasmids used in this study
| Plasmid | Description | Source or reference |
|---|---|---|
| Bluescript KS | Cloning vector | Stratagene |
| Hermes-8 | E. coli-N. gonorrhoeae (ptetM25.2) shuttle vector | 16 |
| pC20 | Promoterless cat gene in SspI site of opa-50 locus | 17 |
| pEMK55 | opa-50 inserted in Hermes-6a | 17 |
| pTR25 | pilC1; library clone | 35 |
| pTR30 | pTR25; ermC in SmaI site | 35 |
| pTR32 | pTR27; cat-1 between SmaI1 and SmaI3 sites | 35 |
| pTR36 | pTR25; cat-1 in SmaI site | 35 |
| pTR45 | pTR27; catlow between XhoI1 and XhoI2 sites | 35 |
| pTR81 | pilC2iv in Bluescript KS | 37 |
| pTR102 | pilC2iv in Hermes-8 | 35 |
| pIS25 | pilC2His6iv a in Bluescript KS | This work |
| pIS26 | pilC2His6iv in Hermes-8 | This work |
| pRH117 | pPAH121; pilEF3 | 11 |
pilC2His6iv, pilC2iv with His6 coding sequence.
Construction of strains and plasmids.
All plasmids used in this work are described in Table 2. All enzymes were used in accordance with manufacturer instructions, and the remaining cloning procedures were carried out by standard methods (40). The basis for the construction of the pilC2His6 (see below) gene was plasmid pTR102, which contains the invariant pilC2 gene in a Hermes vector. This plasmid was used for PCR, which resulted in two fragments. A second PCR with those two fragments resulted in a fragment encoding the N terminus of PilC with six histidines (His6) inserted (pilC2His6) (37). This N-terminal fragment with the histidine insertion was exchanged for the N-terminal portion of PilC encoded by pTR81, generating pIS25.
Transformation and conjugation of N. gonorrhoeae and E. coli.
Transformation and conjugation of gonococci were carried out as described by Rudel et al. (38). E. coli K-12 was transformed by the method of Messing and Vieira (21).
Purification of PilC2His6 protein.
Gonococcal strain N560 was induced overnight on GC agar plates containing tetracycline at 10 μg ml−1 and IPTG at 100 μg ml−1 at 37°C in 5% CO2. E. coli H2627 was grown overnight on LB plates with ampicillin (100 μg ml−1). PilC protein expression was induced in liquid cultures by adding IPTG at 100 μg ml−1 for 2 h at 37°C. The bacteria were pelleted, suspended in 50 mM Tris-Cl (pH 8.0)–150 mM NaCl, and lysed by sonication. The suspension was centrifuged at 4,000 rpm in a Sorvall centrifuge for 20 min at 4°C to separate the bacterial membranes. The membranes were harvested by centrifugation at 35,000 × g for 1 h at 4°C. The PilC2His6 (PilC2 with His6 attached) protein was dissolved by incubating the membranes in 2% N′,N-dimethyldodecylamine-N-oxide (LDAO) in 50 mM Tris-Cl (pH 8.0)–10 mM MgCl2–500 mM NaCl for 45 min at 37°C. After centrifugation at 35,000 × g for 1 h at 4°C, the supernatant was loaded on a nickel-nitrilotriacetic acid-agarose column equilibrated with 2% LDAO in 50 mM Tris-Cl (pH 8.0)–10 mM MgCl2–500 mM NaCl. After a wash with 50 mM imidazole in phosphate-buffered saline (PBS) to eliminate nonspecific binding, the PilC2His6 protein was eluted by a shift of the pH from 8.0 to 4.0 with 10 mM sodium citrate buffer containing 150 mM NaCl.
Purification of pili.
The gonococcal and pseudomonal pili were isolated by the method of Brinton et al. (2). The piliated wild-type strain and the piliated pilC mutant were grown for 18 h on GC agar plates. After harvesting of the bacteria in 50 mM Tris-Cl (pH 8.0)–150 mM NaCl, the bacteria were washed twice and resuspended in 0.15 M carbonate buffer (pH 10.5). The pili were sheared off in a Sorvall Omnimixer at 5,000 rpm for 60 s on ice. The cell debris was removed by centrifugation at 13,000 × g for 30 min at 4°C. The supernatant was dialyzed against PBS (pH 7.4) at 4°C overnight. At this pH, the pili crystallized and could be collected by centrifugation at 15,000 × g for 60 min at 4°C in a Sorvall centrifuge. After resuspension of the pellet in carbonate buffer, the suspension was centrifuged at 20,000 × g for 30 min at 4°C to remove insoluble outer membrane proteins. Afterward, the supernatant was dialyzed against PBS (pH 7.4) overnight at 4°C. The crystallization and solubilization steps were repeated three times to obtain pili of a high purity.
Covaspheres.
The purified PilC2His6 protein and the purified pili were covalently bound to Covaspheres MX fluorescent particles (0.5 μm) (Duke Scientific Corporation) by the method described by the manufacturer. Fluorescent beads (100 μl) were mixed with 20 μg of purified PilC2His6 protein, the same amount of purified pili, or fetuin at a ratio of 1:2. For coupling, the probes were rotated for 1 h at room temperature and pelleted by centrifugation. The supernatant was collected, and the protein concentrations before and after coupling were calculated on a sodium dodecyl sulfate (SDS) gel after silver staining. The unoccupied sites on the Covaspheres were saturated by incubation in 20 mM Tris-Cl (pH 7.5)–1% fetuin for 10 min.
Cell cultures.
Tissue culture reagents were obtained from Gibco-BRL. The epithelial cell lines used in the adherence experiments were ME-180 human cervix carcinoma (ATCC HTB33), Hec-1B human endometrium carcinoma (ATCC HTHB133), RT112 human urinary bladder carcinoma (kindly provided by W. W. Franke, German Cancer Research Center, Heidelberg, Federal Republic of Germany [FRG]), Chang human conjunctiva (ATCC CCL20.2), MDCK (ATCC CCL34), MDBK and PSEK (kindly provided by H.-J. Rziha), and NIH 3T3 mouse fibroblast (ATCC CRL1658). The RT112 cells were routinely maintained in Waymouth’s MB752/1 medium supplemented with 10% fetal calf serum (FCS), the MDBK and NIH 3T3 cells in were grown in Dulbecco minimal essential medium with 10% FCS, and the PSEK cells were grown in minimal essential medium with nonessential amino acids and 5% FCS at 37°C in 10% CO2. All the other epithelial cell lines were cultured in RPMI medium supplemented with 5% FCS at 37°C in 5% CO2. The endothelial cells used were HUVEC (Promocell, Heidelberg, FRG), which were cultured in endothelial cell growth medium (Promocell) at 37°C in 5% CO2. Cultures of primary cornea epithelium were maintained as previously described (44).
Adherence and adherence competition experiments.
For the adherence and adherence competition experiments, epithelial cells were cultivated on glass coverslips in 24-well plates until preconfluent. The HUVEC were placed on glass coverslips coated with 0.2% gelatin to mediate adherence of the endothelial cells to the glass slides. All cells were preincubated for competition experiments by adding purified PilC2His6 protein at 600 ng ml−1 to epithelial and endothelial cells, incubating the mixture for 20 min at 37°C, and washing the mixture twice with PBS-Ca2+-Mg2+. The cells were infected with 5 × 107 bacteria in 0.5 ml of medium without FCS per well for 1 h at 37°C in 5% CO2. To stop the infection and to remove nonbound bacteria, the cells were washed five times with PBS-Ca2+-Mg2+. Then, the cells were fixed with 2% paraformaldehyde in PBS for 30 min and stained with crystal violet. The number of adherent bacteria was established and compared with the adherence of wild-type strain N138, which is set at 100% adherence. Representative photographs were taken to show the adherence patterns of the bacteria. All gonococci used in this assay lacked the expression of the Opa proteins, which would have led to Opa-mediated binding of the bacteria to epithelial cells (19). Opa-mediated adherence used as a control has been described elsewhere (19).
Hemagglutination.
Erythrocytes for agglutination studies were collected from fresh human blood from a healthy adult volunteer by low-speed centrifugation and repeated washing in PBS. The erythrocytes were diluted to concentrations of 1 and 0.5% in round-bottom microtiter plates (Nunc, Wiesbaden, Germany). For hemagglutination, 109 bacteria, 500 ng of purified pili, and the same amount of purified PilC2His6 protein were suspended in 200 μl of a 1% erythrocyte dilution. A volume of 100 μl was removed and diluted with 0.5% erythrocytes in the next well. This step was repeated so that all samples were tested at dilutions of between 1:2 and 1:64. The plates were incubated at room temperature for 2 h, and the hemagglutination titers were determined visually by comparison with positive (N138) and negative (N300) controls.
Immunofluorescence studies.
Antiserum AK217 was produced by immunization of a rabbit with purified native PilC2His6 protein. For immunostaining, preconfluent epithelial cells on glass coverslips were preincubated with 600 ng of purified PilC2His6 protein ml−1 for 20 min at 37°C in 5% CO2. The cells were washed twice with PBS-Ca2+-Mg2+ and fixed in 2% paraformaldehyde in PBS for 30 min at room temperature. All coverslips were incubated with PBS containing 7% FCS for 1 h at room temperature. The coverslips were washed once with PBS and incubated in 200 μl of a 1:700 dilution of AK217 (rabbit anti-PilC serum) in PBS–7% FCS overnight at 4°C. The coverslips were rinsed five times with PBS–0.05% Tween 20 and incubated with a fluorescein isothiocyanate-conjugated secondary antibody at a dilution of 1:1,000.
SDS-polyacrylamide gel electrophoresis and protein staining.
The presence of PilE and PilC in whole bacterial cell lysates or in purification steps was determined by 15 or 12.5% polyacrylamide gel electrophoresis with SDS, respectively, followed by Western blot analysis or silver staining as described previously (1).
RESULTS
Purification of pilin-free PilC protein.
N. gonorrhoeae N560 is a derivative of strain MS11 which produces PilC protein in large quantities upon induction with IPTG (35). N560 contains the pilC2 gene of strain MS11 on the low-copy-number plasmid ptetM25.2 under the control of the Ptrc promoter (35). In order to achieve overproduction, two modifications were introduced into the coding region of pilC2: (i) conservative nucleotide changes in the variable poly-G region to ensure stable gene expression (pilC2iv) (35) and (ii) a DNA sequence encoding His6 to facilitate the purification of the modified protein (PilC2His6) by affinity chromatography through a Ni2+-nitrilotriacetic acid column (10). The latter modification, which generated pIS25, was achieved by substitution of the 5′ region of pilC2iv in pTR81. To produce PilC2His6 in a PilE-free environment, the BamHI-HindIII fragment of pIS25 was subcloned into the Hermes-8 shuttle vector (16), generating pIS26, which was used to transform N219 to generate N879 containing pHIS26, the recombinant ptetM25.2 plasmid encoding PilC2His6. N879 was used as a donor for the conjugative transfer of pHIS26 into N655, a mutant of strain MS11 carrying deletions in pilE and in both pilC genes (35, 38), yielding the final PilC-overproducing strain, N560.
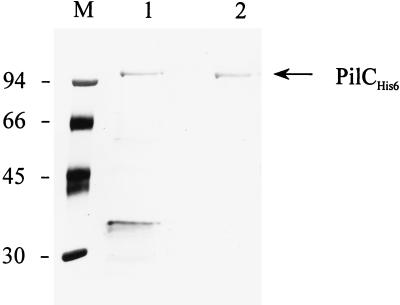
The recombinant PilC2His6 protein was purified from IPTG-induced cultures as described in Materials and Methods. To exclude any contaminating gonococcal proteins or LPS, both individual and pooled fractions were tested on silver-stained gels (Fig. 1). Furthermore, the PilC2His6 protein could also be purified from recombinant E. coli H2627 carrying pIS25. PilC2His6 protein produced in E. coli was purified by essentially the same procedure; however, this preparation was slightly contaminated by some unidentified E. coli proteins (Fig. 1).
FIG. 1.
Demonstration of PilC2His6 proteins purified from N. gonorrhoeae and E. coli. The protein gel shows pure silver-stained PilC2His6 proteins from recombinant E. coli H2627 (lane 1) and from N. gonorrhoeae (lane 2). Lane M, molecular weight markers (in thousands).
The functional integrity of PilC2His6 was tested by genetic complementation of the pilC double mutant N621 with plasmid pHIS26, yielding strain N881.
Binding of purified PilC2His6 to human epithelial cells and competitive inhibition of pilus-mediated adherence of N. gonorrhoeae and N. meningitidis.
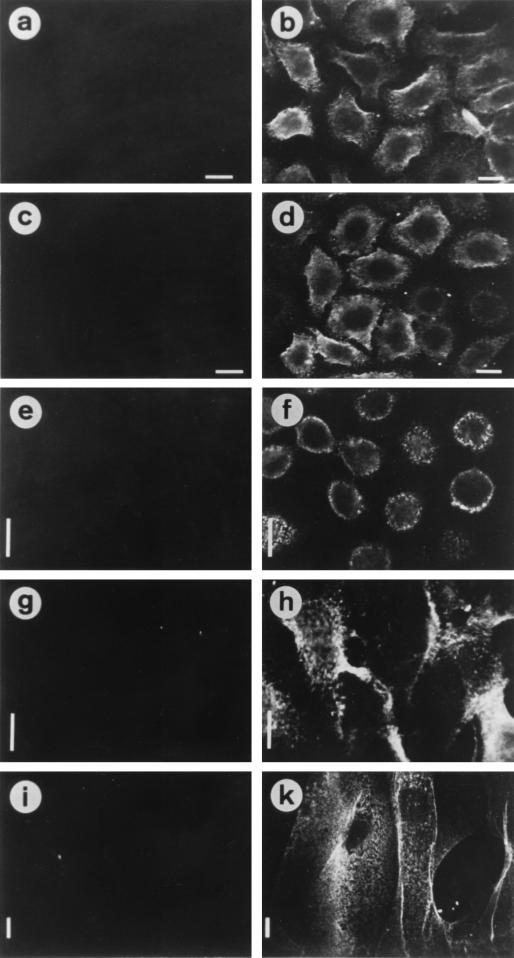
We demonstrated previously the binding of purified PilC2His6 protein to ME-180 human cervix carcinoma epithelial cells but the lack of binding to MDCK cells (37). Here we show that the binding of the recombinant PilC2His6 protein exhibited the same pronounced specificity for epithelial cells irrespective of whether it was derived from recombinant E. coli or from recombinant N. gonorrhoeae. Indeed, PilC2His6 from both preparations bound to ME-180, Hec-1B, and RT112 human epithelial cells, which were shown before to be good substrates for the adherence of piliated gonococci (38) (Fig. 2b, d, f, and h). Furthermore, strong binding was detected with human primary cornea epithelial cells (Fig. 2k), in contrast to the same cell type from sheep, bovine, or porcine origin (data not shown). Also, no binding of the protein to nonhuman cell lines which did not interact with piliated gonococci, such as MDCK, MDBK, PSEK, and NIH 3T3, was observed (data not shown). Thus the PilC2His6 protein purified from E. coli showed the same binding specificity for epithelial cells as the protein prepared from gonococci (Fig. 2d).
FIG. 2.
Binding of purified PilC2His6 proteins to different human epithelial cell types. (Left panels) Untreated epithelial cells. (Right panels) Epithelial cells after treatment with 600 ng of pure PilC2His6 protein from N. gonorrhoeae ml−1, except that panels c and d show cells not treated (c) and treated (d) with PilC2His6 purified from E. coli. The bound proteins were stained with rabbit anti-PilC serum (AK217) and fluorescein isothiocyanate-labelled secondary antibody. Epithelial cells were ME-180 (a to d), Hec-1B (e and f), RT112 (g and h), and human primary cornea epithelial cells (i and k). Bars, 10 μm.
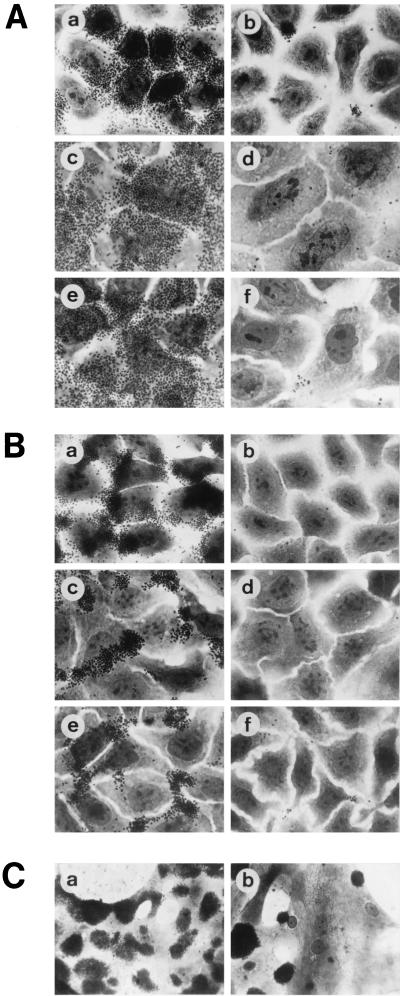
To test whether E. coli PilC2His6 was capable of blocking the binding of piliated N. gonorrhoeae to the epithelial cell receptor(s), we performed adherence competition experiments. PilC protein indeed blocked the binding of piliated N. gonorrhoeae N137 (PilEF3) (Fig. 3A, panel b), N137 (PilEE1), and N200 (PilEA) (data not shown) and of piliated N. meningitidis N862 to several human epithelial cell lines (Fig. 3B, panels b, d, and f). The most efficient binding of piliated wild-type gonococci was observed with human primary cornea epithelial cells (Fig. 3C, panel a). The individual primary cornea epithelial cells differed in their capacities to bind gonococci, probably due to their differentiation status. While some of the cells were completely covert, others bound less of the piliated gonococci. To inhibit the attachment of the wild-type strain N138 (PilEF3) to primary cornea epithelial cells, which have a high affinity for piliated gonococci, an approximately 20-fold-larger amount of purified PilC2His6 protein was required, compared to the amount required to inhibit the binding of N. gonorrhoeae to permanent epithelial cell lines (Fig. 3C, panel b). This finding might indicate a higher density of accessible PilC receptors on the primary cells.
FIG. 3.
(A) PilC purified from E. coli blocks the binding of N. gonorrhoeae to epithelial cells. (a and b) Binding patterns of piliated wild-type N. gonorrhoeae N138 without preincubation (a) and after preincubation (b) with 600 ng of PilC2His6 protein purified from E. coli ml−1 (b). (c to f) Competition of binding of N. gonorrhoeae N138 to human epithelial cells by PilC purified from N. gonorrhoeae. Shown are infected Hec-1B cells without pretreatment (c) and after pretreatment with 600 ng of PilC2His6 protein ml−1 (d) and infected RT112 cells without pretreatment (e) and after pretreatment with 600 ng of PilC2His6 protein ml−1 (f). (B) Competition of binding of N. meningitidis to human epithelial cells by PilC purified from N. gonorrhoeae. Target cells were ME-180 (a and b), Hec-1B (c and d), and RT112 (e and f) without pretreatment (a, c, and e) and after pretreatment with 600 ng of PilC2His6 purified from gonococci ml−1 (b, d, and f). (C) Competition of N. gonorrhoeae N138 binding to primary cornea epithelial cells by purified PilC2His6 protein. Cornea cells were from human origin. Binding of N138 to target cells without pretreatment (a) and after pretreatment with 12 μg of PilC2His6 purified from gonococci ml−1 (b) is shown.
Treatment of the target cells with an unrelated His6-tagged antibody fragment, the elution buffer without PilC protein, or the heat-treated PilC2His6 protein did not affect the attachment of either N. gonorrhoeae or N. meningitidis to any of the epithelial cells, consistent with previous results (37). Treatment of Chang conjunctiva epithelial cells with PilC2His6 had no influence on the non-pilus-mediated adherence conferred by the phase-variable Opa adhesin; for example, the ability of the Opa50-expressing, nonpiliated strain N303 (19) to adhere was not affected (data not shown). Thus, the inhibition of gonococcal adherence by PilC2His6 protein is pilus specific.
Preincubation of the epithelial cells with pili purified from wild-type strains or piliated pilC double mutant N556 (35) at concentrations of about 5 μg/ml or more could not prevent the adherence of wild-type N. gonorrhoeae to epithelial cells (data not shown). Since the amount of PilC in pilus preparations from wild-type strains is below 1% compared with the amount of PilE, the PilC concentration in these experiments was at least 50-fold lower than that required for competitive inhibition of adherence by PilC. This experiment thus provides evidence that PilE is not an efficient competitor for the binding of piliated Neisseria strains.
Purified PilC protein binds to HUVEC and inhibits the pilus-mediated adherence of gonococci and meningococci.
Pili constitute important determinants for the association of N. meningitidis and N. gonorrhoeae with human endothelial cells (26, 28, 48). In order to define the role of PilC in the interaction of Neisseria with endothelial tissue, PilC2His6 binding and adherence inhibition assays similar to those described for epithelial cells were performed. First, several pathogenic Neisseria strains were tested for adherence to HUVEC. Piliated gonococcal strains N137 (PilC+ PilEE1) and N138 (PilC+ PilEF3) and meningococcal strain N862 strongly bound to HUVEC (Fig. 4). In contrast, the piliated pilC double mutant N556 did not interact with HUVEC (Fig. 4e). This finding suggests that PilC is involved in the pilus-dependent adherence of N. gonorrhoeae to HUVEC. Consistently, after treatment of HUVEC with purified PilC2His6 protein at a concentration of 600 ng/ml, the binding of gonococcal as well as meningococcal strains was prevented (Fig. 4b, d, and h). Furthermore, efficient binding of PilC2His6 to HUVEC was demonstrated by immunostaining of bound protein on the cells with the specific PilC antiserum (Fig. 4m). Hence, PilC proteins from different N. gonorrhoeae and N. meningitidis strains constitute endothelial cell-specific adhesins.
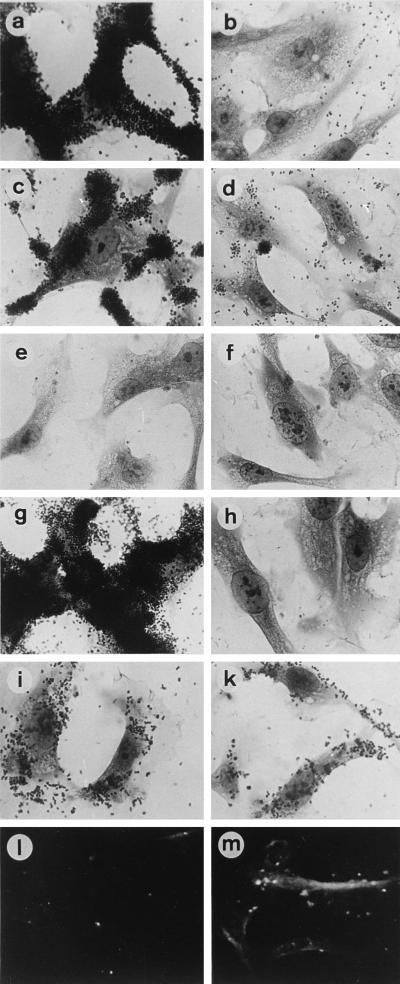
FIG. 4.
Binding of N. gonorrhoeae and N. meningitidis to HUVEC (left panels) is inhibited by the addition of purified PilC2His6 protein (right panels). (a to k) Bacterial strains tested were N. gonorrhoeae N138 (a and b), N. gonorrhoeae N137 (c and d), N. gonorrhoeae pilC double mutant N556 (e and f), N. meningitidis N862 (g and h), and Opa50-expressing N. gonorrhoeae N303 (i and k). (l and m) Binding of purified PilC2His6 protein to HUVEC (m) and a control experiment without purified PilC2His6 protein (l).
PilC is able to mediate the binding of purified pili to human epithelial cells.
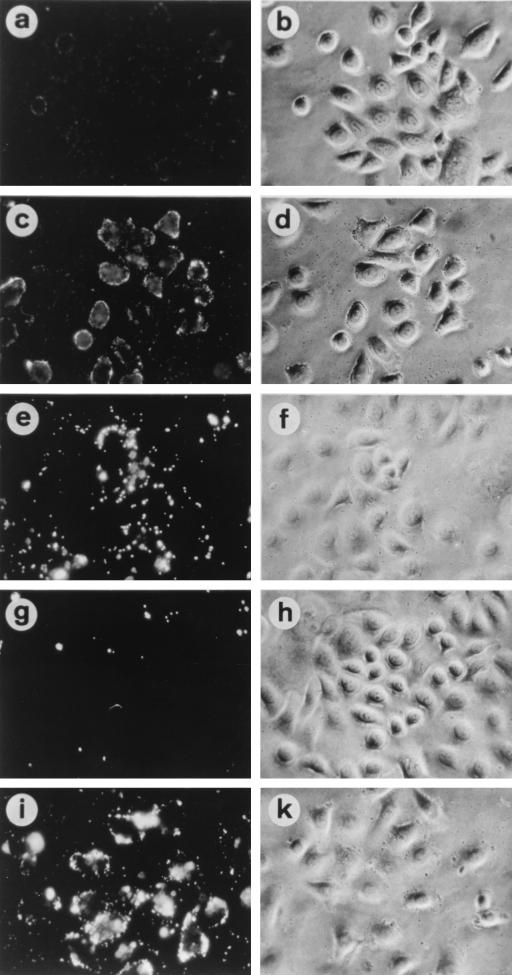
To address the question of whether PilE is able to bind to epithelial cells, we set up an in vitro binding assay with purified components. Pili isolated from wild-type strain N137 and from the piliated pilC double mutant N556 and purified PilC2His6 protein alone were coated on Covasphere MX fluorescent particles and analyzed for binding to ME-180 epithelial cells and MDCK epithelial cells. Covaspheres coated with pili from strain N137 or with isolated PilC2His6 protein bound well to ME-180 cells (Table 3 and Fig. 5), but essentially no binding was observed for MDCK cells, with the exception of apparently dead cells (data not shown). Interestingly, the patterns of binding of the two samples to ME-180 cells appeared to be different. In contrast, Covaspheres coated with strain N556 pili interacted with neither ME-180 cells nor MDCK cells. However, the same Covaspheres coated with N556 pili bound to ME-180 but not MDCK cells when supplemented with 400 ng of purified PilC protein (Fig. 5g and h). In a control experiment, Covaspheres coated with other proteins, such as fetuin, a glycoprotein purified from FCS, showed no binding to any of the cell lines (Fig. 5a and b).
TABLE 3.
Binding of fluorescent particles coated with purified components to ME-180 cells
| Purified protein + supplement | Binding to ME-180 cellsa |
|---|---|
| N137 pili | + |
| N556 pili | − |
| X91 × N138 pili | − |
| X90 pili | − |
| N556 pili + PilC2His6 protein | ++ |
| X91 + PilC2His6 protein | + |
| X90 + PilC2His6 protein | − |
| PilC2His6 protein | + |
| Fetuin | − |
| Fetuin + PilC2His6 protein | − |
++, very strong binding; +, strong binding; −, no binding.
FIG. 5.
Adherence of Covaspheres fluorescent particles to ME-180 cells as seen by fluorescent microscopy (left panels) and light microscopy (right panels). Particles were coated with fetuin (a and b), purified PilC2His6 protein (c and d), pili purified from N. gonorrhoeae N137 (e and f), and pili from the pilC double mutant N556 (g and h). (i and k) Restoration of binding of Covasphere particles coated with pili from N556 after preincubation of the particles with 400 ng of purified PilC2His6 protein ml−1.
To strengthen the evidence obtained for the adherence of PilC to epithelial cells, purified N. gonorrhoeae F3 pili produced in P. aeruginosa X91 (11) were tested in the same assay and compared to a control pilus preparation from nonrecombinant P. aeruginosa X90. N. gonorrhoeae F3 pili from X91 as well as pili from X90 coated on beads were unable to bind to ME-180 cells. However, when PilC protein was added to these beads, X91 pili but not X90 pili adhered strongly to ME-180 cells (Table 3). These experiments suggested that variant pili derived from either N556 or X91 are not able to interact with epithelial cells in the absence of PilC protein. Rather, in this novel in vitro binding assay, PilC protein is an essential component for the binding of pili to human epithelial cells.
PilE is an adhesin for human erythrocytes.
Besides epithelial cell-specific adherence, gonococcal pili are known to cause agglutination of human erythrocytes (3, 15, 38). Since PilC-deficient mutants still agglutinate human erythrocytes, this type of binding might be independent of PilC protein. To elucidate the pilus adhesin specific for erythrocytes, several strains, purified pili, and pure PilC2His6 protein were analyzed in hemagglutination experiments. Recombinant P. aeruginosa X91 expressing N. gonorrhoeae MS11 F3 pili (11) as well as preparations of N. gonorrhoeae F3 pili produced in P. aeruginosa were included in the hemagglutination assay. All piliated N. gonorrhoeae strains as well as the purified pili caused a strong hemagglutination reaction, independent of the particular variant PilE and of the presence or absence of PilC protein (Table 4). Also, the recombinant P. aeruginosa strain producing N. gonorrhoeae F3 pili and purified pili from the recombinant P. aeruginosa strain agglutinated human erythrocytes, whereas the parental P. aeruginosa strain or pilus preparations from this strain did not agglutinate erythrocytes. The addition of pure PilC2His6 protein to human erythrocytes did not result in agglutination (Table 4). Nor could agglutination of erythrocytes by purified pili be inhibited by preincubation of the erythrocytes with PilC2His6 protein purified from N. gonorrhoeae or E. coli.
TABLE 4.
Interactions of different gonococcal variants and purified components with human erythrocytes
| Strain or purified protein | Agglutination of erythrocytes by strain/purified proteina |
|---|---|
| N137 cells or pili | +/+ |
| N138 cells or pili | +/+ |
| N140 cells or pili | +/+ |
| N200 cells or pili | +/+ |
| N300 cells | − |
| N556 cells or pili | +/+ |
| X91 cells or pili | +/+ |
| X90 cells | − |
| N560 PilC2His6 protein | − |
| H2627 PilC2His6 protein | − |
+, positive reaction; −, negative reaction.
In agreement with the results of these experiments, immunofluorescence studies with antisera raised against purified PilC2His6 protein (AK217) confirmed that the PilC protein did not bind to human erythrocytes (data not shown). This result suggests that the two different binding specificities of the gonococcal pilus are elicited by two different pilus proteins: PilC functions as an adhesin for pilus-mediated binding to epithelial and endothelial cells, and PilE probably functions as a hemagglutinin. These biochemical data are thus consistent with our previous genetic data (38).
DISCUSSION
The type 4 pili of N. gonorrhoeae and N. meningitidis constitute key determinants for the adherence of these pathogens to epithelial and endothelial cells and for the agglutination of human erythrocytes (27, 38, 45, 48, 49). Under natural conditions, pili represent the only means for capsulated N. meningitidis to adhere to human mucosal surfaces (45, 49); pili were demonstrated to be essential for the establishment of experimental infections by N. gonorrhoeae (20, 43, 51).
The characterization of the pilus adhesins is complicated by the extreme structural variability of the major pilus subunit PilE (for a review, see references 23 and 42), which influences pilus binding to epithelial cells (18, 27, 38, 45) and, in a different way, also to endothelial cells (45, 48, 49) but not to human erythrocytes (38). One or more of these binding specificities could be due to adhesive domains located in PilE, as already suggested (34, 41, 47). Furthermore, both the N. gonorrhoeae and the N. meningitidis pili were recently found to carry unique glycosylation sites (30, 50), which could exert adhesive properties or modulate the adherence of neisserial pili.
Other experimental approaches indicated that the gonococcal pilus was not a homogeneous structure consisting of a single repeated subunit but rather contained minor proteins (25, 29). One of these pilus-associated proteins, PilC, was cloned and characterized as a pilus assembly factor found in the bacterial outer membrane (12). However, it was possible to assemble pili in the absence of PilC, and such pili had lost the ability to adhere to human epithelial cells (35, 38). Furthermore, in a N. meningitidis strain, pili assembled in the presence of two variant PilC proteins differed dramatically in their binding to human epithelial as well as endothelial cells (26). Only recently were gonococcal PilC proteins shown to represent type 4 pilus tip-located adhesins capable of competing for the pilus receptor on human epithelial cells (37). This observation agrees with the notion that neisserial pili, perhaps in contrast to other known type 4 pili, require a relatively conserved adhesin because of the extreme and unique variability of the major subunit PilE.
The aims of this study were to further assess the role of PilC (besides its accessory function in pilus assembly and natural transformation competence) as a pilus adhesin in the gonococcal pilus and to evaluate its specificity for different targets encountered during neisserial infections in the human host. The production of PilC2His6 protein in recombinant E. coli definitely ruled out the activity of any other neisserial factors in the preparation, such as lipopolysaccharide or other proteins. Attempts to purify a functional PilC2 fusion protein from E. coli inclusion bodies have failed so far (33, 36a), probably because the proper three-dimensional structure of PilC is essential for receptor recognition. PilC proteins contain several cysteine residues which are oxidized in the native molecule to form disulfide bonds. This fact is clearly apparent in the different migrations of PilC1 and PilC2 in SDS-polyacrylamide gels under reducing versus nonreducing conditions (36a). It is therefore not surprising that purified PilC fusion proteins are not functional as adhesins because folding during membrane transport may be an essential step. Consistent with this assumption is that E. coli-derived PilC2His6 purified from membranes exhibited the same specificity for human epithelial cells as did PilC2His6 purified from the N. gonorrhoeae overproducing strain.
As targets for E. coli PilC2His6, human primary cornea epithelial cells and permanent epithelial cell lines were identified, but primary cornea epithelial cells and permanent epithelial cell lines from nonhuman animals and human erythrocytes were not targets. Thus, independent of the bacterial background in which the PilC2His6 protein was produced, it exhibited the same strong species and cell type specificities. Furthermore, the binding affinities of PilC2His6 protein purified from N. gonorrhoeae and E. coli were probably identical, since about the same amounts of PilC2His6 protein were needed in order to competitively inhibit pilus-mediated binding of several different neisserial strains.
The novel in vitro binding assay allowed us to study the binding of cell-free pilus preparations. Covasphere fluorescent particles coated with wild-type pili or purified PilC2His6 protein adhered strongly to epithelial cells, whereas coating of the same particles with PilC-free pili purified from strain N556 or recombinant P. aeruginosa X91 did not result in significant binding. In contrast, at least two variant forms of PilE, PilEN556 and PilEF3, were able to result in adherent pili when produced in PilC+ gonococci (35, 38). The in vitro binding behavior of pilus preparations was thus consistent with the binding of piliated PilC-producing N. gonorrhoeae strains and the inability of piliated PilC-deficient mutants to bind to epithelial cells (37, 38).
The addition of pure PilC2His6 was sufficient to convert the Covasphere particles coated with PilC-deficient pili produced by N. gonorrhoeae or P. aeruginosa from no binding to strong and specific binding. This result might indicate that PilC directly interacts with purified PilE but not with pili from nonrecombinant P. aeruginosa or with fetuin. We observed, however, slightly weaker binding of PilC-complemented X91 pili than of N556 pili. This result might well have depended on different binding efficiencies of variant pilins. We cannot, however, exclude the possibility that additional factors in the gonococcal pili facilitated the proper presentation of adhesive PilC.
In this context, it is also of interest that slightly different binding patterns were observed depending on whether the Covasphere particles were coated with PilC alone or in the context of purified pili (i.e., PilE). This result may suggest that PilE somehow modulates or contributes to the binding of N. gonorrhoeae pili to epithelial cells, perhaps by recognizing a secondary receptor. This secondary receptor may be related to the postulated PilE-specific receptor on human erythrocytes. The receptor for PilC on target cells has not been identified yet. However, the membrane cofactor protein (MCP or CD46) was recently shown to function as a cellular receptor for the pili of both pathogenic Neisseria species (14). Consistent with the phase-variable binding patterns of piliated strains, MCP is expressed on almost every human cell type, with the exception of erythrocytes. This interesting distribution of MCP6 correlates well with the binding specificity of the PilC adhesin, making MCP6 a candidate receptor for PilC.
The same pilus preparations which displayed PilC-dependent binding to epithelial cells efficiently agglutinated human erythrocytes independent of the presence of PilC in vitro. The PilC-independent agglutination of human erythrocytes has been described before, leading to the hypothesis of two different binding properties associated with different binding domains of gonococcal pili (38). Strong evidence for PilE as the hemagglutinin was provided by an analysis of the gonococcal pili formed by a recombinant P. aeruginosa strain. Whereas neither intact wild-type P. aeruginosa nor wild-type pilus preparations agglutinated erythrocytes, a clonal gonococcal PilE protein expressed in the recombinant strain and pilus preparations from the recombinant strain strongly agglutinated erythrocytes. Therefore, the hemagglutinin is located in PilE and likely includes the relatively conserved regions already suggested to be involved in receptor recognition (34, 41).
As already described for pili of Enterobacteriaceae, gonococcal pili, belonging to the type 4 pilus class, represent a further example of pili exhibiting multiple binding specificities conferred by different pilus proteins. For instance, Pap pili of the F7 type contain, in addition to the α-d-Gal-(1-4)-(Gal)-specific adhesin FsoG, the pilus-associated FsoE and FsoF proteins, which bind to fibronectin (52). Similarly, the tip adhesin SfaS of E. coli binds to receptors containing neuraminic acid, whereas the major subunit confers binding to brain sulfate glycolipids, which lack any neuraminic acid (32).
The intriguing question of how PilC-dependent binding to epithelial and endothelial cells is modulated by variant PilE remains to be answered. Virji et al. (45) found several meningococcal PilE variants which bound more strongly to endothelial cells than to epithelial cells. Since we and others (26) were able to demonstrate the involvement of PilC in pilus adherence to both cell types, PilE or other pilus proteins may influence the recognition of the receptor of PilC. A similar phenomenon has been described for the neuraminic acid-specific adhesin SfaS, which is able to cause agglutination of human erythrocytes. The SfaS adhesin is able to acquire two conformations, depending on expressed SfaA. A change in the conformation of the SfaA subunit leads to a change in the conformation of the SfaS adhesin and results in altered pilus receptor specificities (7, 31). Certain domains of PilC may be masked or may be presented differently, depending on the context of the variant pilus proteins. These characteristics may influence the recognition of similar but not identical receptors on epithelial and endothelial cells.
ACKNOWLEDGMENTS
We are grateful to R. Haas for reconstructing the recombinant P. aeruginosa strains, to H.-J. Rziha for cell lines, and to C. Lanz for sequencing analysis.
This work was supported in part by the BMFT (grant 01-KI-8920) and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Blum H, Beier H, Gross H J. Improved silver staining of plant proteins in polyacrylamide gels. Electrophoresis. 1987;8:93–99. [Google Scholar]
- 2.Brinton C C, Bryan J, Dillon J A. Uses of pili in gonorrhoea control: role of bacterial pili in disease, purification and properties of gonococcal pili, and progress in the development of a gonococcal pilus vaccine for gonorrhoea. In: Gotschlich E C, Holmes K K, Sawyer W D, Young F E, editors. Immunology of Neisseria gonorrhoeae. Washington, D.C: American Society for Microbiology; 1978. pp. 155–178. [Google Scholar]
- 3.Buchanan T M, Pearce W A. Pili as a mediator of the attachment of gonococci to human erythrocytes. Infect Immun. 1976;13:1483–1489. doi: 10.1128/iai.13.5.1483-1489.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fussenegger M, Rudel T, Barten R, Ryll R, Meyer T F. Transformation competence and type-4 pilus biogenesis in Neisseria gonorrhoeae—a review. Gene. 1997;192:125–134. doi: 10.1016/s0378-1119(97)00038-3. [DOI] [PubMed] [Google Scholar]
- 5.Gubish E R J, Chen K C, Buchanan T M. Attachment of gonococcal pili to lectin-resistant clones of Chinese hamster ovary cells. Infect Immun. 1982;37:189–194. doi: 10.1128/iai.37.1.189-194.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haas R, Schwarz H, Meyer T F. Release of soluble pilin antigen coupled with gene conversion in Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1987;84:9079–9083. doi: 10.1073/pnas.84.24.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Haas, R., and T. F. Meyer. Unpublished data.
- 7.Hacker J, Kestler H, Hoschutzky H, Jann K, Lottspeich F, Korhonen T K. Cloning and characterization of the S fimbrial adhesin II complex of an Escherichia coli O18:K1 meningitis isolate. Infect Immun. 1993;61:544–550. doi: 10.1128/iai.61.2.544-550.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagblom P, Segal E, Billyard E, So M. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. Nature. 1985;315:156–158. doi: 10.1038/315156a0. [DOI] [PubMed] [Google Scholar]
- 9.Heckels, J. E. 1989. Structure and function of pili of pathogenic Neisseria species. Clin. Microbiol. Rev. 2(Suppl.):66–73. [DOI] [PMC free article] [PubMed]
- 10.Hochuli E, Gillessen D, Kocher H P. Specificity of the immunoadsorbent used for large-scale recovery of interferon alpha-2a. J Chromatogr. 1987;411:371–378. doi: 10.1016/s0021-9673(00)93988-8. [DOI] [PubMed] [Google Scholar]
- 11.Hoyne P A, Haas R, Meyer T F, Davies J K, Elleman T C. Production of Neisseria gonorrhoeae pili (fimbriae) in Pseudomonas aeruginosa. J Bacteriol. 1992;174:7321–7327. doi: 10.1128/jb.174.22.7321-7327.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonsson A B, Nyberg G, Normark S. Phase variation of gonococcal pili by frameshift mutation in pilC, a novel gene for pilus assembly. EMBO J. 1991;10:477–488. doi: 10.1002/j.1460-2075.1991.tb07970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahrs A F, Bihlmaier A, Facius D, Meyer T F. Generalized transposon shuttle mutagenesis in Neisseria gonorrhoeae: a method for isolating epithelial cell invasion-defective mutants. Mol Microbiol. 1994;12:819–831. doi: 10.1111/j.1365-2958.1994.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 14.Kallstrom H, Liszewski M K, Atkinson J P, Jonsson A B. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol Microbiol. 1997;25:639–647. doi: 10.1046/j.1365-2958.1997.4841857.x. [DOI] [PubMed] [Google Scholar]
- 15.Koransky J R, Scales R W, Kraus S J. Bacterial hemagglutination by Neisseria gonorrhoeae. Infect Immun. 1975;12:495–498. doi: 10.1128/iai.12.3.495-498.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kupsch E M, Aubel D, Gibbs C P, Kahrs A F, Rudel T, Meyer T F. Construction of Hermes shuttle vectors: a versatile system useful for genetic complementation of transformable and non-transformable Neisseria mutants. Mol Gen Genet. 1996;250:558–569. doi: 10.1007/BF02174444. [DOI] [PubMed] [Google Scholar]
- 17.Kupsch E M, Knepper B, Kuroki T, Heuer I, Meyer T F. Variable opacity (Opa) outer membrane proteins account for the cell tropisms displayed by Neisseria gonorrhoeae for human leukocytes and epithelial cells. EMBO J. 1993;12:641–650. doi: 10.1002/j.1460-2075.1993.tb05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambden P R, Robertson J N, Watt P J. Biological properties of two distinct pilus types produced by isogenic variants of Neisseria gonorrhoeae P9. J Bacteriol. 1980;141:393–396. doi: 10.1128/jb.141.1.393-396.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makino S, van Putten J P, Meyer T F. Phase variation of the opacity outer membrane protein controls invasion by Neisseria gonorrhoeae into human epithelial cells. EMBO J. 1991;10:1307–1315. doi: 10.1002/j.1460-2075.1991.tb07649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGee, Z. A., D. S. Stephens, L. H. Hoffman, W. F. Schlech, and R. G. Horn. 1983. Mechanisms of mucosal invasion by pathogenic Neisseria. Rev. Infect. Dis. 5(Suppl.):708–714. [DOI] [PubMed]
- 21.Messing J, Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982;19:269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- 22.Meyer T F, Mlawer N, So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982;30:45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- 23.Meyer T F, Pohlner J, van Putten J P. Biology of the pathogenic neisseriae. Curr Top Microbiol Immunol. 1994;192:283–317. doi: 10.1007/978-3-642-78624-2_13. [DOI] [PubMed] [Google Scholar]
- 24.Meyer, T. F., and J. P. van Putten. 1989. Genetic mechanisms and biological implications of phase variation in pathogenic neisseriae. Clin. Microbiol. Rev. 2(Suppl.):139–145. [DOI] [PMC free article] [PubMed]
- 25.Muir L L, Strugnell R A, Davies J K. Proteins that appear to be associated with pili in Neisseria gonorrhoeae. Infect Immun. 1988;56:1743–1747. doi: 10.1128/iai.56.7.1743-1747.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nassif X, Beretti J L, Lowy J, Stenberg P, O’Gaora P, Pfeifer J, Normark S, So M. Roles of pilin and PilC in adhesion of Neisseria meningitidis to human epithelial and endothelial cells. Proc Natl Acad Sci USA. 1994;91:3769–3773. doi: 10.1073/pnas.91.9.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nassif X, Lowy J, Stenberg P, O’Gaora P, Ganji A, So M. Antigenic variation of pilin regulates adhesion of Neisseria meningitidis to human epithelial cells. Mol Microbiol. 1993;8:719–725. doi: 10.1111/j.1365-2958.1993.tb01615.x. [DOI] [PubMed] [Google Scholar]
- 28.Nassif X, So M. Interaction of pathogenic neisseriae with nonphagocytic cells. Clin Microbiol Rev. 1995;8:376–388. doi: 10.1128/cmr.8.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parge H E, Bernstein S L, Deal C D, McRee D E, Christensen D, Capozza M A, Kays B W, Fieser T M, Draper D, So M. Biochemical purification and crystallographic characterization of the fiber-forming protein pilin from Neisseria gonorrhoeae. J Biol Chem. 1990;265:2278–2285. [PubMed] [Google Scholar]
- 30.Parge H E, Forest K T, Hickey M J, Christensen D A, Getzoff E D, Tainer J A. Structure of the fibre-forming protein pilin at 2.6 A resolution. Nature. 1995;378:32–38. doi: 10.1038/378032a0. [DOI] [PubMed] [Google Scholar]
- 31.Parkkinen J, Rogers G N, Korhonen T, Dahr W, Finne J. Identification of the O-linked sialyloligosaccharides of glycophorin A as the erythrocyte receptors for S-fimbriated Escherichia coli. Infect Immun. 1986;54:37–42. doi: 10.1128/iai.54.1.37-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prasadarao N V, Wass C A, Hacker J, Jann K, Kim K S. Adhesion of S-fimbriated Escherichia coli to brain glycolipids mediated by sfaA gene-encoded protein of S-fimbriae. J Biol Chem. 1993;268:10356–10363. [PubMed] [Google Scholar]
- 33.Rahman M, Källström H, Normark S, Jonsson A B. PilC of pathogenic Neisseria is associated with the bacterial cell surface. Mol Microbiol. 1997;25:11–25. doi: 10.1046/j.1365-2958.1997.4601823.x. [DOI] [PubMed] [Google Scholar]
- 34.Rothbard J B, Fernandez R, Wang L, Teng N N, Schoolnik G K. Antibodies to peptides corresponding to a conserved sequence of gonococcal pilins block bacterial adhesion. Proc Natl Acad Sci USA. 1985;82:915–919. doi: 10.1073/pnas.82.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudel T, Boxberger H J, Meyer T F. Pilus biogenesis and epithelial cell adherence of Neisseria gonorrhoeae pilC double knock-out mutants. Mol Microbiol. 1995;17:1057–1071. doi: 10.1111/j.1365-2958.1995.mmi_17061057.x. [DOI] [PubMed] [Google Scholar]
- 36.Rudel T, Facius D, Barten R, Scheuerpflug I, Nonnenmacher E, Meyer T F. Role of pili and the phase-variable PilC protein in natural competence for transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1995;92:7986–7990. doi: 10.1073/pnas.92.17.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a.Rudel, T., and T. F. Meyer. Unpublished data.
- 37.Rudel T, Scheuerpflug I, Meyer T F. Neisseria PilC protein identified as type-4 pilus tip-located adhesin. Nature. 1995;373:357–359. doi: 10.1038/373357a0. [DOI] [PubMed] [Google Scholar]
- 38.Rudel T, van Putten J P, Gibbs C P, Haas R, Meyer T F. Interaction of two variable proteins (PilE and PilC) required for pilus-mediated adherence of Neisseria gonorrhoeae to human epithelial cells. Mol Microbiol. 1992;6:3439–3450. doi: 10.1111/j.1365-2958.1992.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 39.Ryll R R, Rudel T, Scheuerpflug I, Barten R, Meyer T F. PilC of Neisseria meningitidis is involved in class II pilus formation and restores pilus assembly, natural transformation competence and adherence to epithelial cells in PilC-deficient gonococci. Mol Microbiol. 1997;23:879–892. doi: 10.1046/j.1365-2958.1997.2631630.x. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 41.Schoolnik G K, Fernandez R, Tai J Y, Rothbard J, Gotschlich E C. Gonococcal pili. Primary structure and receptor binding domain. J Exp Med. 1984;159:1351–1370. doi: 10.1084/jem.159.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seifert H S. Questions about gonococcal pilus phase and antigenic variation. Mol Microbiol. 1996;21:433–440. doi: 10.1111/j.1365-2958.1996.tb02552.x. [DOI] [PubMed] [Google Scholar]
- 43.Swanson J, Robbins K, Barrera O, Corwin D, Boslego J, Ciak J, Blake M, Koomey J M. Gonococcal pilin variants in experimental gonorrhea. J Exp Med. 1987;165:1344–1357. doi: 10.1084/jem.165.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tjia K F, van Putten J P, Pels E, Zanen H C. The interaction between Neisseria gonorrhoeae and the human cornea in organ culture. An electron microscopic study. Graefes Arch Clin Exp Ophthalmol. 1988;226:341–345. doi: 10.1007/BF02172964. [DOI] [PubMed] [Google Scholar]
- 45.Virji M, Alexandrescu C, Ferguson D J, Saunders J R, Moxon E R. Variations in the expression of pili: the effect on adherence of Neisseria meningitidis to human epithelial and endothelial cells. Mol Microbiol. 1992;6:1271–1279. doi: 10.1111/j.1365-2958.1992.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 46.Virji M, Everson J S, Lambden P R. Effect of anti-pilus antisera on virulence of variants of Neisseria gonorrhoeae for cultured epithelial cells. J Gen Microbiol. 1982;128:1095–1100. doi: 10.1099/00221287-128-5-1095. [DOI] [PubMed] [Google Scholar]
- 47.Virji M, Heckels J E. The role of common and type-specific pilus antigenic domains in adhesion and virulence of gonococci for human epithelial cells. J Gen Microbiol. 1984;300:1089–1095. doi: 10.1099/00221287-130-5-1089. [DOI] [PubMed] [Google Scholar]
- 48.Virji M, Kayhty H, Ferguson D J, Alexandrescu C, Heckels J E, Moxon E R. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol Microbiol. 1991;5:1831–1841. doi: 10.1111/j.1365-2958.1991.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 49.Virji M, Makepeace K, Peak I, Payne G, Saunders J R, Ferguson D J, Moxon E R. Functional implications of the expression of PilC proteins in meningococci. Mol Microbiol. 1995;16:1087–1097. doi: 10.1111/j.1365-2958.1995.tb02334.x. [DOI] [PubMed] [Google Scholar]
- 50.Virji M, Saunders J R, Sims G, Makepeace K, Maskell D, Ferguson D J. Pilus-facilitated adherence of Neisseria meningitidis to human epithelial and endothelial cells: modulation of adherence phenotype occurs concurrently with changes in primary amino acid sequence and the glycosylation status of pilin. Mol Microbiol. 1993;10:1013–1028. doi: 10.1111/j.1365-2958.1993.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 51.Watt P J, Ward M E. The interaction of gonococci with human epithelial cells. In: Robert R B, editor. The gonococcus. New York, N.Y: John Wiley & Sons, Inc.; 1977. pp. 355–368. [Google Scholar]
- 52.Westerlund B, Van Die I, Kramer C, Kuusela P, Holthofer H, Tarkkanen A M, Virkola R, Riegman N, Bergmans H, Hoekstra W. Multifunctional nature of P fimbriae of uropathogenic Escherichia coli: mutations in fsoE and fsoF influence fimbrial binding to renal tubuli and immobilized fibronectin. Mol Microbiol. 1991;5:2965–2975. doi: 10.1111/j.1365-2958.1991.tb01856.x. [DOI] [PubMed] [Google Scholar]