Abstract
This report reviews concepts related to operation of the classic parallel-tube model (PTM) for hepatic disposition and examines two recent proposals of a newly derived equation to describe hepatic clearance (CLH). It is demonstrated that the proposed equation is identical to a re-arrangement of an earlier relationship from Pang and Rowland and provides a means of calculation of intrinsic clearance (CLint,PTM) rather than CLH as posed. We further demonstrate how classic hepatic clearance models with an assumed CLint, while subject to numerous limitations, remain highly useful and necessary in both traditional pharmacokinetics (PK) and physiologically based pharmacokinetic (PBPK) modeling.
Keywords: hepatic clearance, intrinsic clearance, parallel tube model, physiologically based pharmacokinetics, well-stirred model
INTRODUCTION
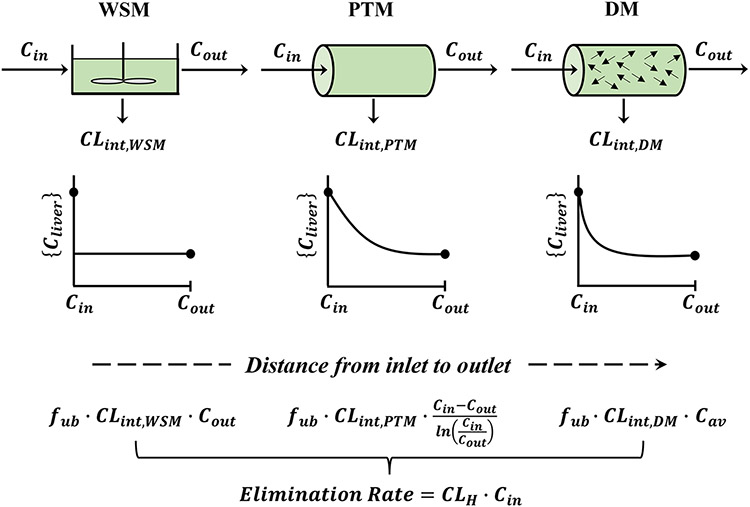
The primary models used in pharmacokinetics to describe hepatic clearance of many drugs are the well-stirred model (WSM), the parallel-tube model (PTM), and the dispersion model (DM) as shown in Fig. 1. The WSM, while greatly simplifying the structure of the liver and other organs, is easy to operate and is extensively used to generally describe hepatic drug disposition. It is primarily the basis of applying physiologically based pharmacokinetic (PBPK) models (1) and for utilizing in vitro metabolic data obtained from hepatocytes and microsomes for making in vivo extrapolations (IVIVE) (2).
Fig. 1.
Basic hepatic models. Representation of the well-stirred model (WSM), parallel-tube model (PTM), and dispersion model (DM) showing the same steady-state input (Cin) and outflow (Cout) drug concentrations in blood. The internal hepatic blood concentrations {Cliver} differ according to model assumptions. In turn, the systemically determined elimination rate calculated from CLH × Cin and dose/AUC will be accompanied by differing intrinsic clearances {CLint} as designated for the three models with fub as the fraction unbound in blood. The complex Cav for the DM falls between Cout and (Cin − Cout)/(ln(Cin/Cout)), and thus, the rank order of expected CLint values is as follows: WSM > DM > PTM. It can be noted that the spatial distribution and exponential decline in blood concentrations in the PTM can be calculated from: where fx is the fractional distance between the inlet and outlet of the liver model
The WSM assumes that the liver is a single, well-stirred compartment and that the concentration of unbound drug in the emergent blood is in equilibrium with the unbound drug within the liver with elimination activity described as intrinsic clearance (3, 4). The PTM assumes that the liver is comprised of an array of identical and parallel tubes with enzymes distributed evenly in each cross-section of the sinusoidal vascular and perivascular space. This model was proposed by Winkler et al. (5, 6). The WSM assumes that the drug concentration in the liver is constant and equal to the emergent drug concentration (Cout), while the PTM assumes that there is an exponentially declining drug concentration from the inlet (Cin) to the outlet Cout. The properties and comparisons of the two models have been well described by Pang and Rowland (7, 8).
The model construct by Winkler et al. (5, 6) described the PTM with convective transport and Michaelis–Menten enzymatic metabolism that, for steady-state linear conditions, was written as follows:
| (1) |
where QH is hepatic blood flow and Vmax/Km are capacity/equilibrium constants for metabolism. Pang and Rowland (7) recognized that Velocity/substrate concentration (Cin) is hepatic clearance (CLH), Vmax/Km is intrinsic clearance (CLint,PTM) for the PTM, and assumed that only fractional unbound drug in blood (fub) is subject to metabolism (viz. the “free drug hypothesis”) as applied in PK for the WSM to write the currently operative steady-state equation for the PTM as follows:
| (2) |
They provided an array of ancillary equations to describe functioning of the PTM compared to the WSM (7) including the relationship:
| (3) |
where CLH can be calculated from perfused organ measurements using EH as the Extraction Ratio = (Cin − Cout)/Cin. They then compared the application of the two models to experimental data for several compounds (8).
ASSESSMENT OF THE KOCHAK-BENET EQUATION
This commentary partly addresses the recent derivations and equations that claim to describe a method of calculation of CLH for the PTM. As developed by Kochak (9) based on an “advection mass transport” paradigm and by Benet et al. (10) based on a flow reactor perspective, it was posed that hepatic clearance (“CLH”) can be calculated from:
| (4) |
Furthermore, Kochak stated that Eq. 4 = QH x E with E described as “a new Extraction Factor” (9) for data obtained from typical organ perfusion experiments where steady-state Cin and Cout are measured. The two sets of authors used different assumptions and derivations to arrive at this equation. The Kochak E creates some confusion as it looks like the traditional EH, but it is not. Neither author compared this equation to the expectations from the classic equations for the PTM (Eq. 2) nor assessed its relevance to in vivo PK or to PBPK models.
The following calculations were first performed to make such comparison. An array of QH and CLint,PTM values were employed to calculate CLH using Eq. 2 with fub assumed = 1. Pang and Rowland (7) showed for the PTM that for fub = 1:
| (5) |
Thus, for assumed values of Cin, expected values of Cout can be obtained. In turn, the Kochak-Benet equation was applied to compare values of their “CLH” (Eq. 4) for each pair of CLint,PTM and QH values. Such calculations are provided in Table I with the values for the established PTM bolded in the denominators. The classic PTM functions as expected with highest CLH values that are limited by QH.1 It can be readily seen that the calculations for the “CLH” from Eq. 4 do not agree with differences as great as 20-fold. Thus, it is evident that the Kochak-Benet equation is not equivalent to the classical PTM for calculating CLH. Moreover, this equation produced “CLH” values that matched the assumed CLint,PTM values, many of which were assigned high values consistent with drugs producing large EH values. Also, it can be observed that when Cin/Cout is larger than 3, then Eq. 4 predicts a “CLH” that exceeds QH, which can only happen for CLint.
Table I.
Comparison of Kochak-Benet (Eq. 4, Numerator) and Classical PTM (Eq. 2, Denominator) Equations for Hepatic Clearance (CLH)
| CLint,PTM | Assigned values of QH |
||||
|---|---|---|---|---|---|
| 300 | 600 | 900 | 1200 | 1500 | |
| → 0 | CLint,PTM | CLint,PTM | CLint,PTM | CLint,PTM | CLint,PTM |
| 300 | 300/190 | 300/236 | 300/255 | 300/265 | 300/272 |
| 600 | 600/259 | 600/379 | 600/438 | 600/472 | 600/495 |
| 900 | 900/285 | 900/466 | 900/569 | 900/633 | 900/677 |
| 1200 | 1200/295 | 1200/519 | 1200/663 | 1200/759 | 1200/826 |
| 1500 | 1500/298 | 1500/551 | 1500/730 | 1500/856 | 1500/948 |
| 3000 | 3000/300 | 3000/596 | 3000/868 | 3000/1101 | 3000/1297 |
| 6000 | 6000/300 | 6000/600 | 6000/899 | 6000/1192 | 6000/1470 |
Numbers have arbitrary values with units of volume/time
Upon further assessment, it can be readily shown that rearrangement of Eq. 5 taking ln(exp(-CLint,PTM/QH)) produces Eq. 4. It is difficult to follow the original derivations for either model that led to Eq. 4 to determine why they produced an outcome that Eq. 4 reflects an intrinsic clearance rather than a systemic clearance (traditional CLH). However, the simple adjustment of the Pang and Rowland equation easily produces Eq. 4. Also, Kochak’s “CLH” value was also shown to serve in place of CLint,PTM for calculation of the bioavailable fraction (F) escaping first-pass effect found previously for the PTM (7).
ADDITIONAL CONSIDERATIONS
There are some incidental concerns with the two publications as well. Kochak’s applications of Eq. 4 to the published data set for chromic phosphate colloid (and other compounds) from hepatic perfusion studies do not provide validation as claimed that Eq. 4 is superior to the QH x EH relationship for CLH. He appears to be indirectly comparing his model’s CLint,PTM with the other’s CLH and an ambiguous correction factor (a) was needed. The fittings of EH versus QH used meaningless quadratic functions instead of the WSM EH = CLint/(QH + CLint) or EH from the PTM exponential in Eq. 2. Preferable is application of such full mechanistic equations for the WSM and PTM that compare EH fittings as carried out by Pang and Rowland (8) who conceded that both models fitted some of these same data equally well (although the WSM was preferred for lidocaine). Such more proper comparisons have been done numerous times since (11). Kochak uses strong language to condemn the structure and operation of the WSM in advocating his version of the misinterpreted PTM. However, the two models have been compared more appropriately in numerous publications since that of Pang and Rowland (7, 8). Sodlii et al. (11) recently demonstrated the frequent similarity in fittings of both models for a diverse array of published data, and they opined at the time that “the simple but unphysiologic well-stirred model is the only model that can describe the trustworthy published available data.”
The Benet publication (10) primarily serves to remind readers that there are many definitions and approaches to assessment of clearance (CL) with the focus on differentiating the well-known calculation of CLH from the elimination rate/plasma concentration ratio versus the various intrinsic clearances (that he redefines) that require operation of the various hepatic models as also shown here (Fig. 1). The former (“one valid definition of clearance”2) allows calculation of CL from Dose/AUC for a fully bioavailable drug. Invoking CLint involves the need for a model for the liver and includes drug binding in blood (fub) and other assumptions if one seeks to describe or connect CLH to enzymatic activity (Vmax/Km or CLint,PTM). Benet et al. (10) compare some of the features of the WSM, PTM, and DM, which have well-elaborated structures and mathematical properties (12). As also shown previously (12), he points out with visualization (similar to Fig. 1) that the models display expected drug concentrations in hepatic blood that can differ considerably. In turn, this indicates that different intrinsic clearances will be needed to produce the same dose/AUC values and QH x EH values. Benet et al. (10) convey that in vitro assessments of CLint are commonly being scaled to in vivo CLH based on the WSM. However, IVIVE has also been performed with the PTM (3) and both models offer similar rough approximations with underprediction of in vivo CLH from in vitro CLint data, particularly for high clearance drugs.
Other concerns with the Benet et al. article (10), besides misrecognition that Eq. 4 is the PTM intrinsic clearance, are that their Eq. 5 is the traditional CLH = QH x EH and they provide a “CLH” equation for the DM with an unsupported configuration in place of the EH term. Thus, posing that “all the models of organ elimination will yield different mechanistic liver clearance values (Eq. 5-7),” while correct in words, offers a mix of systemic, intrinsic, and conjured clearance equations for comparison. If one gives an IV dose of a drug cleared only by the liver, dose/AUC will yield one CLH that is universal (i.e., “the one valid definition”). It is well appreciated (12) that application of the three hepatic models will yield differing intrinsic clearances owing to assumptions for the differing internal and unknowable hepatic drug concentrations {Cliver} and operative structures of the models. The mass balance for steady-state rate of drug elimination by hepatic metabolism is as follows:
| (6) |
with both the generic {CLint} and {Cliver} terms being dependent on the assumed model, while CLH is usually model-independent for a drug fully cleared by the liver (omitting consideration of fub). For example, {Cliver} is Cout for the WSM, while for the PTM:
| (7) |
as shown by Winkler et al. (5, 6) and depicted in Fig. 1. Note that all these concentrations refer to blood concentrations that equilibrate within the clearance organ.
RELEVANCE OF HEPATIC MODELS TO IN VIVO PHARMACOKINETICS
The classic hepatic models have been extensively applied to numerous drugs including in vitro and in vivo PK data for propranolol PK in rat and man, primarily using the WSM. This compound has been found to be well absorbed and subject to metabolism only by the liver. After IV doses in rats, the reported values for systemic blood clearance (viz. CLH) that could be calculated from dose/AUC were 84.7 and 63.2 (13), 71.2 (plasma) (14), and 65.7 mL/min/kg (15). The typical blood/plasma ratio (R) is 1.16 (15) and fraction unbound in plasma (fup) is 0.13 (16).
Several direct assessments of hepatic extraction allow comparisons of systemic versus hepatic versus intrinsic clearances of propranolol in rats. Suzuki et al. (17) measured propranolol in femoral and hepatic vein blood during constant rate infusion of propranolol in rats and found EH = 0.93. Hung et al. (18) found a similar EH of 0.95 in perfused rat livers using an indicator dilution technique. Singh et al. (16) carried out IV and portal vein infusions of propranolol in rats to determine a steady-state EH of 0.73. The mean oral bioavailability (F) of propranolol reported in rats by Shibasaki et al. (15) was 0.228, giving a mean EH value (from EH = 1 − F) of 0.772 (7). With their assessed QH value of 85 mL/min/kg, the CLH can be calculated from QH × EH as 65.6 mL/min/kg, similar to the above listed IV dose/AUC or CLH values and consistent with propranolol being entirely metabolized by the liver. Furthermore, the results from the Shibasaki et al. (15) study allows (2) calculation of CLint values from:
| (8) |
| (9) |
The CLint,WSM value with EH = 0.772 is 288 mL/min/kg and the corresponding CLint,PTM value is 126 mL/min/kg. The larger value for the WSM is expected (Fig. 1) as well as both values being larger than CLH and QH. Use of the EH of 0.93 (17) or 0.95 (18) will produce even larger CLint values.
Of particular note, application of the Kochak-Benet equation (using Cout = 0.228 Cin) to the EH value for propranolol reported in the Shibasaki study (15) also yields a “CLH” value of 126 mL/min/kg, matching CLint,PTM rather than any of the other clearance values. It can be noted that including fub = 0.167 as applied in vivo (16) would produce much higher apparent CLint values. As another means of calculation, the relationship for the PTM that F = exp(−CLint,PTM/QH) described by Pang and Rowland (7) that was also offered by Kochak (9) produces the same PTM clearance value of 126 mL/min/kg for the Shibasaki et al. data (15).
The further relevance of classic clearance concepts can be demonstrated with propranolol studies in man. The EH of propranolol was reported as 0.72 in man at oral doses at and above 30 mg based on PK data from oral and IV dosing (19), producing similar expectations that intrinsic clearances will be larger than QH. Indeed, the in vivo predicted CLint value is reported as 267 for the WSM and 154 for the PTM, with 13.2 for CLH and 21.9 mL/min/kg for QH for propranolol in humans (2, 19). Human PK data for propranolol were used by Gibaldi et al. (20) to first demonstrate how an IV dose/AUC value could be used with the WSM to estimate the first-pass availability (F) of an oral dose of drug. This approach was confirmed for propranolol and has since served as a simple method of using IV data to anticipate oral dose F if there are no product release issues. The value of oral dose/AUC reflects CLint,WSM for drugs such as propranolol while a more complicated equation is needed to calculate CLint,PTM(7). It is common practice to call such dose/AUC the “Oral Dose Clearance” with symbol CL/F in consideration that F may be less than 1 for multiple reasons.
As is common practice in human IVIVE today (2), an early study by Singh et al. (16) used rat hepatocytes to assess in vitro metabolism of propranolol and related it to in vivo results. They obtained an in vitro CLint value based on disappearance of propranolol during an incubation experiment (substrate depletion method), scaled their hepatocyte number to the whole rat liver, used measured in vivo R (0.78) and fup (0.13) values, and applied the WSM with QH = 60 mL/min/kg to calculate the expected in vitro EH. Their value (0.90) was larger than their own in vivo measurement of 0.73, but was closer to that (0.93) of Suzuki et al. (17) and (0.95) from Hung et al. (18). This difference in EH values was attributed to nonlinear metabolism of propranolol (16). Similar approaches are currently used with human hepatocytes and microsomes to predict in vivo clearances (2).
As pointed out by Benet et al. (10), in vivo systemic clearance values measure CLH for a drug fully metabolized by the liver, while in vitro assessments of CLint using hepatocytes or microsomes require a model such as the WSM or PTM for scaling to CLH (2) The use of IVIVE for human PK generally correlates well in terms of spanning low to high clearances over five orders of magnitude. However, using in vitro measurements with both the WSM and PTM systematically underpredicts in vivo clearances by about five-fold (2), perhaps for some of the reasons described in the next section. Nevertheless, there is considerable value in making such predictions with the expectation that they will only be approximate.
RELEVANCE OF MODEL-DEPENDENT CLEARANCES IN PBPK
It can be observed that commonly applied basic PBPK models usually follow the principles of Bischoff and Dedrick (21) who added a Vmax/Km metabolic function acting on Cout to Fick’s Law of Perfusion to describe hepatic distribution and elimination of thiopental. They subsequently invoked clearance terminology to describe various drug elimination processes in a PBPK model for methotrexate (22). Their relationships were predecessors to the simple PBPK differential equation (DE):
| (10) |
where Cliver is the measured and modeled total hepatic drug concentration and Vliver is actual liver volume. When Cin and Cout are plasma concentrations, it is commonplace to substitute Cout = Cliver/Kp, where Kp is an equilibrium tissue/plasma partition coefficient. In addition, the CLint term is usually operated with fraction unbound in plasma as fup × CLint in assuming that the free drug hypothesis applies. The liver and all other organs and tissues in the body are thus essentially described in simple PBPK models by the WSM, which may not be generally appreciated (10). However, PBPK models provide fitted or predicted Cin versus time profiles from which the systemic clearance can be readily calculated as a secondary parameter from dose/AUC with operation of the DE, dAUC/dt = Cin. Such values will be slightly smaller than conventionally calculated clearance values when the initial condition of the plasma concentration DE is dose/blood volume (23). This systemic or whole body clearance is helpful in providing a summation of all clearance processes in the body.
It is readily possible to test various added complexities, including applying Eq. 7 for the PTM, to create and assess alternative liver (and other tissue) models in PBPK modeling. It can be pointed out that PBPK models for organs described partly by Fick’s Law of Perfusion require addition of tissue concentrations as a model-assigned variable with specified assumptions to operate the DE. Since Cout is seldom or even impossible to measure in vivo for individual organs and tissues (how could this be done for bone, skin, muscle, and fat?), the Cliver term reduces the number of variables in the DE. A model-independent CLH × Cin may not even operate in PBPK models to describe hepatic drug concentrations. The fact that PBPK models have been applied successfully and extensively for numerous drugs, often with commercial software, indicates the considerable utility of the WSM in the field. This simple organ model is a major cornerstone of PK that can be augmented when either various complexities are encountered or assumptions are called for.
SOME LIMITATIONS OF THE HEPATIC AND PBPK MODELS
The three basic hepatic models, of course, only apply to drugs that are fully bioavailable and subject to hepatic metabolism and/or biliary excretion and without transporter involvement (24). For the latter situation, both influx and efflux clearances can be added to the basic models (12). Within PBPK models, the hepatic models operate with Cin reflecting about 80% input of blood from the portal vein and the remaining from the hepatic artery thus allowing for first-pass effects for oral or intraperitoneal doses and helping to inform (CLin values. Drugs that undergo breakdown by hydrolysis, esterases, or proteolysis in blood will have CL = Dose/AUC values that are not rate-limited by any blood flow. Numerous drugs undergo reversible metabolism (futile cycling) where dose/AUC reflects a combination of loss and return clearances (25, 26). Similar considerations apply when intestinal secretion and/or biliary excretion occur with enterohepatic cycling (27). Some drugs may efflux slowly from red blood cells (RBC) where the models should either account for such diffusion or employ a QH that is less than blood flow (28). Compounds with low permeability (PS) require model adjustments that account for this property (29).
Perhaps, the greatest uncertainty in operation of hepatic and PBPK models, as well as in pharmacodynamics, is the application of the free drug hypothesis with the use of fup in the WSM or PTM. Albumin-mediated hepatic drug uptake (30) and rapid dissociation of protein-bound drug (31) have been demonstrated. Both the RBC and albumin have been shown with indicator dilution studies to traverse the rat liver in about 1 min (32). Drugs typically bind to their targets with equilibrium dissociation constants (KD) ranging from 10−8 to 10−12 M with a dissociation half-life for koff as small as minutes (33). Drug binding to plasma proteins is much weaker and koff values may be too fast to measure. Also, proteins, perhaps carrying drugs, can slowly enter tissues by convection as recognized in PBPK models for monoclonal antibodies (34). An insightful review by Bowman and Benet (35) observed, “…some highly bound ligands have more efficient [hepatic] uptake than can be explained by their unbound fraction.” Attempts in IVIVE and PBPK to utilize in vitro metabolism data using the WSM or PTM may have additional limitations owing to similar considerations for in vitro nonspecific cell or tissue binding where only the free drug is assumed to be metabolized and the need to extrapolate tissue dilution binding values to the whole organ (36).
In spite of these complexities and uncertainties, the basic hepatic models offer highly useful starting points in PK and PBPK in considering tissue distribution and clearance processes for drugs. Any of these complexities, if supported by experimental data, can be built into these foundational hepatic models.
CONCLUSIONS
The recently developed Kochak-Benet equation reflects CLint,PTM (rather than CLH) for the PTM as confirmed by simulations, re-arrangement of an early equation from Pang and Rowland (7), and assessment of published in vivo PK data for propranolol. Their QH x E equation may find utility as a quick method of calculation of CLint,PTM when typical organ extraction-type data are available. Considerations of the model-dependency of {CLint} as well as {Cliver} provide insights into the assumptions, requirements, and limitations of IVIVE, PK, and PBPK modeling.
Acknowledgements
WJJ acknowledges that he was a reviewer for the Benet et al. (10) publication and regrets that he did not catch the indicated problems at that time. The authors appreciate review comments by Dr. Yoo-Seong Jeong.
Funding
This work was supported by NIH Grant R35 GM131800.
Footnotes
The authors declare no competing interests.
Interestingly, the lowest CLH values are often less than CLint,PTM. It is commonly expected that CLH approaches CLint,PTM when the latter becomes very small. However, this requires that QH > > CLint,PTM as can be found with Eq. 2. A similar phenomenon occurs with the WSM. Of course, adding fub will usually produce lower values of fub x CLint,PTM operative in the more complete models of hepatic clearance. This type of behavior was demonstrated previously in simulations by Winkler et al. (5).
Benet et al. (10) pose that amount eliminated per unit of time/systemic concentration is the “one valid definition of CL.” While this is a correct and mechanistically operative relationship for hepatic metabolism, perhaps a more general definition of a clearance process is velocity/substrate concentration as long appreciated (5). The latter is more useful in recognizing clearance processes (viz. Eq. 1 versus 2), allows for relationships such as Eq. 6, and helps in designation of transport and flow/permeability distribution clearances versus elimination clearances.
References
- 1.Miller NA, Reddy MB, Heikkinen AE, Lukacova V, Parrott N. Physiologically based pharmacokinetic modeling for first-in-human predictions: an updated model building strategy illustrated with challenging industry case studies. Clin Pharmacokin. 2019;58:727–16. [DOI] [PubMed] [Google Scholar]
- 2.Halifax D, Foster JA, Houston JB. Prediction of human metabolic clearance from in vitro systems: retrospective analysis and prospective view. Pharm Res. 2010;27:2150–61. [DOI] [PubMed] [Google Scholar]
- 3.Rowland M, Benet LZ, Graham GG. Clearance concepts in pharmacokinetics. J Pharmacokin Biopharm. 1973;1:123–36. [DOI] [PubMed] [Google Scholar]
- 4.Wilkinson GR, Shand DG. A physiological approach to hepatic drug clearance. Clin Pharmacol Ther. 1975;18:377–90. [DOI] [PubMed] [Google Scholar]
- 5.Winkler K, Keiding S, Tygstrup N, Clearance as a quantitative measure of liver function. In, “The Liver: Quantitative Aspects of Structure and Function”, pp 144–155, Karger-Basel; 1973. [Google Scholar]
- 6.Bass L, Keiding S, Winkler K, Tygstrup N. Enzymatic elimination of substrates flowing through the intact liver. J Theoret Biol. 1976;61:393–409. [DOI] [PubMed] [Google Scholar]
- 7.Pang KS and Rowland M, Hepatic clearance of drugs. I. Theoretical considerations of a “well-stirred” and a “parallel tube” model. Influence of hepatic blood flow, plasma and blood binding, and the hepatocellular enzymatic activity on hepatic drug clearance. J Pharmacokin Biopharm 1977; 5: 625–653. [DOI] [PubMed] [Google Scholar]
- 8.Pang KS and Rowland M, Hepatic clearance of drugs. II. Experimental evidence for acceptance of the “well-stirred” model over the “parallel tube” model using lidocaine in the perfused rat liver in situ preparation, J Pharmacokin Biopharm 1977; 5:655–680. [DOI] [PubMed] [Google Scholar]
- 9.Kochak GM. Critical analysis of hepatic clearance based on an advection mass transfer model and mass balance. J Pharm Sci. 2020;109:2059–69. [DOI] [PubMed] [Google Scholar]
- 10.Benet LZ, Sodhi JK, Makrygiorgos G, Mesbah A. There is only one valid definition of clearance: critical examination of clearance concepts reveals the potential for errors in clinical drug dosing. AAPS J. 2021;23:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sodhi JK, Wang H-J, Benet LZ. Are there any experimental perfusion data that preferentially support the dispersion and parallel-tube models over the well-stirred model of organ elimination? Drug Metab Dispos. 2020;48:537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pang KS, Han YR, Noh K, Lee PI, and Rowland M, Hepatic clearance concepts and misconceptions: why the well-stirred model is still used even though it is not physiological reality? Biochem Pharmacol 2019; 169: 113596. [DOI] [PubMed] [Google Scholar]
- 13.Schneck DW, Pritchard JF, Hayes AH Jr. Studies on the uptake and binding of propranolol by rat tissues. J Pharmacol Exp Ther. 1977;203(3):621–9. [PubMed] [Google Scholar]
- 14.Katayama H, Fujiwara J, Yasuhara M, Okumura K, Hori R. Increased availability of propranolol in rats with uranyl nitrate-induced acute renal failure. J Pharmacobiodyn. 1984;7(8):536–44. [DOI] [PubMed] [Google Scholar]
- 15.Shibasaki S, Asahina M, Kawamata Y, Kojo M, Nishigaki R, Umemura K. The inhibitory effects of cimetidine on elimination and distribution of propranolol in rats. J Pharmacobiodyn. 1989;12(9):549–57. [DOI] [PubMed] [Google Scholar]
- 16.Singh K, Tripp SL, Dunton AW, Douglas FL, Rakhit A. Determination of in vivo hepatic extraction ratio from in vitro metabolism by rat hepatocytes. Drug Metab Dispos. 1991;19:990–6. [PubMed] [Google Scholar]
- 17.Suzuki T, Isozaki S, Ohkuma T, Rikihisa T. Influence of the route of administration on the mean hepatic extraction ratio of propranolol in the rat. J Pharm-Dyn. 1980;3:603–11. [DOI] [PubMed] [Google Scholar]
- 18.Hung DY, Siebert GA, Chang P, Whitehouse MW, Fletcher L, Crawford DHG, Roberts MS. Hepatic pharmacokinetics of propranolol in rats with adjuvant-induced systemic inflammation. Am J Physiol Gastrointest Liver Physiol. 2006;290:G343–51. [DOI] [PubMed] [Google Scholar]
- 19.Shand DG and Rangno RE, The disposition of propranolol. I. Elimination during oral absorption in man. Pharmacology 1972; 7: 159–68. [DOI] [PubMed] [Google Scholar]
- 20.Gibaldi M, Boyes RN, Feldman S. Influence of first-pass effect on availability of drugs on oral administration. J Pharm Sci. 1971;60:1338–40. [DOI] [PubMed] [Google Scholar]
- 21.Bischoff K, Dedrick RL. Thiopental pharmacokinetics. J Pharm Sci. 1968;57:1346–51. [DOI] [PubMed] [Google Scholar]
- 22.Bischoff KB, Dedrick RL, Zaharko DS. Preliminary model for methotrexate pharmacokinetics. J Pharm Sci. 1970;59:149–54. [DOI] [PubMed] [Google Scholar]
- 23.Cao Y, Jusko WJ. Applications of minimal physiologically-based pharmacokinetic models. J Pharmacokin Pharmacodyn. 2012;39:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kusuhara H, Sugiyama Y. In vitro – in vivo extrapolation of transporter-mediated clearance in the liver and kidney. Drug Metab Pharmacokin. 2009;24:37–52. [DOI] [PubMed] [Google Scholar]
- 25.Cheng H, Jusko WJ. Pharmacokinetics of reversible metabolic systems. Biopharm Drug Disp. 1993;14:721–66. [DOI] [PubMed] [Google Scholar]
- 26.Roberts MS, Magnusson BM, Burczynski FJ, Weiss M. Enterohepatic circulation: Physiological, pharmacokinetic and clinical implications. Clin Pharmacokinet. 2002;41:751–90. [DOI] [PubMed] [Google Scholar]
- 27.Zhang D, Wei C, Hop CECA, Wright MR, Hu M, Lai Y, Khojasteh SC, Humphreys WG. Intestinal excretion, intestinal recirculation, and renal tubule reabsorption are underappreciated mechanisms that drive the distribution and pharmacokinetic behavior of small molecule drugs. J Med Chem. 2021;64:7045–59. [DOI] [PubMed] [Google Scholar]
- 28.Chow FS, Piekoszewski W, Jusko WJ. Effect of hematocrit and albumin concentration on hepatic clearance of tacrolimus (FK 506) during rabbit liver perfusion. Drug Metab Disp. 1997;25:610–6. [PubMed] [Google Scholar]
- 29.Jeong Y-S, Yin C-S, Ryu H-M, Noh C-K, Song Y-K, Chung S-J. Estimation of the minimum permeability coefficient in rats for perfusion-limited tissue distribution in whole-body physiologically-based pharmacokinetics. Eur J Pharmaceu Biopharm. 2017;115:1–17. [DOI] [PubMed] [Google Scholar]
- 30.Li N. Badrinarayanan, Isida K, Li X, Roberts J, Wang S, Hayashi M, and Gupta A, Albumin-mediated uptake improves human clearance prediction for hepatic uptake transporter substrates aiding a mechanistic in vitro-in vivo extrapolation (IVIVE) in discovery research. AAPS J. 2021;23:1. [DOI] [PubMed] [Google Scholar]
- 31.Baker M, Parton T. Kinetic determinants of hepatic clearance: plasma protein binding and hepatic uptake. Xenobiotica. 2008;37:1110–34. [DOI] [PubMed] [Google Scholar]
- 32.Pang KS, Sherman IA, Schwab AJ, Geng W, Barker F 3rd, Dlugosz JA, Currier G, Goresky CA. Role of the hepatic artery in the metabolism of phenacetin and acetaminophen: intravital microscopic and multiple indicator dilution study in perfused rat liver. Hepatology. 1994;20:672–83. [PubMed] [Google Scholar]
- 33.Dahl G, Akerud T. Pharmacokinetics and the drug-target residence time concept. Drug Discovery Today. 2013;18:697–707. [DOI] [PubMed] [Google Scholar]
- 34.Cao Y, Balthasar JP, Jusko WJ. Second-generation minimal physiologically-based pharmacokinetic model for monoclonal antibodies. J Pharmacokin Pharmacodyn. 2013;40:597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowman CM, Benet LZ. An examination of protein binding and protein-facilitated uptake relating to in vitro-in vivo extrapolation. Eur J Pharm Sci. 2018;123:502–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jusko WJ, Molins EAG, Ayyar VS. Seeking nonspecific binding: assessing the reliability of tissue dilutions for calculating fraction unbound. Drug Metab Disp. 2020;48:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]