Abstract
The outer membrane of pathogenic Leptospira species grown in culture media contains lipopolysaccharide (LPS), a porin (OmpL1), and several lipoproteins, including LipL36 and LipL41. The purpose of this study was to characterize the expression and distribution of these outer membrane antigens during renal infection. Hamsters were challenged with host-derived Leptospira kirschneri to generate sera which contained antibodies to antigens expressed in vivo. Immunoblotting performed with sera from animals challenged with these host-derived organisms demonstrated reactivity with OmpL1, LipL41, and several other proteins but not with LipL36. Although LipL36 is a prominent outer membrane antigen of cultivated L. kirschneri, its expression also could not be detected in infected hamster kidney tissue by immunohistochemistry, indicating that expression of this protein is down-regulated in vivo. In contrast, LPS, OmpL1, and LipL41 were demonstrated on organisms colonizing the lumen of proximal convoluted renal tubules at both 10 and 28 days postinfection. Tubular epithelial cells around the luminal colonies had fine granular cytoplasmic LPS. When the cellular inflammatory response was present in the renal interstitium at 28 days postinfection, LPS and OmpL1 were also detectable within interstitial phagocytes. These data establish that outer membrane components expressed during infection have roles in the induction and persistence of leptospiral interstitial nephritis.
Leptospirosis is an important global human and veterinary health problem (17, 42). Humans become accidental hosts through exposure to chronically infected wild and domestic animals that serve as reservoir hosts. In the reservoir host, pathogenic Leptospira species disseminate hematogenously to the kidney, where they colonize the apical surface of the proximal renal tubule, which allows shedding in the urine and transmission to new hosts (13, 15, 28, 40, 45). The kidney is also a major target organ in the disease process, especially in accidental hosts. The host inflammatory response to renal tubular infection is interstitial nephritis characterized by a mixed cellular infiltrate consisting of lymphocytes, monocytes, plasma cells, and occasional neutrophils (4). This leptospiral interstitial nephritis results in both acute and chronic kidney damage and loss of renal function.
An important focus of current leptospiral research is identification of outer membrane proteins (OMPs) that are involved in the pathogenesis of leptospirosis. By virtue of their location on the cell surface, leptospiral OMPs are likely to be relevant to an understanding of host-pathogen interactions. In particular, outer membrane and/or surface components expressed by leptospires presumably facilitate colonization of the apical surface of proximal tubular epithelial cells in the kidney. Studies on outer membrane components are also important in vaccine development given the failure of currently available leptospiral vaccines to prevent renal disease in cattle (8–10).
The genes encoding several leptospiral OMPs have been cloned and sequenced, including the transmembrane porin OmpL1 and the lipoprotein OMPs LipL36 and LipL41 (22, 23, 37). While these three OMPs were known to be expressed, along with lipopolysaccharide (LPS), in the outer membrane of cultivated Leptospira species, their in vivo expression and potential relevance in the pathogenesis of disease in the mammalian host were unknown. In this study, we have utilized the complementary approaches of immunoblotting and immunohistochemistry to characterize the expression and distribution of outer membrane antigens in a hamster model of leptospirosis.
(Portions of this work were presented at the 96th General Meeting of the American Society for Microbiology, New Orleans, La., 19 to 23 May 1996.)
MATERIALS AND METHODS
Bacteria.
Virulent Leptospira kirschneri serovar grippotyphosa strain RM52 was originally isolated from material submitted to the Veterinary Diagnostic Laboratory at Iowa State University during an outbreak of swine abortion in 1983 (43), stored in liquid nitrogen (1), and passaged fewer than five times in Johnson-Harris bovine serum albumin–Tween 80 medium (Bovuminar PLM-5 microbiological media; Intergen) (26). Leptospires were enumerated by dark-field microscopy as described by Miller (31).
Gel electrophoresis and immunoblotting.
Leptospiral samples for sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis were solubilized in final sample buffer composed of 62.5 mM Tris hydrochloride (pH 6.8), 10% glycerol, 5% 2-mercaptoethanol, and 2% SDS. Proteins were separated on a 12% gel with a discontinuous buffer system (27) and stained with Coomassie brilliant blue or were transferred to nitrocellulose (Schleicher & Schuell) for immunoblotting. For antigenic detection on immunoblots, the nitrocellulose was blocked with 5% nonfat dry milk in PBS–0.1% Tween 20 (PBS-T) and incubated for 1 h with antiserum in PBS-T. Immunoblots probed initially with rabbit antisera specific for leptospiral outer membrane proteins (diluted 1:5,000) were subsequently probed with protein A conjugated to horseradish peroxidase (diluted 1:5,000; Amersham). Immunoblots probed initially with hamster sera (diluted 1:2,000) were then probed with mouse anti-hamster antibody (diluted 1:10,000; Sigma) and then finally probed with sheep anti-mouse antibody conjugated to horseradish peroxidase (diluted 1:5,000; Sigma). Antigen-antibody binding was detected with the Enhanced Chemiluminescence system (ECL; Amersham). Blots were incubated in ECL reagents for 1 min and then exposed to XAR-5 film (Kodak).
Antisera.
Purified murine monoclonal antibody F71C2 (22 mg/ml), specific for grippotyphosa serovars, has been described previously (25). Reactivity of monoclonal antibody F71C2 with the LPS antigen of L. kirschneri, serovar grippotyphosa strain RM52, has been demonstrated by immunoblotting (23). Antisera with immunoblot specificity for OmpL1, LipL36, and LipL41 were prepared as previously described (22, 23, 37). Briefly, the pRSET plasmid (Invitrogen) containing portions of either the ompL1, lipL36, or lipL41 gene, was transformed into Escherichia coli JM109 (Invitrogen). Expression of the His6 fusion proteins was achieved by isopropyl-β-d-thiogalactopyranoside galactoside (IPTG; Sigma) induction followed by infection with M13/T7 phage containing the T7 polymerase gene driven by the E. coli lac promoter. The His6 fusion proteins were purified by affinity chromatography using Ni2+-nitrilotriacetic acid-agarose (Qiagen). OMP-specific antisera were prepared by immunizing New Zealand White rabbits with the purified His6 fusion proteins.
Immunoprecipitation of leptospiral proteins.
Samples for immunoprecipitation containing 8 × 109 L. kirschneri organisms were resuspended in 1.25 ml of 10 mM Tris HCl (pH 8.0)–10 mM EDTA–1 mM phenylmethylsulfonyl fluoride. To this suspension was added 12.5 μl of 10% protein-grade Triton X-100 (Calbiochem), followed by gentle agitation for 30 min at 4°C. The insoluble material was removed by centrifugation at 16,000 × g for 10 min. To the supernatant was added 0.2 ml of either LipL36 or LipL41 rabbit antiserum and 0.25 ml of a slurry of staphylococcal protein A–Sepharose CL-4B (Sepharose-SpA; Sigma). The suspension was gently agitated for 1 h. The Sepharose-SpA-antibody-antigen complexes were washed twice in 0.01% Triton X-100 in 10 mM Tris HCl (pH 8.0) and resuspended in final sample buffer.
Infection with culture-derived L. kirschneri.
Unless otherwise noted, the hamsters utilized in these studies consisted of approximately equal numbers of male and female Golden Syrian hamsters (Harlan Sprague Dawley). Five-week-old hamsters, in groups of three, were inoculated intraperitoneally (i.p.) with serial 10-fold dilutions of virulent, culture-derived L. kirschneri from a liquid culture. The term “culture-derived” is used to emphasize that these organisms, though virulent, were cultivated in liquid medium and to distinguish them from host-derived organisms (see below). Moribund hamsters were euthanized; liver and kidney tissues were removed, fixed in formalin, and paraffin embedded. Hamsters surviving at 28 days after challenge were euthanized, and their sera were harvested for immunoblot studies; liver and kidney tissues were removed, fixed in formalin, and paraffin embedded. Tissue sections were stained with hematoxylin and eosin (H&E) or silver stain by the technique of Steiner and Steiner (39).
Infection with host-derived L. kirschneri.
Host-derived organisms were obtained from liver tissue from a moribund weanling hamster 10 days after i.p. challenge with culture-derived L. kirschneri. Infected liver tissue was minced and incubated for 5 min in normal rabbit serum. Uninfected adult hamsters (female) and 7-week-old hamsters were then inoculated i.p. with 0.3 ml of the serum containing host-derived L. kirschneri. Hamsters surviving at 28 days after challenge were euthanized, and their sera were harvested for immunoblot studies.
Immunohistochemistry.
Serial 5-μm sections of kidney tissue taken at 10 and 28 days after infection with culture-derived L. kirschneri were cut. Tissue sections were placed on Probond Plus slides. Paraffin was removed from sections with xylene and ethanol by standard procedures. Tissues were treated with 3% hydrogen peroxide in methyl alcohol for 20 min at room temperature to remove endogenous peroxidase activity followed by pretreatment with 0.1% trypsin in 0.1 M Tris HCl (pH 7.6) with 0.1% CaCl2 for 5 min at 37°C. Nonspecific staining of tissue sections was blocked with 10% normal goat or rat serum with incubation at room temperature for 20 min prior to incubation overnight at 4°C with primary antibody. The antibody concentrations used were 1:12,000 for F71C2, 1:6,000 for anti-OmpL1, 1:4,000 for anti-LipL41, and 1:3,000 for anti-LipL36. Controls included no primary antibody, normal rabbit or rat serum, and hyperimmune serum on kidney sections from uninfected hamsters. Unbound primary antibody was removed and tissues were incubated at room temperature for 30 min with biotinylated goat anti-rabbit immunoglobulin (Vector) or monoclonal rat anti-mouse immunoglobulin (Zymed). After the sections were washed, they were incubated for 20 min at room temperature with supersensitive streptavidin-alkaline phosphatase (Biogenex) or streptavidin-horseradish peroxidase (Zymed). Enzyme reactions were developed by using New Fuchsin (Biogenex) or 3,3-diaminobenzidine plus hydrogen peroxide (DAKO). All slides were counterstained with hematoxylin before dehydration in alcohols and Propar (xylene substitute), and coverslips were mounted. Smears of organisms from actively growing cultures were processed like the tissue sections without the removal of paraffin.
RESULTS
Challenge of hamsters with virulent L. kirschneri.
L. kirschneri RM52 produces lethal infection in a high percentage of hamsters (24), although the time to death after i.p. inoculation is typically several days longer than that observed with some other leptospiral strains (16). Four of 21 (19%) 5-week-old hamsters survived to day 28 after challenge with culture-derived L. kirschneri. The 50% lethal dose for the culture-derived organisms given by i.p. inoculation in this experiment was less than 102. However, as shown in Table 1, lethality at high challenge doses appeared to be both delayed and decreased. This finding has been confirmed in separate experiments using larger numbers of hamsters (21) and may represent an immunization effect which occurs when animals are inoculated with large doses of culture-derived organisms. Liver and kidney tissue was collected from animals that succumbed to the acute phase of infection on days 10 and 11 after challenge and during the chronic phase of infection on day 28 after challenge. Serum was collected from the four animals that survived to day 28 after i.p. challenge with culture-derived L. kirschneri.
TABLE 1.
Response of hamsters to i.p. challenge with culture-derived and host-derived L. kirschneri
| Hamsters challenged with: | Age | Challenge dose | No. of animals in group | No. of survivors at day 28 | Days to death of nonsurvivors |
|---|---|---|---|---|---|
| Culture-derived L. kirschneri | 5 wk | 106 | 6 | 3 | 11, 13, 16 |
| 5 wk | 105 | 6 | 1 | 13, 14, 14, 15, 26 | |
| 5 wk | 104 | 3 | 0 | 10, 11, 11 | |
| 5 wk | 103 | 3 | 0 | 10, 10, 10 | |
| 5 wk | 102 | 3 | 0 | 10, 10, 11 | |
| Host-derived L. kirschneria | 7 wk | 9 | 1 | 10, 10, 11, 11, 12, 12, 16, 17 | |
| 6 mo | 4 | 3 | 10 |
Obtained from the liver of a moribund hamster 10 days after i.p. challenge with 103 culture-derived L. kirschneri organisms.
The concentration of host-derived L. kirschneri organisms used to inoculate the second group of hamsters was estimated by dark-field microscopy to be less than 105/ml. One of nine (11%) 7-week-old hamsters and three of four (75%) adult hamsters survived to day 28 after i.p. challenge with host-derived L. kirschneri (Table 1). Serum was collected from the four animals that survived to day 28 after i.p. challenge with the host-derived microorganisms.
Humoral immune response to leptospiral proteins during infection with virulent L. kirschneri.
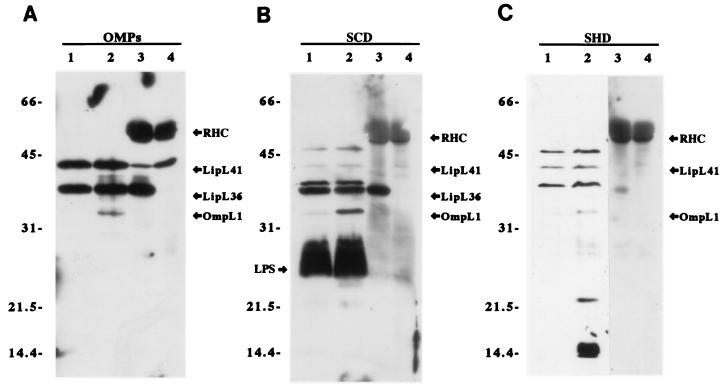
Hamsters were challenged with host-derived L. kirschneri to generate sera which would contain antibodies directed exclusively towards antigens expressed in vivo. Serum from animals surviving infection with host-derived organisms was designated SHD (for serum from animals infected with host-derived L. kirschneri). As a control for immunogenicity of leptospiral proteins, serum was also collected from animals surviving infection with culture-derived organisms and designated SCD (for serum from animals infected with culture-derived L. kirschneri). Immunoblot analysis of leptospiral proteins was performed with sera from all animals that survived to 28 days postinfection (four SCD and four SHD samples). As shown in Fig. 1, both SCD and SHD samples detected a heat-modifiable protein with a molecular mass of 33 kDa, which is consistent with the properties of the porin OmpL1 (36). SHD samples had stronger reactivity with several smaller heat-modifiable antigens with molecular masses of 14, 15, and 22 kDa. Non-heat-modifiable antigens with molecular masses of 37, 41, and 46 kDa were detected by both kinds of sera. However, only SCD samples reacted with the 36-kDa antigen and LPS, present as a diffuse band between apparent molecular masses of 24 and 29 kDa. Reactivity with the lipoproteins LipL36 and LipL41 was confirmed by probing immunoprecipitated native proteins.
FIG. 1.
Immunoblots of leptospiral proteins using antisera from hamsters challenged with culture-derived and host-derived L. kirschneri. Each panel is an immunoblot of leptospiral proteins that were unheated (lanes 1), boiled (lanes 2), and immunoprecipitated with antisera specific for LipL36 (lanes 3) and LipL41 (lanes 4). Panels were probed with a mixture of OmpL1, LipL36, and LipL41 antisera (A), an SCD sample (B), and an SHD sample (C). The SCD and SHD immunoblots shown are the results for serum from one animal from each group but are representative of immunoblot results obtained with sera from the other animals in the SCD and SHD groups. Hamster serum from uninfected littermates was nonreactive (data not shown). Rabbit heavy-chain (RHC) and light-chain immunoglobulin bands are visible in lanes 3 and 4 because these samples are immunoprecipitates.
Histological features of kidney tissue infected with culture-derived L. kirschneri.
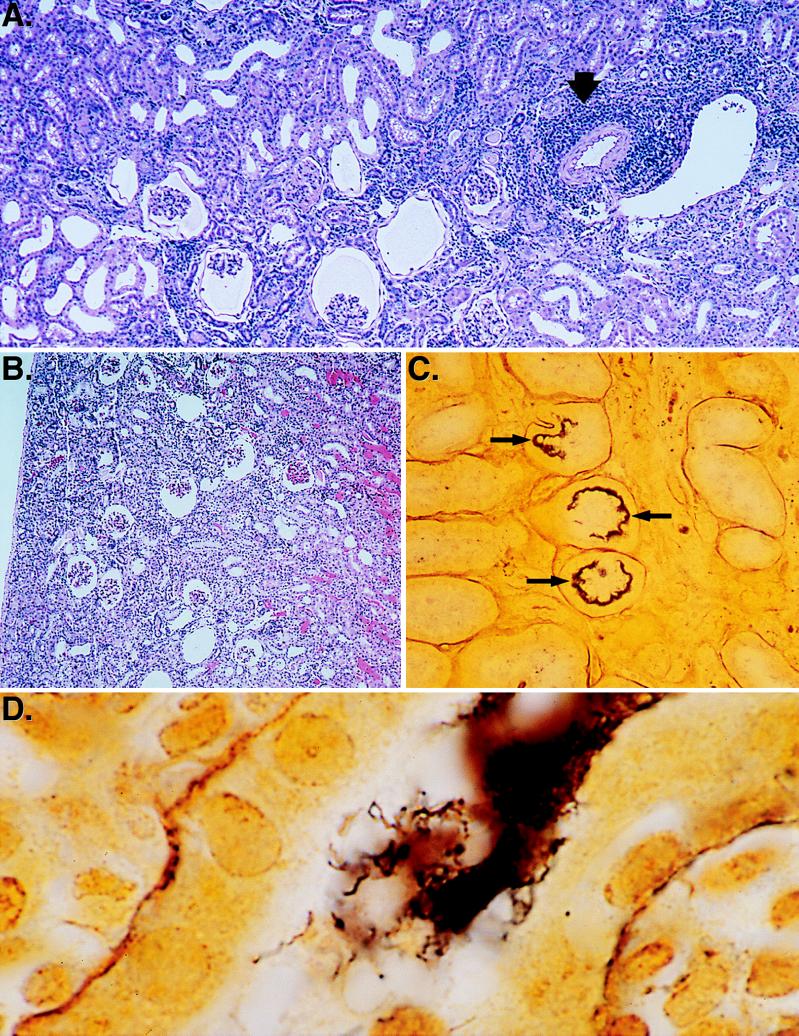
Kidney tissue obtained 10 days after infection revealed that although the cortical and medullary architecture was intact, nearly all the glomeruli were shrunken or contracted (Fig. 2B). The glomerular spaces were enlarged and occasionally showed proteinaceous material without inflammatory cells. Vessels were congested, and tubules contained proteinaceous material mixed with erythrocytes. An early mixed lymphocyte-plasma cell infiltrate was occasionally noted near the larger arteries at the cortical-medullary interface, but no infiltrate was evident in the tissues surrounding the smaller arteries.
FIG. 2.
Histopathology of hamster kidney after infection with L. kirschneri. (A) H&E stain of hamster kidney tissue 28 days after infection showing contracted glomeruli and an interstitial inflammatory infiltrate (arrow). Film magnification, ×25. Final magnification, ×150. (B) H&E stain of hamster kidney tissue 10 days after infection showing contracted glomeruli, vascular congestion, and proteinaceous debris in the tubules. Film magnification, ×25. Final magnification, ×100. (C) Silver stain of hamster kidney tissue 28 days after infection showing a low-power view of renal tubules with (arrows) and without spirochetal involvement. Film magnification, ×100. Final magnification, ×600. (D) Silver stain of hamster kidney tissue 28 days after infection showing a high-power view of the dense accumulation of spirochetes in a renal tubule. Film magnification, ×1,000. Final magnification, ×6,000.
At 28 days after infection, a mixed inflammatory infiltrate, consisting of monocytes, lymphocytes, and plasma cells, was evident (Fig. 2A). The inflammatory infiltrate was prominent in the cortex and surrounding small to medium-sized vessels and was particularly notable adjacent to small arteries and arterioles. Many glomeruli were contracted, and the glomerular space was filled with proteinaceous material. Silver staining of kidney sections obtained on day 28 after infection revealed that occasional tubules in the cortex contained a dense accumulation of spirochetes lining the tubular wall (Fig. 2C). In some fields, these consisted of individual, positively stained spirochetes, as they extended into the luminal space (Fig. 2D).
Immunohistochemistry with immunological reagents specific for leptospiral outer membrane antigens.
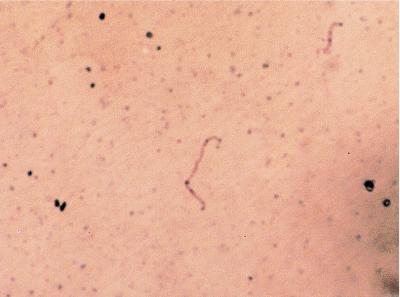
Smears of culture-derived L. kirschneri were positive with antibody F71C2 (data not shown), LipL36 (Fig. 3), and LipL41 (data not shown) antisera, with individual spirochetes discernible. OmpL1 antiserum did not stain organisms prepared in this manner. This was surprising, considering that this same antiserum reacts specifically with OmpL1 upon immunoblotting (22, 36), immunoelectron microscopy (22), and surface immunoprecipitation (21) and probably reflects the reduced sensitivity of immunohistochemistry for smeared organisms and the low number of OmpL1 molecules in the outer membrane (24).
FIG. 3.
Immunohistochemistry of cultivated L. kirschneri. A representative positive smear of cultivated L. kirschneri stained with rabbit polyclonal antiserum specific for LipL36 is shown. Film magnification, ×1,250. Final magnification, ×5,750.
The immunohistochemistry techniques employed in this study were found to increase the sensitivity of antigen detection while preserving tissue integrity. Formalin-fixed paraffin-embedded tissues provided excellent preservation of tissue architecture. The use of charged slides for immunostaining improved tissue integrity by minimizing the loss of tissue and retaining tissue architecture. Permanent indicators such as New Fuchsin permitted dehydration of tissue sections prior to mounting. The cellular definition of dehydrated sections was superior to that of aqueous mounts. Because formalin fixation may result in loss of antigenic sites, trypsin treatment was used as an antigen retrieval technique. Antigen detection was also improved by reagents utilizing the high affinity of avidin-biotin interactions and increased sensitivity of the enzymatic indicators. We found that these approaches resulted in improved localization and detection of antigen.
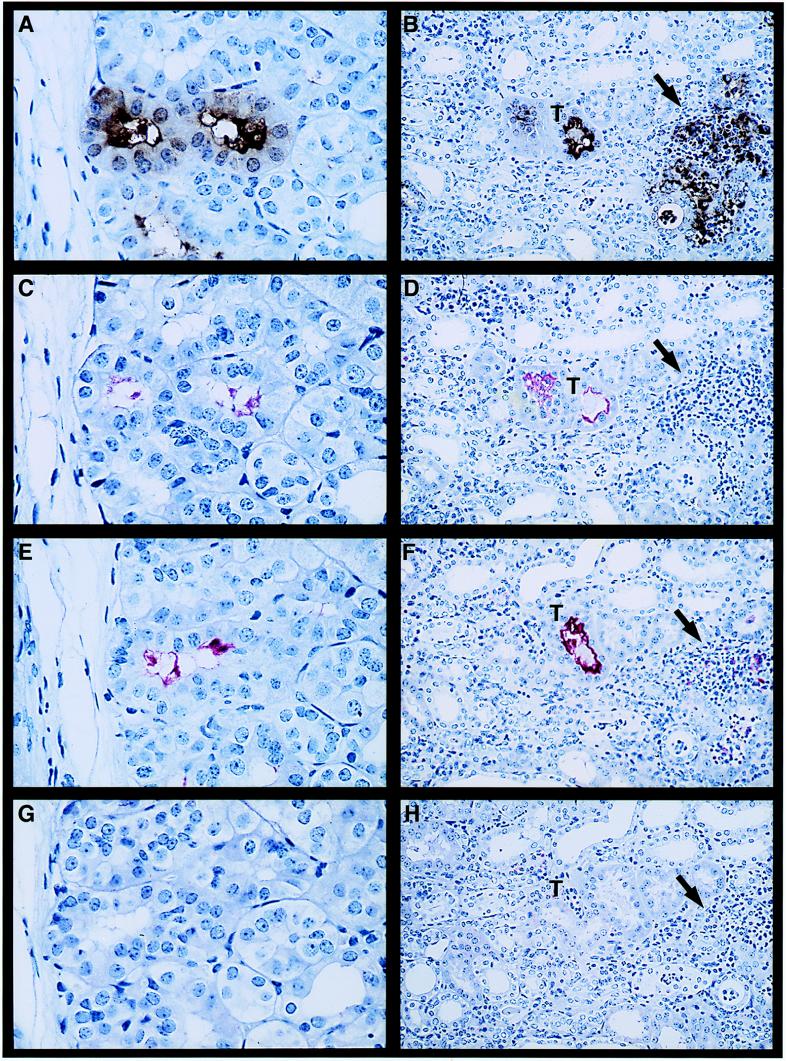
Leptospires within the proximal tubules of kidney sections obtained at 10 days after infection with culture-derived L. kirschneri stained positively for antibody F71C2 (specific for LPS) and for antisera to OmpL1 and LipL41. There was discrete staining of intraluminal colonies distributed throughout the cortex. Demonstration that the same colonies stained positively for all three antisera was achieved by examination of serial sections (Fig. 4A, C, and E). LipL36 antisera did not stain the same sites at concentrations that were positive for staining smears of culture-derived L. kirschneri. Prominent fine granular staining occurred within the cytoplasm of the proximal convoluted tubular epithelial cells around the luminal colonies when sections were stained with antibody F71C2 (Fig. 4A). There was little or no antigen detection in the interstitium in kidney sections obtained 10 days after infection (data not shown).
FIG. 4.
Immunohistochemistry of kidney tissue obtained at 10 days (A, C, E, and G) (film magnification, ×400; final magnification, ×940) and 28 days (B, D, F, and H) (film magnification, ×160; final magnification, ×375) postinfection with virulent L. kirschneri by using the LPS-specific monoclonal antibody F71C2 (A and B) and rabbit polyclonal antisera specific for LipL41 (C and D), OmpL1 (E and F), and LipL36 (G and H). Higher magnification in the day 10 panels was needed to show the details of antigen expression in the renal tubular lumen and the presence of LPS in the cytoplasm of the tubular epithelial cells (A). No antigen was detected in the renal interstitium on day 10. A lower magnification was needed to show the wider distribution of antigen at the day 28 time point. LPS and OmpL1 were detected both in tubules (T) and in the renal interstitum (arrow) at the sites of inflammatory infiltrate (B and F, respectively).
Kidney sections obtained at 28 days after infection were positive for the presence of leptospiral antigen within tubules, and in certain areas, in the interstitium and at sites of interstitial inflammatory cellular infiltrates. Antibodies to LPS, LipL41, and OmpL1 all stained leptospiral colonies within tubules in the renal cortex (Fig. 4B, D, and F), and the colonized tubules were distributed throughout the cortex. LPS reactivity was seen in the interstitium and in areas of interstitial cellular inflammation as coarse or fine granular staining. In some instances, the LPS and OmpL1 antigens were detected apparently within phagocytic cells (Fig. 4B and F). Interstitial OmpL1 reactivity was less prominent than that observed with LPS. LipL41 reactivity was found only within the renal tubules (Fig. 4D). Similar to the results for the day 10 specimens, no reactivity with the LipL36 antiserum was evident. These results suggest that LipL36 is not expressed during leptospiral infection of the kidney.
DISCUSSION
In this study, we have characterized the expression and distribution of selected leptospiral outer membrane components during infection by using the complementary approaches of immunoblotting and immunohistochemistry. Expression of specific leptospiral outer membrane components in vivo has not been previously documented. Cultivated Leptospira species have been known to express the outer membrane components LPS, OmpL1, LipL36, and LipL41 (22–24, 37). However, it has not been known to what extent leptospiral culture conditions recapitulate the in vivo environment or whether cultivated organisms resemble those found in the mammalian host. Most pathogenic bacteria, including Leptospira species, are capable of adapting to disparate environmental conditions. Pathogenic leptospires must successfully negotiate the bloodstream, renal tubular lumen, and in many cases the inanimate environment outside the host to complete their life cycle. Environmental adaptation by pathogenic bacteria involves differential expression of outer membrane components, including proteins and LPS (18, 20).
Our immunoblot studies were designed to evaluate whether leptospiral antigens were expressed in vivo. Serum was generated by challenging hamsters with either host-derived or culture-derived L. kirschneri. Sera from animals challenged with L. kirschneri obtained directly from infected hamster tissue should only contain antibodies to antigens expressed in vivo. These SHD samples reacted with OmpL1, LipL41, and several other less well characterized antigens (Fig. 1C). We were able to classify these new antigens as either heat modifiable (14-, 15-, and 22-kDa) or non-heat modifiable (37- and 46-kDa) antigens. The electrophoretic mobility of most proteins, including LipL36 and LipL41, is non-heat modifiable, indicating that heat is not required for denaturation to occur in sample buffer containing SDS and mercaptoethanol. In contrast, when heat-modifiable electrophoretic mobility is observed, this suggests that a protein has structural characteristics, such as transmembrane beta-sheets or buried disulfide bonds, which are resistant to complete denaturation until heated. Transmembrane OMPs, such as the leptospiral porin OmpL1, constitute a class of proteins whose electrophoretic mobility is frequently heat modifiable (7, 14, 36).
Our immunoblot studies did not detect an antibody response to LipL36 or LPS in hamsters infected with host-derived organisms (Fig. 1C). Immunoblot control studies were conducted with SCD samples (Fig. 1B), showing that these antigens are immunogenic. A lower antibody response to LipL36 in hamsters challenged with host-derived L. kirschneri than in those challenged with culture-derived L. kirschneri has been confirmed by a LipL36 enzyme-linked immunosorbent assay (23). One explanation of the lack of immunoblot reactivity to LipL36 and LPS is a lack of in vivo expression of these antigens. Our immunohistochemistry results indicate that this is the case for LipL36 but not for LPS. The poor antibody response to LPS in hamsters challenged with host-derived organisms may be due to the fact that our immunoblotting strategy detected immunoglobulin G (IgG) antibodies with greater sensitivity than it detected IgM antibodies. This latter explanation is consistent with the findings of previous immunoblot studies using human clinical leptospirosis sera which found that the early humoral immune response to LPS primarily involved IgM antibodies (11, 12).
The differential antibody response in sera from hamsters challenged with culture-derived and host-derived L. kirschneri may also involved effects of the size of the challenge inoculum, antigen dose, hamster age, or the carbohydrate nature of the LPS antigen. The immunoblot studies we report involve sera from four animals surviving challenge with culture-derived L. kirschneri and four animals surviving challenge with host-derived L. kirschneri. The animals surviving challenge with culture-derived organisms were inoculated with 105 to 106 organisms, which is probably greater than the number of host-derived organisms inoculated per hamster. The data presented in Table 1 suggest that there was lower mortality in hamsters challenged with ≥105 culture-derived organisms than in hamsters challenged with <105 culture-derived organisms. We have also observed lower mortality at doses of ≥105 culture-derived organisms in a separate, larger study of 9-week-old hamsters (21). Since this phenomenon does not occur in immunologically immature 3-week-old hamsters (21), we believe that it has an immunological basis, such as a T-cell-independent response occurring only at high challenge doses.
The primary focus of this study was to examine the expression and distribution of leptospiral outer membrane components during infection. Immunohistochemistry has proven to be an extremely useful tool for assessment of in vivo expression and distribution of specific bacterial molecules (6, 33). Immunohistochemistry experiments showed strong reactivity with OmpL1 on in vivo L. kirschneri. Reactivity with LipL41 was less prominent but clearly positive. These data, combined with the evidence that the proteins OmpL1 and LipL41 are exposed on the leptospiral surface (22, 37), indicate that both proteins are potential immunoprotective molecules. Both the immunoblot and immunohistochemical data indicate lack of LipL36 expression in vivo, even though this protein is expressed in relatively abundant amounts by cultivated L. kirschneri (23). As a positive control for the immunohistochemistry procedure, LipL36 was detectable on culture-derived L. kirschneri. However, it is possible that LipL36 is lost in the fixation, embedding, and staining process. Formalin fixation can result in loss of antigenic epitopes due to protein cross-linking. On the other hand, the LPS, OmpL1, and LipL41 antigens serve as positive controls for antigen preservation in the immunohistochemistry techniques used in our study. The environmental signals which regulate leptospiral OMP expression are not understood. The finding of differential LipL36 expression in vivo and in vitro may be a reflection of these regulatory signals.
An alternative explanation for differential expression of LipL36 is the fact that the L. kirschneri RM52 strain is not a clonal population of organisms. However, we do not feel that this explanation is likely. Immunohistochemistry of cultivated L. kirschneri RM52 revealed a relatively homogeneous population with respect to LipL36 expression (Fig. 3). Within each group of hamster sera, there was relatively uniform immunoblot and enzyme-linked immunosorbent assay reactivities to LipL36 and other antigens. It is also worth noting that a low-passage culture was used (passages, <5), which limits the likelihood that subpopulations could develop during cultivation; this is consistent with the fact that a significant number of organisms were virulent and capable of producing a lethal infection. In either case, our results indicate that culture-derived Leptospira species differ from in vivo organisms.
The characterization of specific antigens in our studies is an advance over previous immunohistochemistry studies. Previous studies used antisera that were generated by immunizing rabbits with whole or crude leptospiral preparations, making it impossible to discern specific leptospiral antigens (2, 3, 32, 35, 38, 41). Recently, renal infection in the hamster model of leptospirosis has been characterized by immunohistochemistry using a monoclonal antibody to a 24-kDa component of leptospiral glycolipoprotein; however, the precise nature of this antigen has not been defined (34). The high degree of tissue integrity achieved by the immunohistochemical methods in our study also provides important insights into the distribution of leptospiral antigens during renal infection. Leptospiral antigen had previously been demonstrated by immunohistochemistry both in renal tubules and in interstitial macrophages (2, 3, 32, 34, 35, 38, 41). Three of these studies used fluorescein isothiocyanate-conjugated antisera, which did not allow presentation of both the histopathology and the antigen location in the same image (32, 38, 41). Two other studies used an immunoperoxidase staining procedure (2, 3) that resulted in significant losses in tissue integrity. The finding of leptospiral antigen within macrophages in both our present study and earlier studies raises the question of its origin. The study by Morrison and Wright also demonstrated IgG within the macrophages (32), suggesting that opsonization of leptospiral antigen was involved in the phagocytic process. The explanations offered in these previous studies for how the antigen appeared in the interstitium was that the antigen was either left behind by migrating organisms or represented antigenic debris from killed organisms. However, these explanations are not consistent with the fact that in neither our present study nor the earlier immunohistochemistry studies were discrete organisms visualized within the macrophages.
An important difference between our study and the earlier reports is that we examined the distribution of antigens at different time points. At 10 days after infection, the leptospires had already localized to the tubular lumen, as demonstrated by the finding of intraluminal LPS, OmpL1, and LipL41. At this early time point, LPS was also found throughout the cytoplasm of proximal tubular epithelial cells whose luminal surface was colonized by leptospires, an observation also noted by Scanziani et al. (35). However, cellular infiltrates had not yet appeared in the renal interstitium and little or no interstitial antigen was detectable. LPS was found in the interstitium and within phagocytes at 28 days after infection. OmpL1 was also detected in the interstitium, primarily within phagocytes, at the later time point. However, LipL41 was found exclusively within the tubular lumen. The finding of LPS and OmpL1, but not LipL41, in the interstitium suggests that migration of outer membrane antigens is selective. Another explanation of these data is that LipL41 may be more readily degraded by proteolytic enzymes found in the epithelial cell cytoplasm or in the interstitium of the kidney.
These findings suggest that interstitial antigen may derive in part from leptospires within the tubular lumen. There are a number of potential explanations for how leptospiral outer membrane antigens could cross the tubular epithelial barrier. One explanation is that epithelial cell damage could simply result in increased permeability to leptospiral antigens. Another possibility is intracellular invasion by motile leptospires, a process which appears to occur via endocytic vesicles (13, 30, 44, 46). A third potential mechanism is active transport of leptospiral antigen from the tubular lumen into the interstitium. The proximal tubular location of leptospiral colonization has been confirmed by a number of studies (13, 29, 32, 34, 40, 45). The primary function of the proximal tubular epithelium is to reabsorb luminal contents. The spirochetal outer membrane is labile and can be released as extracellular membrane-bound vesicles, or blebs. Outer membrane blebs have been demonstrated in Borrelia burgdorferi (19), and disassociation of the leptospiral outer membrane from the protoplasmic cylinder has been observed in the formation of leptospiral salt-altered cells (5). By whatever mechanism(s) translocation occurs, leptospiral outer membrane antigens are taken up by phagocytes associated with the interstitial cellular infiltrate that also includes lymphocytes and plasma cells. These data raise the intriguing possibility that translocation of outer membrane components from the renal tubule into the interstitium contributes to the host inflammatory response and the renal damage which is the hallmark of leptospiral interstitial nephritis.
ACKNOWLEDGMENTS
This work was supported by funding from VA Medical Research Funds (D.A.H.), a UCLA School of Medicine Frontiers of Science Award (D.A.H.), and a Public Health Service Grant AI-34431 (to D.A.H.).
We thank S. Haake for helpful suggestions and critical review of the manuscript.
REFERENCES
- 1.Alexander A D, Lessel E F, Evans L B, Franck E, Green S S. Preservation of leptospiras by liquid-nitrogen refrigeration. Int J Syst Bacteriol. 1972;22:165–169. [Google Scholar]
- 2.Alves V A, Gayotto L C, Yasuda P H, Wakamatsu A, Kanamura C T, De Brito T. Leptospiral antigens (L. interrogans serogroup icterohaemorrhagiae) in the kidney of experimentally infected guinea pigs and their relation to the pathogenesis of the renal injury. Exp Pathol. 1991;42:81–93. doi: 10.1016/s0232-1513(11)80051-4. [DOI] [PubMed] [Google Scholar]
- 3.Alves V A F, Vianna M R, Yasuda P H, DeBrito T. Detection of leptospiral antigen in the human liver and kidney using an immunoperoxidase staining procedure. J Pathol. 1987;151:125–131. doi: 10.1002/path.1711510205. [DOI] [PubMed] [Google Scholar]
- 4.Arean V M. The pathologic anatomy and pathogenesis of fatal human leptospirosis (Weil’s disease) Am J Pathol. 1962;40:393–423. [PMC free article] [PubMed] [Google Scholar]
- 5.Auran N E, Johnson R C, Ritzi D M. Isolation of the outer sheath of Leptospira and its immunogenic properties in hamsters. Infect Immun. 1972;5:968–975. doi: 10.1128/iai.5.6.968-975.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barthold S W, Fikrig E, Bockenstedt L K, Persing D H. Circumvention of outer surface protein A immunity by host-adapted Borrelia burgdorferi. Infect Immun. 1995;63:2255–2261. doi: 10.1128/iai.63.6.2255-2261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beher M, Schnaitman C, Pugsley A. Major heat-modifiable outer membrane protein in gram-negative bacteria: comparison with the OmpA protein of Escherichia coli. J Bacteriol. 1980;143:906–913. doi: 10.1128/jb.143.2.906-913.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolin C A, Cassells J A, Zuerner R L, Trueba G. Effect of vaccination with a monovalent Leptospira interrogans serovar hardjo type hardjo-bovis vaccine on type hardjo-bovis infection of cattle. Am J Vet Res. 1991;52:1639–1643. [PubMed] [Google Scholar]
- 9.Bolin C A, Thiermann A B, Handsaker A L, Foley J W. Effect of vaccination with a pentavalent leptospiral vaccine on Leptospira interrogans serovar hardjo type hardjo-bovis infection of pregnant cattle. Am J Vet Res. 1989;50:161–165. [PubMed] [Google Scholar]
- 10.Bolin C A, Zuerner R L, Trueba G. Effect of vaccination with a pentavalent leptospiral vaccine containing Leptospira interrogans serovar hardjo type hardjo-bovis on type hardjo-bovis infection of cattle. Am J Vet Res. 1989;50:2004–2008. [PubMed] [Google Scholar]
- 11.Chapman A J, Adler B, Faine S. Antigens recognised by the human immune response to infection with Leptospira interrogans serovar hardjo. J Med Microbiol. 1988;25:269–278. doi: 10.1099/00222615-25-4-269. [DOI] [PubMed] [Google Scholar]
- 12.Chapman A J, Everard C O, Faine S, Adler B. Antigens recognized by the human immune response to severe leptospirosis in Barbados. Epidemiol Infect. 1991;107:143–155. doi: 10.1017/s0950268800048779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheville N F, Huhn R, Cutlip R C. Ultrastructure of renal lesions in pigs with acute leptospirosis caused by Leptospira pomona. Vet Pathol. 1980;17:338–351. doi: 10.1177/030098588001700308. [DOI] [PubMed] [Google Scholar]
- 14.Exner M M, Doig P, Trust T J, Hancock R E W. Isolation and characterization of a family of porin proteins from Helicobacter pylori. Infect Immun. 1995;63:1567–1572. doi: 10.1128/iai.63.4.1567-1572.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faine S. Leptospira and leptospirosis. Boca Raton, Fla: CRC Press, Inc.; 1994. [Google Scholar]
- 16.Faine S. Virulence in Leptospira. II. The growth in vivo of virulent Leptospira icterohaemorrhagiae. Br J Exp Pathol. 1957;38:8–14. [PMC free article] [PubMed] [Google Scholar]
- 17.Farr R W. Leptospirosis. Clin Infect Dis. 1995;21:1–8. doi: 10.1093/clinids/21.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garon C F, Dorward D, Corwin M D. Structural features of Borrelia burgdorferi—the Lyme disease spirochete: silver staining for nucleic acids. Scanning Electron Microsc. 1989;3:109–155. [PubMed] [Google Scholar]
- 20.Guo L, Lim K B, Gunn J S, Bainbridge B, Darveau R P, Hackett M, Miller S I. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science. 1997;276:250–253. doi: 10.1126/science.276.5310.250. [DOI] [PubMed] [Google Scholar]
- 21.Haake, D. A. Unpublished data.
- 22.Haake D A, Champion C I, Martinich C, Shang E S, Blanco D R, Miller J N, Lovett M A. Molecular cloning and sequence analysis of the gene encoding OmpL1, a transmembrane outer membrane protein of pathogenic Leptospira spp. J Bacteriol. 1993;175:4225–4234. doi: 10.1128/jb.175.13.4225-4234.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haake D A, Martinich C, Summers T A, Shang E S, Pruetz J D, McCoy A M, Mazel M K, Bolin C A. Characterization of leptospiral outer membrane lipoprotein LipL36: downregulation associated with late log-phase growth and mammalian infection. Infect Immun. 1998;66:1579–1587. doi: 10.1128/iai.66.4.1579-1587.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haake D A, Walker E M, Blanco D R, Bolin C A, Miller M N, Lovett M A. Changes in the surface of Leptospira interrogans serovar grippotyphosa during in vitro cultivation. Infect Immun. 1991;59:1131–1140. doi: 10.1128/iai.59.3.1131-1140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrmann J L, Bakoss P, Korver H, Bulu A A, Bellenger E, Terpstra W J, Saint Girons I, Baranton G. A new serovar in the Grippotyphosa serogroup comprising leptospiral isolates from different regions. Int J Syst Bacteriol. 1994;44:362–364. doi: 10.1099/00207713-44-2-362. . (Erratum, 44:597, 1994.) [DOI] [PubMed] [Google Scholar]
- 26.Johnson R C, Harris V G. Differentiation of pathogenic and saprophytic leptospires. I. Growth at low temperatures. J Bacteriol. 1967;94:27–31. doi: 10.1128/jb.94.1.27-31.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Mailloux M, Barnier A. Antileptospiral vaccination. Its interest regarding certain social-professional categories. Méd Maladies Infect. 1976;6:142–146. . (In French.) [Google Scholar]
- 29.Marshall R B. The route of entry of leptospires into the kidney tubule. J Med Microbiol. 1976;9:149–152. doi: 10.1099/00222615-9-2-149. [DOI] [PubMed] [Google Scholar]
- 30.Merien F, Baranton G, Perolat P. Invasion of Vero cells and induction of apoptosis in macrophages by pathogenic Leptospira interrogans are correlated with virulence. Infect Immun. 1997;65:729–738. doi: 10.1128/iai.65.2.729-738.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller J N. Spirochetes in body fluids and tissues: manual of investigative methods. Springfield, Ill: Charles C. Thomas, Publisher; 1971. [Google Scholar]
- 32.Morrison W I, Wright N G. Canine leptospirosis: an immunopathological study of interstitial nephritis due to Leptospira canicola. J Pathol. 1976;120:83–89. doi: 10.1002/path.1711200204. [DOI] [PubMed] [Google Scholar]
- 33.Pepe J C, Wachtel M R, Wagar E, Miller V L. Pathogenesis of defined invasion mutants of Yersinia enterocolitica in a BALB/c mouse model of infection. Infect Immun. 1995;63:4837–4848. doi: 10.1128/iai.63.12.4837-4848.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira M M, Andrade J, Lacerda M D, Batoreu N M, Marchevsky R S, Ribeiro dos Santos R. Demonstration of leptospiral antigens on tissues using monoclonal antibodies and avidin-biotin peroxidase staining. Exp Toxic Pathol. 1997;49:505–511. doi: 10.1016/s0940-2993(97)80155-8. [DOI] [PubMed] [Google Scholar]
- 35.Scanziani E, Luini M, Fabbi M, Pizzocaro P. Comparison between specific immunoperoxidase staining and bacteriological culture in the diagnosis of renal leptospirosis in pigs. Res Vet Sci. 1991;50:229–232. doi: 10.1016/0034-5288(91)90112-2. [DOI] [PubMed] [Google Scholar]
- 36.Shang E S, Exner M M, Summers T A, Martinich C, Champion C I, Hancock R E, Haake D A. The rare outer membrane protein, OmpL1, of pathogenic Leptospira species is a heat-modifiable porin. Infect Immun. 1995;63:3174–3181. doi: 10.1128/iai.63.8.3174-3181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shang E S, Summers T A, Haake D A. Molecular cloning and sequence analysis of the gene encoding LipL41, a surface-exposed lipoprotein of pathogenic Leptospira species. Infect Immun. 1996;64:2322–2330. doi: 10.1128/iai.64.6.2322-2330.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sitprija V, Pipatanagul V, Mertowidjojo K, Boonpucknavig V, Boonpucknavig S. Pathogenesis of renal disease in leptospirosis: clinical and experimental studies. Kidney Int. 1980;17:827–836. doi: 10.1038/ki.1980.95. [DOI] [PubMed] [Google Scholar]
- 39.Steiner G, Steiner G. New simple silver stain for demonstration of bacteria, spirochetes, and fungi in sections of paraffin embedded tissue blocks. J Lab Clin Med. 1944;29:868–871. [Google Scholar]
- 40.Sterling C R, Thiermann A B. Urban rats as chronic carriers of leptospirosis: an ultrastructural investigation. Vet Pathol. 1981;18:628–637. doi: 10.1177/030098588101800508. [DOI] [PubMed] [Google Scholar]
- 41.Taylor P L, Hanson L E, Simon J. Serologic, pathologic, and immunologic features of experimentally induced leptospiral nephritis in dogs. Am J Vet Res. 1970;31:1033–1049. [PubMed] [Google Scholar]
- 42.Thiermann A B. Leptospirosis: current developments and trends. J Am Vet Med Assoc. 1984;184:722–725. [PubMed] [Google Scholar]
- 43.Thiermann A B, McClellan R D, Hill H T. Improved techniques for the isolation of leptospires from swine abortion cases. Ann Proc Am Assoc Vet Lab Diagn. 1984;27:233–244. [Google Scholar]
- 44.Thomas D D, Higbie L M. In vitro association of leptospires with host cells. Infect Immun. 1990;58:581–585. doi: 10.1128/iai.58.3.581-585.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson J C, Manktelow B W. Pathogenesis of renal lesions in haemoglobinaemic and non-haemoglobinaemic leptospirosis. J Comp Pathol. 1989;101:201–214. doi: 10.1016/0021-9975(89)90066-2. [DOI] [PubMed] [Google Scholar]
- 46.Vinh T, Faine S, Adler B. Adhesion of leptospires to mouse fibroblasts (L929) and its enhancement by specific antibody. J Med Microbiol. 1984;18:73–85. doi: 10.1099/00222615-18-1-73. [DOI] [PubMed] [Google Scholar]