ABSTRACT
Background
Serological tests for antibody measurement in leprosy have a series of limitations in discriminating contacts and patients. The present paper intends to evaluate if association of more than one antibody isotype in serum samples may be a useful tool in leprosy diagnosis.
Methods
This study evaluated 395 leprosy contacts and 71 leprosy index cases living in endemic municipalities in Northeastern Brazil. The participants were evaluated according to their anti-phenolic glycolipid antigen-I isotype (PGL-I) profile. Serum anti-PGL-I IgM, IgG, and IgA were measured by indirect ELISA.
Results
A strong association was found for antibody positivity in MB leprosy index cases. The odds ratios were 6.11 (95% CI 3.08 – 12.16) for IgM, 3.31 (1.66 – 6.61) for IgG, and 16.97 (8.39 – 34.2) for IgA. For IgM associated with one or more isotypes, the OR was 21.0 (95% CI 10.11 – 43.64), and for IgG + IgA, the OR was 17.58 (6.23 – 49.54). The highest diagnostic sensitivity of 76.0% (95% CI 61.8 – 86.9) was observed for IgM, and the lowest value was 24.1% (13.0 – 38.2), which was observed for IgG + IgA isotypes. Regarding presumptive positive predictive values, the lowest value was obtained for IgM at 24.7% (95% CI 18.1 – 32.3), and the highest values were observed for IgM+ one or more isotypes and for IgG + IgA isotype at 60.0% (44.3 – 74.3) and 66.7% (41.0 – 86.7), respectively.
Conclusions
The present work demonstrated that by associating two or more positive antibody isotypes, the risk of facing a real case of leprosy may increase.
KEYWORDS: Leprosy contacts, anti-PGL-I, serum IgA, serum IgG, serum IgM
Introduction
Worldwide, 202,185 new cases of leprosy were reported by the World Health Organization (WHO) in 2019 [1]. Of these, 29,936 occurred in the Americas, and 27,864 were reported in Brazil (approximately 93.07% of the total in the Americas) [1]. The three countries with the highest number of new reported cases in that year were India, Brazil, and Indonesia, which accounted for 79.01% of new leprosy cases [1]. Although there has been a progressive decrease in the incidence of leprosy, the data do not seem to be in line with reality. For instance, there are many undiagnosed cases that need to be investigated by active searches in hyperendemic areas [2].
In 2019, Brazil had an average detection rate of 13.23 new cases for every 100,000 inhabitants, and 78.4% were diagnosed with the multibacillary form [3]. In the same year, 1545 new cases were reported in children below 15 years of age [4]. Of these cases, 50 presented with grade 2 disability (3.23%), which is representative of late diagnosis [4]. For this reason, one of the pillars of the global strategy against leprosy has been to reduce the rate of new cases in children with this level of disability [1].
Diagnosis of leprosy in children reflects active transmission of the bacteria in the community and failures in health strategies [5]. According to the epidemiological bulletin on leprosy of the health secretariat of the state of Ceará, Brazil, the state’s detection rates were 17.2 cases per 100,000 inhabitants in 2019, and there were 2.8 cases in children below 15 years of age per 100,000 inhabitants [6]. A new proposal of the WHO includes early detection of new cases, accurate diagnosis, and treatment [7].
However, there is great difficulty in diagnosing leprosy in its earliest stages due to the failure of following up leprosy contacts. People who have close contact with an index case are at risk of acquiring the disease, but there are still individuals who develop the disease without knowing an index case [8]. The monitoring of people who have contact with index cases of leprosy is considered an effective measure for the early diagnosis of new cases [9].
A question that is frequently raised is whether serological tools could be used for detecting those who are prone to developing the disease [10]. It is well documented that seropositive contacts – those who present positive anti-phenolic glycolipid 1 IgM (anti-PGL-I) titers – have 2.7 times higher risk of developing leprosy than those who are seronegative. However, it is also known that less than 45% of anti-PGL-I IgM contacts develop leprosy [11]. Thus, in the present work, we intend to evaluate if association of more than one antibody isotype in serum samples may be a useful tool in leprosy diagnosis. This approach was examined through a cross-sectional study in four municipalities of the northeastern region of Brazil.
Material and methods
Study design – The present work is a cross-sectional study and included children and young people aged 4 to 15 years who were in contact with leprosy index cases. The contacts and index cases were living in municipalities with high, very high, or hyperendemic rates of leprosy. The municipalities were Rio Largo (RL, Alagoas, high endemicity), Santana do Ipanema (SI, Alagoas, very high endemicity), São Gonçalo do Amarante (SGA, Ceará, high endemicity), and Canindé (CAN, Ceará, hyperendemic leprosy rates). Home visits were scheduled for children and young people to be examined by skilled nursing professionals and trained students. In the municipalities of SGA and CAN, the project relied on the Academic League of Stigmatized Diseases, which was coordinated by the PhD nurse Paula Sacha Nogueira. In the municipalities of RL and SL, the participants were evaluated by local skilled nursing professionals at the Specialized Unit in Tuberculosis and Leprosy, Santana do Ipanema, and by nursing students and professionals under supervision of the PhD nurse Clodis Maria Tavares in Rio Largo.
Leprosy contacts – Contacts aged 4–15 years old (N = 395) were evaluated in terms of the presence of lesions (aspect, number) and nerve thickness. Endogen histamine and thermal, pain, and tactile sensitivity were evaluated when necessary. A clinical sociodemographic questionnaire was administered to the participants (see supplementary material appendices 1 and 2).
Children and young people who lived inside the index case’s home were classified as household contacts (HH). Those living up to five houses to the right or left of the index case’s home were considered peridomicilliary contacts (PD). Friends and relatives who spent some time in the index case’s house were also included in the former group. The participants were also classified according to the WHO operational classification of leprosy index cases as paucibacillary (PB) and multibacillary (MB) contacts [12]. At the health center, blood was collected (4 mL) in tubes containing anticoagulant, and after centrifugation, serum samples were aliquoted, transported to the Laboratory of Immunology, UFC, Ceara, and maintained at −20° C until analysis.
Index cases – Leprosy patients (N = 71, aged 10 to 97 years old) were selected according to the registration of the National Notifiable Diseases System (SINAN) of each municipal health secretariat and defined as index cases. They were invited to go to a health center, where a clinical sociodemographic questionnaire (see supplementary material appendix 3) was administered, and venous blood without anticoagulant was collected (4 mL). After obtaining serum, the samples were transported to the Laboratory of Immunology, UFC, Ceará, and maintained at −20° C until analysis.
The index cases were classified in paucibacillary or multibacillary according to the WHO classification [12]. A paucibacillary clinical form was considered when a compromised anatomical region and/or nervous trunk was identified. A multibacillary clinical form was considered when two or more anatomical regions or more than one nervous trunk were affected. Information about treatment was also obtained from the medical records.
Ethical aspects – The project was approved by the National Committee for Research Ethics (process CAAE 11709213.9.0000.5054). After explanation of the project, the guardians of the participants were asked to sign a consent form. Participants aged 8 to 15 years old were also asked to sign a consent form themselves, as were the index cases.
Serological tests – Serum anti-PGL-I antibodies were measured as described by Macedo et al. [13] with slight modifications. Microplates precoated with 5 mg/L of native PGL-I (donated by BEI Resources/ATCC, Manassas, USA) were incubated with 1% fetal bovine serum (FBS, GibcoTM, Brazil) in phosphate buffered saline (PBS, pH 7.4) for 2 h at 37°C in a humid chamber. After washing, serum samples were diluted in PBS with 0.5% FBS (1:200 for IgG and IgM; 1:50 for IgA), added to plates (50 μL per well in duplicate), and incubated for 2 h at 37°C. After washing, the conjugates [peroxidase-labeled anti-IgG (code A0170, Sigma, USA), anti-IgM (code SA5-10293, Thermo, USA), and anti-IgA (code A0295, Sigma, USA) diluted to 1:20000, 1:2500, and 1:12000, respectively] were added to the plates and incubated for 1.5 h at 37°C. After washing, the plates were incubated for 30 min at room temperature with chromogen/substrate solution for ELISA (100 μL per well), which contained tetramethylbenzidine (TMB) (Invitrogen™, USA). The reaction was interrupted by adding 25 μL of 2.5 N sulfuric acid. The analysis was performed at 450 nm and 620 nm (reference wavelength) using an ELISA plate reader (ASYS Expert Plus, Biochrom, UK).
Four wells contained all reagents except for serum and were used as blank samples. An aliquot of pooled normal human serum (NHS) was used as a cutoff sample and tested in all assays in quadruplicate. The NHS pool comprised serum samples from 50 local blood donors, who were seronegative for HIV, Chagas, hepatitis B and C, HTLV, syphilis and did not present leprosy at the time of sample collection [13]. Titration of the conjugates were done in order that the optical density (OD) of the cutoff serum sample did not exceed 0.250. The negative control OD should be below the mentioned value, and the positive control OD should be at least five times above the mentioned value (see supplementary material appendix 4).
Statistical analysis – The data were analyzed using nonparametric tests as the values did not follow a Gaussian distribution (Kolgomorov-Smirnov test). The Kruskall-Wallis test was used to compare antibody levels from three or more groups. The Fisher test was used to analyze the possible association between the frequency of positive or negative serum antibody levels in leprosy index cases and contacts. The odds ratio (OR) and 95% confidence interval were calculated to evaluate the odds of presenting leprosy and 1 or ≥ 2 positive serum antibody isotypes. Diagnostic sensitivity and specificity and positive and negative predictive values were also calculated. All statistical analyses were performed using GraphPad Prism version 6.0, and the level of statistical significance was 5% (p < 0.05).
Results
The study included 395 leprosy contacts in the age group of 4 to 15 years, who were divided in those without lesions (N = 340, named healthy contacts) and those with suspected lesions (N = 55). Among those without lesions (Table 1A), 175 were female children (median age 10 years old), and 165 were male children (median age 9 years old). The participants were classified as HH contacts (N = 67), PD contacts (N = 273), PB contacts (N = 89), and MB contacts (N = 251).
Table 1.
Age range, gender, and clinical forms of leprosy index cases, household (HH) contacts and peridomicilliary (PD) contacts without lesions (healthy contacts), and those with lesions.
| (A) Contacts without lesions (healthy contacts) (N = 340) | |||||||||||||
| Gender |
Age median (range) |
Type of contact | |||||||||||
|
HH (N = 67) |
PD (N = 273) |
||||||||||||
|
PB (n = 14) |
MB (N = 53) |
PB (N = 75) |
MB (N = 198) |
||||||||||
| Female (N = 175) | 10 (4–15 years old) |
7 | 27 | 42 | 99 | ||||||||
| Male (N = 165) |
9 (4–15 years old) |
7 | 26 | 33 | 99 | ||||||||
| (B) Contacts with lesions (N = 55) | |||||||||||||
| Gender |
Age median (range) |
Type of contact | |||||||||||
|
HH (N = 20) |
PD (N = 35) |
||||||||||||
|
PB (N = 14) |
MB (N = 6) |
PB (N = 9) |
MB (N = 26) |
||||||||||
| Female (N = 29) | 11 (4–14 years old) |
5 | 2 | 5 | 17 | ||||||||
| Male (N = 26) | 10 (5–15 years old) |
9 | 4 | 4 | 9 | ||||||||
| (c) Index cases (N = 71) | |||||||||||||
| Gender |
Age median (range) |
Clinical form (n = 71) | |||||||||||
| PB (N = 21) | MB (N = 50) | ||||||||||||
| Female (N = 30) | 46 (10–81 years old) |
12 | 18 | ||||||||||
| Male (N = 41) | 50 (15–97 years old) |
9 | 32 | ||||||||||
Obs. HH (household contacts); PD (peridomicilliary contacts); PB (paucibacillary); MB (multibacillary).
Among those with lesions (Table 1B), there were 29 female children (median age 11 years old) and 26 male children (median age 10 years old). The participants were classified as HH (N = 20), PD (N = 35), PB (N = 23), and MB (N = 32) contacts. Three of them showed nerve thickness. They presented grade 0 disability. The participants with suspected lesions or nerve thickness were referred to the health center to be examined by a physician. After the project was finished, 7 contacts with suspicion got diagnosis of leprosy (6 with the indeterminate clinical form and 1 with the borderline clinical form of leprosy).
Thirty female index cases (median age 46 years old) and 41 male index cases (median age 50 years old) were included in the study (Table 1C). The index cases were classified as having the PB clinical form (N = 21) and MB clinical form (N = 50). Eighteen index cases (25.4%) were under treatment, 12 index cases (16.9%) were recruited one year after treatment, 6 index cases (8.5%) were recruited two years after treatment, and 25 index cases (35.2%) were recruited 3 or more years after treatment. Information regarding treatment from ten index cases was missing in the medical records (14.1%). No statistical differences were found with respect to antibody levels considering the year after treatment (Kruskal-Wallis test, p = 0.61 for IgM, p = 0.075 for IgG and p = 0.61 for IgA, data not shown).
Serum anti-PGL-I titers
Figure 1 presents the interquartile ranges and medians of anti-PGL-I IgM, IgG, and IgA levels in serum samples from MB contacts without lesions (MB healthy contacts; N = 251), MB contacts with suspected lesions (N = 32), and MB leprosy index cases (N = 50). Anti-PGL-I IgM levels were higher in MB leprosy index cases than in MB healthy contacts (Kruskal-Wallis test, p < 0.0001) and MB contacts with lesions (K-W test, p < 0.01). Anti-PGL-I IgG levels were higher in MB leprosy index cases than in healthy contacts (K-W test, p < 0.001). Anti-PGL-I IgA levels were higher in MB leprosy index cases than contacts with or without lesions (K-W test, p < 0.0001).
Figure 1.
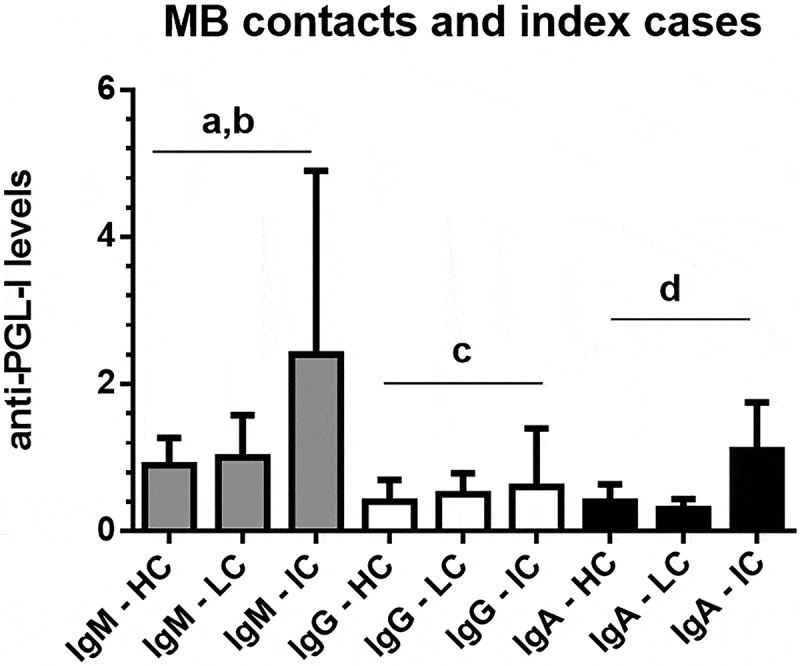
Interquartile ranges and medians of anti-PGL-I IgM, IgG, and IgA levels in serum samples from MB (multibacillary) contacts without lesions/MB healthy contacts (HC; N = 251), MB contacts with suspected lesions (LC; N = 32), and MB leprosy index cases (IC; N = 50). The cutoff values were 1.1 for IgA and 1.2 for IgG and IgM isotypes.
ap<0.0001, Kruskal-Wallis test, anti-PGL-I IgM levels in MB index cases were higher than in MB healthy contacts; bp<0.01, Kruskal-Wallis test, anti-PGL-I IgM levels in MB index cases were higher than in MB contacts with lesions; cp<0.001, Kruskal-Wallis test, anti-PGL-I IgG levels in MB index cases were higher than in MB healthy contacts; dp<0.0001, Kruskal-Wallis test, anti-PGL-I IgA levels in MB index cases were higher than in MB healthy contacts and contacts with lesions.
Figure 2 presents the interquartile ranges and medians of anti-PGL-I IgM, IgG, and IgA levels in serum samples from PB healthy contacts (N = 89), PB contacts with suspected lesions (N = 23), and PB leprosy index cases (N = 21). The only isotype that presented any statistical difference was IgA. Anti-PGL-I IgA levels were higher in PB leprosy index cases than contacts without lesions (K-W test, p < 0.01).
Figure 2.
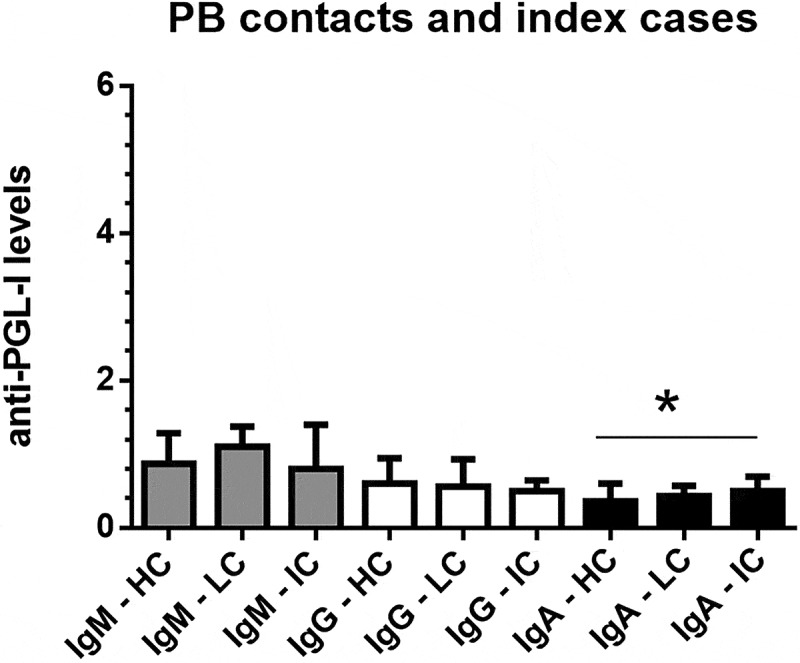
Interquartile ranges and medians of anti-PGL-I IgM, IgG, and IgA levels in serum samples from PB (paucibacillary) contacts without lesions/PB healthy contacts (HC; N = 89), PB contacts with suspected lesions (LC; N = 23), and PB leprosy index cases (IC; N = 21). The cutoff values were 1.1 for IgA and 1.2 for IgG and IgM isotypes.
*p < 0.01, Kruskal-Wallis test, anti-PGL-I IgA levels in PB index cases were higher than in PB healthy contacts.
Figure 3 presents the interquartile ranges and medians of anti-PGL-I IgM, IgG, and IgA levels in serum samples from MB contacts who were household contacts (HH contacts; N = 6), MB peridomicilliary contacts (PD contacts; N = 26), and MB leprosy index cases (N = 50). Anti-PGL-I IgM levels were higher in MB leprosy index cases than in HH and PD multibacillary contacts (K-W test, p < 0.01 and p < 0.0001, respectively). Anti-PGL-I IgG levels were higher in MB leprosy index cases than in HH and PD multibacillary contacts (K-W test, p < 0.0001 and p < 0.01, respectively). Anti-PGL-I IgA levels were higher in MB leprosy index cases than HH and PD multibacillary contacts (K-W test, p < 0.0001).
Figure 3.
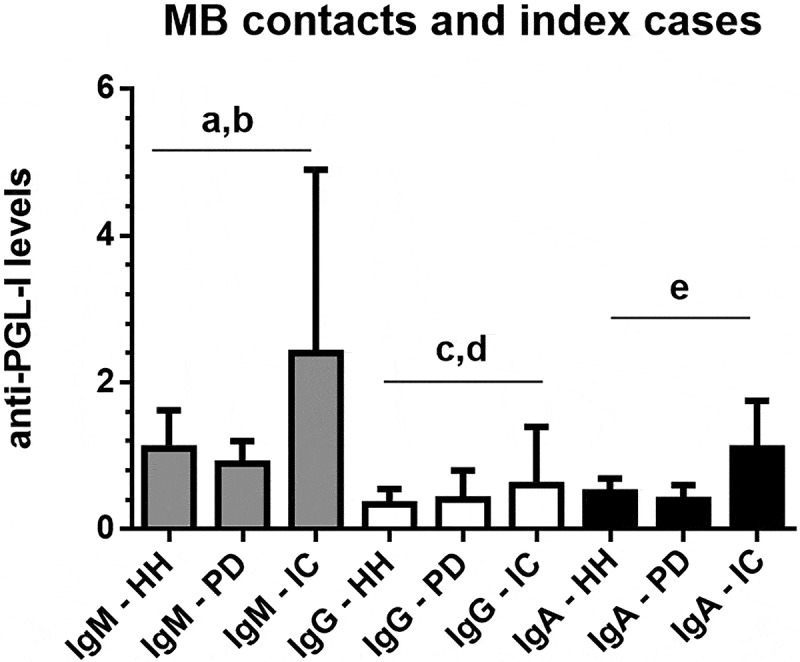
Interquartile ranges and medians of anti-PGL-I IgM, IgG, and IgA levels in serum samples from MB (multibacillary) contacts who were household (HH, N = 6), or MB peridomicilliary contacts (PD, N = 26), and MB leprosy index cases (N = 50). The cutoff values were 1.1 for IgA and 1.2 for IgG and IgM isotypes.
ap<0.0001, Kruskal-Wallis test, anti-PGL-I IgM levels in MB index cases were higher than in PD contacts; bp<0.01, Kruskal-Wallis test, anti-PGL-I IgM levels in MB index cases were higher than in HH contacts; cp<0.0001, Kruskal-Wallis test, anti-PGL-I IgG levels in MB index cases were higher than in HH contacts; dp<0.01, Kruskal-Wallis test, anti-PGL-I IgG levels in MB index cases were higher than in PD contacts; ep<0.0001, Kruskal-Wallis test, anti-PGL-I IgA levels in MB index cases were higher than in HH and PD contacts.
Figure 4 presents the interquartile ranges and medians of anti-PGL-I IgM, IgG, and IgA levels in serum samples from PB household contacts (N = 14), PB peridomicilliary contacts (N = 14), and PB leprosy index cases(N = 21). The only isotype that presented any statistical difference was IgA. Anti-PGL-I IgA levels were higher in PB leprosy index cases than PB peridomicilliary contacts (K-W test, p < 0.05).
Figure 4.
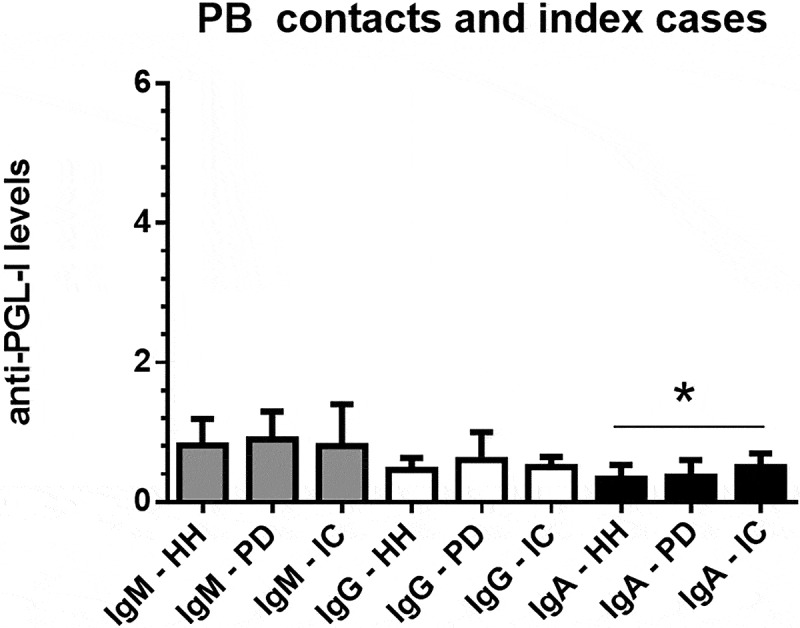
Interquartile ranges and medians of anti-PGL-I IgM, IgG, and IgA levels in serum samples from PB (paucibacillary) household contacts (HH, N = 14), PB peridomicilliary contacts (PD, N = 14), and PB leprosy index cases (N = 21). The cutoff values were 1.1 for IgA and 1.2 for IgG and IgM isotypes.
*p < 0.05, Kruskal-Wallis test, anti-PGL-I IgA levels in PB index cases were higher than in PD contacts.
Frequency of positive anti-PGL-I antibodies
The frequency of positive antibody isotypes was evaluated in leprosy index cases with the PB clinical form (Table 2) and the MB clinical form (Table 3) in association with healthy contacts. No association was found between the positivity of anti-PGL-I and index cases with the PB clinical form, irrespective of the isotype (p = 1.0 for IgM and IgG and p = 0.055 for IgA, Fisher test). Nonetheless, a strong association was found for all antibody isotypes in MB leprosy index cases (p < 0.0001 for IgM and IgA and p = 0.0014 for IgG, Fisher test).
Table 2.
Frequency of positive/negative anti-PGL-I levels in paucibacillary leprosy index cases and healthy contacts.
| Serum anti-PGL1 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IgM |
IgG |
IgA |
IgM + one or more isotypes |
IgG + IgA |
||||||
| Index cases (n = 21) |
Healthy contacts (n = 340) |
Index cases (n = 21) |
Healthy contacts (n = 340) |
Index cases (n = 21) |
Healthy contacts (n = 340) |
Index cases (n = 21) |
Healthy contacts (n = 340) |
Index cases (n = 21) |
Healthy contacts (n = 340) |
|
| Positive | 7 | 116 | 2 | 39 | 4 | 22 | 3 | 18 | 1 | 6 |
| Negative | 14 | 224 | 19 | 301 | 17 | 318 | 18 | 322 | 20 | 334 |
| Fisher test | p = 1.0 | p = 1.0 | p = 0.055 | p = 0.11 | p = 0.35 | |||||
Table 3.
Frequency of positive/negative anti-PGL-I levels in multibacillary leprosy patients and healthy contacts. Parameters of OR, diagnostic sensitivity and specificity, and negative and positive predictive values (and 95% interval confidence) for isolated anti-PGL- I IgA, IgM, IgG, IgM + one or more isotypes, and IgG + IgA antibody levels.
| Serum anti-PGL1 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IgM |
IgG |
IgA |
IgM + one or more isotypes |
IgG + IgA |
||||||
| Index cases (n = 50) |
Healthy contacts (n = 340) |
Index cases (n = 50) |
Healthy contacts (n = 340) |
Index cases (n = 50) |
Healthy contacts (n = 340) |
Index cases (n = 50) |
Healthy contacts (n = 340) |
Index cases (n = 50) |
Healthy contacts (n = 340) |
|
| Positive | 38 | 116 | 15 | 39 | 27 | 22 | 27 | 18 | 12 | 6 |
| Negative | 12 | 224 | 35 | 301 | 23 | 318 | 23 | 322 | 38 | 334 |
| Fisher test | p < 0.0001 | p = 0.0014 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||||
|
Odds ratio (95% CI) |
6.11 (3.08–12.16) |
3.31 (1.66–6.61) |
16.97 (8.39–34.2) |
21.0 (10.11–43.64) |
17.58 (6.23–49.54) |
|||||
| Diagnostic sensitivity (95% CI) | 76.0 (61.8–86.9) |
30.0 (17.8–44.6) |
54.0 (39.3–68.2) |
54.0 (39.3–68.2) |
24.0 (13.0–38.2) |
|||||
|
Diagnostic specificity (95% CI) |
65.9 (60.6–70.9) |
88.5 (84.7–91.7) |
93.5 (90.4–95.9) |
94.7 (91.8–96.8) |
98.2 (96.2–99.4) |
|||||
|
Positive predictive value (95% CI) |
24.7 (18.1–32.3) |
27.8 (16.5–41.6) |
55.1 (40.2–69.3) |
60.0 (44.3–74.3) |
66.7 (41.0–86.7) |
|||||
|
Negative predictive value (95% CI) |
94.9 (91.3–97.4) |
89.6 (85.8–92.6) |
93.3 (90.0–95.7) |
93.3 (90.2–95.7) |
89.8 (86.3–92.7) |
|||||
CI = confidence interval.
Regarding the IgM isotype, the OR was 6.11 (95% CI 3.08–12.16). For the IgG isotype, the OR was 3.31 (95% CI 1.66–6.61). For the IgA isotype, the OR was 16.97 (95% CI 8.39–34.2). When positive IgM was considered together with one or more isotypes (p < 0.0001, Fisher test), the OR was 21.0 (95% CI 10.11–43.64), and when positive IgG and IgA status was analyzed (p < 0.0001, Fisher test), the OR was 17.58 (95% CI 6.23–49.54).
The highest diagnostic sensitivity was observed for the IgM isotype at 76.0% (95% CI 61.8–86.9), and the lowest value was observed for IgG + IgA isotypes at 24.1% (95% CI 13.0–38.2). With respect to diagnostic specificity, the highest value was obtained for IgG + IgA isotypes at 98.2% (95% CI 96.2–99.4), and the lowest value was for the isolated IgM isotype at 65.9% (95% CI 60.6–70.9). Regarding presumptive positive predictive values, the lowest value was obtained for IgM at 24.7% (95% CI 18.1–32.3), and the highest values were observed for IgM + one or more isotypes and for IgG + IgA isotype at 60.0% (95% CI 44.3–74.3) and 66.7% (95% CI 41.0–86.7), respectively. Negative predictive values were high for all the isolated or associated isotypes. For instance, for the IgM isotype, the negative predictive value was 94.9 (95% CI 91.3–97.4), and that for IgM associated with one or more isotypes was 93.3% (95% CI 90.2–95.7).
Discussion
Although leprosy has been eliminated as a public health problem in several countries [14], Brazil is one of the countries that still suffers from a high incidence, late diagnosis, and grade 2 physical disability at diagnosis [15]. Diagnosis in primary care is essentially clinical based on the search for injuries with altered sensitivity and/or thickened nerves [12]. Delays in diagnosis are due to a lack of the patient’ knowledge, as well as a lack of knowledge and skill of health professionals [16]. In addition, a lack of government attention may directly affect the quality of service provided to the population [7]. Another worrying factor is leprosy in children, which represent recent infections and high transmission rates [17]. Furthermore, children with leprosy indicate that they were possibly contacts of undiagnosed and therefore untreated leprosy patients [18].
Diagnostic tests have been developed and established for a multitude of infectious diseases [19], even for those just recently described [20]. Regarding leprosy diagnosis, as it is a disease that has practically no laboratory tests as support tools, there is a risk of the diagnosis being delayed by up to 10 years in Brazil [16], as well as other countries [21]. The only laboratory test available is intradermal smear microscopy, which can have a negative result in cases of paucibacillary clinical forms of leprosy [12]. Therefore, the search for biomarkers that consider the immune response to mycobacteria is extremely relevant [22].
Regarding detection of antibodies against PGL-I antigen, which is specific to the mycobacterium [23], Araújo et al. [24] found in a longitudinal study with 10 years of follow-up that those who were seropositive for anti-PGL-I had a 5.7-fold increased risk of developing leprosy. The authors reported disease confirmation in 2% of the participants. According to Penna et al. (2016), contacts with positive anti-PGL-I have a 3-fold higher risk of developing leprosy than those with negative anti-PGL-I. In their systematic review and meta-analysis, the authors found an overall OR of 3.05 with diagnostic sensitivities ranging of 1.96 to 39.29% and diagnostic specificities of 83.52 to 98.03% [11].
Leturiondo et al. (2021) highlight a fundamental point in that although serological tests have a series of limitations, they can be important to assist in clinical routine (for example, in differentiating leprosy from other dermatological conditions). Assessing endemic controls and patients and considering the paucibacillary and multibacillary clinical forms, the authors found diagnostic sensitivities of 32% and 81%, diagnostic specificities of 81.7% and 99%, positive predictive values of 14.9% and 43.3%, and negative predictive values of 92.9% and 94.6%, respectively [25].
In a study carried out by our group, a strong correlation was demonstrated between the anti-PGL-I IgM and IgA levels (r = 0.74; p < 0.0001), and there was a moderate association between the tests (Kappa coefficient of 0.48) [13]. In the multibacillary leprosy clinical form, diagnostic sensitivities for IgM, IgG, and IgA were 81.3%, 21.9%, and 53.1%, respectively. In the paucibacillary leprosy clinical form, they were 59.1%, 22.7%, and 40.9%, respectively. Diagnostic specificities were 88.2% for IgM and 100% for IgG and IgA. Therefore, at that time, we suggested the inclusion of the IgA isotype in the follow-up of contacts, in addition to the IgG and IgM isotypes [13].
During the same period, we carried out a prospective follow-up study of 69 children and young people between 4 and 15 years of age for 3 years [26]. One striking observation was that the IgG isotype showed a strong association with leprosy (relative risk of 8.5 times). One child who was a peridomicilliary contact presented increased levels of the IgA isotype three years before the appearance of a hypochromic lesion on the back of his neck. In the second year, he showed increased levels of IgG and IgA. Only in the third year of follow-up did he show increased levels of IgM, besides IgG and IgA [26]. This clearly demonstrates the importance of investigating the three antibody isotypes.
In the present work, we carried out a cross-sectional study with samples of index cases of MB and PB clinical forms and healthy contacts (children and young people without suggestive lesions or nerve thickening who lived with or next to index cases). Contacts with suspected lesions and/or nerve thickening were not included in the control group. No significant associations were found for PB leprosy. Instead, based on the data for MB leprosy, the ORs for IgM and IgG were 6.11 and 3.31, respectively. The highest OR values were found for IgA (OR 16.97), for IgM, when it was associated with one or more isotypes (OR 21.0), and for IgG + IgA isotypes (OR 17.58). This means that compared to healthy contacts, MB leprosy index cases show 16.97, 21.0, or 17.58 times the odds of presenting positive isolated IgA isotype, IgM associated with one and more isotypes, or IgG + IgA isotypes, respectively. This represents 2.77-, 3.42- or 2.87- times higher possibilities that leprosy patients will present one of the former biomarkers, respectively, than positive isolated IgM compared to healthy contacts.
A question that constantly arises is why IgM is not only used since it is known that PGL-I is a T-independent antigen [23]. The reason for seeking an association with other biomarkers is that although IgM alone has a higher diagnostic sensitivity than the other isotypes (considering the multibacillary forms of the disease), its diagnostic specificity is not as good. Therefore, it can impair the ability to confirm leprosy since its hypothetical positive predictive value is only 24.7%. Meanwhile, when it is associated with another isotype, there is a significant improvement in its positive predictive value, which rises to 55.1%.
Data from another study [27] corroborate our results, demonstrating that IgA is a biomarker that should be used in the follow-up of contacts and to help in the diagnosis of leprosy. That study found that IgA anti-NDO-HSA (natural octyl disaccharide bound to human serum albumin) presented diagnostic sensitivities of 100% and 95% for the multibacillary and paucibacillary forms of leprosy, as well as diagnostic specificities of 95% and 85%, respectively.
Another question that arises is why molecular tests are not used instead of serological tests. In our recent experience, virtually no blood samples from contacts were positive for M. leprae DNA, and only one patient’s blood sample was positive (unpublished data). Contacts cannot be biopsied for obvious reasons, and nasopharyngeal molecular testing has no association with disease. Therefore, we believe that among the best biomarkers with all their limitations are antibodies, considering not only the IgM isotype but also the main isotypes present in the systemic circulation and associating them with each other and perhaps other molecules of the immune system, such as chemokines. The important idea is that if we associate the isotypes, the probability of disease can increase. In conclusion, when faced with two or more positive antibody isotypes, we may already be closer to a real case of leprosy. This would certainly help in the diagnosis of leprosy.
Acknowledgments
We would like to thank Prof. Paulo César de Almeida, Health Sciences Center, Universidade Estadual do Ceará, Brazil, for reviewing the statistical analysis of the present manuscript.
Funding Statement
This work was financially supported by FUNCAP/PPSUS/SESA, Grant number 3966535/2017, and by MCTI/CNPq/MS-SCTIE, Grant number 403461/2012-0.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].World Health Organization . Global leprosy update, 2019: time to step-up prevention initiatives. Weekly Epidemiological Rec. 2020;95(36):417–440. [Google Scholar]
- [2].Salgado CG, Barreto JG, Da Silva MB, et al. Are leprosy case numbers reliable?. Lancet Infect Dis. 2018. Feb;18(2):135–137. PMID: 29412953. DOI: 10.1016/S1473-3099(18)30012-4. [DOI] [PubMed] [Google Scholar]
- [3].BRASIL, Ministério da Saúde . Secretaria de Vigilância em Saúde. Boletim Epidemiológico: Hanseníase. Brasília (DF), n. Especial; 2020. [cited 2020 Apr 16]. Available from: http://www.aids.gov.br/pt-br/pub/2020/boletim-epidemiologico-de-hanseniase-2020 [Google Scholar]
- [4].World Health Organization . New cases of leprosy in children below 15 years of age. [cited 2021 May 12]. Available from: https://www.who.int/data/gho/data/themes/topics/leprosy-hansens-disease
- [5].Barreto JG, Bisanzio D, Frade MAC, et al. Spatial epidemiology and serologic cohorts increase the early detection of leprosy. BMC Infect Dis. 2015;15:527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].CEARÁ, Governo do Estado . Secretaria de Saúde. Boletim Epidemiológico: Hanseníase. Fortaleza (CE); 2021. [cited 2021 Jan 19]. Available from: https://www.saude.ce.gov.br/wp-content/uploads/sites/9/2018/06/boletim_epidemiologico_hanseniase_20211901_v2.pdf [Google Scholar]
- [7].World Health Organization . Towards zero leprosy. Global Leprosy (Hansen’s Disease) Strategy 2021–2030; 2021. [Accessed 26 May 2021]. Available from: https://apps.who.int/iris/handle/10665/340774
- [8].Bratschi MW, Steinmann P, Wickenden A, et al. Current knowledge on Mycobacterium leprae transmission: a systematic literature review. Lepr Rev. 2015;86(2):142–155. [PubMed] [Google Scholar]
- [9].Salgado CG, Barreto JG, Silva MB, et al. Are leprosy case numbers reliable?. Lancet Infect Dis. 2018;18(2):135–137. [DOI] [PubMed] [Google Scholar]
- [10].Smith WC, Aerts A.. Role of contact tracing and prevention strategies in the interruption of leprosy transmission. Lepr Rev. 2014;85(1):2–17. [PubMed] [Google Scholar]
- [11].Penna MLF, Penna GO, Iglesias PC, et al. Anti-PGL-I positivity as a risk marker for development of leprosy among contacts of leprosy cases: systematic review and meta-analysis. PLOS Negl Trop Dis. 2016;10(5):e0004703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].BRASIL . Ministério da Saúde. Guia prático sobre hanseníase. Brasília-DF; 2017. [Accessed 31 May 2021]. Available from: https://portalarquivos2.saude.gov.br/images/pdf/2017/novembro/22/Guia-Pratico-de-Hanseniase-WEB.pdf [Google Scholar]
- [13].Macedo AC, Guimarães JA, Rodrigues RO, et al. Serum anti-phenolic glycolipid—1 IgA correlates to IgM isotype in leprosy patients: a possiblecandidate for seroepidemiological surveys?. J Clin Lab Anal. 2017;32(3):e22276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].World Health Organization . Task Force on definitions, criteria and indicators for interruption of transmission and elimination of leprosy. New Delhi: World Health Organization, Regional Office for South-East Asia; 2021. Licence: CC BY-NC-SA 3.0 IGO. [cited 2021 Oct 26] Available from: https://apps.who.int/iris/handle/10665/342172 [Google Scholar]
- [15].Salgado CG, Barreto JG, Da Silva MB, et al. What do we actually know about leprosy worldwide?. Lancet Infect Dis. 2016;16(7):778. [DOI] [PubMed] [Google Scholar]
- [16].Henry M, GalAn N, Teasdale K, et al. Factors contributing to the delay in diagnosis and continued transmission of leprosy in Brazil–an explorative, quantitative, questionnaire based study. PLoS Negl Trop Dis. 2016;10(3):e0004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Barreto JG, Frade MAC, Bernardes Filho F, et al. Leprosy in children. Curr Infect Dis Rep. 2017;19(6):23. [DOI] [PubMed] [Google Scholar]
- [18].Lana FC, Fabri Ada C, Lopes FN, et al. Deformities due to leprosy in children under fifteen years old as an indicator of quality of the leprosy control programme in Brazilian municipalities. J Trop Med. 2013;2013:812793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Delforge ML. De l’utilité ou non de la sérologie infectieuse: morceaux choisis [On the usefulness of serology testing in infectious diseases: selected topics]. Rev Med Brux. 2011;32(4):285–288. [PubMed] [Google Scholar]
- [20].West R. Serology and other adaptive immune response testing for COVID-19. The Johns Hopkins University; 2021:1–4. [cited 2021 Oct 26] Available from: https://www.centerforhealthsecurity.org/resources/COVID-19/COVID-19-fact-sheets/200228-Serology-testing-COVID.pdf
- [21].Yaghoobi R, Feily A, Ranjbari N, et al. Lepromatous leprosy: a commonly misdiagnosed disease. ScientificWorldJournal. 2010;10:2348–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].van Hooij A, Geluk A. In search of biomarkers for leprosy by unraveling the host immune response to Mycobacterium leprae. Immunol Rev. 2021;301(1):175–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Spencer JS, Brennan PJ. The role of Mycobacterium leprae phenolic glycolipid I (PGL-I) in serodiagnosis and in the pathogenesis of leprosy. Lepr Rev. 2011;82(4):344–357. [PubMed] [Google Scholar]
- [24].Araujo S, Rezende MMF, Sousa DCR, et al. Risk-benefit assessment of Bacillus Calmette-Guérin vaccination, anti-phenolic glycolipid I serology, and Mitsuda test response: 10-year follow-up of household contacts of leprosy patients. Rev Soc Bras Med Trop. 2015;48(6):739–745. [DOI] [PubMed] [Google Scholar]
- [25].Leturiondo AL, Noronha AB, Do Nascimento MOO, et al. Performance of serological tests PGL1 and NDO-LID in the diagnosis of leprosy in a reference center in Brazil. BMC Infect Dis. 2019;19(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nagao-Dias AT, Macedo AC, Rodrigues RO, et al. Serum anti-PGL-I IgG, IgM, and IgA in a 3-year follow-up study of 4-15-year-old leprosy contacts. Pediatr Infect Dis J. 2019;38(9):e193–198. [DOI] [PubMed] [Google Scholar]
- [27].Silva KKPE, de Oliveira EE, Elias CMM, et al. Serum IgA antibodies specific to M. leprae antigens as biomarkers for leprosy detection and household contact tracking. Front Med (Lausanne). 2021;8:698495. [DOI] [PMC free article] [PubMed] [Google Scholar]


