ABSTRACT
The objectives of this study were to explore global epidemiological characteristics of leprosy, and to provide reference for the construction of prevention strategies for leprosy. Computer retrieval of the study on the epidemiology of leprosy from 2010 to 2020 in Web of Science, PubMed, and SCOPUS databases were summarized. The included studies were assessed for the quality of the AHRQ; the proportions of the study indices were meta-analyzed with Stata 16.0. A random effects model was adopted to merge categories, including sex, type, grade 2 deformity (G2D) and age group for meta-analysis. The subgroup analysis used region as a stratification factor to analyze whether there were differences in the indicators. The meta-analysis included 30 studies totaling 11,353 cases. The global pooled proportion of male to female subjects with leprosy was 63% (95% CI 59%, 66%) to 37% (95% CI 34%, 41%), respectively. The pooled multibacillary proportion and paucibacillary proportion were 69% (95% CI 62%, 76%) and 31% (95% CI 24%, 38%), respectively. The pooled grade 2 deformity (G2D) proportion was 22% (95% CI 15%, 30%). Among age groups, the pooled children proportion was 11% (95% CI 8%, 13%), and the pooled adult proportion was 89% (95% CI 87%, 92%). The subgroup analysis indicated that epidemiological indicators varied from country to country. This study suggested that disparities existed between sex, type, grade 2 deformity (G2D) and age group characteristics of leprosy from country to country.
KEYWORDS: Leprosy, epidemiology, proportion, meta-analysis
1. Introduction
Leprosy is an infectious chronic disease and a neglected tropical disease induced by Mycobacterium leprae, and mainly affects the skin, peripheral nerves, upper respiratory mucosa and eyes[1]. The prolonged physical deformities associated with leprosy get progressively worse with delayed diagnosis and increasing age [2]. While leprosy is a millennial disease, it is a public health and social issue of global concern prevalent in at least 122 countries [3]. The prevalence of leprosy declined from over 5 million cases in the 1980s to less than 129,192 in the late of 2020s [4]. This change was due to leprosy control around the world over the years. Based on the estimated new leprosy infections in 2020 published by World Health Organization (WHO), the top five countries, in sequence, are India, Brazil, Indonesia, Democratic Republic of the Congo, Bangladesh, and the proportion of newly detected leprosy cases with multibacillary leprosy was about 67.3%. In the meanwhile, 38.6% of the new leprosy cases were among females in the world. Considering that leprosy has still not been eradicated, it was essential to further investigate the epidemiological characteristics of leprosy.
Schreuder et al [5]. Conducted a study reporting the epidemiologic trends of leprosy indicating that there appeared to be regional differences in the gender proportion of leprosy patients at the time of diagnosis and treatment, and male leprosy patients were more susceptible to deformities than female. A meta-analysis also pointed out that physical disability was virtually twice as frequent in male patients as with female patients [2]. Nevertheless, this discrepancy was not evident in all countries analyzed. The World Health Organization (WHO) categorized leprosy into multibacillary leprosy (MB) and paucibacillary leprosy (PB). A cross-sectional study in Iran illustrated that 85.7% of leprosy patients were multibacillary leprosy and 14.3% were leprosy patients infected with paucibacillary leprosy [6]. In India, a study noted that newly detected cases of leprosy continued to persist and the grade 2 deformity (G2D) rate was also on the rise among new leprosy cases [7]. A study of leprosy patients in only one tertiary level hospital showed that the proportion of grade 2 deformity was much higher than 10% in Ethiopia [8]. Furthermore, in the study of factors influencing the incidence of leprosy in globally endemic areas, indicated that the apparent deformities have increased year to year from 2005 to 2015 in China [9]. The available studies on grade 2 deformity were all single for a certain country and risk factors without systematic investigation. A systematic review in Brazil pointed that leprosy status of children under 15 years was extremely undesirable and the proportion of disability was also high [10]. A recent review of childhood leprosy in India systematically reported its prevalence status and showed that the incidence in children remained high [11]. Thus, the comprehension of childhood leprosy proportion among new leprosy cases in various countries worldwide is essential.
Simultaneously, the World Health Organization (WHO) also published some raw data, including the number of new cases of women, grade 2 deformity (G2D) and children in different countries. Therefore, the objective of this study was to investigate the epidemiological characteristics of leprosy across regions worldwide by meta-analysis, and to systematically analyze the difference of sex, type, grade 2 deformity (G2D) and age group in different countries, in order to offer a reference point for preventing leprosy and controlling outbreaks of leprosy and a scientific basis for the goal of early and complete elimination of leprosy hazards.
2. Methods
2.1. Search strategy and selection criteria
A literature search was performed on PubMed, Web of Science, and SCOPUS to confirm English language publications with information relating to the global epidemiology of leprosy. The following medical subject headings (MeSH) terms and keywords were used in the search strategy: leprosy, epidemiology, prevalence and incidence. All the databases were searched from 2010 until 2020. Two readers filtered through the results of the search and identified potentially relevant studies based on the title and abstract. Disagreements between the two readers were settled with discussions.
Inclusion criteria were as follows: (1) literature was available in English and reported the epidemiological characteristics of leprosy; (2) the research method was a cross-sectional study or baseline investigations; (3) data were complete; and (4) the diagnosis criteria followed leprosy diagnostic criteria.
Exclusion criteria were as follows: (1) small sample sizes (< 30); (2) repetition; (3) overview; (4) systematic review; (5) reviews or lectures; (6) reported data that overlapped with already included articles; (7) the source of the sample was unclear; and (8) statistical content was not available.
2.2. Data extraction
Data extraction was performed by two reviewers who screened the literature including author details, publication year, geographic location of study, total sample size of leprosy patient cohort, the number of male and female patients, the number of multibacillary (MB) and paucibacillary (PB), the number of grade 2 deformity (G2D), age < 15 years, and age ≥ 15 years.
2.3. Quality assessment
Quality appraisal of the included literatures were carried out using the ‘The Agency for Healthcare Research and Quality (AHRQ)’, which consists of 11 entries [12]. The quality of the included studies was independently assessed by two researchers, with a score of 1 for ‘yes’ and 0 for ‘no or unclear’, for a total of 11 points. Any discrepancies were resolved through consensus-based discussions. Studies were graded according to their scores into low, medium, and high quality, with scores of 0 to 3, 4 to 7 and 8 to 11, respectively.
2.4. Statistical analysis
Depending on the abovementioned inclusion and exclusion criteria, the data on the epidemiological characteristics of leprosy published in domestic and foreign journals were organized based on the requirements of the meta-analysis, and the database was established. Single group rate meta-analysis of each prevalence indicator was estimated using STATA 16.0 for the included studies of the epidemiology of leprosy. When I2 was ≤ 50%, the fixed-effects model was used; otherwise, we used the random-effects model. Sources of heterogeneity were explored by means of a subgroup analysis, and to examine the authenticity of data, a funnel plot was produced using STATA 16.0.
3. Results
3.1. Selection of studies
From the abovementioned search method, a total of 2,242 studies were retrieved from the database, and 1931 studies were excluded as irrelevant and duplicate articles based on the titles and abstracts. After screening the full text based on the inclusion and exclusion criteria, a total of 281 studies were excluded and 30 studies were included in the quantitative synthesis. The detailed flowchart of the search and selection process was shown in Figure 1.
Figure 1.
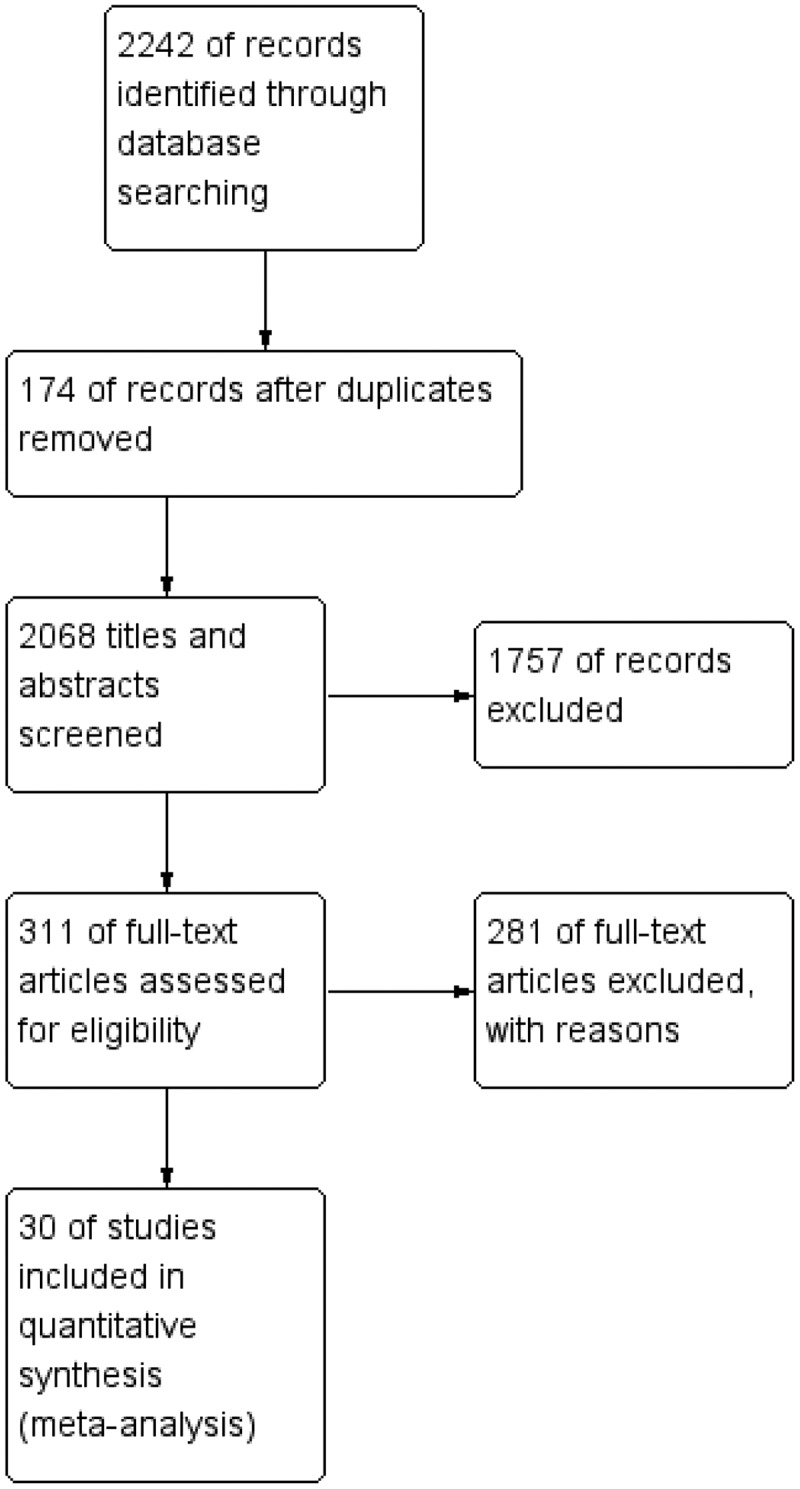
Flow diagram of study selection process.
3.2. Characteristics and quality assessment of included studies
This systematic review included 30 studies occurring between 2010 to 2020, spanning 10 countries and comprising 11,353 leprosy patients. The 10 countries included India (10), Philippines (1), Brazil (7), Ethiopia (2), China (3), Madagascar (1), Iran (1), Saudi Arabia (1), Nigeria (2) and Bangladesh (2). The leprosy patient sample sizes of the included studies ranged from 39 to 4,775, among which, the data of male, female, multibacillary (MB), paucibacillary (PB), grade 2 deformity (G2D), age < 15 years, and age ≥ 15 years were collected. In the quality assessment, quality scores of the included studies ranged from 5 to 9, and contained 16 medium quality and 14 high quality studies. A more detailed description of the studies was shown in Table 1.
Table 1.
Descriptive characteristics of all studies included in meta-analysis
| Author details | Publication year | Country | Cases (n) | Male (n) | Female (n) | MB (n) | PB (n) | G2D (n) | Age<15 (n) | Age≥15 | Quality scores |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vinnarasan et al[27]. | 2018 | India | 39 | 28 | 11 | NA | NA | NA | NA | NA | 5 |
| Scheelbeek et al[28]. | 2013 | Philippines | 204 | 150 | 54 | NA | NA | NA | NA | NA | 5 |
| Soares Dos Santos et al.[29] | 2020 | Brazil | 111 | 64 | 47 | 85 | 26 | NA | NA | NA | 6 |
| Govindharaj et al[30]. | 2016 | India | 65 | 45 | 20 | 46 | 19 | 10 | NA | NA | 6 |
| Sharma et al[31]. | 2017 | India | 97 | 80 | 17 | NA | NA | NA | NA | NA | 5 |
| Abdela et al[3]. | 2020 | Ethiopia | 57 | 44 | 13 | 51 | 6 | 34 | 2 | 55 | 9 |
| Mangeard-Lourme et al.[32] | 2017 | India | 321 | 169 | 152 | 89 | 232 | 7 | 119 | 202 | 9 |
| Sun et al[33]. | 2012 | China | 1324 | 905 | 419 | 1124 | 200 | 298 | 39 | 1285 | 8 |
| Long et al[34]. | 2017 | China | 4775 | 3276 | 1499 | 4041 | 734 | 1134 | 106 | 4669 | 9 |
| Kumar et al[35]. | 2019 | India | 315 | 213 | 102 | NA | NA | NA | NA | NA | 5 |
| Ambrosano et al[36]. | 2018 | Brazil | 41 | 22 | 19 | NA | NA | NA | NA | NA | 5 |
| Zhang et al[37]. | 2020 | China | 40 | 28 | 12 | 33 | 7 | 14 | NA | NA | 7 |
| Suttels et al[38]. | 2016 | Madagascar | 87 | 53 | 34 | 68 | 19 | 16 | 10 | 77 | 9 |
| Mansori et al[39]. | 2017 | Iran | 122 | 72 | 50 | 113 | 9 | NA | NA | NA | 6 |
| Assiri et al[40]. | 2014 | Saudi Arabia | 56 | 47 | 9 | 27 | 29 | 6 | NA | NA | 8 |
| de Oliveira et al[41]. | 2019 | Brazil | 71 | 42 | 29 | NA | NA | 1 | NA | NA | 7 |
| Shukla et al[42]. | 2015 | India | 358 | 173 | 185 | 133 | 225 | 6 | 37 | 321 | 9 |
| Rodrigues et al[43]. | 2020 | Brazil | 40 | 21 | 19 | NA | NA | NA | NA | NA | 5 |
| Kumar et al[44]. | 2015 | India | 70 | 35 | 35 | 32 | 38 | 11 | 19 | 51 | 9 |
| Ganesan et al[45]. | 2018 | India | 171 | 85 | 86 | 24 | 147 | 147 | NA | NA | 7 |
| John et al[46]. | 2017 | Nigeria | 61 | 24 | 37 | 52 | 9 | 8 | 7 | 54 | 9 |
| Nazario et al[47]. | 2017 | Brazil | 360 | 196 | 164 | 285 | 75 | NA | 7 | 353 | 8 |
| Arif et al[48]. | 2019 | India | 220 | 148 | 72 | 161 | 59 | NA | NA | NA | 7 |
| Mowla et al[49]. | 2015 | Bangladesh | 99 | NA | NA | 69 | 30 | 22 | 5 | 94 | 8 |
| Mowla et al[50]. | 2017 | Bangladesh | 177 | 134 | 43 | 131 | 46 | NA | NA | NA | 6 |
| Bandeira et al[51]. | 2017 | Brazil | 41 | 24 | 17 | 26 | 15 | NA | NA | NA | 6 |
| Darlong et al[52]. | 2020 | India | 100 | 60 | 40 | 67 | 33 | 13 | 45 | 55 | 8 |
| Shumet et al[8]. | 2015 | Ethiopia | 513 | 328 | 185 | 509 | 4 | 132 | 25 | 488 | 8 |
| Martins et al[53]. | 2016 | Brazil | 434 | 206 | 228 | 292 | 141 | 50 | NA | NA | 7 |
| Chukwu et al[54]. | 2018 | Nigeria | 984 | 565 | 419 | 946 | 38 | 281 | 72 | 912 | 8 |
NA: not available
3.3. Sex characteristics of leprosy
This systemic review incorporated 30 studies, 27 of which contained the sex distribution of leprosy cases. Altogether, the 27 studies contained a sample size of 11,091 leprosy patients, among which, the reported male proportion ranged from 39.3% to 83.9%. The pooled proportion of males to females was 63% (95% CI 59%-66%) to 37% (95% CI 34%-41%), respectively. The result of the degree of heterogeneity inconsistency (I2) was 91.3% (P < 0.001) (Figure 2, Figure 3).
Figure 2.
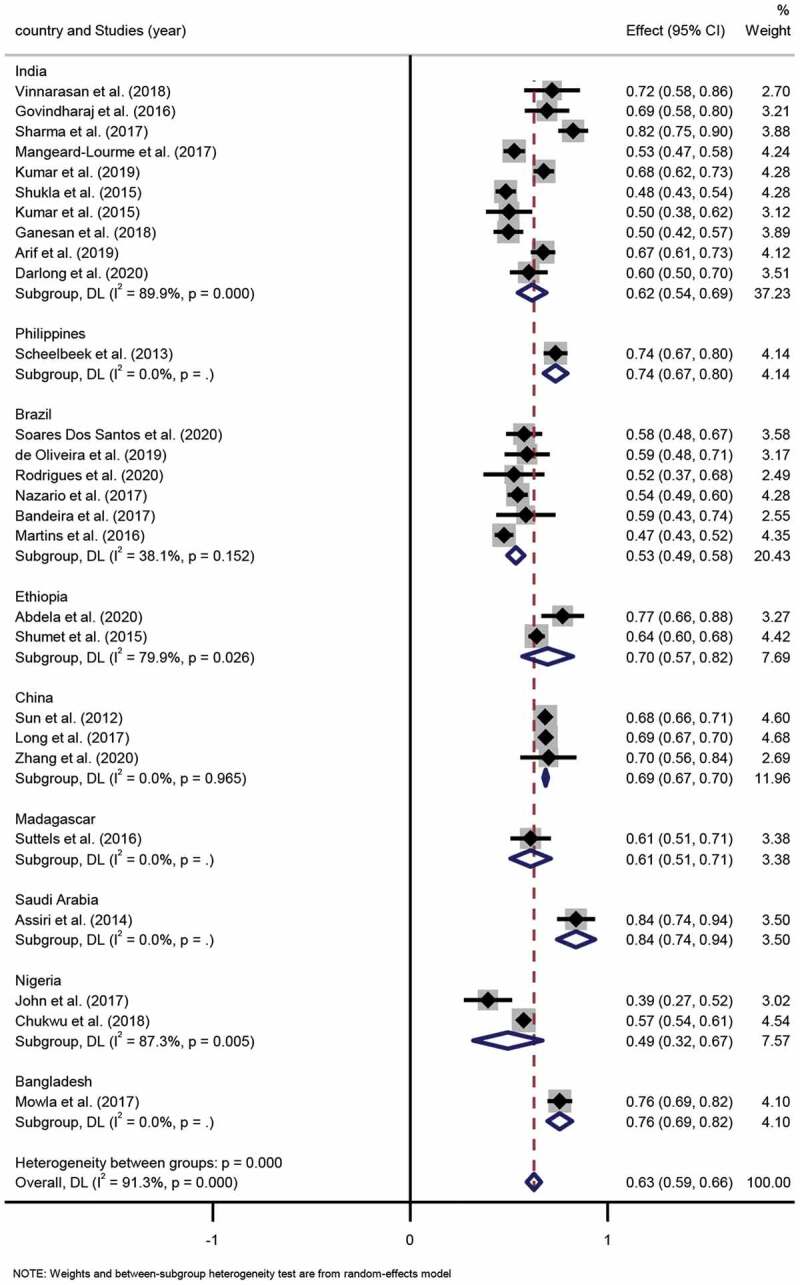
Forest plot of proportion of male patients with leprosy from studies conducted in different countries.
Figure 3.
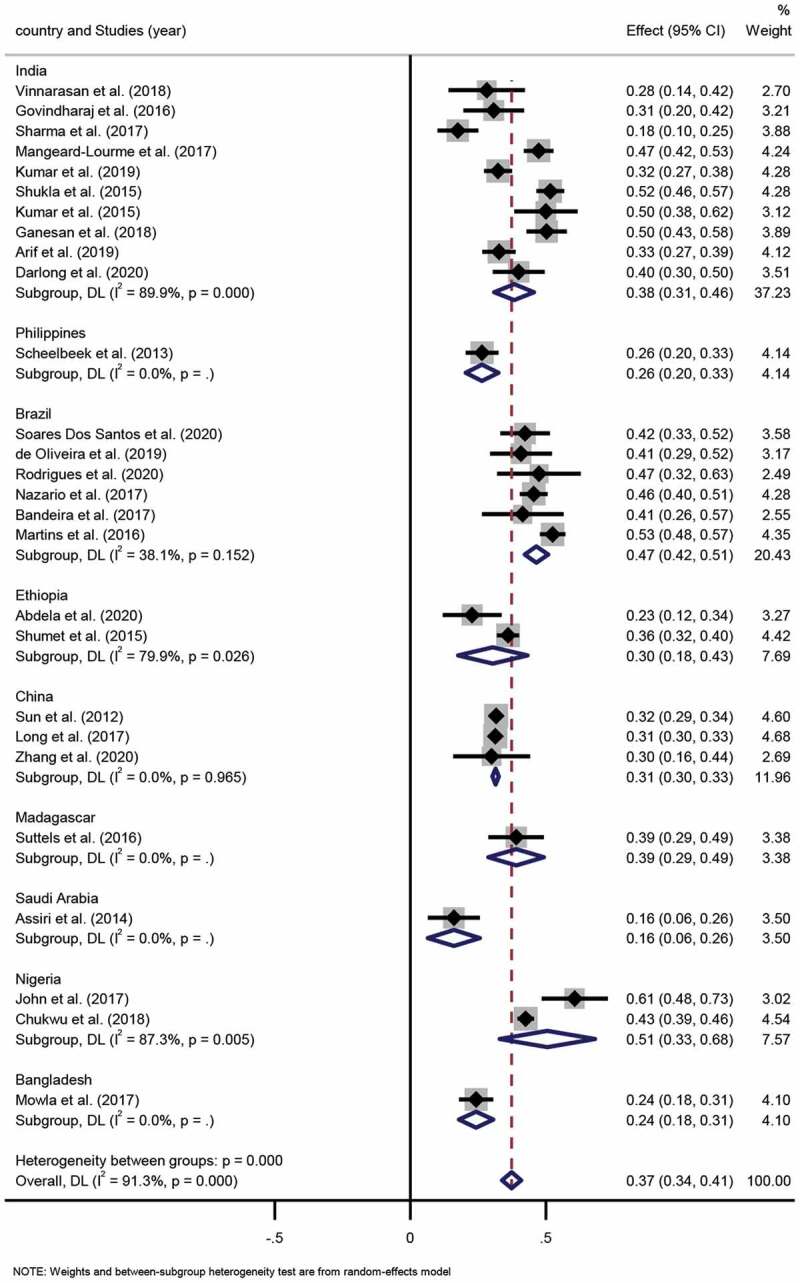
Forest plot of proportion of female patients with leprosy from studies conducted in different countries.
Subgroup analysis. Ten studies were conducted in India, and the pooled proportion of males to females was 62% (95% CI 54%-69%; I2=89.9%, p = 0.000) to 38% (95% CI 31%-46%; I2 =89.9%, p = 0.000), respectively. Six studies took place in Brazil, among which, the pooled proportion of males to females was 53% (95% CI 49%-58%; I2=38.1%, p = 0.152) to 47% (95% CI 42%-51%; I2=38.1%, p = 0.152), respectively. Two studies were conducted in Ethiopia, and the pooled proportion of males to females was 70% (95% CI 57%-82%; I2=79.9%, p = 0.026) to 30% (95% CI 18%-43%; I2 =79.9%, p = 0.026), respectively. Three studies were performed in China, in which the pooled proportion of males to females was 69% (95% CI 67%-70%; I2=0.0%, p = 0.965) to 31% (95% CI 30%-33%; I2=0.0%, p = 0.965). Two studies were performed in Nigeria, in which the pooled proportion of males to females was 49% (95% CI 32%-67%; I2=87.3%, p = 0.005) to 51% (95% CI 33%-68%; I2=87.3%, p = 0.005), respectively. Differences in the pooled proportion of males and females across the various countries were statistically significant (p < 0.001).
3.4. Type characteristics of leprosy
According to WHO standards, leprosy was divided into multibacillary (MB) and paucibacillary (PB) [6]. For this systemic review, 22 studies included the number of MB and PB, which included a total of 10,424 leprosy patients. The MB proportion across these studies ranged widely from 14% to 89%, and similarly, the PB proportion across these studies ranged from 10% to 86%. The pooled proportion of MB and PB was 69% (95% CI 62%-76%) and 31% (95% CI 24%-38%), respectively. The degree of heterogeneity inconsistency (I2) was 99.3% (P < 0.001) (Figure 4, Figure 5).
Figure 4.
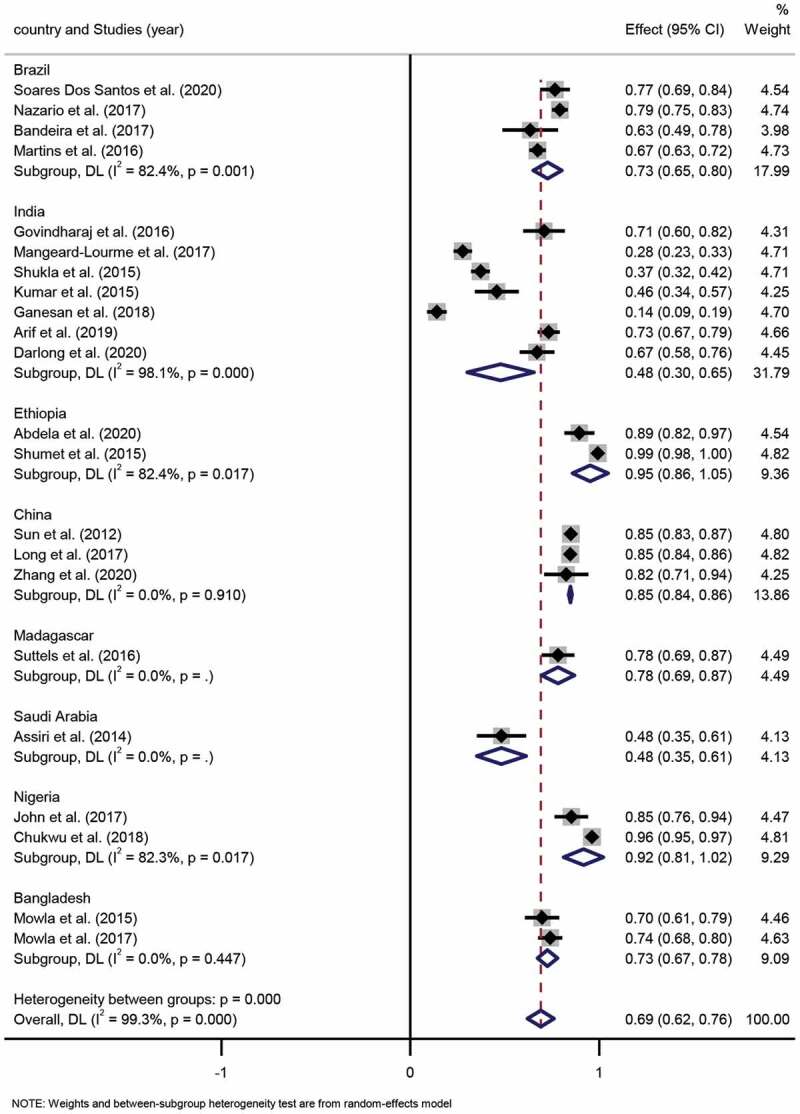
Forest plot of proportion of MB patients from studies conducted in different countries.
Figure 5.
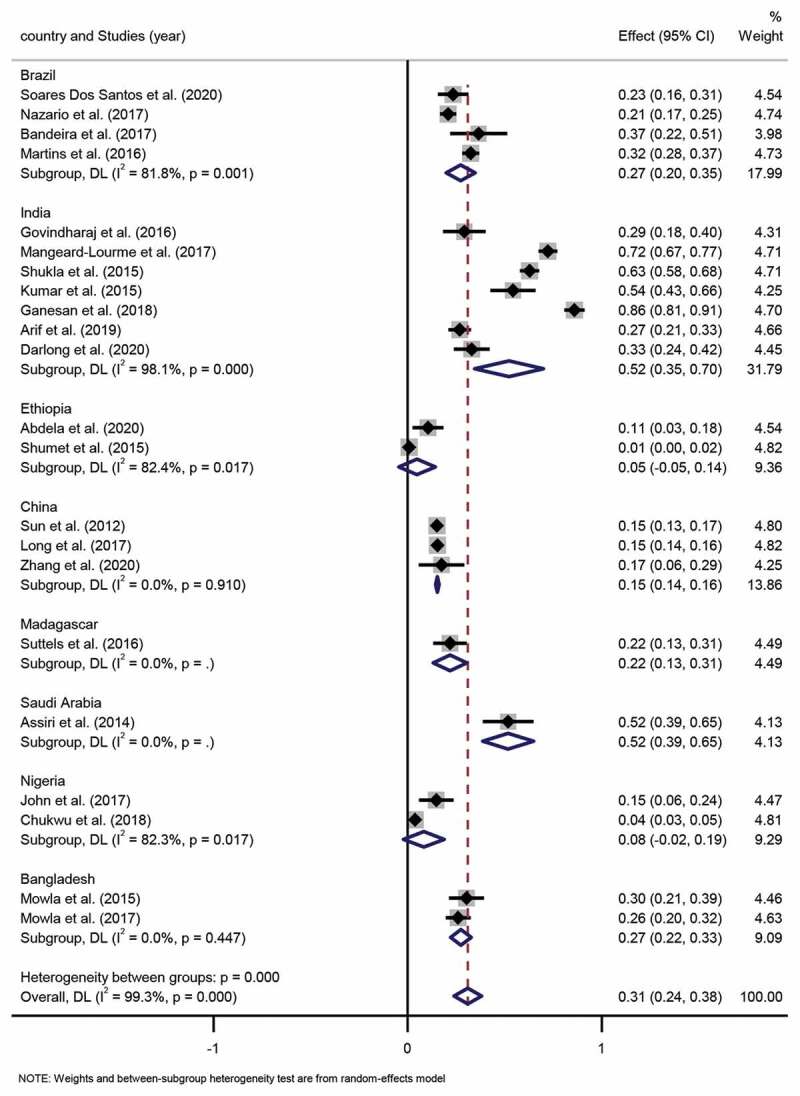
Forest plot of proportion of PB patients from studies conducted in different countries.
Subgroup analysis. Four studies were based in Brazil, and the pooled proportion of MB and PB proportion was 73% (95% CI 65%-80%; I2=82.4%, p = 0.001) and 27% (95% CI 20%-35%; I2 =82.4%, p = 0.001), respectively. Seven studies were conducted in India with a pooled MB and PB proportion of 48% (95% CI 30%-65%; I2=98.1%, p = 0.000) and 52% (95% CI 35%-70%; I2 =98.1%, p = 0.000), respectively. Two studies were executed in Ethiopia, for which the pooled proportion of MB and PB was 95% (95% CI 86%-105%; I2=82.4%, p = 0.017) and 5% (95% CI −5%-14%; I2=82.4%, p = 0.017), respectively. Three studies were conducted in China, among which, the pooled proportion of MB and PB was 85% (95% CI 84%-86%; I2=0.0%, p = 0.910) and 15% (95% CI 14%-16%; I2=0.0%, p = 0.910), respectively. Two Nigerian studies indicated that the pooled proportion of MB and PB was 92% (95% CI 81%-102%; I2=82.3%, p = 0.017) and 8% (95% CI −2%-19%; I2=82.3%, p = 0.017), respectively. Lastly, two studies carried out in Bangladesh had a pooled proportion of MB and PB of 73% (95% CI 67%-78%; I2=0.0%, p = 0.447) and 27% (95% CI 22%-33%; I2=0.0%, p = 0.447), respectively. Differences in pooled MB and PB proportion estimates across the various countries were statistically significant (p < 0.001).
3.5. Characteristics of leprosy grade 2 deformity (G2D)
With reference to the leprosy disability grading method (WHO, 1997), disability level was categorized as 0, 1 and 2. Due to leprosy grade 2 deformity (G2D) with visible deformity or damage, which caused significant mental and life misery to leprosy patients, but also generated great economic pressure on individuals, families and the government, with significant indirect economic burden on daily life exceeded the direct economic burden. Pooled G2D proportion was estimated from 18 studies which altogether included 2,190 G2D leprosy patients. The pooled proportion of G2D was determined to be 22% (95% CI 15%-30%; I2=99.1%; P < 0.001). (Figure 6)
Figure 6.
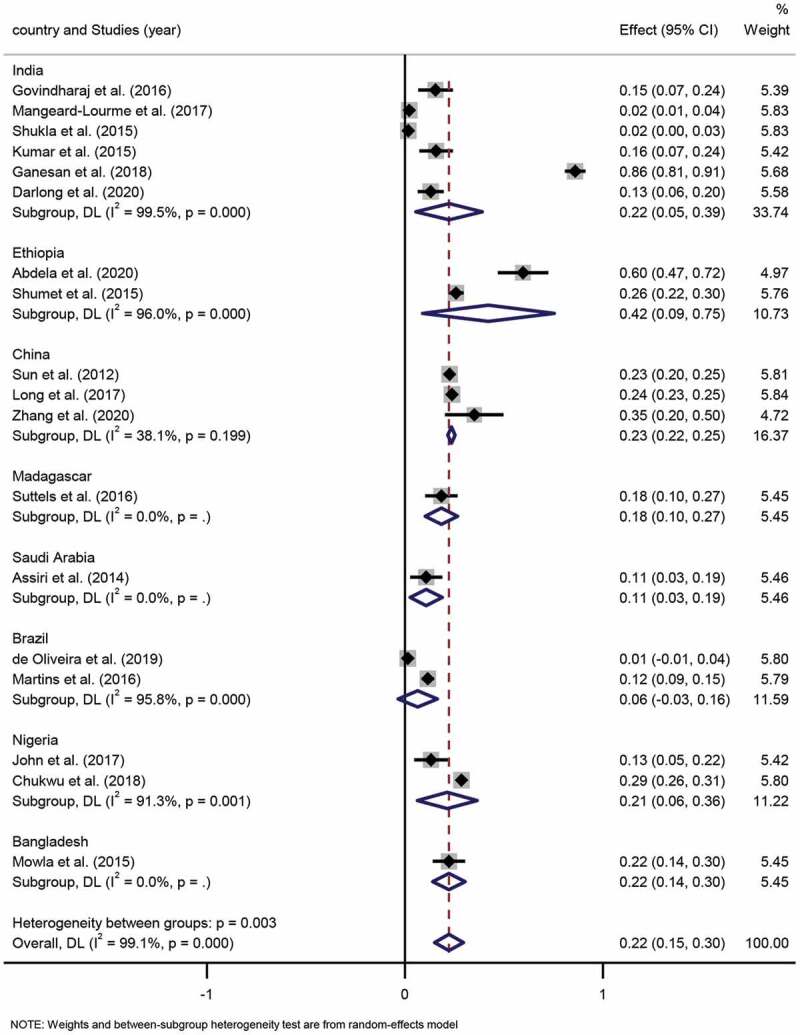
Forest plot of proportion of G2D patients from studies conducted in different countries.
Subgroup analysis. Six studies were conducted in India, and the pooled G2D proportion was determined as 22% (95% CI 5%-39%; I2=99.5%, p = 0.000). Two studies involved Ethiopia, among which, the pooled proportion estimate for the studies conducted in G2D was 42% (95% CI 9%-75%; I2=96.0%, p = 0.000). Three studies included China, and the pooled G2D proportion estimate for China region was determined as 23% (95% CI 22%-25%; I2=38.1%, p = 0.199). Two studies were related to Brazil, in which the pooled G2D proportion was identified as 6% (95% CI −3%-16%; I2=95.8%, p = 0.000). Two studies performed in Nigeria, for which the pooled G2D proportion was ascertained as 21% (95% CI 6%-36%; I2=91.3%, p = 0.001). Differences in pooled G2D proportion estimates in the various countries were statistically significant (p < 0.001).
3.6. Age group characteristics of leprosy
The World Health Organization (WHO) has published that the new cases of children leprosy (age< 15 years) were an indicator of the spread of leprosy in the community [13]. For studies involving age group characteristics of leprosy, which together a total of 9,109 leprosy patients, the children and adult proportion across these included studies ranged from 2% to 37% and 63% to 98%, respectively. The pooled proportion of children to adults was 11% (95% CI 8%-13%) to 89% (95% CI 87%-92%), respectively. The result of national heterogeneity for the degree of inconsistency (I2) was 96.4% (P < 0.001). (Figure 7, Figure 8), indicating significant differences in the ratio of children to adults across countries.
Figure 7.
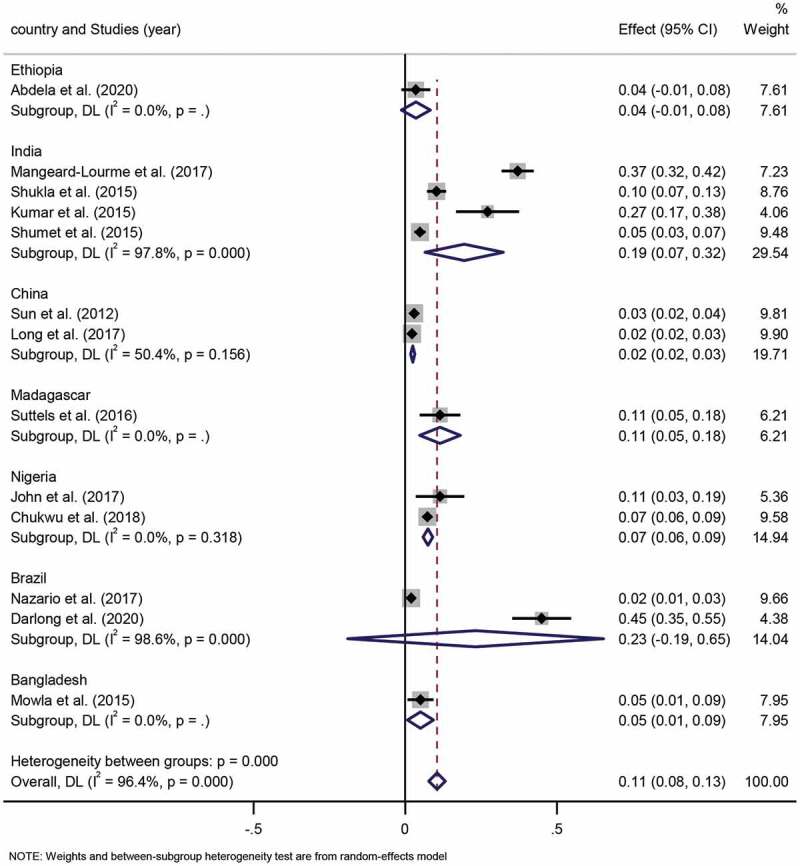
Forest plot of proportion of children patients from studies conducted in different countries.
Figure 8.
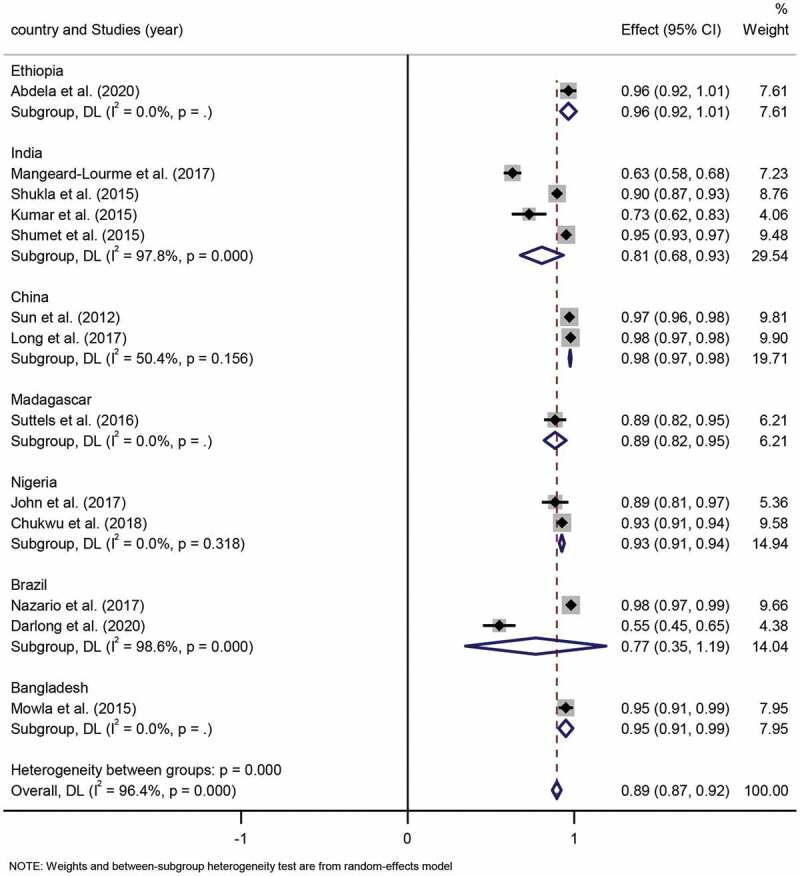
Forest plot of proportion of adult patients from studies conducted in different countries.
Subgroup analysis. Four studies were based in India, and the pooled proportion of children to adults was 19% (95% CI 7%-32%; I2=97.8%, p = 0.000) to 81% (95% CI 68%-93%; I2=97.8%, p = 0.000), respectively. Two studies conducted in China had a pooled proportion of children to adults of 2% (95% CI 2%-3%; I2=50.4%, p = 0.156) to 98% (95% CI 97%-98%; I2=50.4%, p = 0.156), respectively. Two studies conducted in Nigeria, the pooled proportion of children to adults was 7% (95% CI 6%-9%; I2=0.0%, p = 0.318) to 93% (95% CI 91%-94%; I2=0.0%, p = 0.318), respectively. Two studies were performed in Brazil, among which, the pooled children and adult proportion was 23% (95% CI −19%%-65%; I2=98.6%, p = 0.000) to 77% (95% CI 35%-119%; I2 =98.6%, p = 0.000). Differences in pooled children and adult proportion estimates in the various countries were statistically significant (p < 0.001).
3.7. Heterogeneity assessment
The funnel plot indicated that there was significant publication bias in the characteristic of type, G2D, and age group for the meta-analysis, which a direct observation revealed an asymmetrical display, but the sex characteristic meta-analysis funnel plot was in symmetry (Figure 9).
Figure 9.
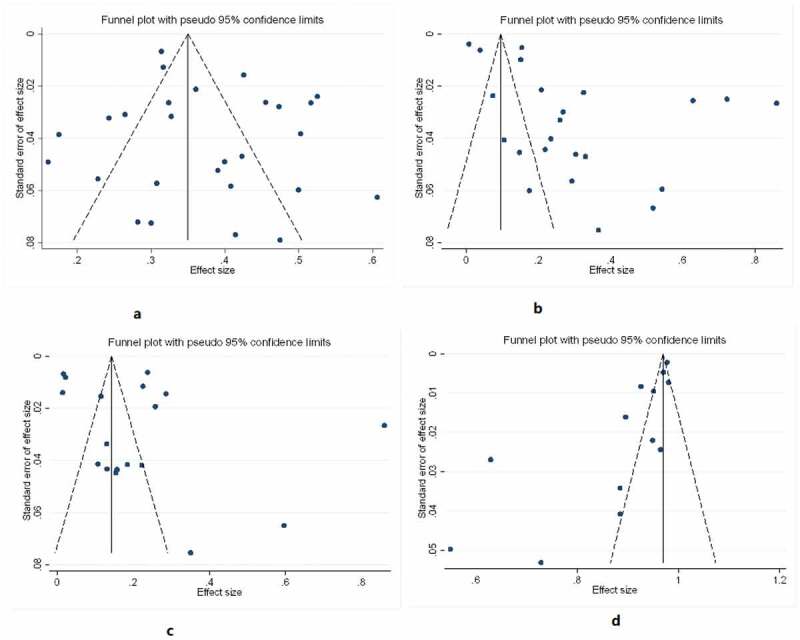
A funnel plot of the included studies reporting the epidemiology of leprosy in different countries.
4. Discussion
The studies involved in our meta-analysis indicated that the pooled proportion of female leprosy proportion was 37%, which was similar to the data reported at the WHO (36.8%), and the pooled multibacillary leprosy proportion, G2D proportion and children leprosy proportion were 69%, 22% and 11%, respectively, which was a little higher than the figures reported at the WHO (67.3%, 17.3% and 6.8%, respectively). Even so, considering that a significant proportion of the studies included in this meta-analysis examined the literature concerning populations in high prevalence countries, the proportion of sex, type, G2D and children leprosy could have been more modest if the included studies had been more comprehensive.
The sex distribution of leprosy analysis demonstrated the proportion of leprosy was significantly higher among males than females. In addition, the subgroup analysis indicated statistical differences in leprosy gender distribution between countries. The pooled proportion of females was highest in Nigeria, followed by Brazil, India, China and Ethiopia. The results of this study indicated that there are geographical discrepancies in the sex ratio of leprosy, which was consistent with Liu et al [14]. Globally, approximately 35–37% of all reported new cases of leprosy are female, but a study has shown that some countries showed very few cases in women, potentially owing to female under-diagnosis [15]. A cadence study of emerging Leprosy Cases in Bangladesh and Ethiopia revealed a sex ratio M:F of 1.66, which was attributed to low morbidity consciousness among women and poor access to health services, resulting in delayed access to care [16]. The reasons for this phenomenon are that men are more activated socially and therefore have more access to infectious agents than women, while some scholars believe that men have greater opportunities to access health services than women. In addition, low status, illiteracy, and other cultural issues may also contribute to gender disparities.
The pooled multibacillary (MB) proportion in this meta-analysis was 69%, which was higher than Sarode et al [15]. illustrated. The result of the subgroup analysis in the type characteristic of leprosy indicated that the proportion of multibacillary (MB) was much higher than the proportion of paucibacillary (PB) in Brazil, Ethiopia, China and Nigeria, whereas in India, the finding was the opposite. Some studies have elaborated that multibacillary (MB) leprosy is more susceptible to mouth and nose fluid transmission in the population, and multibacillary leprosy was related to a higher potential for complementary conditions [17,18]. One study indicated that multibacillary leprosy was somewhat more frequent in Asia, but paucibacillary leprosy was more dominant in Africa [1]. The high proportion of multibacillary in leprosy and its consequences have drawn attention to the need for enhancing the monitoring and treatment of individuals at high risk for multibacillary leprosy.
The result of the pooled proportion of G2D was 22%, which is generally the consistency with those reported by others [19]. Currently, the global proportion of G2D from WHO reported in 2019 was 17.3%, and the indicator reflecting the early detection of cases is the number of new cases with G2D, which tangentially furnishes information, for instance, the perception of leprosy in the community or the degree of quality of leprosy control services [7,20]. This subgroup analysis showed that the pooled G2D proportion in Ethiopia was relatively higher than in other countries, and the bias of this result was due to the data derived from Abdela et al [3]. The WHO Global Leprosy Strategy was undergoing constant change, and aimed to accelerate action toward a leprosy-free world by focusing on early detection of new cases to reduce the risk of disabilities in 2010–2020 [21]. In this respect, our results provided information on G2D characteristics, and interventions should be targeted for early detection and reduction of grade 2 disability in endemic countries. Simultaneously, a study pointed out that using early diagnosis and multidrug treatment prevented disabilities [22].
The pooled proportion of children leprosy cases in this study was 11%, and could be seen in the forest plot of age group that India and Brazil had higher cases than in other countries, which was a bias caused by the selection of articles and the economic status of the country. Studies have shown that the clinical signs of childhood leprosy are sometimes atypical, leading to delays in diagnosis and consequent disability, and age was associated with the duration of the disease and delay in diagnosis [23,24]. In addition, researchers pointed that the proportion of child new leprosy cases indicated that Mycobacterium leprae infection was still spreading [25]. In one study [26], the proportion of children leprosy cases was still high in India, which was consistent with our studies. Therefore, in endemic countries, it is essential to keep children under observation and provide early diagnosis to those who are at risk of contracting leprosy as a way of achieving zero disability among children with leprosy.
5. Limitations
Limitations of this study included (i) the heterogeneity of the included studies was high, probably due to the different regions, time of the included literature and the large differences in data quality, which affected the accuracy of the study results; (ii) possible biases in selection and information, sample representativeness, and, as only English-language literature were included, this may also have led to publication bias; and (iii) there might be omissions in the included studies.
6. Conclusions
Despite these limitations, this study demonstrated the global epidemiology of leprosy from 2010 to 2020. Therefore, early and accurate detection of new cases of childhood leprosy in high-risk populations is of great significance, as well as focused monitoring of susceptible males, thus enabling early detection, diagnosis and treatment, which can reduce the delay period and G2D proportion. Limitations in the quality and number of included studies indicated that these findings need to be confirmed by additional high-quality studies.
Acknowledgments
This work was supported by the grants from Research Project of China Disabled Persons’ Federation - on assistive technology(2021CDPFAT-46).
Correction Statement#
These authors contributed equally to this work.This article has been republished with minor changes. These changes do not impact the academic content of the article.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Suzuki K, Akama T, Kawashima A, et al. Current status of leprosy: epidemiology, basic science and clinical perspectives. J DERMATOL. 2012;39(2):121–129. [DOI] [PubMed] [Google Scholar]
- [2].de Paula HL, de Souza CDF, Silva SR, et al. Risk Factors for Physical Disability in Patients With Leprosy: a Systematic Review and Meta-analysis. JAMA DERMATOL. 2019;155(10):1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Abdela SG, Diro E, Zewdu FT, et al. Delayed diagnosis and ongoing transmission of leprosy in the post-elimination era in Boru Meda hospital, Ethiopia. J INFECT DEV COUNTR. 2020;14S(6SI):10S–15S. [DOI] [PubMed] [Google Scholar]
- [4].Mushtataq S. Leprosy in the post-elimination phase: so near and yet so far. GIORN ITAL DERMAT V. 2020;155(3):269–279. [DOI] [PubMed] [Google Scholar]
- [5].Schreuder PAM, Noto S, Richardus JH.. Epidemiologic trends of leprosy for the 21st century. CLIN DERMATOL. 2016;34(1):24–31. [DOI] [PubMed] [Google Scholar]
- [6].Ghavidel M, Taghanaki HRB, Samiee A, et al. Characterization of New Leprosy Cases in Northeast of Iran within the Last 15 Years. Iran J Med Sci. 2018;43(4):416–420. [PMC free article] [PubMed] [Google Scholar]
- [7].Kumar A, Karotia D. Accelerating towards a Leprosy Free India through innovative approaches in the National Leprosy Eradication Programme (NLEP), India. LEPROSY REV. 2020;91(2):145–154. [Google Scholar]
- [8].Shumet T, Demissie M, Bekele Y. Prevalence of Disability and Associated Factors among Registered Leprosy Patients in All Africa Tb and Leprosy Rehabilitation and Training Centre (ALERT), Addis Ababa, Ethiopia. Ethiop J Health Sci. 2015;25(4):313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li Y, Shakya S, Long H, et al. Factors Influencing Leprosy Incidence: a Comprehensive Analysis of Observations in Wenshan of China, Nepal, and Other Global Epidemic Areas. Front Public Health. 2021;9:666307 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Araujo Vieira MC, Nery JS, Paixao ES, et al. Leprosy in children under 15 years of age in Brazil: a systematic review of the literature. PLOS NEGLECT TROP D. 2018;12(e000678810): e0006788 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pradhan S, Nayak BP, Dash G. Childhood leprosy: a review. INDIAN J PAEDIATRIC DERMATOL. 2019;20(2):112–116. [Google Scholar]
- [12].Fasano A, Catassi C. Celiac Disease. NEW ENGL J MED. 2012;367(25):2419–2426. [DOI] [PubMed] [Google Scholar]
- [13].Arunraghav P, Herakal K. Leprosy in elderly and children among new cases - A 3-year retrospective study. Indian Dermatol Online J. 2021;12(2):294–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu YY, Yu MW, Ning Y, et al. A study on gender differences in newly detected leprosy cases in Sichuan, China, 2000-2015. INT J DERMATOL [Journal Article]. 2018;57(12):1492–1499. 2018-12-01. [DOI] [PubMed] [Google Scholar]
- [15].Sarode G, Sarode S, Anand R, et al. Epidemiological aspects of leprosy. DM-DIS MON. 2020;66(7): 100899. [DOI] [PubMed] [Google Scholar]
- [16].Van Veen Natasja HJ, Abraham M, RJ H. The relationship between detection delay and impairment in leprosy control: a comparison of patient cohorts from Bangladesh and Ethiopia. LEPROSY REV. 2006;77(4):356–65 . [PubMed] [Google Scholar]
- [17].Bratschi MW, Steinmann P, Wickenden A, et al. Current knowledge on Mycobacterium leprae transmission: a systematic literature review. LEPROSY REV. 2015;86(2):142–155. [PubMed] [Google Scholar]
- [18].McCormick CD, Lea J, Stryjewska BM, et al. Trends of leprosy and multibacillary infection in the state of Georgia since the early 1900s. PLOS NEGLECT TROP D. 2019;13(e000771310):e0007713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ghavidel M, Taghanaki HRB, Samiee A, et al. Characterization of new leprosy cases in northeast of Iran within the last 15 years. Iran J Med Sci. 2018;43(4):416–420. 2018-01-01. [PMC free article] [PubMed] [Google Scholar]
- [20].Gnimavo RS, Djossou P, Sopoh GE, et al. Trends of the leprosy control indicators in Benin from 2006 to 2018. BMC PUBLIC HEALTH [Journal Article]. 2020;20(1):1254. 2020-08-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gillini L, Cooreman E, Pandey B, et al. Implementing the Global Leprosy Strategy 2016-2020 in Nepal: lessons learnt from active case detection campaigns. LEPROSY REV. 2018;89(1):77–82. [Google Scholar]
- [22].Azizi MH, Bahadori M. A history of leprosy in Iran during the 19th and 20th centuries. ARCH IRAN MED. 2011;14(6):425–430. [PubMed] [Google Scholar]
- [23].Darlong J, Govindharaj P, Darlong F, et al. A study of untreated leprosy affected children reporting with Grade 2 disability at a referral centre in West Bengal, India. LEPROSY REV. 2017;88(3):298–305. [Google Scholar]
- [24].Moschioni C, Antunes CM, Grossi MA, et al. Risk factors for physical disability at diagnosis of 19,283 new cases of leprosy. Rev Soc Bras Med Trop [Comparative Study; Journal Article]. 2010;43(1):19–22. 2010-01-01. [DOI] [PubMed] [Google Scholar]
- [25].Smith CS, Aerts A, Kita E, et al. Time to define leprosy elimination as zero leprosy transmission? LANCET INFECT DIS. 2016;16(4):398–399. [DOI] [PubMed] [Google Scholar]
- [26].Ramasamy S, Kumar A, Govindharaj P. Screening household contacts of children diagnosed with leprosy in a tertiary referral centre, Chhattisgarh State, India. LEPROSY REV. 2018;89(2):117–123. [Google Scholar]
- [27].Vinnarasan M, Vinothiney K, Gopalan B, et al. A CLINICO-EPIDEMIOLOGICAL STUDY OF PAEDIATRIC LEPROSY IN A TERTIARY CARE CENTRE. J Evol Med Dent Sci. 2018;7(21):2558–2561. [Google Scholar]
- [28].Scheelbeek PFD, Balagon MVF, Orcullo FM, et al. A Retrospective Study of the Epidemiology of Leprosy in Cebu: an Eleven-Year Profile. PLOS NEGLECT TROP D. 2013;7(9):e2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dos Santos MA S, De Aquino JL B, Pegas E, et al. Analysis of clinical aspects of leprosy patients between 2010-2017 at a reference center in Campinas. AN BRAS DERMATOL. 2020;95(2):252–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Govindharaj P, Darlong J, John AS, et al. Children and adolescents’ attitude towards having leprosy in a high endemic district of India. LEPROSY REV. 2016;87(1):42–52. [PubMed] [Google Scholar]
- [31].Sharma P, Shah A, Chauhan R. CLINICO-EPIDEMIOLOGICAL STUDY OF NEW LEPROSY CASES IN A RURAL TERTIARY CARE CENTRE IN CENTRAL INDIA. J Evol Med Dent Sci. 2017;6(14):1077–1079. [Google Scholar]
- [32].Mangeard-Lourme J, Singh A, Singh RK, et al. Enhanced active case-finding, identifying leprosy cases missed by recent detection campaigns in Munger District, Bihar, India. LEPROSY REV. 2017;88(4):452–462. [Google Scholar]
- [33].Sun P, Yu M, Yan L, et al. Epidemiological analysis on leprosy in China,2010. Acta Universitatis Medicinalis Nanjing. 2012;32(2):155–159. [Google Scholar]
- [34].Long S, Yu M, Yan L, et al. Epidemiological features of leprosy in China from 2011 to 2015. Chin J Dermatol. 2017;50(6):400–403. [Google Scholar]
- [35].Kumar GA, Rani MS, Gowthami IS, et al. EPIDEMIOLOGICAL PROFILE OF LEPROSY CASES ATTENDING TERTIARY CARE HOSPITAL IN VISAKHAPATNAM. J Evol Med Dent Sci. 2019;8(8):527–531. [Google Scholar]
- [36].Ambrosano L, Dos Santos MA S, Abrahao Machado EC F, et al. Epidemiological profile of leprosy reactions in a referral center in Campinas (SP), Brazil, 2010-2015. AN BRAS DERMATOL. 2018;93(3):460–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang Q, Li G, Li C, et al. Epidemiological situation of leprosy in a province in China: a long time to diagnosis and a high rate of deformity. BMC PUBLIC HEALTH. 2020;20(1). DOI: 10.1186/s12889-020-09933-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Suttels V, Lenaerts T. Epidemiology and spatial exploratory analysis of leprosy in the district of Toliara, Madagascar. LEPROSY REV. 2016;87(3):305–313. [Google Scholar]
- [39].Mansori K, Ayubi E, Nasehi M, et al. Epidemiology of Leprosy in Iran from 2005 to 2015. Tanaffos. 2017;16(2):144–148. [PMC free article] [PubMed] [Google Scholar]
- [40].Assiri A, Yezli S, Tayeb T, et al. Eradicating leprosy in Saudi Arabia: outcome of a ten-year surveillance (2003-2012). TRAVEL MED INFECT DI. 2014;12(6B):771–777. [DOI] [PubMed] [Google Scholar]
- [41].de Oliveira MF, Antunes DE, Dos Santos DF, et al. Evaluation of the cutaneous sensation of the face in patients with different clinical forms of leprosy. PLOS ONE. 2019;14(3):e0213842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shukla LK, Patel RN, Patel SV, et al. Evaluation of the effect of Block Level Awareness Campaign on performance indicators of National Leprosy Elimination Program in Vadodara district, Gujarat, India. INDIAN J DERMATOL VE. 2015;81(3):257–262. [DOI] [PubMed] [Google Scholar]
- [43].Rodrigues TSV, Gomes LC, Cortela DCB, et al. Factors associated with leprosy in children contacts of notified adults in an endemic region of Midwest Brazil. J PEDIAT-BRAZIL. 2020;96(5):593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kumar MS, Padmavathi S, Shivakumar M, et al. Hidden leprosy cases in tribal population groups and how to reach them through a collaborative effort. LEPROSY REV. 2015;86(4):328–334. [PubMed] [Google Scholar]
- [45].Ganesan DK, Muthunarayanan L. Is disability in leprosy still a burden? A cross-sectional study in a rural block in Tamil Nadu, India. T ROY SOC TROP MED H. 2018;112(1):31–35. [DOI] [PubMed] [Google Scholar]
- [46].John S, Tyndall JA, Okoye V, et al. Leprosy among the Fulani nomadic pastoralist population of Adamawa State, Nigeria. LEPROSY REV. 2017;88(3):364–372. [Google Scholar]
- [47].Nazario AP, Ferreira J, Schuler-Faccini L, et al. Leprosy in Southern Brazil: a twenty-year epidemiological profile. REV SOC BRAS MED TRO. 2017;50(2):251–255. [DOI] [PubMed] [Google Scholar]
- [48].Arif T, Amin SS, Adil M, et al. Leprosy in the post-elimination era: a clinico-epidemiological study from a northern Indian tertiary care hospital. Acta Dermatovenerol Alp Pannonica Adriat. 2019;28(1):7–10. [PubMed] [Google Scholar]
- [49].Mowla MR, Ara S, Tripura S. Leprosy profiles in post-elimination stage: a tertiary care hospital experience. INT J DERMATOL. 2015;54(12):1407–1413. [DOI] [PubMed] [Google Scholar]
- [50].Mowla MR, Ara S, Mizanur Rahman AFM, et al. Leprosy reactions in postelimination stage: the Bangladesh experience. J EUR ACAD DERMATOL. 2017;31(4):705–711. [DOI] [PubMed] [Google Scholar]
- [51].Bandeira SS, Pires CA, Simoes Quaresma JAS. Nerve Damage in Young Patients with Leprosy Diagnosed in an Endemic Area of the Brazilian Amazon: a Cross-Sectional Study. The Journal of Pediatrics. 2017;185:143–148. [DOI] [PubMed] [Google Scholar]
- [52].Darlong J, Govindharaj P. Parents’ attitude towards their children and adolescents affected by leprosy in an endemic district in West Bengal, India. Leprosy Review. 2020;91(3):282–290. [Google Scholar]
- [53].Martins RJ, Gomes Carloni ME O, Saliba Moimaz SA, et al. Sociodemographic and epidemiological profile of leprosy patients in an endemic region in Brazil. REV SOC BRAS MED TRO. 2016;49(6):777–780. [DOI] [PubMed] [Google Scholar]
- [54].Chukwu JN, Ekeke N, Nwafor CC, et al. Worsening of the disability grade during leprosy treatment: prevalence and its determinants in Southern Nigeria. T ROY SOC TROP MED H. 2018;112(11):492–499. [DOI] [PubMed] [Google Scholar]


