Abstract
Objectives
Concentrations of amyloid‐β peptides (Aβ42/Aβ40) and neurofilament light (NfL) can be measured in plasma or cerebrospinal fluid (CSF) and are associated with Alzheimer’s disease brain pathology and cognitive impairment. This study directly compared plasma and CSF measures of Aβ42/Aβ40 and NfL as predictors of cognitive decline.
Methods
Participants were 65 years or older and cognitively normal at baseline with at least one follow‐up cognitive assessment. Analytes were measured with the following types of assays: plasma Aβ42/Aβ40, immunoprecipitation‐mass spectrometry; plasma NfL, Simoa; CSF Aβ42/Aβ40, automated immunoassay; CSF NfL plate‐based immunoassay. Mixed effects models evaluated the global cognitive composite score over a maximum of 6 years as predicted by the fluid biomarkers.
Results
Analyses included 371 cognitively normal participants, aged 72.7 ± 5.2 years (mean ± standard deviation) with an average length of follow‐up of 3.9 ± 1.6 years. Standardized concentrations of biomarkers were associated with annualized cognitive change: plasma Aβ42/Aβ40, 0.014 standard deviations (95% confidence intervals 0.002 to 0.026); CSF Aβ42/Aβ40, 0.020 (0.008 to 0.032); plasma Nfl, −0.018 (−0.030 to −0.005); and CSF NfL, −0.024 (−0.036 to −0.012). Power analyses estimated that 266 individuals in each treatment arm would be needed to detect a 50% slowing of decline if identified by abnormal plasma measures versus 229 for CSF measures.
Interpretation
Both plasma and CSF measures of Aβ42/Aβ40 and NfL predicted cognitive decline. A clinical trial that enrolled individuals based on abnormal plasma Aβ42/Aβ40 and NfL levels would require only a marginally larger cohort than if CSF measures were used.
Introduction
The pathological hallmarks of Alzheimer’s disease (AD)—amyloid plaques, neurofibrillary tangles, and neuronal degeneration—accumulate for decades prior to the onset of dementia symptoms. 1 , 2 The presence of AD pathology can be assessed using cerebrospinal fluid (CSF) assays or neuroimaging techniques such as positron emission tomography (PET) or nuclear magnetic resonance imaging (MRI). These biomarkers can stratify an individual's risk for future cognitive decline and/or identify high‐risk individuals for recruitment into clinical trials. 3 , 4 , 5 Individuals with subtle evidence of cognitive change, even if clinically normal, are at high risk of developing dementia. 6 , 7
Unfortunately, lumbar punctures and brain imaging are burdensome and expensive, limiting their use, especially when serial assessments are required. Recently, high‐performance blood‐based AD biomarkers have been developed that provide practical advantages over CSF or PET imaging, including a reduction in burden and cost. Multiple studies have demonstrated that plasma Aβ42/Aβ40 as measured by high precision assays has high correspondence with amyloid PET imaging. 8 , 9 , 10 , 11 A variety of plasma p‐tau isoforms have been identified that have high correspondence with amyloid PET, discriminate AD from other disorders, and predict progression to dementia. 12 , 13 , 14 Finally, plasma neurofilament light chain (NfL) correlates with brain imaging metrics, AD risk factors, and cognitive performance 15 , 16 , 17 , 18 , 19 . Together, these results demonstrate that plasma Aβ42/Aβ40, p‐tau isoforms, and NfL reflect underlying AD pathology and may be useful prognostic biomarkers of disease pathology. Importantly, analytes may have differential performance based on the disease stage of the cohort under investigation. In cohorts of cognitively normal individuals, single molecule array (Simoa) measures of p‐tau181 may be less accurate in the detection of PET amyloid status whereas mass spectrometry measures of Aβ42/Aβ40 perform relatively well. 20 , 21
Although multiple studies have examined the relationship of cognitive decline with either plasma Aβ42/Aβ40 22 , 23 , 24 or plasma NfL, 15 , 25 , 26 , 27 it is unclear whether plasma measures of Aβ42/Aβ40 or NfL perform similarly to CSF measures of these analytes. Further, there are major differences in assay performance, especially for plasma Aβ42/Aβ40 assays, 28 , 29 and there have not been any well‐powered studies of cognitive decline as predicted by high‐performance measurements of plasma Aβ42/Aβ40. To date, few studies have directly compared plasma and CSF biomarkers in predicting cognitive change in a single cohort, and they have overall shown similarity between CSF and plasma in the ability to predict decline. Mielke et al. (2019) 15 compared CSF to plasma NfL and found that although effect sizes were similar across the two analytes, only plasma NfL (not CSF) was significantly associated with cognition. Conversely, Verberk et al. (2020) 23 found that plasma and CSF markers of amyloid performed similarly to one another, whereas only CSF markers of total tau predicted cognitive change (plasma total tau did not). Finally, using model fit comparisons, Cullen et al. (2021) 25 found that CSF measures outperformed plasma measures in predicting longitudinal decline, although this analysis used a lower performance measure of plasma Aβ42/Aβ40.
Given the variable performance of fluid biomarker assays that limited the interpretation of previous studies, we aimed to evaluate cognitive decline as predicted by the best available measures of each analyte. We used plasma Aβ42/Aβ40 measurements from a high‐performance immunoprecipitation‐mass spectrometry assay and CSF Aβ42/Aβ40 measurements from a fully automated immunoassay. Plasma NfL was measured with Single Molecule (Simoa) technology, and a well‐validated plate‐based immunoassay was used to measure CSF NfL. We examined how these measures predicted cognitive decline in initially cognitively normal older participants. We hypothesized that lower Aβ42/Aβ40 and higher NfL would predict cognitive decline. Moreover, we hypothesized that the degree of decline associated with plasma and CSF measures would be similar. Finally, we hypothesized that combining Aβ42/Aβ40 and NfL would improve models of cognitive decline. 30
Methods
Participants
This study analyzed biofluid samples and clinical/cognitive data from research participants enrolled in studies of memory and aging at the Charles F. and Joanne Knight Alzheimer Disease Research Center (ADRC) at Washington University in St. Louis. To be included in the study, participants were required to be at least 65 years of age, be rated as clinically normal at the baseline sample collection, have existing data on plasma and CSF Aβ42/Aβ40 and NfL from samples collected at the same visit, have a cognitive assessment near the time of fluid collection, and have at least one follow‐up cognitive assessment.
Clinical and cognitive assessments
The presence and severity of dementia were evaluated with the Clinical Dementia Rating® (CDR®) scale. 31 A CDR of 0 is cognitively normal with an absence of dementia symptoms; a CDR of 0.5, 1, 2, and 3 indicate very mild, mild, moderate, and severe dementia, respectively. All participants in the present study were rated as CDR 0 at baseline when plasma and CSF samples were obtained.
A comprehensive cognitive battery was administered. Since the cognitive battery has changed slightly over time, a global cognitive composite was formulated using tests that have been administered to all participants in the same format over the past two decades: the Free and Cued Selective Reminding Test, 32 Category Fluency for Animals, 33 Trailmaking Parts A and B, 34 WMS‐R associate learning test, 35 and Digit Symbol Substitution test from the Wechsler Adult Intelligence Scale‐Revised. 36 These tests cover a wide range of cognitive domains, exhibit adequate psychometric properties (e.g., minimal ceiling or floor effects) and are broadly similar to tests used in composite cognitive endpoints in ongoing or recently completed clinical trials. 37 , 38 Outcomes of each of the six tests were z‐scored to the sample at baseline, scaled such that lower scores indicated worse performance, and then averaged to form the global cognitive composite. If a participant was missing two or more of the component tests, the time point was removed prior to analysis.
Standard protocol approvals, registrations, and patient consents
Written informed consent was obtained from all participants and their study partners. All procedures were approved by Washington University's Human Research Protection Office.
Plasma and CSF collection and processing
CSF and blood samples were collected at approximately 8 am following overnight fasting as previously described. 8 , 39 Plasma Aβ42 and Aβ40 were measured in the C2N Diagnostics commercial laboratory with an immunoprecipitation‐mass spectrometry assay (St. Louis, MO, USA). 40 Plasma NfL was measured with Quanterix Nf‐Light assay kits on an HD‐X analyzer. Concentrations of CSF Aβ40, Aβ42, total tau (t‐tau), and tau phosphorylated at 181 (p‐tau181) were measured by chemiluminescent enzyme immunoassay using a fully automated platform (LUMIPULSE G1200, Fujirebio, Malvern, PA, USA). CSF NfL was measured via commercial ELISA kit (UMAN Diagnostics, Umeå, Sweden). APOE genotype was determined by genotyping rs7412 and rs429358 with Taqman genotyping technology. 41
Statistical analysis
Baseline age and the biomarkers were z‐scored to the sample at baseline (see values in Table 1) to facilitate comparisons between the different biomarkers. Plasma and CSF NfL both were highly skewed and thus were transformed with the natural logarithm to approximate normality prior to calculation of the z‐score. Effects were reported as a mean estimate with an associated 95% confidence interval. All analyses were conducted using the lme4 package 42 in the R statistical computing environment 43 version 4.0.5.
Table 1.
Participant characteristics at the time of baseline plasma/CSF collection.
|
All participants N = 371 |
Amyloid negative N = 229 |
Amyloid Positive N = 142 |
p‐value = | ||||
|---|---|---|---|---|---|---|---|
| Characteristic | Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 72.7 | 5.2 | 71.8 | 4.9 | 74.1 | 5.4 | <0.001 |
| Education (years) | 16.1 | 2.6 | 16.1 | 2.5 | 16.0 | 2.6 | 0.57 |
| Race | 0.001 | ||||||
| Black or African American | 34 | NA | 30 | NA | 4 | NA | |
| White | 337 | NA | 199 | NA | 138 | NA | |
| Sex (% Female) | 51% | NA | 48% | NA | 56% | NA | 0.16 |
| APOE ε4 status (ε4 carrier) | 35% | NA | 23% | NA | 54% | NA | <0.001 |
| Mini‐Mental State Exam | 29.1 | 1.3 | 29.1 | 1.2 | 29.0 | 1.4 | 0.47 |
| Interval between clinical visit and sample collection (years) | 0.24 | 0.2 | 0.23 | 0.2 | 0.24 | 0.18 | 0.90 |
| Number of visits* | 5.4 | 2.7 | 5.3 | 2.7 | 5.5 | 2.7 | 0.49 |
| Cognitive follow‐up (years) complete dataset | 5.1 | 2.9 | 5.0 | 2.9 | 5.2 | 2.8 | 0.44 |
| Cognitive follow‐up (years) limited dataset | 3.9 | 1.6 | 3.8 | 1.6 | 4.0 | 1.5 | 0.15 |
| CSF Aβ42/Aβ40 | 0.07 | 0.02 | 0.09 | 0.01 | 0.05 | 0.01 | <0.001 |
| Plasma Aβ42/Aβ40 | 0.10 | 0.01 | 0.1 | 0.007 | 0.09 | 0.007 | <0.001 |
| CSF total tau (pg/ml) | 355.5 | 220.2 | 288.0 | 179.9 | 464.3 | 235.8 | <0.001 |
| CSF p‐tau181 (pg/ml) | 46.8 | 28.9 | 34.3 | 11.8 | 67.0 | 36.2 | <0.001 |
| CSF NfL (pg/ml) | 878.1 | 488.5 | 841.0 | 551.8 | 937.9 | 357.9 | 0.04 |
| CSF NfL (log) | 6.7 | 0.4 | 6.6 | 0.4 | 6.8 | 0.4 | <0.001 |
| Plasma NfL (pg/ml) | 14.0 | 6.8 | 12.8 | 6.4 | 15.9 | 7.0 | <0.001 |
| Plasma NfL (log) | 2.5 | 0.4 | 2.5 | 0.4 | 2.7 | 0.4 | <0.001 |
Note: Amyloid positivity was defined by a CSF Aβ42/Aβ40 of <0.0673. Continuous variables were tested using two‐sample t‐tests and categorical variables were tested with a chi‐square test.
The first set of analyses used the complete dataset to explore the relationships between biomarkers and cognition. The global cognitive composite was modeled using the following terms: age at baseline, self‐identified race, APOE ε4 carrier status (ε4 allele non‐carrier was the reference group), self‐identified sex (male was the reference group), years of education, years since baseline (hereafter referred to as “time”), a given biomarker (plasma Aβ42/Aβ40, plasma NfL, CSF Aβ42/Aβ40, or CSF NfL), and the interaction between the biomarker and time. Given the relatively long follow‐up available for some participants (up to 12 years in a few cases), a time‐squared (time 2 ) term was included to allow for nonlinear trajectories. All models included a random intercept and random slope of time. Random slopes of the time‐squared term were not included due to issues with model convergence.
The second set of analyses using a limited dataset were performed to evaluate cognitive change over a shorter time period, as would be more relevant to clinical trials. These analyses included only cognitive assessments obtained within 6 years of the initial sample collection. Models did not include the time 2 term, to facilitate model interpretation and comparisons. To examine the effect of combining Aβ42/Aβ40 and NfL, additional models were examined that included both analytes within a given modality (i.e., plasma or CSF), and all two and three‐way interactions between the biomarkers and time. CSF Aβ42/Aβ40 status (positive is <0.0673) was based on the value with the highest combined sensitivity and specificity for amyloid PET status in the entire Knight ADRC. 44 Plasma Aβ42/Aβ40 status (positive is <0.101) was based on the value with the highest combined sensitivity and specificity with CSF status in the current cohort, which ensured the cut‐offs for plasma and CSF were comparable. There is no validated reference standard to establish NfL cut‐offs, so positive NfL status was defined as the value greater than one standard deviation above the sample mean of the log‐transformed variable, which corresponded to >1,193 pg/ml for CSF NfL and >19.5 pg/ml for plasma NfL. For a sensitivity analysis, a cut‐off of 1.5 standard deviations above the mean was examined (Appendix 1). The estimated rates of change over 6 years for participants who were in the biomarker‐positive groups were used in a power analysis to determine the number of participants that would be needed to detect a 50% slowing in the rate of cognitive change across a 6‐year clinical trial with 80% power.
Data availability policy
Data are available to qualified investigators upon request to the Knight ADRC (https://knightadrc.wustl.edu/Research/ResourceRequest.htm).
Results
Participant characteristics: A total of 373 participants met inclusion criteria for the study. All individuals self‐identified their race as either Black/African American or non‐Hispanic White, except one participant who identified their race as “other.” Due to the importance of controlling for potential differences associated with race, this participant was removed prior to analysis. Additionally, one person had a several‐year gap between their initial assessment and subsequent follow‐up so they were also removed, leaving 371 individuals in the study cohort. In the final cohort that included all follow‐up cognitive assessments (complete dataset), the participants had an average baseline age of 72.7 ± 5.2 years (mean ± standard deviation), 51% were female, 35% were APOE ε4 carriers, and 38% were amyloid positive (Table 1). In the complete dataset, the average number of visits was 5.4 ± 2.7 and the average length of follow‐up was 5.1 ± 2.9 years with a range from 0.9 to 12.3 years. In the limited dataset, which included only cognitive assessments obtained within 6 years of sample collection, the average number of visits was 4.4 ± 1.5 and the average length of follow‐up was 3.9 years ±1.6. All other variables were the same across the complete and limited datasets. Plasma Aβ42/Aβ40 was correlated with CSF Aβ42/Aβ40 (Spearman's rho = 0.63, CI = 0.56 to 0.69, p < 0.001) and plasma NfL was correlated with CSF NfL (Spearman's rho = 0.49, CI = 0.41 to 0.57, p < 0.001).
Global cognition as a function of time, age, APOE ε4 carrier status, sex, and race
Analyses of the complete dataset were performed to explore the relationships between biomarkers and cognition. Regardless of which biomarker the model included, the effects of time, age, APOE ε4 carrier status, sex, and race were consistent (Figs. S1–S2; Tables S1–S4). Performance on the global composite declined as a function of time‐squared (time2, p < 0.0001), demonstrating that the rate of cognitive decline accelerates over time. As has previously been reported in our cohort, baseline performance on the cognitive composite was lower for older individuals (p < 0.001) and for men (p < 0.001). 45 After controlling for the other covariates, APOE ε4 carrier status did not influence baseline cognition. In these analyses, African American participants had lower baseline scores, potentially due to biases in clinical/cognitive testing instruments, differences in social determinants of health or other factors that may be associated with racial group. 46 , 47 , 48 , 49 Two‐way interactions between race and time, and three‐way interactions between race, time, and biomarker levels were evaluated and found to be not significant, so these terms were not included in the final models.
Global cognition as a function of CSF and plasma Aβ42/Aβ40
In the complete dataset that included all follow‐up cognitive assessments, higher (more normal) CSF Aβ42/Aβ40 was associated with better performance on the global cognitive composite at baseline (β = 0.10 (95% confidence intervals 0.04 to 0.17), p = 0.003, Table S1). There was a significant interaction between CSF Aβ42/Aβ40 and time2 (β = 0.004 (0.003 to 0.006), p < 0.001), whereby participants with lower (more abnormal) CSF Aβ42/Aβ40 declined more quickly, and this rate of decline accelerated over time (see Fig. 1A). In contrast to the model for CSF Aβ42/Aβ40, there was no significant association between plasma Aβ42/Aβ40 and cognition at the baseline assessment (p = 0.06, Table S2). There was a significant association between plasma Aβ42/Aβ40 and time 2 (β = 0.002 (0.000 to 0.004), p = 0.016, see Figure 1B), whereby participants with lower (more abnormal) plasma Aβ42/Aβ40 declined more quickly.
Figure 1.
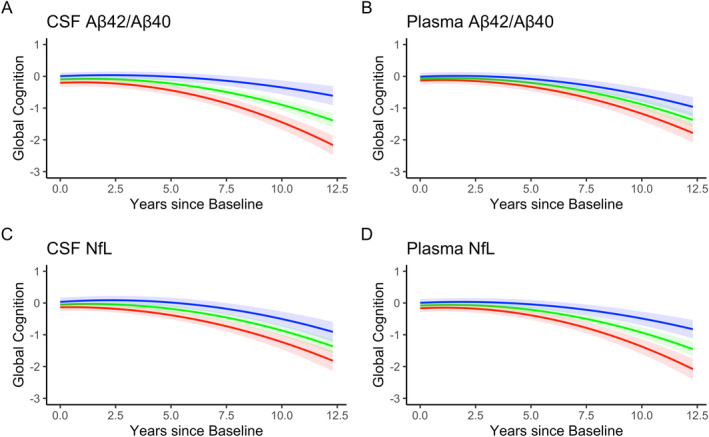
Associations between each biomarker and global cognitive decline in the complete data set. Trend lines represent “abnormal” (red line, 1 standard deviation below the mean for Aβ42/Aβ40 and one standard deviation above the mean for NfL), “average” (green line, mean value), and “above normal” (blue line, 1 standard deviation above the mean for Aβ42/Aβ40 and one standard deviation below the mean for NfL) for each biomarker. These are descriptive terms for visualization only and do not reflect clinical or pathological cut points. The quadratic rate of change was significant in all models and the biomarkers moderated the quadratic term in all models except for CSF NfL.
Analyses were also performed using the limited dataset that only included cognitive assessments within 6 years of sample collection to increase the relevance to clinical trials and facilitate model interpretation (Figs. S3–S5). As in the complete dataset, higher (more normal) CSF Aβ42/Aβ40 levels were associated with better baseline cognitive performance (β = 0.09 (0.03 to 0.16), p = 0.006, Table 2). CSF Aβ42/Aβ40 levels were also associated with the linear rate of cognitive change (β = 0.020 (0.008 to 0.032), p = 0.001, Table 2) such that for every 1 standard deviation decrease in the Aβ42/Aβ40 ratio, cognition declined by an additional 0.020 standard deviations per year. Plasma Aβ42/Aβ40 was not significantly associated with baseline cognitive performance, but lower (more abnormal) plasma Aβ42/Aβ40 was associated with cognitive decline (β = 0.014 (0.002 to 0.026), p = 0.02, Table 3).
Table 2.
Mixed model for the global cognitive composite in the limited dataset (≤6 years of follow‐up) as predicted by CSF Aβ42/Aβ40. Age and CSF Aβ42/Aβ40 were standardized.
| Predictors | Estimates | Std. Error | df | CI | p |
|---|---|---|---|---|---|
| (Intercept) | −0.829 | 0.206 | 370.515 | −1.234 to −0.424 | <0.001 |
| Age [Years] | −0.199 | 0.032 | 370.538 | −0.262 to −0.137 | <0.001 |
| Education [Years] | 0.047 | 0.012 | 370.605 | 0.023 to 0.070 | <0.001 |
| Race [Black] | −0.594 | 0.107 | 374.482 | −0.804 to −0.384 | <0.001 |
| APOE ε4 status (ε4 carrier) | 0.064 | 0.069 | 370.709 | −0.072 to 0.200 | 0.353 |
| Sex [Female] | 0.292 | 0.061 | 370.706 | 0.171 to 0.412 | <0.001 |
| Time | −0.020 | 0.006 | 251.317 | −0.032 to −0.008 | 0.001 |
| CSF Aβ42/Aβ40 | 0.094 | 0.034 | 370.668 | 0.027 to 0.161 | 0.006 |
| Time * CSF Aβ42/Aβ40 | 0.020 | 0.006 | 260.175 | 0.008 to 0.032 | 0.001 |
| Random effects | |||||
| σ2 | 0.06 | ||||
| τ00 id | 0.30 | ||||
| τ11 id.time | 0.01 | ||||
| ρ01 id | 0.17 | ||||
| ICC | 0.86 | ||||
| N id | 371 | ||||
| Observations | 1637 | ||||
| Marginal R2/Conditional R2 | 0.203/0.887 | ||||
Table 3.
Mixed model for the global cognitive composite in the limited dataset (≤6 years of follow‐up) as predicted by plasma Aβ42/Aβ40. Age and plasma Aβ42/Aβ40 were standardized.
| Predictors | Estimates | Std. Error | df | CI | p |
|---|---|---|---|---|---|
| (Intercept) | −0.816 | 0.208 | 370.479 | −1.225 to −0.408 | <0.001 |
| Age [Years] | −0.216 | 0.031 | 370.833 | −0.277 to −0.155 | <0.001 |
| Education [Years] | 0.048 | 0.012 | 370.596 | 0.024 to 0.071 | <0.001 |
| Race [Black] | −0.583 | 0.108 | 374.460 | −0.796 to −0.370 | <0.001 |
| APOE ε4 status (ε4 carrier) | 0.011 | 0.066 | 370.815 | −0.119 to 0.141 | 0.868 |
| Sex [Female] | 0.267 | 0.062 | 370.724 | 0.144 to 0.389 | <0.001 |
| Time | −0.021 | 0.006 | 247.306 | −0.033 to −0.008 | 0.001 |
| Plasma Aβ42/Aβ40 | 0.052 | 0.033 | 369.660 | −0.012 to 0.116 | 0.114 |
| Time * Plasma Aβ42/Aβ40 | 0.014 | 0.006 | 240.178 | 0.002 to 0.026 | 0.021 |
| Random effects | |||||
| σ2 | 0.06 | ||||
| τ00 id | 0.30 | ||||
| τ11 id.time | 0.01 | ||||
| ρ01 id | 0.18 | ||||
| ICC | 0.86 | ||||
| N id | 371 | ||||
| Observations | 1637 | ||||
| Marginal R2/Conditional R2 | 0.186/0.886 | ||||
Global cognition as a function of CSF and plasma NfL
In the complete dataset, participants with higher (more abnormal) CSF NfL performed worse (β = −0.09 (−0.16 to −0.02), p = 0.016, Table S3) on the global cognitive composite at baseline; there was a similar association with plasma NfL (β = −0.09 (−0.16 to −0.02), p = 0.011, Table S4). There was a significant interaction between CSF NfL and linear time (β = −0.019 (−0.04 to −0.001), p = 0.038, Fig. 1C) but not time2 (p = 0.36). Plasma NfL interacted with time 2 but not time (β = −0.004 (−0.006 to −0.002), p < 0.001, Fig. 1D).
In the limited dataset, there was an association between worse baseline cognitive performance and higher (more abnormal) CSF NfL (β = −0.08 (−0.15 to −0.011), p = 0.023) and plasma NfL (β = −0.07 (−0.14 to −0.01), p = 0.03). A faster rate of cognitive decline was associated with higher (more abnormal) CSF NfL (β = −0.024 (−0.036 to −0.012), p < 0.001, Table 4) and plasma NfL (β = −0.018 (−0.030 to ‐ 0.005), p = 0.005, Table 5).
Table 4.
Mixed model for the global cognitive composite in the limited dataset (≤6 years of follow‐up) as predicted by CSF NfL. Age was standardized. CSF NfL was transformed with the natural logarithm and then standardized.
| Predictors | Estimates | Std. Error | df | CI | p |
|---|---|---|---|---|---|
| (Intercept) | −0.836 | 0.207 | 370.819 | −1.242 to −0.429 | <0.001 |
| Age [Years] | −0.189 | 0.034 | 370.167 | −0.255 to −0.122 | <0.001 |
| Education [Years] | 0.050 | 0.012 | 370.904 | 0.027 to 0.074 | <0.001 |
| Race [Black] | −0.581 | 0.107 | 375.046 | −0.791 to −0.370 | <0.001 |
| APOE ε4 status (ε4 carrier) | −0.020 | 0.063 | 371.166 | −0.145 to 0.105 | 0.754 |
| Sex [Female] | 0.238 | 0.064 | 371.535 | 0.112 to 0.364 | <0.001 |
| Time | −0.021 | 0.006 | 251.864 | −0.033 to −0.009 | 0.001 |
| CSF NfL | −0.080 | 0.035 | 369.351 | −0.150 to −0.011 | 0.023 |
| Time * CSF NfL | −0.024 | 0.006 | 255.508 | −0.036 to −0.012 | <0.001 |
| Random effects | |||||
| σ2 | 0.06 | ||||
| τ00 id | 0.30 | ||||
| τ11 id.time | 0.01 | ||||
| ρ01 id | 0.18 | ||||
| ICC | 0.86 | ||||
| N id | 371 | ||||
| Observations | 1637 | ||||
| Marginal R2/Conditional R2 | 0.207/0.889 | ||||
Table 5.
Mixed model for the global cognitive composite in the limited dataset (≤6 years of follow‐up) as predicted by plasma NfL. Age was standardized. Plasma NfL was transformed with the natural logarithm and then standardized.
| Predictors | Estimates | Std. Error | df | CI | p |
|---|---|---|---|---|---|
| (Intercept) | −0.857 | 0.207 | 370.365 | −1.265 to −0.449 | <0.001 |
| Age [Years] | −0.183 | 0.034 | 370.117 | −0.250 to −0.115 | <0.001 |
| Education [Years] | 0.050 | 0.012 | 370.585 | 0.026 to 0.074 | <0.001 |
| Race [Black] | −0.558 | 0.106 | 375.162 | −0.767 to −0.349 | <0.001 |
| APOE ε4 status (ε4 carrier) | −0.012 | 0.063 | 370.760 | −0.137 to 0.113 | 0.850 |
| Sex [Female] | 0.292 | 0.061 | 370.629 | 0.171 to 0.413 | <0.001 |
| Time | −0.021 | 0.006 | 248.359 | −0.034 to −0.009 | 0.001 |
| Plasma NfL | −0.077 | 0.034 | 372.746 | −0.144 to −0.010 | 0.025 |
| Time * Plasma NfL | −0.018 | 0.006 | 270.812 | −0.030 to −0.005 | 0.005 |
| Random effects | |||||
| σ2 | 0.06 | ||||
| τ00 id | 0.30 | ||||
| τ11 id.time | 0.01 | ||||
| ρ01 id | 0.21 | ||||
| ICC | 0.86 | ||||
| N id | 371 | ||||
| Observations | 1637 | ||||
| Marginal R2/Conditional R2 | 0.193/0.888 | ||||
Combining Aβ42/Aβ40 and NfL in plasma and CSF
Using the limited dataset to facilitate interpretation of results, models of global cognition including either plasma or CSF measures were examined that included both Aβ42/Aβ40 and NfL, as well as the two‐way interaction between Aβ42/Aβ40 and NfL, and the three‐way interaction between Aβ42/Aβ40, NfL, and time. The interactions between Aβ42/Aβ40 and NfL, and between Aβ42/Aβ40, NfL, and time, were not significant for either CSF (p = 0.08) or plasma models (p = 0.93).
For the model including both CSF Aβ42/Aβ40 and CSF NfL, Aβ42/Aβ40 was significantly associated with global cognition at baseline (β = 0.07 (0.01 to 0.14), p = 0.04), as was NfL (β = −0.07 (−0.14 to −0.003), p = 0.04). Both CSF Aβ42/Aβ40 by time (β = 0.015, (0.003 to 0.027), p = 0.02), and CSF NfL by time (β = −0.023, (−0.035 to −0.01), p < 0.001) were significantly associated with global cognition, suggesting that each biomarker is independently associated with cognitive decline (Fig. 2). For the model including both plasma Aβ42/Aβ40 and plasma NfL, only NfL was associated with cognition at baseline (β = −0.07, (−0.14 to −0.005), p = 0.04). There was a trend toward an association between global cognition and plasma Aβ42/Aβ40 by time (β = 0.012, (−0.000 to 0.024), p = 0.06) and there was a significant association between global cognition and plasma NfL by time (β = −0.016, 95% CI −0.028 to −0.003, p = 0.012), again suggesting that each biomarker is independently associated with cognitive decline.
Figure 2.
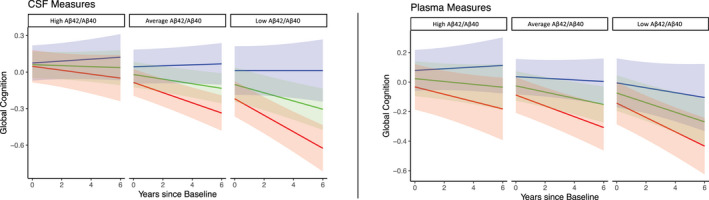
Model predicted rates of change as a function of Aβ42/Aβ40 and NfL in the CSF or plasma in the limited dataset. Trend lines represent “abnormal” NfL (red line, one standard deviation above the mean), “average” (green line, mean value), and “normal” NfL (blue line, one standard deviation below the mean). Panels represent above normal, “High” Aβ42/Aβ40 (one standard deviation above the mean), average, or “abnormal”, “Low” Aβ42/Aβ40 (one standard deviation below the mean). These are descriptive terms for visualization only and do not reflect clinical or pathological cut points.
Power analysis
Based on estimated mean and standard deviations of annual change in the limited dataset for individuals classified as positive on different biomarkers (see Table 6), 695 plasma Aβ42/Aβ40 positive participants per treatment arm would be needed to detect a 50% slowing in cognitive decline over 6 years, whereas 502 CSF Aβ42/Aβ40 positive participants per treatment arm would be needed to detect the same effect. If participants were required to be positive on both plasma Aβ42/Aβ40 and plasma NfL, only 266 participants would be needed. If participants were CSF Aβ42/Aβ40 and CSF NfL positive, only 229 individuals would be needed.
Table 6.
Number of participants, estimated rates of change, and sample size needed to detect 50% slowing of decline for each biomarker category.
| Positive based on | N out of total 372 participants | Estimated annual rate (and SD)** | Effect size (mean/SD) | n per arm* |
|---|---|---|---|---|
| Plasma Aβ42/Aβ40 | 211 (57%) | −0.031 (0.103) | −0.300 | 695 |
| CSF Aβ42/Aβ40 | 142 (38%) | −0.039 (0.111) | −0.354 | 502 |
| Plasma NfL | 52 (14%) | −0.037 (0.111) | −0.331 | 574 |
| CSF NfL | 53 (14%) | −0.081 (0.155) | −0.519 | 233 |
| Plasma Aβ42/Aβ40 and NfL | 37 (10%) | −0.057 (0.118) | −0.487 | 266 |
| CSF Aβ42/Aβ40 and NfL | 32 (8.6%) | −0.118 (0.225) | −0.525 | 229 |
n per arm refers to the sample size needed to detect 50% reduction in cognitive decline with 80% power for a 6‐year trial.
Effect sizes were estimated from a linear mixed effect model on the limited dataset.
Cognitive domain analyses
The complete dataset was used to model three cognitive sub‐domains: episodic memory, executive function, and language (see Tables S5–S16 ). In general, the plasma and CSF biomarkers were not associated with decline in the language or executive function domains, however, there were significant associations between both Aβ42/Aβ40 and NfL in predicting decline in episodic memory.
Discussion
The primary goal of this study was to directly compare plasma versus CSF measures of Aβ42/Aβ40 and NfL as predictors of decline on a global cognitive composite in an initially cognitively normal older cohort. We found that both plasma and CSF measures of Aβ42/Aβ40 and NfL independently predicted decline in a global cognitive composite. This result suggests that plasma measures of these analytes may identify individuals at high risk of cognitive decline, which is highly relevant to AD clinical trials.
Despite the overall similar findings in plasma and CSF measures, at least in the models of the limited dataset where linear trajectories were more easily compared across analytes, the CSF analytes yielded larger estimates of decline than the associated plasma markers. If selection of participants for a research study were based on either plasma Aβ42/Aβ40 or Nfl, substantially more participants would be required to enroll in a clinical trial compared to if screening were performed with CSF measures. However, if selection were based on both plasma Aβ42/Aβ40 and NfL, the trial would require only a marginal increase in sample size relative to screening based on both CSF markers.
This study used a high precision assay for plasma Aβ42/Aβ40, and currently, available plasma Aβ42/Aβ40 assays have widely varying performance. The small difference between normal and abnormal levels of plasma Aβ42/Aβ40 requires a high precision assay to observe the robust effects seen in this study. In a head‐to‐head comparison of several plasma Aβ42/Aβ40 assays, immunoprecipitation‐coupled mass spectrometry methods (as used in the current study) significantly outperformed other methods in discriminating individuals with abnormal amyloid based on CSF28. The use of a low‐performance plasma Aβ42/Aβ40 assay would likely result in inferior performance of plasma Aβ42/Aβ40 relative to CSF Aβ42/Aβ40.
Although the Amyloid‐Tau‐Neurodegeneration (ATN) model of Alzheimer’s disease 3 might suggest that measures of tauopathy and neurodegeneration would be more tightly correlated with cognitive decline than amyloid, 30 , 50 we found that plasma and CSF Aβ42/Aβ40 were associated with cognitive decline to a similar degree as NfL. Notably, NfL is a relatively non‐specific biomarker of neuroaxonal injury, and it may be elevated in some individuals with non‐AD conditions that may or may not cause progressive cognitive decline. Therefore, NfL may indicate risk of cognitive decline from one of many etiologies, including dementia caused by mixed etiologies. 51 , 52 Importantly, the variance explained by each biomarker was independent; each biomarker was a significant predictor, and the interaction of Aβ42/Aβ40, NfL, and time was not significant.
Our study has significant strengths including a relatively large cohort with measures of Aβ42/Aβ40 and NfL in plasma and CSF samples that were collected at the same session, enabling a head‐to‐head comparison of the analytes. However, several limitations should be noted. Specifically, our sample is highly educated, and the majority of participants identified their race as White, and therefore our results may not readily generalize to the larger population. Moreover, because we defined the “baseline” session as the first biomarker assessment, participants may have had cognitive data available prior to the start of this study. Cognitive practice effects manifest differently in individuals with preclinical AD 53 , and the influence of such practice effects were not examined in the present study.
CSF and PET‐based measures of amyloid, tauopathy, and neurodegeneration have been available for many years. However, high costs, burdens, and perceived invasiveness make frequent acquisition of these measures difficult. High‐performance plasma biomarkers have recently been developed but their ability to track with and predict cognitive change has not been fully described. Our results demonstrate that for studies of moderate length, either plasma or CSF markers predict changes of similar magnitude. These results should encourage more widespread use of plasma markers to reduce costs and minimize the burden on research participants and accelerate the development of effective AD treatments.
Author Contributions
AJA and SES: Design and conceptualization of the study, analysis of data, drafting of the manuscript; YL: Design and conceptualization of the study, analysis of data; RH, KV, JH, PV, TW, MM, KMK, AMF, CX, DMH, JCM, RJB: Acquisition and analysis of data.
Conflicts of Interest
A.J. Aschenbrenner reports no disclosures relevant to this manuscript. S. E. Schindler has analyzed blood‐based biomarker data provided by C2N Diagnostics to Washington University. The PrecivityAD™ test is licensed by C2N Diagnostics and Washington University will receive royalties from this test, but Dr Schindler will not receive personal compensation from it; R.L. Henson reports no disclosures relevant to the manuscript; K Volluz reports no disclosures relevant to the manuscript; J. Hassenstab reports no disclosures relevant to this manuscript; P. Verghese is an employee of C2N Diagnostics, which offers the PrecivityAD™ test described in this paper; T. West is an employee of C2N Diagnostics, which offers the PrecivityAD™ test described in this paper; M.R. Meyer is an employee of C2N Diagnostics, which offers the PrecivityAD™ test described in this paper; K.M. Kirmess is an employee of C2N Diagnostics, which offers the PrecivityAD™ test described in this paper; Y. Li reports no disclosures relevant to the manuscript; A.M. Fagan has received research funding from Biogen, Centene, Fujirebio, and Roche Diagnostics. She is a member of the scientific advisory boards for Roche Diagnostics, Genentech, and Diadem. She consults for DiamiR and Seimens Healthcare Diagnostics Inc.; R.J. Bateman (RJB) and DM Holtzman (DMH) co‐founded C2N Diagnostics. Washington University, Dr. Bateman, and Dr. Holtzman have equity ownership interest in C2N Diagnostics and receive royalty income based on technology (stable isotope labeling kinetics and blood plasma assay) licensed by Washington University to C2N Diagnostics. RJB and DMH receive income from C2N Diagnostics for serving on the scientific advisory board. Washington University, with Dr. Bateman and Dr. Holtzman as co‐inventors, have submitted the US provisional patent application “Plasma Based Methods for Detecting CNS Amyloid Deposition.” RJB consults for Roche, Genentech, AbbVie, Pfizer, Boehringer‐Ingelheim, and Merck; DMH consults for Genentech, Denali, and Cajal Neurosciences C. Xiong consults for Diadem; J.C. Morris, MD is the Chair of the Research Strategy Council of the Cure Alzheimer's Fund.
Supporting information
Table S1. Mixed model for the global cognitive composite in the complete dataset as predicted by CSF Aβ42/Aβ40. Age and CSF Aβ42/Aβ40 were standardized.
Table S2. Mixed model for the global cognitive composite in the complete dataset as predicted by plasma Aβ42/Aβ40. Age and plasma Aβ42/Aβ40 were standardized.
Table S3. Mixed model for the global cognitive composite in the complete dataset as predicted by CSF NfL. Age was standardized. CSF NfL was transformed with the natural logarithm and then standardized.
Table S4. Mixed model for the global cognitive composite in the complete dataset as predicted by plasma NfL. Age was standardized. Plasma NfL was transformed with the natural logarithm and then standardized.
Table S5. Mixed model for the executive function composite in the complete dataset as predicted by CSF Aβ42/Aβ40. Age and CSF Aβ42/Aβ40 were standardized.
Table S6. Mixed model for the executive function composite in the complete dataset as predicted by plasma Aβ42/Aβ40. Age and plasma Aβ42/Aβ40 were standardized.
Table S7. Mixed model for the executive function composite in the complete dataset as predicted by CSF NfL. Age was standardized. CSF NfL was transformed with the natural logarithm and then standardized.
Table S8. Mixed model for the executive function composite in the complete dataset as predicted by plasma NfL. Age was standardized. Plasma NfL was transformed with the natural logarithm and then standardized.
Table S9. Mixed model for the episodic memory composite in the complete dataset as predicted by CSF Aβ42/Aβ40. Age and CSF Aβ42/Aβ40 were standardized.
Table S10. Mixed model for the episodic memory composite in the complete dataset as predicted by plasma Aβ42/Aβ40. Age and plasma Aβ42/Aβ40 were standardized.
Table S11. Mixed model for the episodic memory composite in the complete dataset as predicted by CSF NfL. Age was standardized. CSF NfL was transformed with the natural logarithm and then standardized.
Table S12. Mixed model for the episodic memory composite in the complete dataset as predicted by plasma NfL. Age was standardized. Plasma NfL was transformed with the natural logarithm and then standardized.
Table S13. Mixed model for the language composite in the complete dataset as predicted by CSF Aβ42/Aβ40. Age and CSF Aβ42/Aβ40 were standardized.
Table S14. Mixed model for the language composite in the complete dataset as predicted by plasma Aβ42/Aβ40. Age and plasma Aβ42/Aβ40 were standardized.
Table S15. Mixed model for the language composite in the complete dataset as predicted by CSF NfL. Age was standardized. CSF NfL was transformed with the natural logarithm and then standardized.
Table S16. Mixed model for the language composite in the complete dataset as predicted by plasma NfL. Age was standardized. Plasma NfL was transformed with the natural logarithm and then standardized.
Figure S1. Model predicted rates of change for each of the four biomarkers with raw data in the complete dataset. Trend lines represent “abnormal” (red line, 1 standard deviation below the mean for Aβ42/Aβ40 and one standard deviation above the mean for NfL), “average” (green line, mean value), and “above normal” (blue line, 1 standard deviation above the mean for Aβ42/Aβ40 and one standard deviation below the mean for NfL) for each biomarker. These are descriptive terms for visualization only and do not reflect clinical or pathological cut points. The quadratic rate of change was significant in all models and all biomarkers moderated the quadratic trend except for CSF NfL. Circles represent raw cognitive data points and points from individual participants are connected with black lines.
Figure S2. Model predicted rates of change for each of the four biomarkers with model predicted values in the complete dataset. Trend lines represent “abnormal” (red line, 1 standard deviation below the mean for Aβ42/Aβ40 and one standard deviation above the mean for NfL), “average” (green line, mean value), and “above normal” (blue line, 1 standard deviation above the mean for Aβ42/Aβ40 and one standard deviation below the mean for NfL) for each biomarker. These are descriptive terms for visualization only and do not reflect clinical or pathological cut points. The quadratic rate of change was significant in all models and all biomarkers moderated the quadratic trend except for CSF NfL. Circles represent model predicted cognitive data points and points from individual participants are connected with black lines.
Figure S3. Model predicted rates of change for each of the four biomarkers with raw data in the limited (≤6 years of follow‐up) dataset. Trend lines represent “abnormal” (red line, 1 standard deviation below the mean for Aβ42/Aβ40 and one standard deviation above the mean for NfL), “average” (green line, mean value), and “above normal” (blue line, 1 standard deviation above the mean for Aβ42/Aβ40 and one standard deviation below the mean for NfL) for each biomarker. These are descriptive terms for visualization only and do not reflect clinical or pathological cut points. Circles represent raw cognitive data points and points from individual participants are connected with black lines.
Figure S4. Model predicted rates of change for each of the four biomarkers with model predicted values in the limited (<6 years of follow‐up) dataset. Trend lines represent “abnormal” (red line, 1 standard deviation below the mean for Aβ42/Aβ40 and one standard deviation above the mean for NfL), “average” (green line, mean value), and “above normal” (blue line, 1 standard deviation above the mean for Aβ42/Aβ40 and one standard deviation below the mean for NfL) for each biomarker. These are descriptive terms for visualization only and do not reflect clinical or pathological cut points. Circles represent model predicted cognitive data points and points from individual participants are connected with black lines.
Figure S5. Model predicted rates of change for each of the four biomarkers in the limited (<=6 years of follow‐up) dataset. Trend lines represent “abnormal” (red line, 1 standard deviation below the mean for Aβ42/Aβ40 and one standard deviation above the mean for NfL), “average” (green line, mean value), and “above normal” (blue line, 1 standard deviation above the mean for Aβ42/Aβ40 and one standard deviation below the mean for NfL) for each biomarker. These are descriptive terms for visualization only and do not reflect clinical or pathological cut points.
Acknowledgments
We would like to express our gratitude to the research volunteers who participated in the studies from which these data were obtained and their supportive families. We thank the Clinical, Fluid Biomarker, and Imaging Cores at the Knight Alzheimer Disease Research Center for sample and data collection.
This study was supported by National Institute on Aging grants R01AG070941 (SE Schindler), P30AG066444 (JC Morris), P01AG003991 (JC Morris), P01AG026276 (JC Morris), and R01AG053550 (C Xiong). C2N Diagnostics provided the plasma Aβ42/Aβ40 assays for this study at no cost.
Appendix 1. Sensitivity power analysis using 1.5 SDs to determine NfL positivity.
| Positive based on |
N out of total 372 participants |
Estimated annual rate (and SD)** |
Effect size (mean / SD) |
n per arm* |
|---|---|---|---|---|
| Plasma Aβ42/Aβ40 | 211 (57%) | −0.031 (0.103) | ‐0.300 | 695 |
| CSF Aβ42/Aβ40 | 142 (38%) | −0.039 (0.111) | ‐0.354 | 502 |
| Plasma NfL | 30 (8%) | −0.076 (0.182) | ‐0.418 | 360 |
| CSF NfL | 25 (6.7%) | −0.108 (0.192) | ‐0.564 | 199 |
| Plasma Aβ42/Aβ40 and NfL | 22 (5.9%) | −0.080 (0.201) | ‐0.399 | 393 |
| CSF Aβ42/Aβ40 and NfL | 13 (3.5%) | −0.134 (0.288) | ‐0.464 | 293 |
n per arm refers to the sample size needed to detect 50% reduction in cognitive decline with 80% power for a 6‐year trial.
Effect sizes were estimated from a linear mixed effect model on the limited dataset.
NfL cutoffs were determined using 1.5 standard deviations above the group mean.
Funding Statement
This work was funded by National Institute on Aging grants P01AG003991, P01AG026276, P30AG066444, R01AG053550, and R01AG070941.
References
- 1. Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367(9):795‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Price JL, McKeel DW, Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30(7):1026‐1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jack CR, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87(5):539‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jack CR, Wiste HJ, Weigand SD, et al. Age‐specific and sex‐specific prevalence of cerebral β‐amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50–95 years: a cross‐sectional study. Lancet Neurol. 2017;16(6):435‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roe CM, Fagan AM, Grant EA, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80(19):1784‐1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vos SJ, Xiong C, Visser PJ, et al. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12(10):957‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schindler SE, Bollinger JG, Ovod V, et al. High‐precision plasma β‐amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019;93(17):e1647‐e1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid‐β biomarkers for Alzheimer's disease. Nature. 2018;554(7691):249‐254. [DOI] [PubMed] [Google Scholar]
- 10. West T, Kirmess KM, Meyer MR, et al. A blood‐based diagnostic test incorporating plasma Aβ42/40 ratio, ApoE proteotype, and age accurately identifies brain amyloid status: findings from a multi cohort validity analysis. Mol Neurodegener. 2021;16(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ovod V, Ramsey KN, Mawuenyega KG, et al. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017;13(8):841‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Janelidze S, Mattsson N, Palmqvist S, et al. Plasma P‐tau181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med. 2020;26(3):379‐386. [DOI] [PubMed] [Google Scholar]
- 13. Ashton NJ, Pascoal TA, Karikari TK, et al. Plasma p‐tau231: a new biomarker for incipient Alzheimer's disease pathology. Acta Neuropathol. 2021;141(5):709‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma Phospho‐tau217 for Alzheimer disease vs other neurodegenerative disorders. Jama. 2020;324(8):772‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mielke MM, Syrjanen JA, Blennow K, et al. Plasma and CSF neurofilament light: relation to longitudinal neuroimaging and cognitive measures. Neurology. 2019;93(3):e252‐e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lewczuk P, Ermann N, Andreasson U, et al. Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer's disease. Alz Res Therapy. 2018;10(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benedet AL, Leuzy A, Pascoal TA, et al. Stage‐specific links between plasma neurofilament light and imaging biomarkers of Alzheimer's disease. Brain. 2020;143(12):3793‐3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quiroz YT, Zetterberg H, Reiman EM, et al. Plasma neurofilament light chain in the presenilin 1 E280A autosomal dominant Alzheimer's disease kindred: a cross‐sectional and longitudinal cohort study. Lancet Neurol. 2020;19(6):513‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He L, Morley JE, Aggarwal G, et al. Plasma neurofilament light chain is associated with cognitive decline in non‐dementia older adults. Sci Rep. 2021;11(1):13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keshavan A, Pannee J, Karikari TK, et al. Population‐based blood screening for preclinical Alzheimer's disease in a British birth cohort at age 70. Brain. 2021;144(2):434‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schindler SE, Karikari TK, Ashton NJ, et al. Effect of race on prediction of brain amyloidosis by plasma Aβ42/Aβ40, phosphorylated tau, and neurofilament light. Neurology. 2022;99(3):e245‐e257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sullivan KJ, Blackshear C, Simino J, et al. Association of midlife plasma amyloid‐β Levels with cognitive impairment in late life: the ARIC neurocognitive study. Neurology. 2021;97(11):e1123‐e1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Verberk IMW, Hendriksen HMA, van Harten AC, et al. Plasma amyloid is associated with the rate of cognitive decline in cognitively normal elderly: the SCIENCe project. Neurobiol Aging. 2020;89:99‐107. [DOI] [PubMed] [Google Scholar]
- 24. Giudici KV, de Souto BP, Guyonnet S, et al. Assessment of plasma amyloid‐β 42/40 and cognitive decline among community‐dwelling older adults. JAMA Netw Open. 2020;3(12):e2028634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cullen NC, Leuzy A, Janelidze S, et al. Plasma biomarkers of Alzheimer's disease improve prediction of cognitive decline in cognitively unimpaired elderly populations. Nat Commun. 2021;12(1):3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beydoun MA, Noren Hooten N, Beydoun HA, et al. Plasma neurofilament light as a potential biomarker for cognitive decline in a longitudinal study of middle‐aged urban adults. Transl Psychiatry. 2021;11(1):436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bangen KJ, Thomas KR, Weigand AJ, et al. Elevated plasma neurofilament light predicts a faster rate of cognitive decline over 5 years in participants with objectively‐defined subtle cognitive decline and MCI. Alzheimers Dementia. 2021;17(10):1756‐1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Janelidze S, Teunissen CE, Zetterberg H, et al. Head‐to‐head comparison of 8 plasma amyloid‐β 42/40 assays in Alzheimer disease. JAMA Neurol. 2021;78(11):1375‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zicha S, Bateman RJ, Shaw LM, et al. Comparative analytical performance of multiple plasma Aβ42 and Aβ40 assays and their ability to predict positron emission tomography amyloid positivity. Alzheimers Dement. 2022;alz.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aschenbrenner AJ, Gordon BA, Benzinger TLS, Morris JC, Hassenstab JJ. Influence of tau PET, amyloid PET, and hippocampal volume on cognition in Alzheimer disease. Neurology. 2018;91(9):e859‐e866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412.2‐2412‐a. [DOI] [PubMed] [Google Scholar]
- 32. Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;38(6):900‐903. [DOI] [PubMed] [Google Scholar]
- 33. Goodglass H, Kaplan E. Boston diagnostic AphasiaExamination booklet, III, ORAL EXPRESSION, J. animal naming (fluency in controlled association). An Introduction to Model‐Based Cognitive Neuroscience. Lea & Febiger; 1983. [Google Scholar]
- 34. Armitage SG. An analysis of certain psychological tests used for the evaluation of brain injury. Psychol Monogr. 1945;60(1):1‐48. [Google Scholar]
- 35. Wechsler D. Manual: Wechsler Memory Scale‐ Revised. Psychological Corporation; 1987. [Google Scholar]
- 36. Wechsler D. Manual: Wechsler Adult Intelligence Scale‐ Revised. Psychological Corporation; 1981. [Google Scholar]
- 37. Bateman RJ, Benzinger TL, Berry S, et al. The DIAN‐TU next generation Alzheimer's prevention trial: adaptive design and disease progression model. Alzheimers Dement. 2017;13(1):8‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sperling RA, Rentz DM, Johnson KA, et al. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014;6(228):228fs13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schindler SE, Gray JD, Gordon BA, et al. Cerebrospinal fluid biomarkers measured by Elecsys assays compared to amyloid imaging. Alzheimer's Dementia. 2018;14(11):1460‐1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kirmess KM, Meyer MR, Holubasch MS, et al. The PrecivityAD™ test: accurate and reliable LC‐MS/MS assays for quantifying plasma amyloid beta 40 and 42 and apolipoprotein E proteotype for the assessment of brain amyloidosis. Clin Chim Acta. 2021;519:267‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cruchaga C, Kauwe JSK, Mayo K, et al. SNPs associated with cerebrospinal fluid Phospho‐tau levels influence rate of decline in Alzheimer's disease. PLoS Genet. 2010;6(9):e1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed‐Effects Models Using lme4 . J Stat Software. 2015;67(1):1‐48. [Google Scholar]
- 43. R Core Team . R: A Language and Environment for Statisical Computing [internet]. R Foundation for Statistical Computing; 2021. Available from: https://www.R‐project.org/ [Google Scholar]
- 44. Volluz KE, Schindler SE, Henson RL, et al. Correspondence of CSF biomarkers measured by Lumipulse assays with amyloid PET [Internet]. Alzheimer's Dementia. 2021;17(S5) [cited 2022 Jun 30]. Available from:. 10.1002/alz.051085 [DOI] [Google Scholar]
- 45. Schindler SE, Jasielec MS, Weng H, et al. Neuropsychological measures that detect early impairment and decline in preclinical Alzheimer disease. Neurobiol Aging. 2017;56:25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Meeker KL, Wisch JK, Hudson D, et al. Socioeconomic status mediates racial differences seen using the at(n) framework. Ann Neurol. 2021;89(2):254‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fyffe DC, Mukherjee S, Barnes LL, Manly JJ, Bennett DA, Crane PK. Explaining differences in episodic memory performance among older African Americans and whites: the roles of factors related to cognitive reserve and test bias. J Int Neuropsychol Soc. 2011;17(4):625‐638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wright RS, Waldstein SR, Gerassimakis CS, et al. Multiple influences on cognitive function among urban‐dwelling African Americans. J Racial Ethn Health Disparities. 2019;6(4):851‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Crane PK, Narasimhalu K, Gibbons LE, et al. Composite scores for executive function items: demographic heterogeneity and relationships with quantitative magnetic resonance imaging. J Int Neuropsychol Soc. 2008;14(5):746‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, Owen C, Aldea P, Su Y, Hassenstab J, Cairns NJ, Holtzman DM, Fagan AM, Morris JC, Benzinger TLS, Ances BM Tau and Aβ imaging, CSF measures, and cognition in Alzheimer's disease. Sci Transl Med 2016;8(338):338ra66‐338ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mattsson N, Insel PS, Palmqvist S, et al. Cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimer's disease. EMBO mol Med. 2016;8(10):1184‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ashton NJ, Janelidze S, Al Khleifat A, et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat Commun. 2021;12(1):3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hassenstab J, Ruvolo D, Jasielec M, Xiong C, Grant E, Morris JC. Absence of practice effects in preclinical Alzheimer's disease. Neuropsychology. 2015;29(6):940‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Mixed model for the global cognitive composite in the complete dataset as predicted by CSF Aβ42/Aβ40. Age and CSF Aβ42/Aβ40 were standardized.
Table S2. Mixed model for the global cognitive composite in the complete dataset as predicted by plasma Aβ42/Aβ40. Age and plasma Aβ42/Aβ40 were standardized.
Table S3. Mixed model for the global cognitive composite in the complete dataset as predicted by CSF NfL. Age was standardized. CSF NfL was transformed with the natural logarithm and then standardized.
Table S4. Mixed model for the global cognitive composite in the complete dataset as predicted by plasma NfL. Age was standardized. Plasma NfL was transformed with the natural logarithm and then standardized.
Table S5. Mixed model for the executive function composite in the complete dataset as predicted by CSF Aβ42/Aβ40. Age and CSF Aβ42/Aβ40 were standardized.
Table S6. Mixed model for the executive function composite in the complete dataset as predicted by plasma Aβ42/Aβ40. Age and plasma Aβ42/Aβ40 were standardized.
Table S7. Mixed model for the executive function composite in the complete dataset as predicted by CSF NfL. Age was standardized. CSF NfL was transformed with the natural logarithm and then standardized.
Table S8. Mixed model for the executive function composite in the complete dataset as predicted by plasma NfL. Age was standardized. Plasma NfL was transformed with the natural logarithm and then standardized.
Table S9. Mixed model for the episodic memory composite in the complete dataset as predicted by CSF Aβ42/Aβ40. Age and CSF Aβ42/Aβ40 were standardized.
Table S10. Mixed model for the episodic memory composite in the complete dataset as predicted by plasma Aβ42/Aβ40. Age and plasma Aβ42/Aβ40 were standardized.
Table S11. Mixed model for the episodic memory composite in the complete dataset as predicted by CSF NfL. Age was standardized. CSF NfL was transformed with the natural logarithm and then standardized.
Table S12. Mixed model for the episodic memory composite in the complete dataset as predicted by plasma NfL. Age was standardized. Plasma NfL was transformed with the natural logarithm and then standardized.
Table S13. Mixed model for the language composite in the complete dataset as predicted by CSF Aβ42/Aβ40. Age and CSF Aβ42/Aβ40 were standardized.
Table S14. Mixed model for the language composite in the complete dataset as predicted by plasma Aβ42/Aβ40. Age and plasma Aβ42/Aβ40 were standardized.
Table S15. Mixed model for the language composite in the complete dataset as predicted by CSF NfL. Age was standardized. CSF NfL was transformed with the natural logarithm and then standardized.
Table S16. Mixed model for the language composite in the complete dataset as predicted by plasma NfL. Age was standardized. Plasma NfL was transformed with the natural logarithm and then standardized.
Figure S1. Model predicted rates of change for each of the four biomarkers with raw data in the complete dataset. Trend lines represent “abnormal” (red line, 1 standard deviation below the mean for Aβ42/Aβ40 and one standard deviation above the mean for NfL), “average” (green line, mean value), and “above normal” (blue line, 1 standard deviation above the mean for Aβ42/Aβ40 and one standard deviation below the mean for NfL) for each biomarker. These are descriptive terms for visualization only and do not reflect clinical or pathological cut points. The quadratic rate of change was significant in all models and all biomarkers moderated the quadratic trend except for CSF NfL. Circles represent raw cognitive data points and points from individual participants are connected with black lines.
Figure S2. Model predicted rates of change for each of the four biomarkers with model predicted values in the complete dataset. Trend lines represent “abnormal” (red line, 1 standard deviation below the mean for Aβ42/Aβ40 and one standard deviation above the mean for NfL), “average” (green line, mean value), and “above normal” (blue line, 1 standard deviation above the mean for Aβ42/Aβ40 and one standard deviation below the mean for NfL) for each biomarker. These are descriptive terms for visualization only and do not reflect clinical or pathological cut points. The quadratic rate of change was significant in all models and all biomarkers moderated the quadratic trend except for CSF NfL. Circles represent model predicted cognitive data points and points from individual participants are connected with black lines.
Figure S3. Model predicted rates of change for each of the four biomarkers with raw data in the limited (≤6 years of follow‐up) dataset. Trend lines represent “abnormal” (red line, 1 standard deviation below the mean for Aβ42/Aβ40 and one standard deviation above the mean for NfL), “average” (green line, mean value), and “above normal” (blue line, 1 standard deviation above the mean for Aβ42/Aβ40 and one standard deviation below the mean for NfL) for each biomarker. These are descriptive terms for visualization only and do not reflect clinical or pathological cut points. Circles represent raw cognitive data points and points from individual participants are connected with black lines.
Figure S4. Model predicted rates of change for each of the four biomarkers with model predicted values in the limited (<6 years of follow‐up) dataset. Trend lines represent “abnormal” (red line, 1 standard deviation below the mean for Aβ42/Aβ40 and one standard deviation above the mean for NfL), “average” (green line, mean value), and “above normal” (blue line, 1 standard deviation above the mean for Aβ42/Aβ40 and one standard deviation below the mean for NfL) for each biomarker. These are descriptive terms for visualization only and do not reflect clinical or pathological cut points. Circles represent model predicted cognitive data points and points from individual participants are connected with black lines.
Figure S5. Model predicted rates of change for each of the four biomarkers in the limited (<=6 years of follow‐up) dataset. Trend lines represent “abnormal” (red line, 1 standard deviation below the mean for Aβ42/Aβ40 and one standard deviation above the mean for NfL), “average” (green line, mean value), and “above normal” (blue line, 1 standard deviation above the mean for Aβ42/Aβ40 and one standard deviation below the mean for NfL) for each biomarker. These are descriptive terms for visualization only and do not reflect clinical or pathological cut points.
Data Availability Statement
Data are available to qualified investigators upon request to the Knight ADRC (https://knightadrc.wustl.edu/Research/ResourceRequest.htm).


