Introduction
Bipolar disorder (BD) is often characterized by cognitive deficits that persist during remission of acute symptoms1. There can be substantial individual variability in cognitive abilities, but up to 60% of remitted patients demonstrate reduced performance in at least one cognitive domain relative to healthy comparisons2,3. One of the most pronounced difficulties in BD is inhibitory control4,5, the ability to suppress contextually inappropriate responses and behaviors6. Inhibitory control difficulties are associated with functional problems, such as greater likelihood of unemployment7, and even suicidality8. The most effective treatments for mood symptoms are not adequate for ameliorating inhibitory deficits, and hence, it is essential to develop a better understanding of underlying biological mechanisms that might offer further targets for treatments.
Inhibitory control is most often studied in the laboratory using “cold cognition” paradigms, which refers to the exertion of cognitive control during affectively neutral tasks9. However, in the real world, inhibitory control is also critical for implementation of emotion regulation strategies, the inhibition of unhelpful forms of thought and cognitive biases, and refraining from impulsive or risky behaviors such as substance use or self-injury10–15. These different aspects and applications of inhibition may be correlated at a high level in contributing a common “executive functioning” factor, but may also have dissociable components and underlying mechanisms16,17. This presents multiple pathways via which failure to exert inhibitory control and integrate inhibition with the detection, evaluation, and filtering of emotional or affectively laden information, may confer vulnerability to mood episode recurrence. Indeed, self-reported negative cognitive style has been linked to recurrence of major depressive episodes18. It is therefore plausible that failure to inhibit negative processing biases may also increase risk for recurrent mood episode, particularly depression, which is among the most chronic and unresolved clinical challenges in bipolar disorder19. Therefore, from a treatment target perspective, it is of value to know what biomarkers are uniquely or specifically related to “cold cognition” versus those that may have implications for dual cognitive-emotional processes. This can be accomplished by relating biomarkers to emotionally-salient versions of traditionally cold inhibitory control tasks, such as the Affective Go/No-Go, which uses negative, positive, and neutral words as different target response conditions20 in place of the traditional Go/No-Go inhibitory control task which lacks affective overlay. Additionally, given that most existing work assessing the relevance of inflammation to affective processing in mood disorders has focused on reward processing and motivation21, affective inhibition represents an untapped and novel domain to test for the specificity (or the range) of its effects.
A leading potential biological mechanism of affective inhibition dysfunction in BD involves immune dysregulation22. Broadly, the hypothesis is that peripheral inflammatory markers that are triggered in response to stress enter the brain through active transport, interaction with circumventricular organs, or via afferent pathways23. In turn, this may activate central nervous system inflammatory processes, including brain microglia and neurotransmitter expression, as well as oxidative stress and decreased neuroplasticity, that are thought to contribute to structural and functional brain changes associated with cognitive and behavioral phenotypes23. In particular, frontrostriatal inhibitory neurocircuitry, which includes dorsal components implicated in inhibitory control and ventral components implicated in motivational salience, as well as frontolimbic threat processing circuitry, may be particularly vulnerable to the effects of peripheral inflammation21. This is possibly because of a high density of glial receptors in these regions and proximity to circumventricular organs as mechanisms of crossover from peripheral to central inflammatory activation24. It was previously reported in the primary study of the present cohort that peripheral inflammation is associated with broad cognitive dysfunction, including several aspects of executive functioning that are thought to reflect frontal-subcortical systems function25. However, there are no prior studies to our knowledge that address whether peripheral inflammation is also implicated in cognitive-emotional processing, involving both cognitive and affective processing demands, in BD.
To address this gap in the literature, we evaluated the association between C-reactive protein (CRP) levels and affective inhibition accuracy and response times for negative, positive, and neutral stimuli on an affective go/no-go paradigm. We hypothesized that increased CRP would be associated with reduced negative target detection and a response bias (faster response times) during the negative target condition. CRP is an acute phase reactant that is upregulated by proinflammatory cytokines within the innate immune system26. We selected CRP as the focal measure of inflammation because it is one of the most studied markers of immune health across many fields of medicine, it is elevated in BD independent of mood state27, and, due to its relatively long half-life, it is more robust than other commonly measured cytokines to acute changes or measurement confounds affecting sample concentration than are other commonly measured cytokines, including diurnal variation, and dietary, exercise, and sleep patterns28. We also acknowledge that CRP is a non-specific marker of inflammation; that is, many different stimuli can lead to its elevation28. However, there are few existing studies linking peripheral inflammation and cognitive-emotional processing, particularly in bipolar disorder. As such, our goal was to first establish if there is a relationship between a broad measure of inflammation (e.g., CRP) and cognitive-emotional processing, which could then guide whether further explication of the molecular antecedents involved in the instantiation of a chronic-low grade inflammatory response is warranted, as these may constitute targets for medication treatment.
Methods
Participants
Participants with DSM-V BD I and II (n = 119, age range = 18–65, Table 1 for detail) were recruited from the Icahn School of Medicine at Mount Sinai Hospital. The participants included in the current study represent a subset of that previously reported25, who were selected based on having data available for both the Affective Go/No-Go Task20 and CRP. Only 8 healthy control participants had data on both parameters and due to this small sample size, were excluded from further analysis. Diagnosis of BD was determined by the Structured Clinical Interview for DSM-V (SCID-529). Illness features including age at onset, number and type of prior episodes, BD and psychotic subtype, rapid cycling and comorbid axis I diagnoses (current and lifelong) were also derived from the SCID-5.
Table 1.
Sample Demographic and Clinical Characteristics (n = 119)
| Variable | Mean | SD |
|---|---|---|
| Age | 47.49 | 9.9 |
| Education | 14.04 | 2.42 |
| No. Psychotropic Medications | 1.61 | 1.32 |
| Illness Duration | 24.1 | 10.62 |
| No. Hospitalizations | 3.44 | 4.39 |
| No. Mood Episodes | 23.37 | 16.55 |
| Manic Episodes | 7.36 | 8.41 |
| Depressive Episodes | 12.49 | 11.23 |
| No. Suicide Attempts | 0.85 | 2.11 |
| Weeks Since Last Mood Episode | 62.83 | 106.93 |
| N | % | |
| Sex (Female) | 49 | 41.2 |
| Ethnicity (Hispanic/Latino) | 24 | 20.2 |
| Race | ||
| Black | 65 | 54.6 |
| White | 51 | 42.9 |
| Asian | 1 | 0.8 |
| American Indian/Alaska Native | 0 | 0 |
| Other | 2 | 1.7 |
| More than one race | 0 | 0 |
| BD Type I | 92 | 77.3 |
| Current Smoker | 48 | 40.3 |
| Lifetime Psychosis | 47 | 39.5 |
| Mean | SD | |
| Negative | ||
| D Prime | 1.37 | 1.10 |
| Hits RT | 555.98 | 95.59 |
| Commissions RTa | 533.12 | 123.67 |
| Positive | ||
| D Prime | 1.54 | 1.13 |
| Hits RTb | 554.18 | 80.39 |
| Commissions RT | 533.56 | 130.16 |
| Neutral | ||
| D Prime | 1.53 | 1.13 |
| Hits RT | 558.78 | 80.33 |
| Commissions RTc | 537.19 | 115.61 |
n = 118 due to 1 subject with 0% commissions rate
n = 117 due to 2 subjects with 0% hit rate
n = 117 due to 2 subjects with 0% commissions rate
All participants were outpatients and affectively stable, defined as a Clinical Global Impressions Rating Score ≤ 330. Exclusion criteria included inability to consent, history of head trauma, neurological disorder, or childhood Attention Deficit/Hyperactivity Disorder or learning disability. We chose not to exclude individuals with an adult-onset ADHD diagnosis if it was made after the onset of BD due to the confounding of this diagnosis in the context of a serious psychiatric illness with cognitive implications. Thus, there may be some individuals in this sample with a true adult-onset ADHD but none who met criteria for ADHD during childhood, which is when a clearer and less confounded diagnosis is best made. Additionally, exclusion criteria included current diagnosis of minor or major neurocognitive disorder, substance use disorder within the past three months, an active and unstable medical problem that may interfere with cognition, medication with known adverse cognitive effects (i.e., topiramate, tricyclics, and anticholinergics), agents that enhance cognition (e.g., amphetamine, dopamine agonists), and electroconvulsive therapy (ECT) in the past year. Benzodiazepine use was permitted, but participants were required to refrain from use within 4 hours of testing.
Affective Inhibition
Participants performed the Affective Go/No-Go task from the Cambridge Neuropsychological Test Automated Battery (CANTAB)20. This is a continuous performance task that consists of 20 blocks, with two practice blocks followed by 18 testing blocks, where for each block, a target word category and a distracter word category were designated. Then, a series of words were rapidly presented at the center of the screen, and participants were instructed to make a button-press response to words from the target category, while withholding the response to words from the distractor category. There were three categories of words: ‘Positive’, ‘Negative’, and ‘Neutral’. Illustratively (Figure 1), if the target category for a block is ‘Negative’, and distractor category is ‘Positive’, the participants should press the button upon seeing the word ‘Crying’ and withhold the response upon seeing ‘Fun’. Each of the 6 possible target-distracter category combinations were repeated 3 times and order of presentation was counterbalanced across participants. There were 18 trials per block and each word was displayed for 300 msec with a 900 msec inter-stimulus interval (ISI). Participants were instructed to respond as quickly and as accurately as possible.
Figure 1.
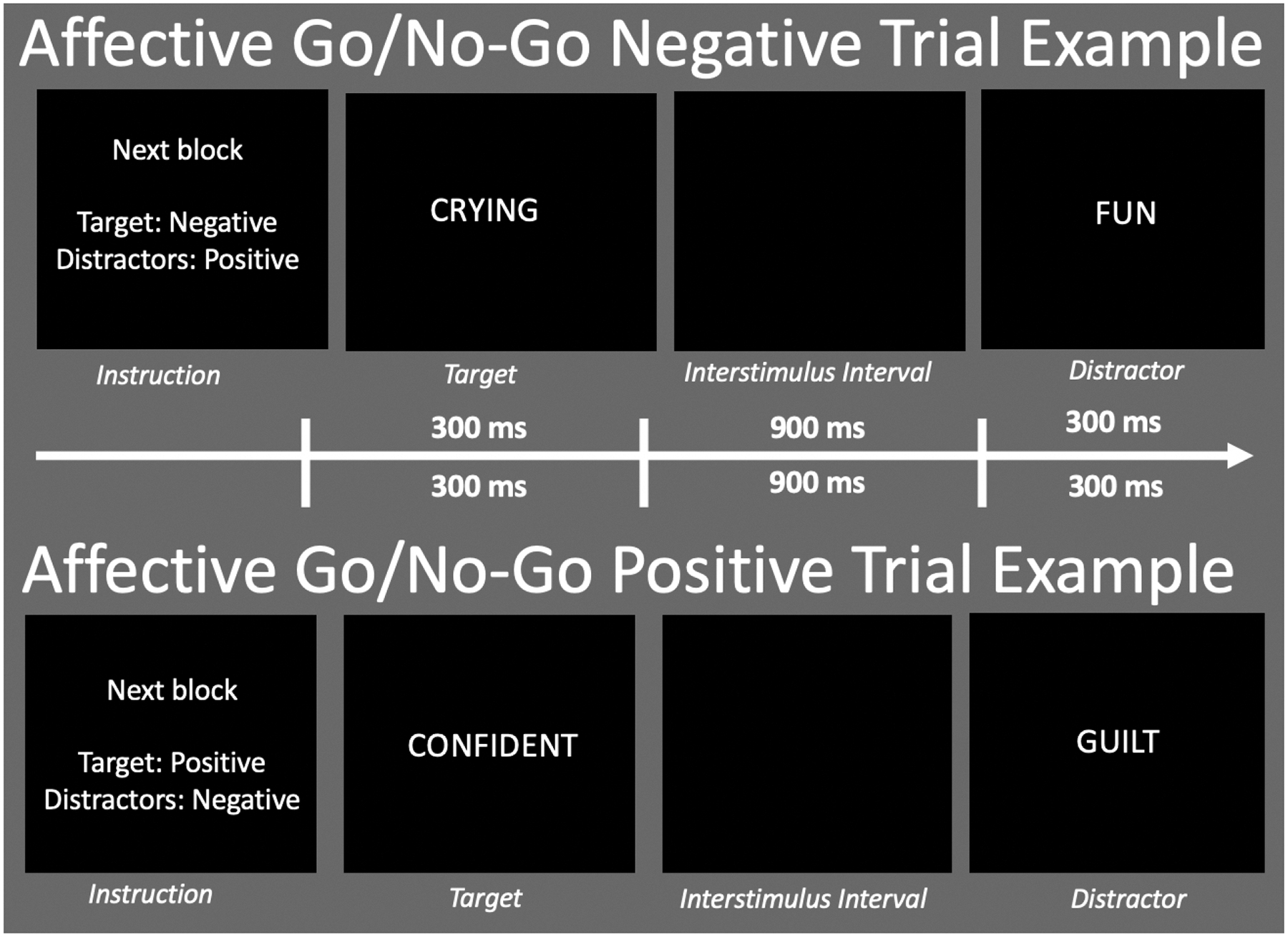
This is a schematic of the Affective Go/No-Go Task Diagram. In the first representation, the target valence is negative. When a word with negative valence is displayed, the participant presses the response button (“Go” condition”). When a word with a positive valence is presented, the participant must withhold a response (“No Go”). In the second representation, the target valence is positive. When a word with positive valence is displayed, the participant presses the response button (“Go” condition”). When a word with a negative valence is presented, the participant must withhold a response (“No Go”).
For each block, the number and percent of target omissions (misses) and commissions (false alarms) were calculated, as well as average response times for target hits and commissions. To evaluate participants’ overall accuracy in discrimination of ‘Positive’, ‘Negative’, and ‘Neutral’ words, d’ was calculated for each target category, using Z(hit rate) – Z(false alarm rate). D’ ranges from 0 (no discrimination) to infinity (perfect discrimination). For blocks where hit or commission rates were either 100% or 0%, effective limits were set of 1-(1/(2*n)) or 1/(2*n), respectively, where n is the number of targets or distractors for the trial. No other behavioral thresholds or accuracy cut-offs were used to optimally model the full range of dimensional performance associations with CRP in the entire sample.
Blood Biomarker Analyses
A research nurse obtained a non-fasting sample of ~16 ml of blood in a serum separator by venipuncture from all participants. The serum samples were stored at −80 °C until batch analyses. The Genital Tract Biology Laboratory at Brigham and Women’s Hospital analyzed CRP on electrochemiluminescence (ECL) multiplex-based assay platform using S600 Meso Scale Discovery (MSD) reader (MSD, Gaithersburg, MD). The lab is accredited by the College of American Pathologists. The ECL assays are highly sensitive with a 5-log scale of linearity starting at 2.5 pg/ml. Each sample was diluted to fit the linearity range. Raw readings were transformed by reader software into concentrations and these values were entered in an Excel database. A split quality control sample was run on each ECL assay plate to ascertain reproducibility of measurement showing a 5.6% inter-assay coefficient of variation.
Statistical Analyses
All analyses were performed in the statistical program SPSS (IBM SPSS Statistics version 24). Analyses comparing the current sample with data available for the measures of interest to participants from the overall study were done with a Student’s t or Chi-squared tests, as appropriate. Accuracy and response times across each target category (‘Positive’, ‘Negative’, and ‘Neutral’) were compared with repeated measures ANOVA. CRP concentrations were not normally distributed (Skewness statistic = 6.31, SE = .22), but achieved a normalized distribution with transformation. Log transformed CRP values were used in subsequent correlational and regression analyses.
Pearson’s bivariate correlations were used to assess the association of CRP with d’ prime and reaction times for ‘Positive’, ‘Negative’, and ‘Neutral’ conditions. Significant bivariate correlations were subsequently subjected to multivariable regression to adjust for potential confounds. In a first step, because residual mood symptoms could be implicated in bias for emotionally valanced words31, HDRS and YMRS scores were entered as covariates. In a subsequent step, we added additional demographic and clinical characteristics that could plausibly covary with either inflammation or affective cognition, including age, sex, race, education, smoking status, number of psychotropic medications, duration of BD illness, number of mood episodes, and number of psychiatric hospitalizations. Body mass index was only recorded for n = 22 patients in this sample and due to this lack of data could not be adjusted for in analyses. Finally, a stepwise regression was conducted to isolate which affective inhibition variables (e.g., negative, positive, and neutral d’ prime, hits response time, and commissions response time) that best explained variance in CRP.
Results
Affective Inhibition Performance
Affective inhibition performance data is summarized in Table 1 and Figure 2. There was a significant difference for detection accuracy between target word conditions, F(1.87, 220.32) = 4.10, p = .020. Post-hoc tests indicated that detection of negative target words was lower compared to both positive (p = .023) and neutral words (p = .028), whereas there was no difference between positive and neutral target words (p = .880). Response times did not significantly differ by target word category for either hits, F(1.83, 212.46) = .61, p = .532, or commissions, F(1.99, 229.22) = .24, p = .784.
Figure 2.
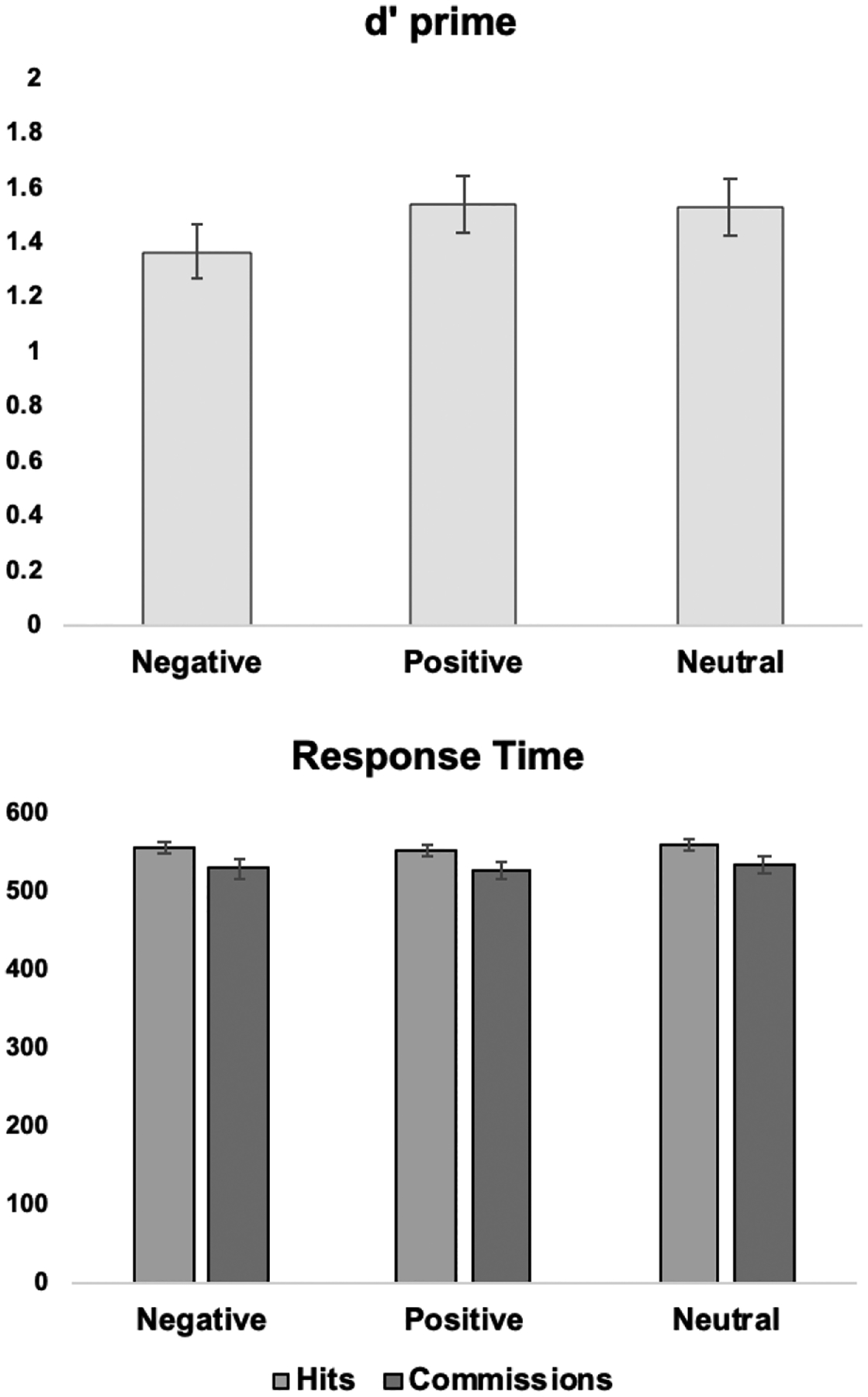
Average detection accuracy and response times during Affective Go/No-go performance
Bivariate correlations between target detection accuracy and response times are reported in Table 2. Greater negative target detection was associated with longer negative hits response times (r = .26, p = .004) and negative commissions response times (r = .24, p = .008). Greater positive target detection was associated with longer positive hits response times (r = .22, p = .015), but not positive commissions response times (r = .08, p = .387). Neutral target detection was not significantly associated with response times, neither for hits (r = .17, p = .065) nor commissions (r = .07, p = .466).
Table 2.
Bivariate correlations between target detection accuracy and response times
| Condition/Variable | Negative | Positive | Neutral | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Negative | D Prime | Hits RT | Commissions RTa |
D Prime | Hits RTb | Commissions RT |
D Prime | Hits RT | Commissions RTc |
| D Prime | -- | ||||||||
| Hits RT | .26** | -- | |||||||
| Commissions RTa | .24** | .73** | |||||||
| Positive | |||||||||
| D Prime | .73** | .24** | .12 | -- | |||||
| Hits RTb | .23* | .76** | .63** | .22* | -- | ||||
| Commissions RT | .16 | .57** | .56** | .08 | .70** | ||||
| Neutral | |||||||||
| D Prime | .77** | .23* | .20* | .84** | .18* | .14 | -- | ||
| Hits RT | .18* | .76** | .60** | .16 | .82** | .60** | .17 | -- | |
| Commissions RTc | .14 | .64** | .53** | .09 | .71** | .55** | .07 | .75** | -- |
p< .05,
p <.01
n = 118 due to 1 subject with 0% commissions rate
n = 117 due to 2 subjects with 0% hit rate
n = 117 due to 2 subjects with 0% commissions rate
Associations of CRP and Affective Inhibition Performance
Univariate associations between CRP and affective inhibition target detection and response times are displayed in Figure 3. CRP was significantly associated with reduced negative target detection (r = −.25, p = .007), faster negative hits response time (r = −.26, p = .006), and faster negative commissions response time (r = −.23, p = .012). CRP was not significantly associated with positive target detection (r = −.15, p = .111), positive hits response times (r = −.13, p = .151), but was related to faster positive commissions response time (r = −.25, p = .006). CRP was not significantly associated with neutral target detection (r = −.18, p = .054), neutral hits response time (r = −.14, p = .124), or neutral commissions response time (r = −.13, p = .155).
Figure 3.
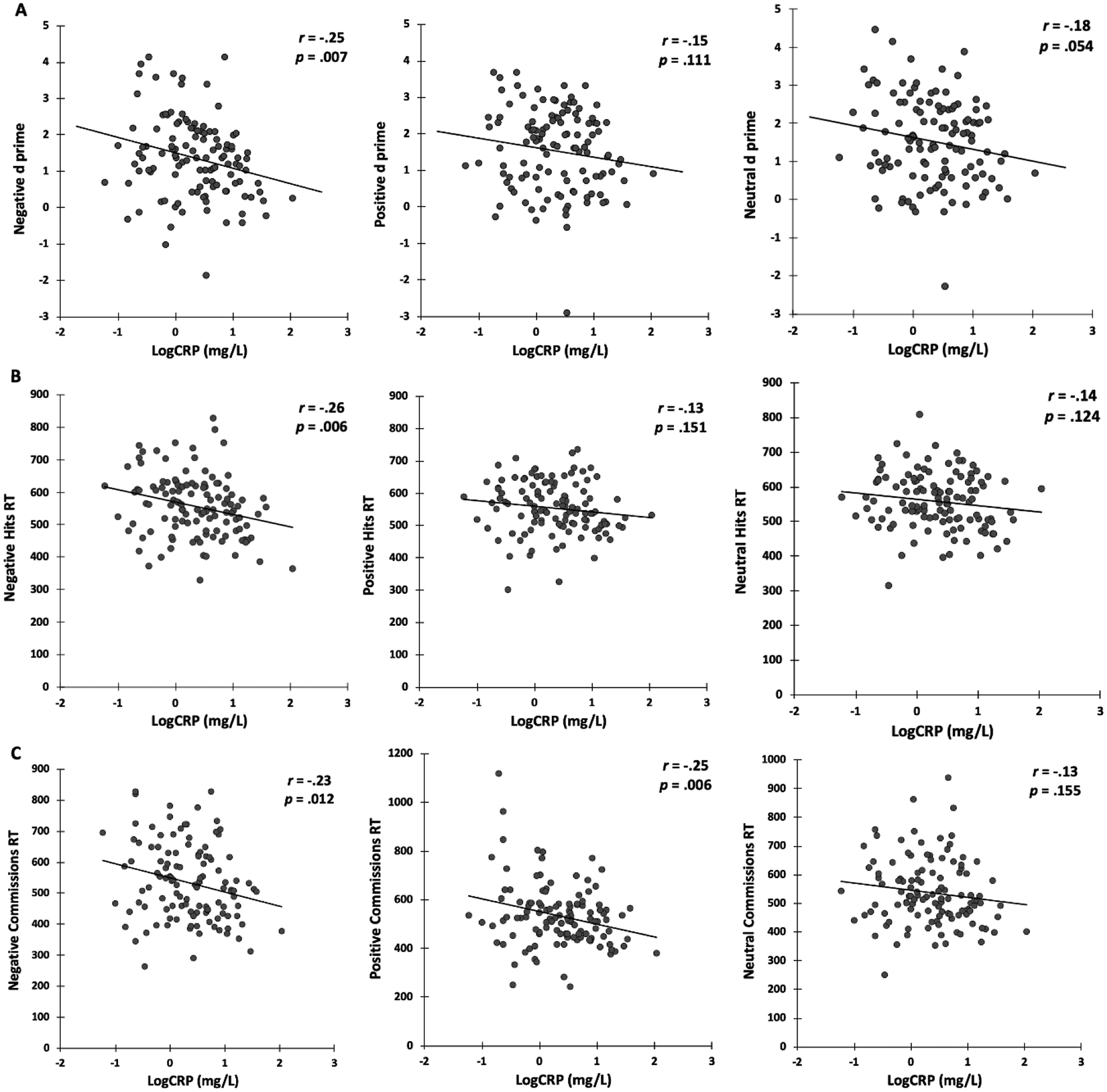
Univariate associations of CRP and affective inhibition performance measures of: A) d prime; B) Hits RT (Response Times); C) Commissions RT. Negative Commissions RT, n = 118 due to 1 subject with 0% commissions rate. Positive Hits RT, n = 117 due to 2 subjects with 0% hit rate. Neutral Commissions RT, n = 117 due to 2 subjects with 0% commissions rate.
In multivariate regression models, the associations of CRP with reduced negative target detection, faster negative hits and commissions response times, and faster positive commissions response times remained significant model terms after adjustment for both depression and mania symptoms and all additional demographic and clinical covariates (Table 3). However, the overall regression models were significant only for negative target detection and negative hits response times.
Table 3.
Multivariable Modeling of CRP and Affective Inhibition Performance
| Negative D Prime | Negative Hits Response Times | Negative Commissions Response Timesa | Positive Commissions Response Times | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | F or t | Sig | R2 | B | SE | F or t | Sig | R2 | B | SE | F or t | Sig | R2 | B | SE | F or t | Sig | R2 | ||
| Step 1 | 3.459 | 0.019 | 0.084 | 3.743 | 0.013 | 0.090 | 2.188 | 0.093 | 0.055 | 2.900 | 0.038 | 0.071 | |||||||||
| Constant | 2.647 | 0.542 | 4.880 | 0.000 | 694.781 | 46.464 | 14.953 | 0.000 | 689.672 | 62.528 | 11.030 | 0.000 | 716.003 | 64.417 | 11.115 | 0.000 | |||||
| logCRP | −0.395 | 0.159 | −2.482 | 0.015 | −36.057 | 13.628 | −2.646 | 0.009 | −43.822 | 18.261 | −2.400 | 0.018 | −49.706 | 18.893 | −2.631 | 0.010 | |||||
| HDRS | −0.015 | 0.017 | −0.881 | 0.380 | −0.619 | 1.466 | −0.422 | 0.674 | −0.193 | 1.948 | −0.099 | 0.921 | −0.519 | 2.033 | −0.255 | 0.799 | |||||
| YMRS | 0.047 | 0.028 | 1.678 | 0.096 | −4.330 | 2.417 | −1.791 | 0.076 | −2.516 | 3.216 | −0.782 | 0.436 | −3.725 | 3.351 | −1.111 | 0.269 | |||||
| Step 2 | 2.739 | 0.002 | 0.257 | 2.257 | 0.012 | 0.222 | 1.491 | 0.133 | 0.160 | 1.130 | 0.343 | 0.125 | |||||||||
| Constant | 2.229 | 1.008 | 2.211 | 0.029 | 659.458 | 88.715 | 7.433 | 0.000 | 660.543 | 120.313 | 5.490 | 0.000 | 709.198 | 129.090 | 5.494 | 0.000 | |||||
| logCRP | −0.382 | 0.161 | −2.378 | 0.019 | −46.677 | 14.138 | −3.302 | 0.001 | −54.21 | 19.293 | −2.81 | 0.006 | −51.155 | 20.572 | −2.487 | 0.015 | |||||
| HDRS | −0.017 | 0.017 | −1.005 | 0.317 | −1.175 | 1.515 | −0.776 | 0.440 | −1.662 | 2.053 | −0.809 | 0.420 | −0.481 | 2.204 | −0.218 | 0.828 | |||||
| YMRS | 0.019 | 0.028 | 0.694 | 0.489 | −5.368 | 2.449 | −2.192 | 0.031 | −4.035 | 3.335 | −1.21 | 0.229 | −4.380 | 3.563 | −1.229 | 0.222 | |||||
| Age | −0.035 | 0.013 | −2.793 | 0.006 | −0.390 | 1.107 | −0.353 | 0.725 | −0.467 | 1.500 | −0.312 | 0.756 | −0.513 | 1.61 | −0.319 | 0.751 | |||||
| Sex | 0.467 | 0.214 | 2.182 | 0.031 | 36.921 | 18.812 | 1.963 | 0.052 | 43.95 | 25.731 | 1.708 | 0.091 | 14.877 | 27.374 | 0.543 | 0.588 | |||||
| Race | −0.004 | 0.117 | −0.035 | 0.972 | −17.276 | 10.276 | −1.681 | 0.096 | −27.991 | 14.182 | −1.974 | 0.051 | −11.482 | 14.953 | −0.768 | 0.444 | |||||
| Education | 0.097 | 0.044 | 2.195 | 0.030 | 5.936 | 3.882 | 1.529 | 0.129 | 6.315 | 5.280 | 1.196 | 0.234 | 4.538 | 5.649 | 0.803 | 0.424 | |||||
| Smoking Status | −0.233 | 0.214 | −1.093 | 0.277 | −23.632 | 18.789 | −1.258 | 0.211 | 4.714 | 25.463 | 0.185 | 0.853 | −5.105 | 27.34 | −0.187 | 0.852 | |||||
| Lifetime Psychosis | −0.054 | 0.202 | −0.266 | 0.791 | −5.934 | 17.803 | −0.333 | 0.740 | −7.021 | 24.196 | −0.29 | 0.772 | −17.085 | 25.906 | −0.659 | 0.511 | |||||
| No. Medications | −0.044 | 0.079 | −0.560 | 0.577 | −15.68 | 6.978 | −2.247 | 0.027 | −21.405 | 9.474 | −2.259 | 0.026 | −11.024 | 10.154 | −1.086 | 0.280 | |||||
| Illness Duration | 0.010 | 0.012 | 0.882 | 0.380 | 1.208 | 1.044 | 1.156 | 0.250 | 0.749 | 1.417 | 0.528 | 0.598 | 0.447 | 1.52 | 0.294 | 0.769 | |||||
| No. Hospitalizations | 0.012 | 0.023 | 0.500 | 0.618 | 2.170 | 2.036 | 1.066 | 0.289 | 3.021 | 2.77 | 1.091 | 0.278 | 4.092 | 2.963 | 1.381 | 0.170 | |||||
| No. Mood Episodes | 0.002 | 0.006 | 0.251 | 0.803 | −0.445 | 0.553 | −0.805 | 0.423 | 0.108 | 0.749 | 0.144 | 0.885 | −1.155 | 0.805 | −1.435 | 0.154 | |||||
n = 118 due to 1 subject with 0% commissions rate
In a stepwise regression including all affective inhibition variables as predictors of CRP level, negative hits response times was retained as the sole variable in a significant model predicting CRP (R2 = .23, F(1, 113) = 6.20, p = .014). Specifically, longer response times were associated with lower CRP (b = −.002, se = .001, p = .014). Tolerance for excluded variables ranged from .42 - .95.
Discussion
The results of the current study indicate that the peripheral marker of inflammation, CRP, was associated with affective inhibition performance in a cohort of euthymic individuals with BD. Specifically, increased CRP was significantly associated with reduced negative target discriminability, which was also significantly reduced compared to positive and neutral target conditions. Additionally, higher CRP levels were associated with faster response times for both negative hits and commissions, and when compared against each other in a stepwise regression, faster negative target response times explained the single-most variance in elevated CRP of all the affective inhibition variables evaluated. Notably, each of these associations were observed after adjusting for several potential confounds to inflammation in bipolar disorder, such as residual mood symptoms, illness course characteristics (medications, psychosis, chronicity), demographic characteristics, and smoking status. Such analyses lend confidence that the observed association between CRP and affective inhibition exists beyond what can be accounted for by demographic and clinical variables that are often disproportionately associated with either BD or inflammation. Taken together, these results add to the existing body of literature which has identified associations between peripheral inflammation and cognitive dysfunction25, by raising the possibility that peripheral inflammation is also implicated in the integration of cognitive control with emotional processing in patients with BD, particularly for evaluation of negative stimuli.
It is important to draw attention to the fact that CRP was associated with reduced negative target word discrimination – which reflects the combination of both low accuracy in correctly identifying negative words and the tendency to incorrectly label positive or neutral words as negative. One potential interpretation is that individuals with BD may be affectively primed to interpret a range of emotionally charged or neutral stimuli as negative and increased immune activation could be involved in the response to this negative affective bias. The observations that increased CRP was associated with faster response times for negative targets and that negative target response times were the sole predictor of CRP levels retained in a stepwise regression, aligns with this possibility and suggests that inflammation may relate to a negativity bias defined by a tendency to more quickly evaluate or judge incoming information as negative. Alternatively, it is also possible that individuals who are less accurate at identifying negative stimuli might mount a stronger or lasting CRP response to regulate or manage unclear emotional inputs.
It is notable that CRP was associated with reduced negative, but not positive or neutral target discrimination. This pattern is suggestive of the possibility that an association of inflammation with reduced inhibitory control is most relevant when attention is primed for a negative, as compared to positive or emotionally ambiguous stimulus, thereby increasing the overall inhibitory demand32,33. Indeed, the variance in negative affective target detection explained by CRP (R2 = .26) is comparable to variance in a composite of non-emotional cognitive measures explained by CRP (R2 = .25) in a previous study of an overlapping sample25, where both studies adjusted for similar confounding variables. Mechanistically, as reflected in structural34–38 and functional34,39 MRI studies of BD, peripheral inflammation may disrupt the frontal executive neural circuitry thought to be critical for top-down regulation when emotional sensitivity is most heightened, such as when processing negative information. However, while not statistically significant, the pattern and direction of association of CRP with positive and neutral target conditions was similar, suggesting there may be a lower-level vulnerability between inflammation and inhibition broadly speaking, that crosses a critical threshold when required to identify and integrate negative stimuli. Accordingly, whereas prior studies have largely examined the associations of inflammation with cognition, reward and emotion processing, or mood symptoms separately, there are likely key domains of intersection and interaction amongst these constructs that will be important to continue to dissect to determine which patients and what areas of dysfunction are most vulnerable to the effects of inflammation.
A primary limitation of the study is the cross-sectional nature of the design. Although there are some promising experimental studies linking cytokine administration or systemic inflammatory challenge to both cognitive and affective processing deficits24, these have not evaluated changes in affective inhibition specifically. Neither have any longitudinal studies focused on this domain, which will be required to fully parse the directionality of the associations observed herein. Moreover, although meta-analysis implicates CRP elevations in euthymic BD and affective inhibition deficits are reported in prior case-control studies, lack of healthy control data precluded our evaluation of whether or not the magnitude of the link between CRP and affective inhibition differs in BD. Importantly, our findings were robust to adjustment for residual mood symptoms and several clinical and demographic features, including many possible confounds to the effects of inflammation. However, we did not collect information on body mass index from all participants and were unable to apply that correction in the current analysis. Additionally, although CRP is generally less vulnerable to diurnal variation than some other cytokines28,40, we did not have information regarding the time of the blood draw and could not demonstrate this quantitatively in the current sample. Equally, there are several other potential sources of inflammation that we did not monitor in our current sample, including low-grade infections, limited physical activity, poor diet, and related cardiovascular risk factors, which can disproportionately affect individuals with BD41–44. Indeed, diets high in fiber and rich in fruits and vegetables45, as well as exercise46 have been associated with lower CRP, plausibly through antioxidative properties that reduce oxidative stress45 and reduced body mass index46. Both factors could contribute to temporary fluctuations in CRP that we were unable to adjust for statistically in the current study. It is important in future work to systematically track timing of these activities and their correlation with time of bio-sample acquisition to assess for any covariance. Of note, poor diet, and exercise habits, in addition to shared genetic risk, are factors implicated in the disproportionate incidence of cardiovascular disease in BD47,48. Therefore, it will be an especially important area of future inquiry to interrogate the interplay of shared genetic risk for cardiovascular disease and BD, and development of inflammation, cerebrovascular disease, and cognitive and neuropsychiatric sequelae over time. Likewise, we did not formally test for pregnancy nor systematically assess for breastfeeding or menopause in female participants, which can also introduction variation in CRP. Lastly, the correlation of peripheral CRP with cognitive-affective behavioral performance theoretically assumes there are mechanisms by which CRP influences central nervous system function. For instance, activate transport, blood brain barrier permeability, circumventricular organs, afferent vagal nerve fibers, or monocyte infiltration are plausible49, but can only be speculated in the present study.
Despite these limitations, the present study advances knowledge on circulating immune function in BD. It adds to an existing body of evidence demonstrating associations between inflammation and cognition or reward sensitivity and motivation separately, by raising the possibility that inflammation is also implicated in the integration of cognitive-affective processing. Our findings also have generated the hypothesis that this association may be more pronounced when stimuli are of negative valence. In future work, additions to study design, such as the inclusion of a control group and longitudinal assessment of broad and specific inflammatory markers, as well as performance-based measures of cognitive and affective processing, will be paramount for determining whether these initial findings observed here align with the notion of an integrated neuroimmune network hypothesis50. The neuroimmune network hypothesis suggests that the effects of low-grade inflammation are unlikely to be specific to any one behavioral construct, but rather may have nuanced and interacting influences across multiple domains of clinical risk, including affective processing, cognition, threat sensitivity, and reward. As deficits in each of these areas may come online during different stages of development or be amplified during different stages of the disease process, longitudinal studies assessing the course of these relationships will be especially important for identifying optimal windows for potential intervention or modulation of systemic inflammatory activation in BD.
References
- 1.Mann-Wrobel MC, Carreno JT, Dickinson D. Meta-analysis of neuropsychological functioning in euthymic bipolar disorder: an update and investigation of moderator variables. Bipolar Disord. 2011;13(4):334–342. [DOI] [PubMed] [Google Scholar]
- 2.Van Rheenen TE, Lewandowski KE, Tan EJ, et al. Characterizing cognitive heterogeneity on the schizophrenia-bipolar disorder spectrum. Psychol Med. 2017;47(10):1848–1864. [DOI] [PubMed] [Google Scholar]
- 3.Burdick KE, Russo M, Frangou S, et al. Empirical evidence for discrete neurocognitive subgroups in bipolar disorder: clinical implications. Psychol Med. 2014;44(14):3083–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langenecker SA, Saunders EF, Kade AM, Ransom MT, McInnis MG. Intermediate: cognitive phenotypes in bipolar disorder. J Affect Disord. 2010;122(3):285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 2009;113(1–2):1–20. [DOI] [PubMed] [Google Scholar]
- 6.Crane NA, Verges A, Kamali M, et al. Developing Dimensional, Pandiagnostic Inhibitory Control Constructs With Self-Report and Neuropsychological Data. Assessment. 2020;27(4):787–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan KA, Vederman AC, Kamali M, et al. Emotion perception and executive functioning predict work status in euthymic bipolar disorder. Psychiatry Research. 2013;210(2):472–478. [DOI] [PubMed] [Google Scholar]
- 8.Minzenberg MJ, Lesh TA, Niendam TA, et al. Control-related frontal-striatal function is associated with past suicidal ideation and behavior in patients with recent-onset psychotic major mood disorders. J Affect Disord. 2015;188:202–209. [DOI] [PubMed] [Google Scholar]
- 9.Tsubota N, Yoshimura M, Hatt K, Yanagawa M. [Successful use of an omental pedicled flap for obliterating empyema associated with a large bronchial fistula]. Nihon Kyobu Geka Gakkai Zasshi. 1990;38(3):499–502. [PubMed] [Google Scholar]
- 10.Langenecker SA, Jacobs RH, Passarotti AM. Current neural and behavioral dimensional constructs across mood disorders. Current behavioral neuroscience reports. 2014;1(3):144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joormann J, Quinn ME. Cognitive processes and emotion regulation in depression. Depression and anxiety. 2014;31(4):308–315. [DOI] [PubMed] [Google Scholar]
- 12.Joormann J, Vanderlind WM. Emotion regulation in depression: The role of biased cognition and reduced cognitive control. Clinical Psychological Science. 2014;2(4):402–421. [Google Scholar]
- 13.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the new York Academy of Sciences. 2012;1251:E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pe ML, Raes F, Koval P, Brans K, Verduyn P, Kuppens P. Interference resolution moderates the impact of rumination and reappraisal on affective experiences in daily life. Cognition & emotion. 2013;27(3):492–501. [DOI] [PubMed] [Google Scholar]
- 15.Malooly AM, Genet JJ, Siemer M. Individual differences in reappraisal effectiveness: the role of affective flexibility. Emotion. 2013;13(2):302. [DOI] [PubMed] [Google Scholar]
- 16.Friedman NP, Miyake A. Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex. 2017;86:186–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman NP, Miyake A. The relations among inhibition and interference control functions: a latent-variable analysis. J Exp Psychol Gen. 2004;133(1):101–135. [DOI] [PubMed] [Google Scholar]
- 18.Stange JP, Adams AM, O’Garro-Moore JK, et al. Extreme cognitions in bipolar spectrum disorders: associations with personality disorder characteristics and risk for episode recurrence. Behav Ther. 2015;46(2):242–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baldessarini RJ, Vázquez GH, Tondo L. Bipolar depression: a major unsolved challenge. Int J Bipolar Disord. 2020;8(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robbins TW, James M, Owen AM, et al. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: Implications for theories of executive functioning and cognitive aging. Journal of the International Neuropsychological Society. 1998;4(5):474–490. [DOI] [PubMed] [Google Scholar]
- 21.Felger JC. Imaging the Role of Inflammation in Mood and Anxiety-related Disorders. Curr Neuropharmacol. 2018;16(5):533–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leboyer M, Soreca I, Scott J, et al. Can bipolar disorder be viewed as a multi-system inflammatory disease? J Affect Disord. 2012;141(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berk M, Kapczinski F, Andreazza AC, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neuroscience & biobehavioral reviews. 2011;35(3):804–817. [DOI] [PubMed] [Google Scholar]
- 24.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millett CE, Perez-Rodriguez M, Shanahan M, et al. C-reactive protein is associated with cognitive performance in a large cohort of euthymic patients with bipolar disorder. Molecular Psychiatry. 2019. [DOI] [PubMed] [Google Scholar]
- 26.Dantzer R Cytokine, sickness behavior, and depression. Immunology and Allergy Clinics. 2009;29(2):247–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandes BS, Steiner J, Molendijk ML, et al. C-reactive protein concentrations across the mood spectrum in bipolar disorder: a systematic review and meta-analysis. The Lancet Psychiatry. 2016;3(12):1147–1156. [DOI] [PubMed] [Google Scholar]
- 28.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. The Journal of clinical investigation. 2003;111(12):1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.First MB. Structured Clinical Interview for the DSM (SCID). In: The Encyclopedia of Clinical Psychology. 1–6. [Google Scholar]
- 30.Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28–37. [PMC free article] [PubMed] [Google Scholar]
- 31.Joormann J, Quinn ME. Cognitive processes and emotion regulation in depression. Depress Anxiety. 2014;31(4):308–315. [DOI] [PubMed] [Google Scholar]
- 32.Carretié L Exogenous (automatic) attention to emotional stimuli: a review. Cognitive, Affective, & Behavioral Neuroscience. 2014;14(4):1228–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kellermann TS, Sternkopf MA, Schneider F, et al. Modulating the processing of emotional stimuli by cognitive demand. Social Cognitive and Affective Neuroscience. 2011;7(3):263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tu PC, Li CT, Lin WC, Chen MH, Su TP, Bai YM. Structural and functional correlates of serum soluble IL-6 receptor level in patients with bipolar disorder. J Affect Disord. 2017;219:172–177. [DOI] [PubMed] [Google Scholar]
- 35.Chen MH, Chang WC, Hsu JW, et al. Correlation of proinflammatory cytokines levels and reduced gray matter volumes between patients with bipolar disorder and unipolar depression. J Affect Disord. 2019;245:8–15. [DOI] [PubMed] [Google Scholar]
- 36.Benedetti F, Poletti S, Hoogenboezem TA, et al. Inflammatory cytokines influence measures of white matter integrity in Bipolar Disorder. J Affect Disord. 2016;202:1–9. [DOI] [PubMed] [Google Scholar]
- 37.Bai YM, Chen MH, Hsu JW, et al. A comparison study of metabolic profiles, immunity, and brain gray matter volumes between patients with bipolar disorder and depressive disorder. J Neuroinflammation. 2020;17(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen MH, Kao ZK, Chang WC, et al. Increased Proinflammatory Cytokines, Executive Dysfunction, and Reduced Gray Matter Volumes In First-Episode Bipolar Disorder and Major Depressive Disorder. J Affect Disord. 2020;274:825–831. [DOI] [PubMed] [Google Scholar]
- 39.Tang G, Chen P, Chen G, et al. Inflammation is correlated with abnormal functional connectivity in unmedicated bipolar depression: an independent component analysis study of resting-state fMRI. Psychol Med. 2021:1–11. [DOI] [PubMed] [Google Scholar]
- 40.Rifai N, Ridker PM. Proposed cardiovascular risk assessment algorithm using high-sensitivity C-reactive protein and lipid screening. Clinical chemistry. 2001;47(1):28–30. [PubMed] [Google Scholar]
- 41.Kilbourne AM, Cornelius JR, Han X, et al. Burden of general medical conditions among individuals with bipolar disorder. Bipolar Disord. 2004;6(5):368–373. [DOI] [PubMed] [Google Scholar]
- 42.Berk M, Williams LJ, Jacka FN, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marshe VS, Pira S, Mantere O, et al. C-reactive protein and cardiovascular risk in bipolar disorder patients: A systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79(Pt B):442–451. [DOI] [PubMed] [Google Scholar]
- 44.SayuriYamagata A, Brietzke E, Rosenblat JD, Kakar R, McIntyre RS. Medical comorbidity in bipolar disorder: The link with metabolic-inflammatory systems. J Affect Disord. 2017;211:99–106. [DOI] [PubMed] [Google Scholar]
- 45.Kuczmarski MF, Mason MA, Allegro D, Zonderman AB, Evans MK. Diet quality is inversely associated with C-reactive protein levels in urban, low-income African-American and white adults. J Acad Nutr Diet. 2013;113(12):1620–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fedewa MV, Hathaway ED, Ward-Ritacco CL. Effect of exercise training on C reactive protein: a systematic review and meta-analysis of randomised and non-randomised controlled trials. Br J Sports Med. 2017;51(8):670–676. [DOI] [PubMed] [Google Scholar]
- 47.Firth J, Solmi M, Wootton RE, et al. A meta-review of “lifestyle psychiatry”: the role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry. 2020;19(3):360–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopresti AL, Jacka FN. Diet and Bipolar Disorder: A Review of Its Relationship and Potential Therapeutic Mechanisms of Action. J Altern Complement Med. 2015;21(12):733–739. [DOI] [PubMed] [Google Scholar]
- 49.Rosenblat JD, McIntyre RS. Bipolar Disorder and Immune Dysfunction: Epidemiological Findings, Proposed Pathophysiology and Clinical Implications. Brain Sci. 2017;7(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nusslock R, Miller GE. Early-Life Adversity and Physical and Emotional Health Across the Lifespan: A Neuroimmune Network Hypothesis. Biol Psychiatry. 2016;80(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]


