Abstract
Interpretation of axonal damage biomarker Neurofilament Light chain (NfL) concentrations is difficult due to the lack of age‐specific and disease‐specific reference values. We here developed an interactive interface to support interpretation of NfL results in human body fluids. We used NfL values of 1698 individuals without a neurological disorder, aged 19–85 years, and patients with MS and dementias. Percentile regression estimates per diagnosis populate interactive graphs, alongside NfL background information (available on: https://mybiomarkers.shinyapps.io/Neurofilament). This accessible interface provides reference for interpretation of the individual patient results for clinicians. It showcases an adaptable method to support interpretation of age‐dependent biomarkers in neurology.
Introduction
Neurofilament light chain (NfL) is a biofluid biomarker of neuroaxonal damage. NfL levels are altered in multiple neurological diseases, with disease‐specific elevations and a strong, non‐linear age relationship. 1 , 2 , 3 Therefore, a single reference value or cut‐off cannot be defined, which hampers use of NfL results in clinical practice. Age‐specific interpretations have been proposed, but none of the previous studies included a large age range with both cerebrospinal fluid (CSF) and blood, or multiple diseases. 4 , 5 , 6 , 7 , 8 To increase the interpretability of test results, we developed an online interactive interface to provide reference values in relation to age and clinical context that was evaluated by clinicians. Here, we present this interactive support interface for NfL results in different body fluids by age and in context of several major neurological disorders.
Subjects and Methods
Reference sample controls and patients
We included data of healthy individuals that served as a reference population and of patients with several neurodegenerative diseases to serve as the patient population. The NfL data were selected based on availability at Amsterdam UMC. 9 , 10 , 11 , 12 , 13 , 14 The patients included in the reference population were either diagnosed with subjective cognitive complaints during clinic work‐up in the Alzheimer center Amsterdam, which included brain imaging, neuropsychological testing, or as symptomatic controls, 15 following a clinical work‐up with a lumbar puncture to exclude MS, an infection, or metastases. 10 , 15 Reference samples were also included from general population volunteers during the yearly reference value blood draw‐event of Clinical Chemistry and control participants from cohort studies, who provided self‐report for the absence of a neurological disorder. 13 , 14 The patient population consisted of patients from the MS Center Amsterdam and Amsterdam Dementia Cohort diagnosed with multiple sclerosis, Alzheimer's dementia, frontotemporal dementia, dementia with Lewy bodies. 9 , 10 About one‐third of the MS patients was under treatment of interferon, although we did not see a difference in NfL levels between treated and untreated patients. All individuals provided informed consent according to local IRB approvals.
NfL measurements
CSF, serum, and EDTA plasma were collected and biobanked at −80°C between 2000 and 2017 according to standardized biobanking protocols. 16 , 17 NfL levels were analyzed at the Amsterdam UMC Neurochemistry laboratory with the single molecular array (Simoa®) NF‐light™ Advantage kit (Quanterix, USA) on the HD‐1 or HDx analyzer according to the user's manual (n = 1205 serum, n = 282 plasma, and n = 494 CSF), with the homebrew NfL Simoa assay (n = 133 serum and n = 120 plasma) on the HD‐1 analyzer, 18 or with the NF‐light® enzyme‐linked immunosorbent assay (ELISA; UmanDiagnostics, Sweden; n = 376 CSF samples). All three assays employ the same pair of antibodies. Results of the homebrew and ELISA assay were aligned to the results obtained with the commercial Simoa assay (details in File S1). Paired serum and plasma results were strongly correlated (Pearson's rho = 0.92 [0.91–0.94], p‐value <2 × 10−16), with a mean ratio of serum to plasma of 0.99, upon which both matrices were together included as “blood (EDTAplasma/serum).” One value below the LOD was imputed at the LOD.
Statistical methods
NfL levels were log‐transformed. Age‐specific percentile ranges were calculated for the reference group and per diagnosis by simple quantile regression with age as the predictor over the available age range per group. The regression output formed for the 5th, 10th, 25th, 50th, 75th, 90th, and 95th percentile formed the input for the interactive graph in the interface. The controls were split at age 50 years to conform the earlier reported infliction point for NfL increase in serum. 5 For the median NfL levels in blood, the formula below 50 years was: NfLpredicted = 21.622+(age×0.023), and over 50 years was: NfL = 20.445+(age×0.047). For blood, the age‐adjusted z‐scores can be approximated based on median percentile, yielding the following formulas for below 50 years: z‐score = [log2(NfLvalue) − (1.622 + (age × 0.023))]/0.699, and for 50 years and over: z‐score = [log2(NfLvalue) − (0.445 + (age × 0.047))]/0.706. We used the quantreg package in R. 19
Online interface approach and clinician input
We developed an interactive interface to support the interpretation of NfL levels. We consulted neurologists with sub‐specialties in multiple sclerosis and cognitive disorders and held a questionnaire about the interface and NfL in clinical practice among 31 neurologists, psychiatrists, and geriatricians. Those results informed the content of the interface (full report in File S1). We used the Shiny package in R. 20
Results
Reference values by age
For the presented version 2.5.1, we used data from 1698 individuals, aged 19 to 87 years, 45% female (Table 1). NfL data were available for 833 individuals without a neurological disorder and 75 patients with multiple sclerosis, 293 patients with Alzheimer's disease dementia, 373 patients with frontotemporal dementia, and 124 patients with dementia with Lewy bodies (Fig. 1A and B). These data formed the input for the fitted 5th to 95th percentile values per age and disorder for the support interface. In the reference control population, blood NfL values (median [5th–95th percentile]) ranged from 5 [3–10] pg/mL at age 30 to 19 [10–37] pg/mL at age 80 (Table 2). CSF NfL values ranged in the reference control population from a median [5th–95th percentile] of 212 [98–620] pg/mL at age 30 to 1243 [812–2104] pg/mL at age 80.
Table 1.
Population characteristics.
| Reference values controls | Alzheimer's dementia | Dementia with Lewy bodies | Fronto‐temporal dementia | Multiple Sclerosis | |
|---|---|---|---|---|---|
| N | 833 | 293 | 124 | 373 | 75 |
| Age, years mean ± SD | 54 ± 14 | 64 ± 8 | 69 ± 7 | 63 ± 8 | 46 ± 9 |
| Age, years range | 19–85 | 37–81 | 52–85 | 26–87 | 25–68 |
| Female, N (%) | 408 (49%) | 156 (53%) | 12 (10%) | 161 (43%) | 36 (48%) |
| Blood NfL, pg/mL mean ± SD | 10 ± 7 | 19 ± 12 | 20 ± 18 | 39 ± 57 | 11 ± 12 |
| Blood NfL, pg/mL range | 2–113 | 5–123 | 5–160 | 2–608 | 2–69 |
| CSF NfL, pg/mL mean ± SD | 723 ± 500 | 1337 ± 1108 | 1270 ± 1106 | 3137 ± 2393 | 1579 ± 2213 |
| CSF NfL, pg/mL range | 157–4722 | 189–13,111 | 183–7499 | 548–17,925 | 465–17,558 |
SD, standard deviation; NfL, Neurofilament light chain; Blood, EDTAplasma or serum; CSF, cerebrospinal fluid.
Figure 1.
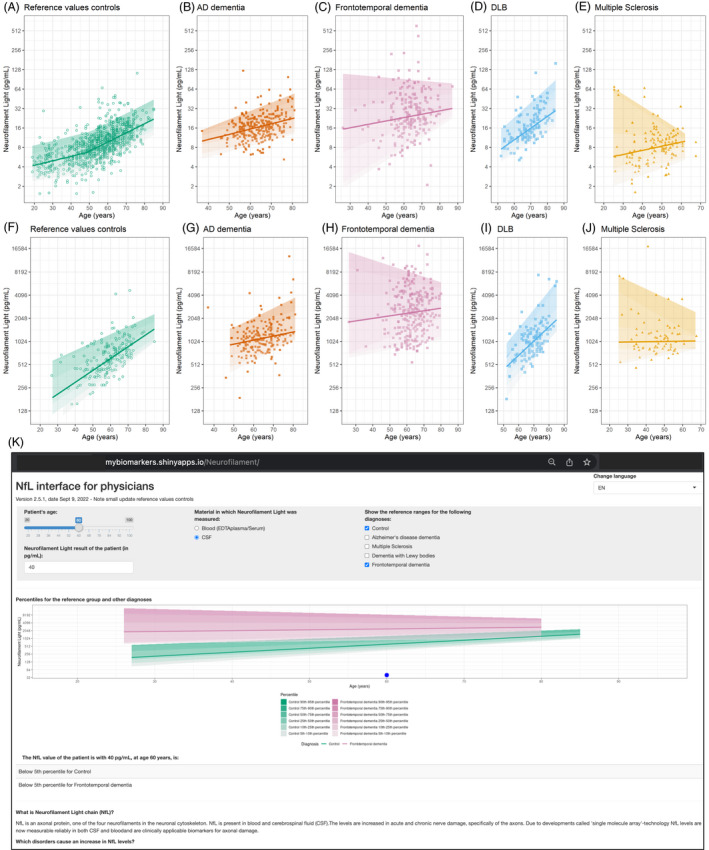
Neurofilament Light chain data and online interface. Visual presentation of NfL values underlying the online interface in (A–E) blood (EDTAplasma/serum) and (F–J) cerebrospinal fluid are presented for the reference value controls and several disorders. (K) Screenshot of v2.5 interface Neurofilament Light chain. Interface accessible by copying this link in a web browser: https://mybiomarkers.shinyapps.io/Neurofilament.
Table 2.
Reference percentiles table Neurofilament Light chain in blood.
| Age | 5th percentile (pg/mL) | 10th percentile (pg/mL) | 25th percentile (pg/mL) | 50th percentile (median) (pg/mL) | 75th percentile (pg/mL) | 90th percentile (pg/mL) | 95th percentile (pg/mL) |
|---|---|---|---|---|---|---|---|
| 20 | 2 | 3 | 3 | 4 | 5 | 6 | 9 |
| 25 | 3 | 3 | 4 | 5 | 6 | 7 | 9 |
| 30 | 3 | 3 | 4 | 5 | 6 | 8 | 10 |
| 35 | 3 | 3 | 4 | 5 | 7 | 9 | 11 |
| 40 | 3 | 4 | 4 | 6 | 8 | 10 | 12 |
| 45 | 3 | 4 | 5 | 6 | 9 | 11 | 13 |
| 50 | 4 | 4 | 5 | 7 | 9 | 11 | 14 |
| 55 | 4 | 5 | 6 | 8 | 10 | 14 | 16 |
| 60 | 5 | 6 | 8 | 10 | 12 | 16 | 19 |
| 65 | 6 | 7 | 9 | 11 | 15 | 19 | 23 |
| 70 | 7 | 9 | 10 | 14 | 17 | 22 | 27 |
| 75 | 9 | 10 | 12 | 16 | 20 | 26 | 32 |
| 80 | 10 | 12 | 14 | 19 | 24 | 31 | 37 |
| 85 | 12 | 15 | 17 | 22 | 28 | 36 | 44 |
Blood = EDTAplasma or serum.
Reference value interface
All reference values per included diagnosis are available in the interface. The interface is publicly accessible since July 2021, and currently on v2.5.1, Sept 2022 (Fig. 1C, https://mybiomarkers.shinyapps.io/Neurofilament). When the age of the patient and the NfL value is entered at the top of the screen, the value for the patient is graphically represented relative to the reference control population. In addition, relevant disease group ranges can be included in the graph by ticking the diagnoses boxes. For differential diagnostic purpose, the entered NfL value is represented in a table as percentile for healthy controls, as well as percentile for disease context. An overview table with important reference values is included at the bottom of the tool. The interface contains background information on NfL. Informed by the feedback of clinicians, we included information on NfL in neurological disorders and several potential pitfalls for NfL value interpretation. The clinicians also made suggestions that could not be implemented, such as on the clinical inference and integration in clinical protocols and the electronic health record (EHR) because it requires specialty consensus of more data. The NfL interpretation support interface is versioned and is suitable for further refinement, for example, additional data input, translation, and visualization.
Discussion
The interactive NfL interface provides clinical users a tool for the interpretation of the individual patient results in blood and CSF, against age‐dependent reference ranges and in the context of several major neurological diseases. The publicly available interface allows for updates of reference data, for example, extension of age ranges, additional disease groups, and other biomarkers. Moreover, the accompanying information can be adapted and presented in additional languages. A strength of the interactive interface is the accessible, intuitive presentation of complex information to guide clinicians in the diagnostic or monitoring process.
Our results are very comparable to earlier reported NfL Simoa reference values and population reports. 5 , 6 , 21 Our values tend to be slightly lower at the 90th and 95th percentile, which might be due to a stringent selection of most participants to be neurologically screened in contrast to general population data. The increase of NfL levels in blood of around 2.5–5 percent per year that increases by age seems robust between studies. Within the included neurological diseases, blood NfL levels seemed to show a stronger age‐related increase than CSF NfL levels, corroborating previous work. 2 , 8
Methodologically, involving a diverse group of physicians during the development of the interface is a strength. This also informed the background information and use of percentiles. NfL outliers strongly contribute to the differential diagnostics, for example, between dementia types, whereas outlier information is lost when abnormality status is based on single cut‐offs. Using a percentile presentation (or normalized scores) was also the choice in the parallel developed recent publication of NfL reference values for MS studies. 5 A limitation in our data currently used to build our v2.5 tool is that we did not have the information on body mass index and estimated glomular filtration rate (eGFR) available for the patients, which have been shown to affect NfL slightly. 5 In addition, even though we excluded all patients with diagnosed neurological disorders from the reference group, some individuals might have subclinical neurological diseases or abnormal CSF AD biomarker levels. Future studies could further refine the data, for example, excluding control individuals with abnormal CSF AD biomarkers or prodromal disorders based on follow‐up, or taking into account mixed pathologies. Another limitation is that the interface uses single‐site measurements only. However, inter‐laboratory variation of serum NfL is <10% with the Simoa NF‐light assay, 22 therefore we consider generalization of the NfL interface feasible. The Simoa NF‐light recently received breakthrough device designation in MS from the FDA, which will further progress clinical use. 22 A next step would be the inclusion of data from other biomarkers. The developed approach can be applied to any biomarker test where a single cut‐off does not suffice.
In conclusion, we provide reference values for blood and CSF for NfL interpretation for controls and neurological disorders in a simple to use and interactive interface.
Author Contributions
Lisa Vermunt: Conceptualization, data curation, funding acquisition, formal analysis, methodology, visualization, writing – original draft preparation. Marco Otte: Formal analysis, methodology, writing – review & editing. Inge M. W. Verberk: Data curation, investigation, writing – review & editing. Joep Killestein: Investigation, writing – review & editing. Afina W. Lemstra: Investigation, writing – review & editing. Wiesje M. van der Flier: Investigation, writing – review & editing. Yolande A. L. Pijnenburg: Investigation, writing – review & editing. Everard G. B. Vijverberg: Investigation, writing – review & editing. Femke H. Bouwman: Investigation, writing – review & editing. Gido Gravesteijn: Investigation, writing – review & editing. Wilma D. J. van de Berg: Investigation, writing – review & editing. Philip Scheltens: Investigation, Writing – Review & Editing. Argonde C. van Harten: Investigation, writing – review & editing. Eline A. J. Willemse: Conceptualization, data curation, funding acquisition, formal analysis, investigation, visualization, writing – original draft preparation. Charlotte E. Teunissen: Conceptualization. funding acquisition, supervision, writing – original draft preparation.
Conflict of Interest
M. Otte, I. M.W. Verberk, Afina W. Lemstra, Yolande A.L. Pijnenburg, F.H. Bouwman, A.C. van Harten, E.A. J. Willemse, G. Gravesteijn report no disclosures relevant to the manuscript. L. Vermunt is advisory consultant for Roche (fees paid to Amsterdam UMC). J. Killestein has accepted speaker and consulting fees from Merck, Biogen, TEVA, Sanofi, Genzyme, Roche, and Novartis. E.G.B. Vijverberg is advisory consultant for several pharmaceutical companies in the field of neurodegeneration. W.M. van der Flier has (had) a collaboration contract, performed contract research, received grants, was invited speaker, or was consultant for Biogen MA, Boehringer Ingelheim, Life‐MI, AVID, Roche BV, Fujifilm, Combinostics, Eisai, Danone, ADx Neurosciences, Oxford Health Policy Forum CIC, Roche, and WebMD Neurology (fees paid to Amsterdam UMC). P. Scheltens is employed by LSP and Amsterdam UMC. P. Scheltens has received consultancy fees (paid to Amsterdam UMC) from AC Immune, Brainstorm Cell, EIP, ImmunoBrain Checkpoint, Genentech, Novartis, Novo Nordisk. He is PI of studies with AC Immune, FUJI‐film/Toyama, UCB, and Vivoryon (fees paid to Amsterdam UMC). C.E. Teunissen CT has a collaboration contract with ADx Neurosciences, performed contract research or received grants from Probiodrug, AC Immune, Biogen‐Esai, CogRx, Toyama, Janssen prevention center, Boehringer, AxonNeurosciences, Fujirebio, EIP farma, PeopleBio, and Roche (fees paid to Amsterdam UMC).
Supporting information
File S1. Methods/materials on transformations between assays and Results questionnaire implementation blood test Neurofilament light.
Acknowledgment
This project received funding from the Selfridges Group Foundation administered by the Weston Brain Institute and ZonMw Verspreiding en implementatie impuls (VIMP #7330502061).
Funding Information
This project received funding from the Selfridges Group Foundation administered by the Weston Brain Institute and ZonMw Verspreiding en implementatie impuls (VIMP #7330502061).
Funding Statement
This work was funded by Weston Brain Institute ; ZonMw grant VIMP / 7330502061; ZonMw Verspreiding en implementatie impuls grant 7330502061.
References
- 1. Khalil M, Pirpamer L, Hofer E, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun. 2020;11:812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Preische O, Schultz SA, Apel A, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer's disease. Nat Med. 2019;25:277‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14:577‐589. [DOI] [PubMed] [Google Scholar]
- 4. Buhmann C, Lezius S, Potter‐Nerger M, Gerloff C, Kuhle J, Choe CU. Age‐adjusted serum neurofilament predicts cognitive decline in Parkinson's disease (MARK‐PD). Mov Disord. 2022;37:435‐436. [DOI] [PubMed] [Google Scholar]
- 5. Benkert P, Meier S, Schaedelin S, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol. 2022;21:246‐257. [DOI] [PubMed] [Google Scholar]
- 6. Harp C, Thanei GA, Jia X, et al. Development of an age‐adjusted model for blood neurofilament light chain. Ann Clin Transl Neurol. 2022;9:444‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ashton NJ, Janelidze S, Al Khleifat A, et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat Commun. 2021;12:3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bridel C, van Wieringen WN, Zetterberg H, et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta‐analysis. JAMA Neurol. 2019;76:1035‐1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verberk IMW, Koel‐Simmelink M, Twaalfhoven H, et al. Ultrasensitive immunoassay allows measurement of serum neurofilament heavy in multiple sclerosis. Mult Scler Relat Disord. 2021;50:102840. [DOI] [PubMed] [Google Scholar]
- 10. van der Flier WM, Pijnenburg YA, Prins N, et al. Optimizing patient care and research: the Amsterdam dementia cohort. J Alzheimers Dis. 2014;41:313‐327. [DOI] [PubMed] [Google Scholar]
- 11. Meeter LHH, Vijverberg EG, Del Campo M, et al. Clinical value of neurofilament and phospho‐tau/tau ratio in the frontotemporal dementia spectrum. Neurology. 2018;90:e1231‐e1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Willemse EAJ, Scheltens P, Teunissen CE, Vijverberg EGB. A neurologist's perspective on serum neurofilament light in the memory clinic: a prospective implementation study. Alzheimers Res Ther. 2021;13:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gravesteijn G, Rutten JW, Verberk IMW, et al. Serum neurofilament light correlates with CADASIL disease severity and survival. Ann Clin Transl Neurol. 2019;6:46‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oosterveld LP, Verberk IMW, Majbour NK, et al. CSF or serum neurofilament light added to alpha‐synuclein panel discriminates Parkinson's from controls. Mov Disord. 2020;35:288‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Teunissen C, Menge T, Altintas A, et al. Consensus definitions and application guidelines for control groups in cerebrospinal fluid biomarker studies in multiple sclerosis. Mult Scler. 2013;19:1802‐1809. [DOI] [PubMed] [Google Scholar]
- 16. Teunissen CE, Petzold A, Bennett JL, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology. 2009;73:1914‐1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Willemse EA, Koel‐Simmelink MJ, Durieux‐Lu S, van der Flier WM, Teunissen CE. Standard biobanking conditions prevent evaporation of body fluid samples. Clin Chim Acta. 2015;442:141‐145. [DOI] [PubMed] [Google Scholar]
- 18. Kuhle J, Barro C, Andreasson U, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med. 2016;54:1655‐1661. [DOI] [PubMed] [Google Scholar]
- 19. Koenker R. Quantile Regression in R: a Vignette. 2019.
- 20.Accessed December 5, 2021. https://shiny.rstudio.com/ [online]. https://shiny.rstudio.com/
- 21. de Wolf F, Ghanbari M, Licher S, et al. Plasma tau, neurofilament light chain and amyloid‐beta levels and risk of dementia; a population‐based cohort study. Brain. 2020;143:1220‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Quanterix Granted Breakthrough Device Designation from U.S . FDA for NfL test for MS|Quanterix [online]. https://www.quanterix.com/press‐releases/quanterix‐granted‐breakthrough‐device‐designation‐from‐us‐fda‐for‐nfl‐test‐for‐multiple‐sclerosis/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1. Methods/materials on transformations between assays and Results questionnaire implementation blood test Neurofilament light.


