Abstract
Acinar cell carcinoma (ACC) is a rare pancreatic malignancy with distinctive clinical, molecular, and morphological features. The long-term survival of ACC patients is substantially superior to that of pancreatic adenocarcinoma patients. As there are no significant patient series about ACCs, our understanding of this illness is mainly based on case reports and limited patient series. Surgical resection is the treatment of choice for patients with the disease restricted to one organ; however, with recent breakthroughs in precision medicine, medicines targeting the one-of-a-kind molecular profile of ACC are on the horizon. There are no standard treatment protocols available for people in which a total surgical resection to cure the condition is not possible. As a result of shared genetic alterations, ACCs are chemosensitive to agents with activity against pancreatic adenocarcinomas and colorectal carcinomas. The role of neoadjuvant or adjuvant chemoradiotherapy has not been established. This article aims to do a comprehensive literature study and present the most recent information on acinar cell cancer.
Keywords: Acinar cell carcinoma, Pancreas, Imaging, Immunohistochemical stains, Molecular features, Surgery, Chemotherapy
Core Tip: Acinar cell carcinoma (ACC) of the pancreas is a rare, malignant neoplasm that accounts for a small percentage of all pancreatic neoplasms. Our understanding of this disease remains unclear as there are no large series of patients with ACC. This review article aims to conduct a comprehensive literature review and present current knowledge about ACC.
INTRODUCTION
The rising prevalence of abdominal imaging has resulted in an uptick in historically rare pancreatic cancer cases. The exocrine pancreas primarily comprises acinar and ductal cells[1]. Although acinar tissue accounts for most of the pancreatic organ, pancreatic neoplasms that mainly exhibit acinar differentiation are rare. The expression of various pancreatic enzymes, such as chymotrypsin, trypsin, and lipase, leads to acinar differentiation in the exocrine pancreas[1]. Most acinar neoplasms are solid, malignant, and not well-recognized[2]. Acinar cell cystadenoma is the sole benign acinar neoplasm documented; uncommon benign cystic, malignant cystic, and mixed carcinomas also exist.
Acinar cell carcinoma (ACC) of the pancreas is a rare, malignant tumor composed of cells with morphological resemblance to acinar cells and with evidence of exocrine enzyme synthesis by the neoplastic cells, accounting for 1%-2% of all pancreatic neoplasms[2-4]. ACC of the pancreas is a high-grade malignancy with no apparent site preference; it can occur in any section of the pancreas, but the head is the most common location.
Currently, there is no definitive course of treatment for ACC; however, vigorous surgical resection with negative margins has been linked to improved long-term survival[5]. Particularly with the use of computerized tomography (CT) and magnetic resonance imaging (MRI), as well as with the confirmation of fine needle aspiration (FNA) biopsies, early recognition and diagnosis are of particular relevance. Knowledge of these lesions' morphologic and immunohistochemical characteristics is also essential for a precise clinical diagnosis. In addition, extensive molecular analyses of acinar neoplasms have identified genomic alterations that may be amenable to specific therapies, thereby increasing the likelihood of widespread clinical molecular testing of these tumors.
This article aims to do a comprehensive literature study and present the most recent information on acinar cell cancer.
EPIDEMIOLOGY
ACC of the pancreas accounts for 1%-2% of all adult exocrine pancreatic neoplasms and 15% of pediatric neoplasms. ACC occurs most frequently in white males and is usually bimodal in age, with peaks in childhood at 8-15 years of age and adulthood with a peak incidence at 60 and is rare between the ages of 20 and 40. ACC has a 3.6 male-to-female ratio. No known racial relationships exist[1,6,7].
CLINICAL FEATURES
Most ACC s exhibit vague symptoms, which include weight loss (45%), abdominal pain (60%), back pain (50%), nausea and vomiting (20%), melena (12%), weakness, anorexia, and diarrhea (8%) are typical non-specific symptoms of ACC[8,9]. In distinction to ductal adenocarcinoma, ACC infrequently obstructs the bile duct and causes jaundice only in 12% of patients. ACC causes a mass effect and displaces those organs but does not infiltrate[10].
Some patients experience paraneoplastic disease known as "Schmid's Triad" or lipase hypersecretion syndrome due to increased lipase levels reaching over 10000 U/dL[11,12]. Elevated lipase may be the first sign of ACC. Patients may present with multiple nodular foci of subcutaneous fat necrosis, polyarthralgia may occur due to fat necrosis in the cancellous bone, and may have peripheral blood eosinophilia[13,14]. On imaging, fat necrosis within the bone can appear as a focal area of sclerosis. Paraneoplastic syndrome may occur following tumor recurrence. Most patients have hepatic metastases at presentation; however, some may present with only a bulky pancreatic mass. The serum lipase levels may normalize after surgical resection of the tumor, and patients may have symptomatic relief. Lipase may be used as a tumor marker in these patients. Alpha-fetoprotein (AFP) blood levels can be elevated in young patients[15].
MACROSCOPY (GROSS FEATURES)
On FNA, an abundance of cells with fluctuating degrees of acinar differentiation but no endocrine or ductal cells are seen. The aspirates consist primarily of cohesive fragments creating acini, cellular cords, or solid nests of neoplastic epithelium, composed of clusters of uniform cells with smoothly contoured, eccentric nuclei containing one or two prominent nuclei nucleoli[16,17].
ACCs can occur in any region of the pancreas and, on average, are 10 to 11 cm in size at the time of diagnosis[18-20]. The tumors are well circumscribed and have a soft, fleshy, tan to red appearance[2]. They occasionally exhibit lobulated appearances, necrosis, and cystic degeneration[20]. On occasion, the neoplasm is found attached to the surface of the pancreas. Involvement of the duct system by ACCs can sometimes be observed grossly as polypoid or fingerlike projections extending from the primary tumor mass toward the duct[21].
PATHOLOGY
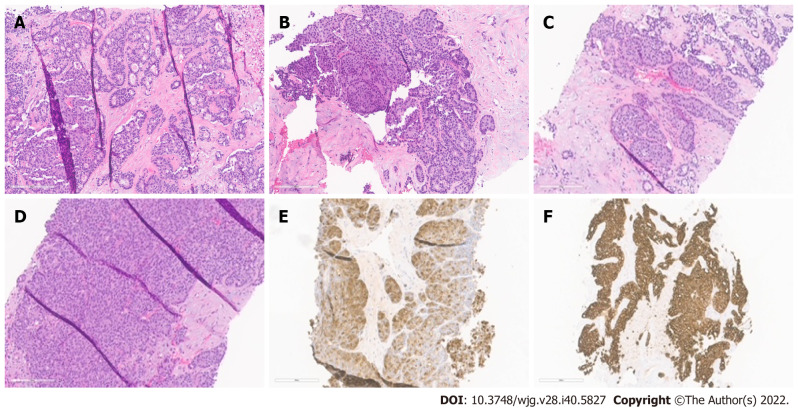
ACCs are cellular neoplasms with minimal stroma on microscopic examination. Multiple architectural patterns, including acinar (Figure 1A), solid (Figure 1B), glandular (Figure 1C), and trabecular (Figure 1D), can be observed. Solid and acinar patterns are the most prevalent. The acinar pattern is characterized by structures resembling normal acini, with small lumina and cells arranged in a monolayer with basally located nuclei. Large sheets of cells depict the solid pattern without lumina. Acinar structures with the dilated lumen characterize the less common granular pattern. The trabecular pattern, which resembles the architecture of well-differentiated neuroendocrine tumors, consists of more pronounced palisading with thinner, interlacing ribbons of cells. Multiple architectural patterns are visible within a single tumor.
Figure 1.
Histopathological patterns. A: Acinar cell carcinoma with the predominant acinar pattern. The acinar pattern is characterized by structures resembling normal acini, with small lumina and cells arranged in a monolayer with basally located nuclei; B: Acinar cell carcinoma with the predominant glandular pattern. Acinar structures with dilated lumina characterize the glandular pattern; C: Acinar cell carcinoma with the predominant trabecular pattern. The trabecular pattern is characterized by ribbons of cells resembling those of pancreatic neuroendocrine tumors; D: Acinar cell carcinoma with a predominant solid pattern. Large sheets of cells characterize the solid pattern without lumina; E: Acinar cell carcinoma, immunohistochemical staining for trypsin; F: Acinar cell carcinoma, immunohistochemical staining for cytokeratin 7.
IMMUNOHISTOCHEMISTRY
The immunohistochemical labeling of enzyme production from pancreatic cells aids in confirming the diagnosis of ACC (Table 1). There are commercially available antibodies for chymotrypsin, trypsin, lipase, and amylase, with the first three being the most frequently used in clinical settings (Figure 1E). These enzymes exhibit varying degrees of specificity and sensitivity. In approximately 95% of cases, trypsin and chymotrypsin reactivity can be demonstrated, and this combination is reportedly the most sensitive for diagnosing ACC[10,22].
Table 1.
Immunohistochemical profile from the differential diagnosis
|
Tumor type
|
PCK
|
Trypsin/chymotrypsin/BCL-10
|
BCL-10
|
B-Catenin
|
Synaptophysin
|
Chromogranin
|
Others
|
| ACC | + | + | + | +/- | -/+ | -/+ | Amylase. Lipase. PAS |
| PDAC | + | - | - | - | - | - | P53, DPC4 loss |
| SPN | -/+ | - | - | +1 | - | - | PR, CD56, Alpha 1-antitrypsin |
| PanNET | + | - | - | - | + | + | CD56, PAX83, ISL13 |
| PBL | + | + | - | +2 | - | - | Alpha fetoprotein, CEA |
Nuclear.
Nuclear/Cytoplasmic and positive in squamous morules.
PAX8 and ISL1 are specific markers for PanNETs.
ACC: Acinar cell carcinoma; PCK: Pancytokeratin; PanNET: Pancreatic neuroendocrine tumors; PBL: Pancreatoblastoma; SPN: Solid pseudopapillary neoplasm; PDAC: Pancreatic ductal carcinoma; PAS: Periodic acid-Schiff; ISL 1: Islet 1; CEA: Carcinoembryonic antigen.
ACC is also positive for CK8 and CK18, and as a result, they will label for AE1/AE3 and CAM5.2. CK7 (Figure 1F) and CK19, which are usually expressed in ductal adenocarcinomas, are generally missing in ACC but may be expressed in a subgroup of ACCs[15,23]. Focal labeling for chromogranin and synaptophysin is observed in a substantial proportion of ACCs, displaying the need to assess with further clear-cut acinar markers, especially in small samples, to avoid misidentifying an ACC as a neuroendocrine tumor or mixed acinar-neuroendocrine differentiation[10,24,25]. Recent research on histological and cytological samples has proven that the monoclonal antibody directed against the COOH-terminal region of the BCL10 protein, which identifies the COOH-terminal portion of CEL, is a highly specific and sensitive approach for detecting ACCs[26,27].
MOLECULAR FEATURES
The genetic alterations driving ACC have been thoroughly characterized and sequenced. The outcomes of these studies have emphasized the diversity of modified genes and pathways among various tumors, as well as the marked genomic instability in a subset of instances, including at the base pairs and chromosomes level. Several studies analyzed ACC for mutations in gene changed in pancreatic ductal adenocarcinoma (PDAC), the most prevalent subtype of pancreatic cancer, before the wide availability of whole genome and exome. Several of these studies reported uncommon mutations in TP53, KRAS, and SMAD4, in addition to the unusual loss of SMAD4 expression in acinar neoplasms, while others reported no modifications in these genes[28-33]. In these studies, the most prevalent ACC mutations affected WNT signalings, such as mutations in APC that rendered inactive and mutations in CTNNB1 that rendered it active; these alterations were found in roughly 20% of ACCs[30]. Recent sequencing studies of the entire exome have highlighted the diversity of mutated genes in the ACC[34,35]. Several other studies have shown substantial chromosomal gains and losses in ACCs and altered regions in various carcinomas; nevertheless, the target genes (if any) in these regions have not been detected[29,30,36,37].
Multiple potentially targetable genetic alterations in pancreatic ACC have been identified. A subset of cases exhibits genomic instability due to mutations in genes involved in DNA repairs, such as ATM, BRCA1, BRCA2, PALB2, and MSH2[34,35]. According to various studies, microsatellite instability has been documented in fluctuating proportions of ACC, ranging from 7% to 14%[30,35,38]. Also, platinum agents and PARP inhibitors treat tumors with mutations in BRCA pathway genes[39,40]. Even though it has not yet been proven in major clinical trials, preliminary findings indicate that tumors with BRAF fusions react to MEK inhibitors, providing ACC with targeted therapy[41-43]. In a subgroup of ACCs, MYC abnormalities, including chromosome 8 polysomy and gene amplification, have been identified recently[44].
DIAGNOSIS
Laboratory analysis
Besides an increase in serum lipase levels associated with the syndrome of lipase hypersecretion, serum tumor markers are not consistently elevated in ACC. Even in the absence of lipase hypersecretion syndrome, lipase might be high. There have been numerous AFP increases, especially in younger patients[2]. Typically, Serum tumor markers such as carbohydrate antigen 19-9, alpha-fetoprotein, and carcinoembryonic antigen are expressed.
Radiological features (imaging)
In recent years, the incidence of uncommon pancreatic cancers has grown, likely due to the increased use of cross-sectional imaging[45]. In most instances, pancreatic cancers are incidentally found by CT. MRI is often used as a secondary test for further characterization. ACC of the pancreas is a well-defined, primarily oval or round exophytic mass in cross-sectional imaging[46,47]. Rarely have cystic variations been described. Typically, it manifests as a dense, largely solid tumor devoid of noticeable cystic alterations[46,48]. One-third of patients may exhibit calcifications, and the majority of the tumor increases constantly, but less so than the adjacent pancreatic parenchyma[49]. The tumor displays heterogeneous enhancement after intravenous infusion of contrast material[46]. Other studies also concluded that when confronted with a large mass with an ovoid shape, exophytic, well-circumscribed arising from the pancreas, with slight and persistent enhancement after contrast administration, and without appreciable biliary or pancreatic dilation, the diagnosis of ACC should be considered[18,47,50]. Another study stated that MRI is better than CT for identifying tumor margination, intratumoral bleeding, tissue invasion, and ductal dilation[51]. On the other hand, CT is more sensitive to detecting central calcification and is commonly utilized due to its accessibility and quickness[50,51]. CT and MRI accurately depict most ACC imaging characteristics, and the two modalities correlate well[51].
Differential diagnosis
Pancreatic endocrine neoplasms that are well-differentiated, solid pseudopapillary neoplasms (SPNs), mixed acinar neoplasms, pancreatoblastoma (PBL), and PDACs should be included in the differential diagnosis (Table 2). Well-differentiated endocrine neoplasm, also commonly known as pancreatic neuroendocrine tumors (PanNET), is the entity most widely confused and misdiagnosed[2]. Both neoplasms typically contain a high number of cells and little amount of stroma, are capable of forming cell sheets and can consist of medium-sized, spherical, monomorphic cells with a microglandular or solid pattern of growth[2]. In addition, the red cytoplasmic zymogen and neurosecretory granules from ACC and PanNETs, respectively, are similar with appropriate differentiation. PanNETs are associated with a more favorable prognosis, making differentiation crucial. ACCs are distinguished from PanNETs by their primary acinar development, cytoplasmic granulation, basally oriented nuclei, and individual conspicuous nucleoli. However, hyalinized stroma, plasmacytoid cell appearance, and a "salt and pepper" chromatin pattern are indicative of PanNET[6]. Immunohistochemistry is advantageous, but it must be utilized with care. Using general neuroendocrine markers such as synaptophysin and chromogranin A alone may be unsafe because several ACCs contain neuroendocrine cells, which are particularly abundant in 30% of cases, as stated before[10,24]. Therefore, the demonstration of acinar cell products is mandatory if an ACC is suspected.
Table 2.
Summary of pertinent differential diagnostic features
|
Differential diagnoses
|
Age/sex
|
Imaging/gross findings
|
Histology
|
Prognosis
|
| ACC | Predominant in males; mean age of 62 yr | Solid, well-circumscribed, bulky tumors; hemorrhage and necrosis are also frequent | Predominant acinar or solid architecture; uniform cells; basally located nuclei; eosinophilic granular cytoplasm; prominent single nucleoli. minimal stroma | Overall aggressive, with high rates of recurrence and metastasis |
| PDAC | Slightly higher in males; 6th-8th decade of life | Solid, poorly defined mass | Large, medium, or small malignant ducts with a tubular pattern; desmoplastic stroma. Processes of mitosis and necrosis | Poor survival rates |
| PanNET | Even distribution between the genders; more prevalent in adults; mean age of 40 | Solid, well-circumscribed. 5% are cystic | Variable architectural patterns; uniform cells; oval or spherical nuclei; granular cytoplasm; undetected nucleoli; minimal stroma | Relatively languid, but with variable results |
| SPN | Almost exclusively female; average age of 28 | Well-defined and encased with cystic degeneration | Pseudopapillae; cells with hyaline/myxoid stroma surrounding vessels; large cytoplasmic hyaline globules; nuclear groove | Overall low malignant potential: The majority are successfully treated surgically |
| PBL | First decade of life, mean age of 4; adults can be affected | Partially encapsulated, frequently lobulated, and substantial | Solid and acinar structure; cellular stroma; keratinization of squamoid nests; heterologous mesenchymal elements | Aggressive; better outcomes for children |
ACC: Acinar cell carcinoma; PanNET: Pancreatic neuroendocrine tumors; PBL: Pancreatoblastoma; SPN: Solid pseudopapillary neoplasm; PDAC: Pancreatic ductal carcinoma
SPNs present as massive, dense pancreatic masses that are clinically identical to ACC; however, SPNs virtually entirely affect women of young age[52]. Cytological smears are characterized by delicately branched vasculature with lightly adherent cells generating pseudopapillary structures, a homogenous population of microscopic cells with cuboidal formation, and a unique hyaline myxoid matrix[52]. Additionally, cytoplasmic hyaline globules are occasionally visible. Moreover, the SPNs nuclei are often normochromic, with numerous indentations, and lack the conspicuous nucleoli found in acinar cells[53]. Typically, SPNs exhibit nuclear immunoreactivity for β-catenin and high expression of CD10, which are also detectable in roughly 10% and 60% of ACCs, respectively. As a result, these two markers must be utilized in conjunction with markers specific to acinar cells, such as trypsin and BCL10[53-55].
PBL is another entity with stroma-poor acinar differentiation that must be evaluated in the differential diagnosis. Similar to ACCs, upon the appearance, PBLs are typically large, with an average size of 10 cm ranging from 1.5 to 20 cm[56]. This uncommon tumor shares nearly all of the cytologic and clinical characteristics of ACC, notably in the population of younger age, where it is frequently observed[2,57]. Cytologically, the neoplasms may be indistinguishable because they both display a predominant acinar-cell differentiation[2]. Heterologous components and squamous nests, such as osseous and cartilage development, characterize PBL[2]. In addition to immunochemistry and clinicopathologic data, the heterogeneous cytologic elements of the types of the tumor-cell present, including a combination of squamoid, mesenchymal, and glandular cells, are a general clue to diagnosing PBL[58]. Histologically, the presence of squamoid nests in PBL is the distinguishing characteristic of the two entities.
ACCs typically lack the characteristic cytomorphologic features of PDACs, allowing these two tumors to be distinguished easily. PDACs typically exhibit "drunk honeycomb" and "tombstone cells" arrangements alongside nuclear heterogeneity and nuclear silhouette abnormalities[59]. This is in contrast to the relatively homogeneous polygonal cells of ACCs, which are arranged in three-dimensional arrays and tissue fragments[1]. ACCs lack the large desmoplastic stroma that describes PDACs and display an overall solid and acinar architecture, whereas PDACs feature a prominent desmoplastic stroma[10,59,60].
STAGING FOR ACINAR PANCREATIC CARCINOMA
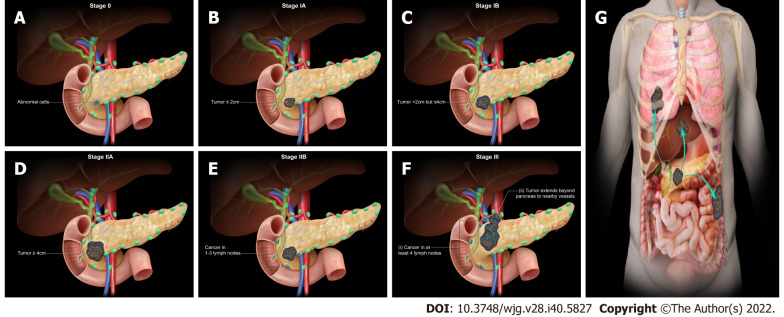
The staging system for acinar pancreatic cancer is undergoing continuous development. Clinical staging is determined by resectability, which is heavily impacted by surgical expertise. The agreed guidelines for the resectability of surgical procedures continue to be refined (e.g., National Comprehensive Cancer Network, MD Anderson Cancer Center, American Hepato-Pancreato-Biliary Association, and International Hepato-Pancreato-Biliary Association). Traditionally, they are categorized as resectable, borderline resectable, locally progressed, and metastatic. To begin with, resectable tumors lack vascular involvement. Next, the borderline resectable tumors involve vascular, local structures, or other signs indicating a high risk of R1 resection. Local invasions (particularly vascular involvement) prevent surgical intervention for locally progressed malignancies. Lastly, metastatic cancer has spread to other organs beyond the main pancreatic tumor. The American Joint Committee on Cancer (AJCC) has classified staging by TNM (tumor, node, metastasis) classification using the consensus guidelines for resectability (Table 3 and Figure 2)[61]. Imaging cases with different AJCC TNM staging were added in chronological order (Figures 3 - 7) to appreciate the differences in changes.
Table 3.
American Joint Committee on Cancer 8th Edition cancer staging for exocrine pancreatic tumor such as pancreatic acinar cell carcinoma
|
Stage
|
TNM
|
Description
|
| 0 | Tis | Carcinoma in situ1 |
| N0 | No regional lymph node metastases | |
| M0 | No distant metastasis | |
| IA | T1 | T1 = Tumor is ≤ 2 cm in any direction |
| T1a = Tumor is ≤ 0.5 cm in any direction | ||
| T1b = Tumor is > 0.5 cm and < 1 cm in any direction | ||
| T1c = Tumor is 1–2 cm in any direction | ||
| N0 | No regional lymph node metastases | |
| M0 | No distant metastasis | |
| IB | T2 | Tumor is > 2 cm and ≤ 4 cm in any direction |
| N0 | No regional lymph node metastases | |
| M0 | No distant metastasis | |
| IIA | T3 | Tumor is > 4 cm in any direction |
| N0 | No regional lymph node metastases | |
| M0 | No distant metastasis | |
| IIB | T1 | T1 = Tumor is ≤ 2 cm in any direction |
| T1a = Tumor is ≤ 0.5 cm in any direction | ||
| T1b = Tumor is > 0.5 cm and < 1 cm in any direction | ||
| T1c = Tumor is 1–2 cm in any direction | ||
| N1 | Metastasis in one to three regional lymph nodes | |
| M0 | No distant metastasis | |
| T2 | Tumor is > 2 cm and ≤ 4 cm in any direction | |
| N1 | Metastasis in one to three regional lymph nodes | |
| M0 | No distant metastasis | |
| T3 | Tumor is > 4 cm in any direction | |
| N1 | Metastasis in one to three regional lymph nodes | |
| M0 | No distant metastasis | |
| III | T1 | T1 = Tumor is ≤ 2 cm in any direction |
| T1a = Tumor is ≤ 0.5 cm in any direction | ||
| T1b = Tumor is > 0.5 cm and < 1 cm in any direction | ||
| T1c = Tumor is 1–2 cm in any direction | ||
| N2 | Metastasis in four or more regional lymph nodes | |
| M0 | No distant metastasis | |
| T2 | Tumor is > 2 cm and ≤ 4 cm in any direction | |
| N2 | Metastasis in four or more regional lymph nodes | |
| M0 | No distant metastasis | |
| T3 | Tumor is > 4 cm in any direction | |
| N2 | Metastasis in four or more regional lymph nodes | |
| M0 | No distant metastasis | |
| T4 | Regardless of tumor size, the cancer has grown outside the pancreas, into the nearby large blood vessels2 | |
| Any N | NX = Regional lymph nodes cannot be assessed | |
| N0 = No regional lymph node metastases | ||
| N1 = Metastasis in one to three regional lymph nodes | ||
| N2 = Metastasis in four or more regional lymph nodes | ||
| M0 | No distant metastasis | |
| IV | Any T | TX = Primary tumor cannot be assessed |
| T0 = No evidence of primary tumor | ||
| Tis = Carcinoma in situ1 | ||
| T1 = Tumor is ≤ 2 cm in any direction | ||
| T1a = Tumor is ≤ 0.5 cm in any direction | ||
| T1b = Tumor is > 0.5 cm and < 1 cm in any direction | ||
| T1c = Tumor is 1–2 cm in any direction | ||
| T2 = Tumor is > 2 cm and ≤ 4 cm in any direction | ||
| T3 = Tumor is > 4 cm in any direction | ||
| T4 = Regardless of tumor size, the cancer has grown outside the pancreas, into the nearby large blood vessels2 | ||
| Any N | NX = Regional lymph nodes cannot be assessed | |
| N0 = No regional lymph node metastases | ||
| N1 = Metastasis in one to three regional lymph nodes | ||
| N2 = Metastasis in four or more regional lymph nodes | ||
| M1 | Distant metastasis |
Included in this category are high-grade pancreatic intraepithelial neoplasia, intraductal papillary mucinous neoplasm with high-grade dysplasia, intraductal tubulopapillary neoplasm with high-grade dysplasia, and mucinous cystic neoplasm with high-grade dysplasia.
Celiac axis, superior mesenteric artery, and/or common hepatic artery are involved.
T: Primary tumor; N: Regional lymph node; M: Distant metastasis.
Figure 2.
American Joint Committee on Cancer 8th Edition cancer staging for exocrine pancreatic tumor such as pancreatic acinar cell carcinoma. A: Stage 0 (TNM: Tis, N0, M0). Tis = Carcinoma in situ. N0 = No regional lymph node metastases. M0 = No distant metastasis; B: Stage IA (TNM: T1, N0, M0). T1 = Tumor ≤ 2 cm in greatest dimension. N0 = No regional lymph node metastases. M0 = No distant metastasis; C: Stage IB (TNM: T2, N0, M0). T2 = Tumor > 2 cm and ≤ 4 cm in greatest dimension. N0 = No regional lymph node metastases. M0 = No distant metastasis; D: Stage IIA (TNM: T3, N0, M0). T3 = Tumor > 4 cm in greatest dimension. N0 = No regional lymph node metastases. M0 = No distant metastasis; E: Stage IIB (TNM: T 1/2/3, N1, M0). T 1/2/3 = Tumor ≤ 2 cm > 4 cm in greatest dimension. N1 = Metastasis in one to three regional lymph nodes. M0 = No distant metastasis; F: Stage III (TNM: T 1/2/3, N2, M0 or T4, Any N, M0). T 1/2/3 = Tumor ≤ 2 cm > 4 cm in greatest dimension. N2 = Metastasis in four or more regional lymph nodes. T4 = Tumor involves celiac axis, superior mesenteric artery, and common hepatic artery, regardless of size; G: Stage IV (TNM: Any T, Any N, M1). M1 = Distant metastasis. T = Primary tumor; N = Regional lymph node; M = Distant metastasis.
Figure 3.
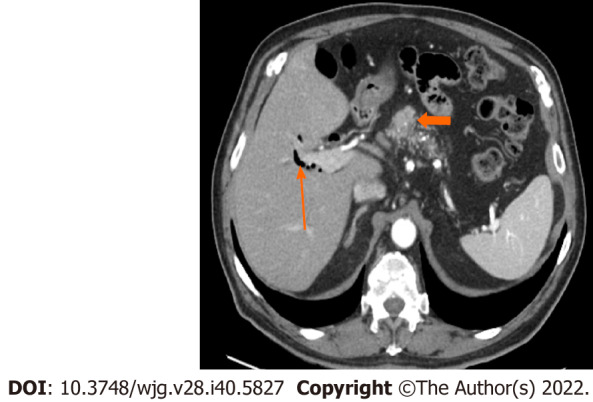
Stage IA (T1, N0, M0). A 77-year-old male patient with acinar cell carcinoma. Axial post-contrast portal-venous phase computed tomography image shows a solid mass (short arrows) measuring 1.8 cm × 1.6 cm and involving the region of the pancreatic head/body. Incidental findings are calcifications seen throughout the pancreas (likely related to changes in chronic pancreatitis) and mild dilatation of the biliary tree with pneumobilia (long arrow).
Figure 7.
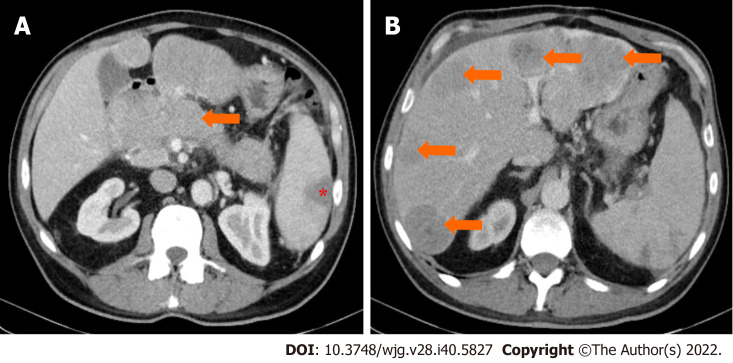
Stage IV. A 64-year-old patient with acinar cell carcinoma. A: Axial post-contrast image port venous phase shows a pancreatic head mass measuring about 3.2 cm × 2.8 cm (arrow); B: Axial post-contrast image port venous phase shows multiple bilobar variable-sized hepatic metastatic lesions (arrows). An incidental finding is an area of splenic infarction (asterisk in image A).
Figure 4.
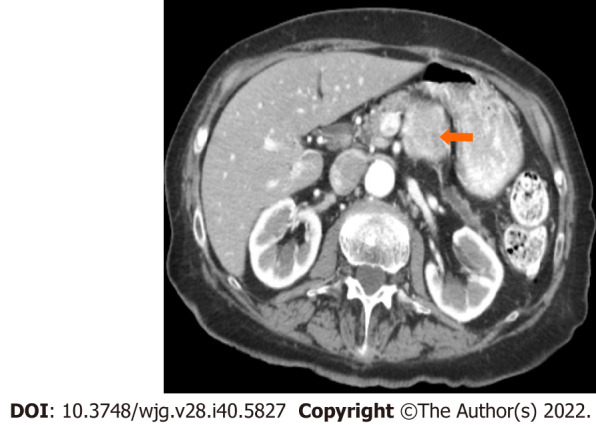
Stage IB (T2, N0, M0). An 89-year-old male patient with acinar cell carcinoma. Axial post-contrast computed tomography arterial phase image shows a large mass in the body of the pancreas (arrow) measuring 3.3 cm × 3 cm. No regional adenopathy is identified.
Figure 5.
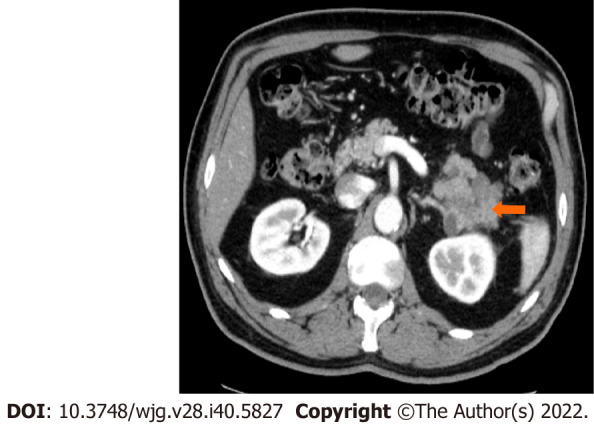
Stage IIA (T3, N0, M0). A 66-year-old patient with acinar cell carcinoma. Axial post-contrast computed tomography image in the arterial phase shows a solid lobulated mass measuring 6.5 cm × 5.4 cm arising from the tail of the pancreas (arrow). The mass is heterogeneous in density with areas of low-density and solid-enhancing areas. No regional or distant lymphadenopathy was detected.
Figure 6.
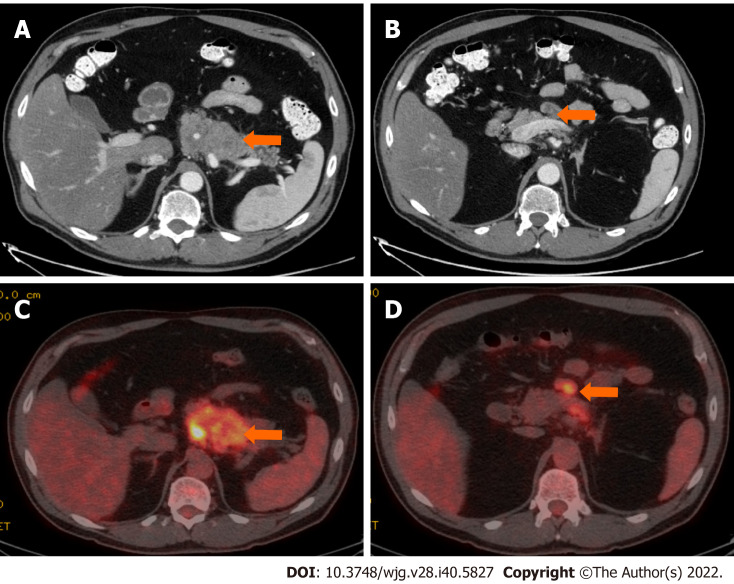
Stage IIB (T3, N1, M0). A 61-year-old patient with acinar cell carcinoma. A: Axial post-contrast computed tomography (CT) image in the portovenous phase shows an infiltrative mass arising from the pancreatic body and tail (arrow); B: axial post-contrast CT images in the portovenous phase shows an enlarged mesenteric lymph node (arrow) measuring 2.6 cm × 1.2 cm; C: Axial positron emission tomography/CT image in the portovenous phase shows hypermetabolic pancreatic body and tail mass (arrow); D: Axial post-contrast CT images in the portovenous phase shows hypermetabolic enlarged mesenteric lymph node (arrow). Pathology of the mass revealed Acinar cell carcinoma with a metastatic mesenteric lymph node.
PROGNOSIS, FACTORS OF PREDICTION, SPREAD, AND METASTASES
ACCs are exceptionally malignant neoplasms; relatively 50% of patients have metastasis during diagnosis[62]. Initial studies indicate that the prognosis for ACCs is better than that for ductal carcinomas but worse than that for PanNETs[5,63,64]. Previous research using population-based cancer databases and epidemiological methods indicated that individuals diagnosed with ACCs have a median overall survival time of roughly 47 mo for patients with localized disease and 14 mo for patients with metastatic disease, with a 5-year survival rate ranging from 36.2% to 72.8% for resected cancers[5,64,65]. A recent study shows that ACC has a better prognosis than adenocarcinoma. They found that the prognosis was deteriorating with a decrease of 25% in a 5-year survival rate to previously reported rates[66]. However, data from a few studies published and anecdotal reports hint that some patients with ACCs have a greater survival rate than expected; unfortunately, no parameters are available to identify this group of patients[67,68].
Rarely do patients with ACC are diagnose with lung, cervical lymph nodes, and ovary metastases. Metastatic illness is typically detected in regional lymph nodes and the liver[69]. Patients presenting as their first disease symptom with distant metastases are infrequent. It has been proposed that ACCs in patients younger than 20 may be less aggressive than in adults[10]. The most significant prognostic indicator is the tumor stage, with patients without lymph nodes or distant metastases having a better prognosis[5,70]. In addition, a poor prognosis is also associated with being male, being over 60, and having a tumor larger than 10 cm[5].
TREATMENT
Current, surgical, radiotherapeutic, and chemotherapeutic treatments used for PDAC are typically used to treat ACC (Table 4). Surgery is regarded as the optimal treatment modality for ACCs that are regionally circumscribed and resectable. Holen et al[8] found that median survival for surgically resected cases was 36 mo, compared to 14 mo for those without surgical resection[8]. Another systematic research by Glazer et al[71] indicated that the total median survival rate for patients with ACC who had resection was approximately 47 mo[71]. More aggressive therapeutic regimens must be sought when surgery is unavailable for locally advanced and metastatic diseases.
Table 4.
Acinar cell carcinoma treatment options
|
Clinical stage
|
Treatment options
|
| Resectable or borderline resectable | Neoadjuvant therapy1 |
| Surgery | |
| Postoperative chemotherapy | |
| Postoperative chemoradiation therapy | |
| Locally advance | Chemotherapy2 |
| Chemoradiation therapy | |
| Surgery | |
| Palliative surgery | |
| Recurrent or metastatic | Chemotherapy2 |
Chemotherapy with or without chemoradiation therapy given before surgery.
With or without target therapy.
Due to the rareness of ACC and the absence of sufficient randomized trials comparing different treatment approaches, the role of adjuvant therapy remains debatable. Table 5 enlists characteristics of studies on various regimes used for treating ACC. In a study at Memorial Sloan-Kettering Cancer Center, systemic chemotherapy for ACC was determined to be ineffective[8]. Following surgical resection, there are no clear treatment guidelines, and most adjuvant therapies are individualized with variable response rates. Lack of prospective data to create therapy guidelines and genetic variation in the APC gene/β-catenin pathway observed in pancreatic acinar cells, ACC patients are frequently treated with chemotherapeutic agents known to be active against PDAC or colorectal cancer[72]. Most study participants received combination chemotherapy protocols based on gemcitabine or fluoropyrimidine[11,73]. If the ACC cannot be resected, the patient should undergo neoadjuvant or palliative 5-FU chemotherapy[70,74]. Combination fluoropyrimidine-based chemotherapies have increased disease control rates[8,11,73,75-77]. Glazer et al[71] systematic review showed that patients with a high-performance status typically receive folinic acid/fluorouracil/oxaliplatin or folinic acid/fluorouracil/irinotecan. In contrast, patients with a lower performance status typically receive gemcitabine/protein-bound paclitaxel[71].
Table 5.
Characteristics of studies on various acinar cell carcinoma treatment regimes
|
Ref.
|
Year of publication
|
ACC sample size
|
Type of treatment
|
No. of patients
|
Conclusion
|
| Holen et al[8] | 2002 | 39 | Resection | 9 | A high recurrence rate following complete surgical resection suggests that micrometastases are present even in localized disease, and that adjuvant therapies may be indicated. Chemotherapy and radiation are ineffective, however, and novel treatments are required |
| RT alone | 22 | ||||
| Fluoropyrimidine-based chemotherapy and RT | 1 | ||||
| Fluoropyrimidine-based chemotherapy | 7 | ||||
| Kitagami et al[65] | 2007 | 115 | Resection | 88 | To improve prognosis, surgical resection should be pursued if possible. If ACC cannot be resected or recurs, chemotherapy is likely to be beneficial. A multidisciplinary treatment centered on the role of surgery must be developed |
| Palliative operation | 12 | ||||
| Exploratory laparotomy | 4 | ||||
| Other treatment1 | 11 | ||||
| Seth et al[11] | 2008 | 14 | Resection | 10 | When feasible, surgical resection is the optimal first-line treatment for resectable ACC due to its superior survival, which can be further improved by the addition of a planned neoadjuvant and/or adjuvant chemoradiation regimen |
| Resection, mixed chemotherapy2 and RT | 4 | ||||
| Wisnoski et al[64] | 2008 | 672 | Resection | 266 | ACC surgical resection appears to improve survival, and the findings support an aggressive strategy for resectable disease. In order to define the role of chemoradiation in the palliative, adjuvant, and neoadjuvant settings, additional research is required |
| Other treatment1 | 406 | ||||
| Schmidt et al[5] | 2008 | 865 | Resection | 190 | In these favorable pancreatic cancers, aggressive surgical resection with negative margins is associated with long-term survival. Second, cancer registries lack certain information, such as the specific type of chemotherapy administered and radiation therapy details. Consequently, institutional and multi-institutional reports of ACC continue to be essential for performing a more comprehensive analysis of the presentation, pathology, natural history, and treatment-related outcomes of ACC |
| Resection and chemotherapy | 33 | ||||
| Resection and RT | 10 | ||||
| Resection and chemoradiation | 100 | ||||
| Other treatment1 | 532 | ||||
| Matos et al[75] | 2009 | 17 | Resection | 12 | ACC requires aggressive surgical resection. Importantly, some patients with locally advanced ACC have responded to a neoadjuvant approach allowing resection of a downstaged tumor; therefore, a combined modality approach should be considered for these patients |
| Mixed chemotherapy2 | 3 | ||||
| Mixed chemotherapy2 and RT | 2 | ||||
| Seki et al[76] | 2009 | 4 | Gemcitabine-based chemotherapy | 1 | A partial response suggested that fluoropyrimidine-based chemotherapy may have some activity against this tumor. To confirm the efficacy of fluoropyrimidine in treating pancreatic ACC, prospective clinical trials are required |
| Fluoropyrimidine-/gemcitabine-based chemotherapy | 3 | ||||
| Lee et al[78] | 2010 | 29 | Resection | 12 | In Korea, the clinical characteristics of ACC include a young age, a large size, a location in the tail, and nonspecific tumour markers. ACC should always be actively treated with surgery, regardless of its size |
| Resection, mixed chemotherapy2 and RT | 10 | ||||
| Mixed chemotherapy2 and RT | 1 | ||||
| Other treatment1 | 6 | ||||
| Butturini et al[73] | 2011 | 9 | Resection | 2 | Using multiple chemotherapy regimens and regional treatments sequentially for recurrent disease allowed for 45-, 85-, and 52-mo post-primary survival. Long-term survival and clinical benefit may be possible with repeated surgery, neoadjuvant and adjuvant chemoradiation therapies, and locoregional therapy |
| Resection and gemcitabine-based chemotherapy | 7 | ||||
| Hartwig et al[79] | 2011 | 17 | Resection | 13 | ACC of the pancreas is a relatively uncommon tumor entity for which resection may lead to long-term survival, even in the presence of limited metastatic disease. Optimized adjuvant treatment protocols are required to improve the long-term survival of ACC patients |
| Resection and gemcitabine-based chemotherapy | 4 | ||||
| Lowery et al[77] | 2011 | 20 | Gemcitabine-based chemotherapy | 20 | Observed efficacy of combination chemotherapy in metastatic patients. ACC supports the use of combination therapies based on gemcitabine or 5-fluorouracil and incorporating irinotecan, a platinum analog, or docetaxel in patients with advanced disease. A potential association between germline mutations in DNA mismatch repair genes and ACC warrants further evaluation |
| Zheng et al[80] | 2015 | 15 | Resection | 12 | Clinicians generally regard pancreatic acinar cell carcinoma as a low-grade malignancy due to its unique clinical features. Positive sentiments towards ACC should be held |
| Resection and gemcitabine-based chemotherapy | 3 | ||||
| Kruger et al[81] | 2016 | 15 | Resection | 3 | In contrast to PDAC, gemcitabine alone does not appear to have significant activity in ACC. Based on the findings, advanced ACC should be treated with chemotherapy regimens containing 5-FU and/or a platinum compound (such as oxaliplatin). Undetermined is whether this observation also applies to adjuvant chemotherapy administered after surgical resection of ACC |
| Resection and gemcitabine-based chemotherapy | 8 | ||||
| Chemoradiation | 1 | ||||
| Mixed chemotherapy2 | 3 | ||||
| Seo et al[82] | 2017 | 20 | Resection | 9 | Compared to PDAC, patients with resectable pancreatic ACC had a favorable prognosis after curative resection. Although adjuvant chemotherapy was not associated with improved survival in this study, it is unknown whether this was due to a selection bias or the ineffectiveness of 5-FU monotherapy in pancreatic ACC. On the basis of molecular analysis utilizing innovative genetic analytic tools, additional research on effective adjuvant chemotherapy is required |
| Resection and 5-fluorouracil-based chemotherapy | 9 | ||||
| Resection and gemcitabine-based chemotherapy | 1 | ||||
| Resection and etoposide plus cisplatin-based chemotherapy | 1 | ||||
| Pishvaian et al[83] | 2020 | 12 | Mixed chemotherapy2 | 12 | Molecularly guided treatments targeting oncogenic drivers and the DNA damage response and repair pathway require further prospective evaluation, based on these real-world findings |
| Zong et al[84] | 2020 | 11 | Resection | 4 | For pancreatic acinar cell carcinoma, surgery is a potentially curative treatment contributing to long-term survival. It has been confirmed that adjuvant systemic therapy, including chemotherapy and chemoradiotherapy, significantly improves survival compared to surgery alone for resectable ACC. To investigate the role and protocol of perioperative and palliative treatments, additional research with a large sample size is required |
| Resection and gemcitabine-based chemotherapy | 4 | ||||
| Resection and capecitabine | 1 | ||||
| Resection and mixed chemotherapy2 | 2 | ||||
| Xu et al[85] | 2022 | 22 | Resection | 6 | Although the value of adjuvant chemotherapy remains obscure, fluoropyrimidine-based chemotherapy merits consideration. Fluorouracil-based chemotherapy, such as FOLFIRINOX, may be the preferred treatment for patients with metastasis, but additional research is required due to the small sample size in this study |
| Resection and S1- based chemotherapy | 3 | ||||
| Resection and SOX- based chemotherapy | 2 | ||||
| Resection and fluoropyrimidine-based chemotherapy | 3 | ||||
| Resection and AG- based chemotherapy | 1 | ||||
| Resection and gemcitabine-based chemotherapy | 7 | ||||
| Chen et al[86] | 2022 | 26 | Resection | 11 | After radical resection, patients with ACC had a longer overall survival than those with PDAC. ACC is also an aggressive tumor with a similar recurrence-free survival trend to PDAC, necessitating multidisciplinary treatment for resectable ACC disease |
| Resection and adjuvant chemotherapy | 15 |
Not specified in manuscript.
Treatment using more than one anticancer drug including FOLFIRNOX (5-FU + oxaliplatin + leucovorin + irinotecan), CAPOX (oxaliplatin + capecitabine), and S1 (tegafur/gimeracil/potassium).
ACC: Acinar cell carcinoma; PDAC: Pancreatic ductal adenocarcinoma; RT: Radiotherapy; 5-FU: 5-fluorouracil; SOX: S1 + Oxaliplatin; AG: Albumin-bound paclitaxel + gemcitabine; FOLFIRNOX: 5-FU + oxaliplatin + leucovorin + irinotecan.
Conventional fractionation and hypofractionated stereotactic body radiotherapy are used to "down-stage" or convert a borderline resectable tumor to one that is resectable[73]. Patients who could potentially benefit from resection can be identified through the absence of ACC development following neoadjuvant radiotherapy. Several studies reported a "major response" rate from 25% to 35% of these individuals[8,11].
CONCLUSION
Pancreatic acinar neoplasms are a molecularly and morphologically heterogeneous group of diseases far less prevalent than ductal neoplasms. Due to its low surgical resectability, high rate of recurrence, and high frequency of metastases at diagnosis, pancreatic ACC has been regarded as cancer with a poor prognosis. At least a subset of the neoplastic cells exhibits acinar differentiation, as defined morphologically (granular cytoplasm, single prominent nucleoli) or immunohistochemically. The molecular system underlying the advancement of ACCs has been elucidated, revealing potentially targetable mutations that offer optimism for developing additional curative options. ACC usually appears as a bulky mass that does not obstruct the pancreatic or bile duct and does not cause jaundice. Elevated lipase levels, subcutaneous nodules, and the presence of bone infarct should help clinch a diagnosis of ACC. Also, these tumors have large lymph nodes at presentation. In the future, molecular diagnostics to detect lesions susceptible to targeted therapy will likely play an important role in patient care.
ACKNOWLEDGEMENTS
We thank Kelly Kage for the beautiful illustrations.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: August 25, 2022
First decision: September 8, 2022
Article in press: October 14, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hiyoshi M, Japan; Nithiyaraj E, India; Rama N, Portugal S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
Contributor Information
Luis Fernando Calimano-Ramirez, Department of Radiology, University of Florida College of Medicine Jacksonville, Jacksonville, FL 32209, United States. luis.calimanoramirez@jax.ufl.edu.
Taher Daoud, Department of Diagnostic Radiology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, United States.
Dheeraj Reddy Gopireddy, Department of Diagnostic Radiology, University of Florida College of Medicine Jacksonville, Jacksonville, FL 32209, United States.
Ajaykumar C Morani, Department of Diagnostic Radiology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, United States.
Rebecca Waters, Department of Pathology and Lab Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, United States.
Kazim Gumus, Department of Research and Diagnostic Radiology, University of Florida College of Medicine Jacksonville, Jacksonville, FL 32209, United States.
Albert Russell Klekers, Department of Diagnostic Radiology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, United States.
Priya R Bhosale, Department of Diagnostic Radiology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, United States.
Mayur K Virarkar, Department of Diagnostic Radiology, University of Florida College of Medicine Jacksonville, Jacksonville, FL 32209, United States.
References
- 1.Toll AD, Hruban RH, Ali SZ. Acinar cell carcinoma of the pancreas: clinical and cytomorphologic characteristics. Korean J Pathol. 2013;47:93–99. doi: 10.4132/KoreanJPathol.2013.47.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klimstra DS. Nonductal neoplasms of the pancreas. Mod Pathol. 2007;20 Suppl 1:S94–112. doi: 10.1038/modpathol.3800686. [DOI] [PubMed] [Google Scholar]
- 3.Béchade D, Desjardin M, Salmon E, Désolneux G, Bécouarn Y, Evrard S, Fonck M. Pancreatic Acinar Cell Carcinoma. Case Rep Gastroenterol. 2016;10:174–180. doi: 10.1159/000445867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fontenot J, Spieler B, Hudson C, Boulmay B. Pancreatic acinar cell carcinoma--literature review and case report of a 56-year-old man presenting with abdominal pain. Radiol Case Rep. 2020;15:39–43. doi: 10.1016/j.radcr.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt CM, Matos JM, Bentrem DJ, Talamonti MS, Lillemoe KD, Bilimoria KY. Acinar cell carcinoma of the pancreas in the United States: prognostic factors and comparison to ductal adenocarcinoma. J Gastrointest Surg. 2008;12:2078–2086. doi: 10.1007/s11605-008-0705-6. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhary P. Acinar Cell Carcinoma of the Pancreas: A Literature Review and Update. Indian J Surg. 2015;77:226–231. doi: 10.1007/s12262-014-1049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lack EE, Cassady JR, Levey R, Vawter GF. Tumors of the exocrine pancreas in children and adolescents. A clinical and pathologic study of eight cases. Am J Surg Pathol. 1983;7:319–327. doi: 10.1097/00000478-198306000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Holen KD, Klimstra DS, Hummer A, Gonen M, Conlon K, Brennan M, Saltz LB. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol. 2002;20:4673–4678. doi: 10.1200/JCO.2002.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Sridharan V, Mino-Kenudson M, Cleary JM, Rahma OE, Perez K, Clark JW, Clancy TE, Rubinson DA, Goyal L, Bazerbachi F, Visrodia KH, Qadan M, Parikh A, Ferrone CR, Casey BW, Fernandez-Del Castillo C, Ryan DP, Lillemoe KD, Warshaw AL, Krishnan K, Hernandez-Barco YG. Pancreatic acinar cell carcinoma: A multi-center series on clinical characteristics and treatment outcomes. Pancreatology. 2021 doi: 10.1016/j.pan.2021.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Klimstra DS, Heffess CS, Oertel JE, Rosai J. Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases. Am J Surg Pathol. 1992;16:815–837. doi: 10.1097/00000478-199209000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Seth AK, Argani P, Campbell KA, Cameron JL, Pawlik TM, Schulick RD, Choti MA, Wolfgang CL. Acinar cell carcinoma of the pancreas: an institutional series of resected patients and review of the current literature. J Gastrointest Surg. 2008;12:1061–1067. doi: 10.1007/s11605-007-0338-1. [DOI] [PubMed] [Google Scholar]
- 12.Burns WA, Matthews MJ, Hamosh M, Weide GV, Blum R, Johnson FB. Lipase-secreting acinar cell carcinoma of the pancreas with polyarthropathy. A light and electron microscopic, histochemical, and biochemical study. Cancer. 1974;33:1002–1009. doi: 10.1002/1097-0142(197404)33:4<1002::aid-cncr2820330415>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 13.Borowicz J, Morrison M, Hogan D, Miller R. Subcutaneous fat necrosis/panniculitis and polyarthritis associated with acinar cell carcinoma of the pancreas: a rare presentation of pancreatitis, panniculitis and polyarthritis syndrome. J Drugs Dermatol. 2010;9:1145–1150. [PubMed] [Google Scholar]
- 14.Radin DR, Colletti PM, Forrester DM, Tang WW. Pancreatic acinar cell carcinoma with subcutaneous and intraosseous fat necrosis. Radiology. 1986;158:67–68. doi: 10.1148/radiology.158.1.3940400. [DOI] [PubMed] [Google Scholar]
- 15.La Rosa S, Sessa F, Capella C. Acinar Cell Carcinoma of the Pancreas: Overview of Clinicopathologic Features and Insights into the Molecular Pathology. Front Med (Lausanne) 2015;2:41. doi: 10.3389/fmed.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samuel LH, Frierson HF Jr. Fine needle aspiration cytology of acinar cell carcinoma of the pancreas: a report of two cases. Acta Cytol. 1996;40:585–591. doi: 10.1159/000333921. [DOI] [PubMed] [Google Scholar]
- 17.Ishihara A, Sanda T, Takanari H, Yatani R, Liu PI. Elastase-1-secreting acinar cell carcinoma of the pancreas. A cytologic, electron microscopic and histochemical study. Acta Cytol. 1989;33:157–163. [PubMed] [Google Scholar]
- 18.Raman SP, Hruban RH, Cameron JL, Wolfgang CL, Kawamoto S, Fishman EK. Acinar cell carcinoma of the pancreas: computed tomography features--a study of 15 patients. Abdom Imaging. 2013;38:137–143. doi: 10.1007/s00261-012-9868-4. [DOI] [PubMed] [Google Scholar]
- 19.Chiou YY, Chiang JH, Hwang JI, Yen CH, Tsay SH, Chang CY. Acinar cell carcinoma of the pancreas: clinical and computed tomography manifestations. J Comput Assist Tomogr. 2004;28:180–186. doi: 10.1097/00004728-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Thompson ED, Wood LD. Pancreatic Neoplasms With Acinar Differentiation: A Review of Pathologic and Molecular Features. Arch Pathol Lab Med. 2020;144:808–815. doi: 10.5858/arpa.2019-0472-RA. [DOI] [PubMed] [Google Scholar]
- 21.Fabre A, Sauvanet A, Flejou JF, Belghiti J, Palazzo L, Ruszniewski P, Degott C, Terris B. Intraductal acinar cell carcinoma of the pancreas. Virchows Arch. 2001;438:312–315. doi: 10.1007/s004280000342. [DOI] [PubMed] [Google Scholar]
- 22.Ordóñez NG, Mackay B. Acinar cell carcinoma of the pancreas. Ultrastruct Pathol. 2000;24:227–241. doi: 10.1080/01913120050176680. [DOI] [PubMed] [Google Scholar]
- 23.Mustafa S, Hruban RH, Ali SZ. Acinar cell carcinoma of the pancreas: a clinicopathologic and cytomorphologic review. J Am Soc Cytopathol. 2020;9:586–595. doi: 10.1016/j.jasc.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Yantiss RK, Chang HK, Farraye FA, Compton CC, Odze RD. Prevalence and prognostic significance of acinar cell differentiation in pancreatic endocrine tumors. Am J Surg Pathol. 2002;26:893–901. doi: 10.1097/00000478-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Kim JY, Brosnan-Cashman JA, Kim J, An S, Lee KB, Kim H, Park DY, Jang KT, Oh YH, Hruban RH, Heaphy CM, Hong SM. Pancreatic acinar cell carcinomas and mixed acinar-neuroendocrine carcinomas are more clinically aggressive than grade 1 pancreatic neuroendocrine tumours. Pathology. 2020;52:336–347. doi: 10.1016/j.pathol.2020.01.437. [DOI] [PubMed] [Google Scholar]
- 26.La Rosa S, Franzi F, Marchet S, Finzi G, Clerici M, Vigetti D, Chiaravalli AM, Sessa F, Capella C. The monoclonal anti-BCL10 antibody (clone 331.1) is a sensitive and specific marker of pancreatic acinar cell carcinoma and pancreatic metaplasia. Virchows Arch. 2009;454:133–142. doi: 10.1007/s00428-008-0710-x. [DOI] [PubMed] [Google Scholar]
- 27.Hosoda W, Sasaki E, Murakami Y, Yamao K, Shimizu Y, Yatabe Y. BCL10 as a useful marker for pancreatic acinar cell carcinoma, especially using endoscopic ultrasound cytology specimens. Pathol Int. 2013;63:176–182. doi: 10.1111/pin.12045. [DOI] [PubMed] [Google Scholar]
- 28.Hoorens A, Lemoine NR, McLellan E, Morohoshi T, Kamisawa T, Heitz PU, Stamm B, Rüschoff J, Wiedenmann B, Klöppel G. Pancreatic acinar cell carcinoma. An analysis of cell lineage markers, p53 expression, and Ki-ras mutation. Am J Pathol. 1993;143:685–698. [PMC free article] [PubMed] [Google Scholar]
- 29.de Wilde RF, Ottenhof NA, Jansen M, Morsink FH, de Leng WW, Offerhaus GJ, Brosens LA. Analysis of LKB1 mutations and other molecular alterations in pancreatic acinar cell carcinoma. Mod Pathol. 2011;24:1229–1236. doi: 10.1038/modpathol.2011.83. [DOI] [PubMed] [Google Scholar]
- 30.Abraham SC, Wu TT, Hruban RH, Lee JH, Yeo CJ, Conlon K, Brennan M, Cameron JL, Klimstra DS. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am J Pathol. 2002;160:953–962. doi: 10.1016/s0002-9440(10)64917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore PS, Orlandini S, Zamboni G, Capelli P, Rigaud G, Falconi M, Bassi C, Lemoine NR, Scarpa A. Pancreatic tumours: molecular pathways implicated in ductal cancer are involved in ampullary but not in exocrine nonductal or endocrine tumorigenesis. Br J Cancer. 2001;84:253–262. doi: 10.1054/bjoc.2000.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terhune PG, Memoli VA, Longnecker DS. Evaluation of p53 mutation in pancreatic acinar cell carcinomas of humans and transgenic mice. Pancreas. 1998;16:6–12. doi: 10.1097/00006676-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Rigaud G, Moore PS, Zamboni G, Orlandini S, Taruscio D, Paradisi S, Lemoine NR, Klöppel G, Scarpa A. Allelotype of pancreatic acinar cell carcinoma. Int J Cancer. 2000;88:772–777. doi: 10.1002/1097-0215(20001201)88:5<772::aid-ijc14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 34.Furukawa T, Sakamoto H, Takeuchi S, Ameri M, Kuboki Y, Yamamoto T, Hatori T, Yamamoto M, Sugiyama M, Ohike N, Yamaguchi H, Shimizu M, Shibata N, Shimizu K, Shiratori K. Whole exome sequencing reveals recurrent mutations in BRCA2 and FAT genes in acinar cell carcinomas of the pancreas. Sci Rep. 2015;5:8829. doi: 10.1038/srep08829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiao Y, Yonescu R, Offerhaus GJ, Klimstra DS, Maitra A, Eshleman JR, Herman JG, Poh W, Pelosof L, Wolfgang CL, Vogelstein B, Kinzler KW, Hruban RH, Papadopoulos N, Wood LD. Whole-exome sequencing of pancreatic neoplasms with acinar differentiation. J Pathol. 2014;232:428–435. doi: 10.1002/path.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taruscio D, Paradisi S, Zamboni G, Rigaud G, Falconi M, Scarpa A. Pancreatic acinar carcinoma shows a distinct pattern of chromosomal imbalances by comparative genomic hybridization. Genes Chromosomes Cancer. 2000;28:294–299. doi: 10.1002/1098-2264(200007)28:3<294::aid-gcc7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 37.Dewald GW, Smyrk TC, Thorland EC, McWilliams RR, Van Dyke DL, Keefe JG, Belongie KJ, Smoley SA, Knutson DL, Fink SR, Wiktor AE, Petersen GM. Fluorescence in situ hybridization to visualize genetic abnormalities in interphase cells of acinar cell carcinoma, ductal adenocarcinoma, and islet cell carcinoma of the pancreas. Mayo Clin Proc. 2009;84:801–810. doi: 10.4065/84.9.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu K, Peng W, Zhou Z. The CT findings of pancreatic acinar cell carcinoma in five cases. Clin Imaging. 2013;37:302–307. doi: 10.1016/j.clinimag.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algül H, O'Reilly EM, McGuinness D, Cui KY, Schlienger K, Locker GY, Kindler HL. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019;381:317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chmielecki J, Hutchinson KE, Frampton GM, Chalmers ZR, Johnson A, Shi C, Elvin J, Ali SM, Ross JS, Basturk O, Balasubramanian S, Lipson D, Yelensky R, Pao W, Miller VA, Klimstra DS, Stephens PJ. Comprehensive genomic profiling of pancreatic acinar cell carcinomas identifies recurrent RAF fusions and frequent inactivation of DNA repair genes. Cancer Discov. 2014;4:1398–1405. doi: 10.1158/2159-8290.CD-14-0617. [DOI] [PubMed] [Google Scholar]
- 42.Ross JS, Wang K, Chmielecki J, Gay L, Johnson A, Chudnovsky J, Yelensky R, Lipson D, Ali SM, Elvin JA, Vergilio JA, Roels S, Miller VA, Nakamura BN, Gray A, Wong MK, Stephens PJ. The distribution of BRAF gene fusions in solid tumors and response to targeted therapy. Int J Cancer. 2016;138:881–890. doi: 10.1002/ijc.29825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee NV, Lira ME, Pavlicek A, Ye J, Buckman D, Bagrodia S, Srinivasa SP, Zhao Y, Aparicio S, Rejto PA, Christensen JG, Ching KA. A novel SND1-BRAF fusion confers resistance to c-Met inhibitor PF-04217903 in GTL16 cells through [corrected] MAPK activation. PLoS One. 2012;7:e39653. doi: 10.1371/journal.pone.0039653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.La Rosa S, Bernasconi B, Vanoli A, Sciarra A, Notohara K, Albarello L, Casnedi S, Billo P, Zhang L, Tibiletti MG, Sessa F. c-MYC amplification and c-myc protein expression in pancreatic acinar cell carcinomas. New insights into the molecular signature of these rare cancers. Virchows Arch. 2018;473:435–441. doi: 10.1007/s00428-018-2366-5. [DOI] [PubMed] [Google Scholar]
- 45.Fitzgerald TL, Hickner ZJ, Schmitz M, Kort EJ. Changing incidence of pancreatic neoplasms: a 16-year review of statewide tumor registry. Pancreas. 2008;37:134–138. doi: 10.1097/MPA.0b013e318163a329. [DOI] [PubMed] [Google Scholar]
- 46.Tatli S, Mortele KJ, Levy AD, Glickman JN, Ros PR, Banks PA, Silverman SG. CT and MRI features of pure acinar cell carcinoma of the pancreas in adults. AJR Am J Roentgenol. 2005;184:511–519. doi: 10.2214/ajr.184.2.01840511. [DOI] [PubMed] [Google Scholar]
- 47.Bhosale P, Balachandran A, Wang H, Wei W, Hwang RF, Fleming JB, Varadhachary G, Charnsangavej C, Tamm E. CT imaging features of acinar cell carcinoma and its hepatic metastases. Abdom Imaging. 2013;38:1383–1390. doi: 10.1007/s00261-012-9970-7. [DOI] [PubMed] [Google Scholar]
- 48.Lalwani N, Mannelli L, Ganeshan DM, Shanbhogue AK, Dighe MK, Tiwari HA, Maximin S, Monti S, Ragucci M, Prasad SR. Uncommon pancreatic tumors and pseudotumors. Abdom Imaging. 2015;40:167–180. doi: 10.1007/s00261-014-0189-7. [DOI] [PubMed] [Google Scholar]
- 49.Hu S, Hu S, Wang M, Wu Z, Miao F. Clinical and CT imaging features of pancreatic acinar cell carcinoma. Radiol Med. 2013;118:723–731. doi: 10.1007/s11547-012-0908-5. [DOI] [PubMed] [Google Scholar]
- 50.Tian L, Lv XF, Dong J, Zhou J, Zhang Y, Xi SY, Zhang R, Xie CM. Clinical features and CT/MRI findings of pancreatic acinar cell carcinoma. Int J Clin Exp Med. 2015;8:14846–14854. [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu MY, Pan KT, Chu SY, Hung CF, Wu RC, Tseng JH. CT and MRI features of acinar cell carcinoma of the pancreas with pathological correlations. Clin Radiol. 2010;65:223–229. doi: 10.1016/j.crad.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 52.Klöppel G, Kosmahl M. Cystic lesions and neoplasms of the pancreas. The features are becoming clearer. Pancreatology. 2001;1:648–655. doi: 10.1159/000055876. [DOI] [PubMed] [Google Scholar]
- 53.Abraham SC, Klimstra DS, Wilentz RE, Yeo CJ, Conlon K, Brennan M, Cameron JL, Wu TT, Hruban RH. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol. 2002;160:1361–1369. doi: 10.1016/s0002-9440(10)62563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.La Rosa S, Adsay V, Albarello L, Asioli S, Casnedi S, Franzi F, Marando A, Notohara K, Sessa F, Vanoli A, Zhang L, Capella C. Clinicopathologic study of 62 acinar cell carcinomas of the pancreas: insights into the morphology and immunophenotype and search for prognostic markers. Am J Surg Pathol. 2012;36:1782–1795. doi: 10.1097/PAS.0b013e318263209d. [DOI] [PubMed] [Google Scholar]
- 55.Notohara K, Hamazaki S, Tsukayama C, Nakamoto S, Kawabata K, Mizobuchi K, Sakamoto K, Okada S. Solid-pseudopapillary tumor of the pancreas: immunohistochemical localization of neuroendocrine markers and CD10. Am J Surg Pathol. 2000;24:1361–1371. doi: 10.1097/00000478-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Ohike N, Morohoshi T. Exocrine Pancreatic Neoplasms of Nonductal Origin: Acinar Cell Carcinoma, Pancreatoblastoma, and Solid-Pseudopapillary Neoplasm. Surg Pathol Clin. 2011;4:579–588. doi: 10.1016/j.path.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Klimstra DS, Wenig BM, Adair CF, Heffess CS. Pancreatoblastoma. A clinicopathologic study and review of the literature. Am J Surg Pathol. 1995;19:1371–1389. doi: 10.1097/00000478-199512000-00005. [DOI] [PubMed] [Google Scholar]
- 58.Reid MD, Bhattarai S, Graham RP, Pehlivanoglu B, Sigel CS, Shi J, Saqi A, Shirazi M, Xue Y, Basturk O, Adsay V. Pancreatoblastoma: Cytologic and histologic analysis of 12 adult cases reveals helpful criteria in their diagnosis and distinction from common mimics. Cancer Cytopathol. 2019;127:708–719. doi: 10.1002/cncy.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conrad R, Castelino-Prabhu S, Cobb C, Raza A. Cytopathology of the pancreatobiliary tract-the agony, and sometimes, the ease of it. J Gastrointest Oncol. 2013;4:210–219. doi: 10.3978/j.issn.2078-6891.2012.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sigel CS, Klimstra DS. Cytomorphologic and immunophenotypical features of acinar cell neoplasms of the pancreas. Cancer Cytopathol. 2013;121:459–470. doi: 10.1002/cncy.21279. [DOI] [PubMed] [Google Scholar]
- 61.Board PATE. Pancreatic Cancer Treatment (Adult) (PDQ®). 2022. [Google Scholar]
- 62.Kryklyva V, Haj Mohammad N, Morsink FHM, Ligtenberg MJL, Offerhaus GJA, Nagtegaal ID, de Leng WWJ, Brosens LAA. Pancreatic acinar cell carcinoma is associated with BRCA2 germline mutations: a case report and literature review. Cancer Biol Ther. 2019;20:949–955. doi: 10.1080/15384047.2019.1595274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.La Rosa S, Klersy C, Uccella S, Dainese L, Albarello L, Sonzogni A, Doglioni C, Capella C, Solcia E. Improved histologic and clinicopathologic criteria for prognostic evaluation of pancreatic endocrine tumors. Hum Pathol. 2009;40:30–40. doi: 10.1016/j.humpath.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 64.Wisnoski NC, Townsend CM Jr, Nealon WH, Freeman JL, Riall TS. 672 patients with acinar cell carcinoma of the pancreas: a population-based comparison to pancreatic adenocarcinoma. Surgery. 2008;144:141–148. doi: 10.1016/j.surg.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 65.Kitagami H, Kondo S, Hirano S, Kawakami H, Egawa S, Tanaka M. Acinar cell carcinoma of the pancreas: clinical analysis of 115 patients from Pancreatic Cancer Registry of Japan Pancreas Society. Pancreas. 2007;35:42–46. doi: 10.1097/mpa.0b013e31804bfbd3. [DOI] [PubMed] [Google Scholar]
- 66.Duorui N, Shi B, Zhang T, Chen C, Fang C, Yue Z, Wu P, Wu Z, Huang X, Li M. The contemporary trend in worsening prognosis of pancreatic acinar cell carcinoma: A population-based study. PLoS One. 2020;15:e0243164. doi: 10.1371/journal.pone.0243164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Armstrong MD, Von Hoff D, Barber B, Marlow LA, von Roemeling C, Cooper SJ, Travis P, Campbell E, Paz-Fumagalli R, Copland JA, Colon-Otero G. An effective personalized approach to a rare tumor: prolonged survival in metastatic pancreatic acinar cell carcinoma based on genetic analysis and cell line development. J Cancer. 2011;2:142–152. doi: 10.7150/jca.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suzuki A, Sakaguchi T, Morita Y, Oishi K, Fukumoto K, Inaba K, Takehara Y, Baba S, Suzuki S, Konno H. Long-term survival after a repetitive surgical approach in a patient with acinar cell carcinoma of the pancreas and recurrent liver metastases: report of a case. Surg Today. 2010;40:679–683. doi: 10.1007/s00595-009-4128-0. [DOI] [PubMed] [Google Scholar]
- 69.Nasser F, Motta Leal Filho JM, Affonso BB, Galastri FL, Cavalcante RN, Martins DLN, Segatelli V, Yamaga LYI, Gansl RC, Tranchesi Junior B, Macedo ALV. Liver Metastases in Pancreatic Acinar Cell Carcinoma Treated with Selective Internal Radiation Therapy with Y-90 Resin Microspheres. Case Reports Hepatol. 2017;2017:1847428. doi: 10.1155/2017/1847428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Distler M, Rückert F, Dittert DD, Stroszczynski C, Dobrowolski F, Kersting S, Grützmann R. Curative resection of a primarily unresectable acinar cell carcinoma of the pancreas after chemotherapy. World J Surg Oncol. 2009;7:22. doi: 10.1186/1477-7819-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glazer ES, Neill KG, Frakes JM, Coppola D, Hodul PJ, Hoffe SE, Pimiento JM, Springett GM, Malafa MP. Systematic Review and Case Series Report of Acinar Cell Carcinoma of the Pancreas. Cancer Control. 2016;23:446–454. doi: 10.1177/107327481602300417. [DOI] [PubMed] [Google Scholar]
- 72.Ordóñez NG. Pancreatic acinar cell carcinoma. Adv Anat Pathol. 2001;8:144–159. doi: 10.1097/00125480-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 73.Butturini G, Pisano M, Scarpa A, D'Onofrio M, Auriemma A, Bassi C. Aggressive approach to acinar cell carcinoma of the pancreas: a single-institution experience and a literature review. Langenbecks Arch Surg. 2011;396:363–369. doi: 10.1007/s00423-010-0706-2. [DOI] [PubMed] [Google Scholar]
- 74.Antoine M, Khitrik-Palchuk M, Saif MW. Long-term survival in a patient with acinar cell carcinoma of pancreas. A case report and review of literature. JOP. 2007;8:783–789. [PubMed] [Google Scholar]
- 75.Matos JM, Schmidt CM, Turrini O, Agaram NP, Niedergethmann M, Saeger HD, Merchant N, Johnson CS, Lillemoe KD, Grützmann R. Pancreatic acinar cell carcinoma: a multi-institutional study. J Gastrointest Surg. 2009;13:1495–1502. doi: 10.1007/s11605-009-0938-z. [DOI] [PubMed] [Google Scholar]
- 76.Seki Y, Okusaka T, Ikeda M, Morizane C, Ueno H. Four cases of pancreatic acinar cell carcinoma treated with gemcitabine or S-1 as a single agent. Jpn J Clin Oncol. 2009;39:751–755. doi: 10.1093/jjco/hyp085. [DOI] [PubMed] [Google Scholar]
- 77.Lowery MA, Klimstra DS, Shia J, Yu KH, Allen PJ, Brennan MF, O'Reilly EM. Acinar cell carcinoma of the pancreas: new genetic and treatment insights into a rare malignancy. Oncologist. 2011;16:1714–1720. doi: 10.1634/theoncologist.2011-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee JH, Lee KG, Park HK, Lee KS. [Acinar cell carcinoma of the pancreas in Korea--clinicopathologic analysis of 27 patients from korean literature and 2 cases from our hospital--] Korean J Gastroenterol. 2010;55:245–251. doi: 10.4166/kjg.2010.55.4.245. [DOI] [PubMed] [Google Scholar]
- 79.Hartwig W, Denneberg M, Bergmann F, Hackert T, Hinz U, Strobel O, Büchler MW, Werner J. Acinar cell carcinoma of the pancreas: is resection justified even in limited metastatic disease? Am J Surg. 2011;202:23–27. doi: 10.1016/j.amjsurg.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 80.Zheng C, Lang M, Xu C, Li N, Ren H, Hao J. Diagnosis and treatment analysis of 15 pancreatic acinar cell carcinoma patients. Chin J Clin Oncol. 2015;42:287–291. [Google Scholar]
- 81.Kruger S, Haas M, Burger PJ, Ormanns S, Modest DP, Westphalen CB, Kleespies A, Angele MK, Hartwig W, Bruns CJ, Kirchner T, Werner J, Heinemann V, Boeck S. Acinar cell carcinoma of the pancreas: a rare disease with different diagnostic and therapeutic implications than ductal adenocarcinoma. J Cancer Res Clin Oncol. 2016;142:2585–2591. doi: 10.1007/s00432-016-2264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seo S, Yoo C, Kim KP, Ryoo BY, Chang HM, Hong SM, Lee JH, Song KB, Hwang DW, Kim KH, Hwang S, Kim SC. Clinical outcomes of patients with resectable pancreatic acinar cell carcinoma. J Dig Dis. 2017;18:480–486. doi: 10.1111/1751-2980.12505. [DOI] [PubMed] [Google Scholar]
- 83.Pishvaian MJ, Blais EM, Brody JR, Lyons E, DeArbeloa P, Hendifar A, Mikhail S, Chung V, Sahai V, Sohal DPS, Bellakbira S, Thach D, Rahib L, Madhavan S, Matrisian LM, Petricoin EF 3rd. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol. 2020;21:508–518. doi: 10.1016/S1470-2045(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zong Y, Qi C, Peng Z, Shen L, Zhou J. Patients With Acinar Cell Carcinoma of the Pancreas After 2005: A Large Population Study. Pancreas. 2020;49:781–787. doi: 10.1097/MPA.0000000000001573. [DOI] [PubMed] [Google Scholar]
- 85.Xu JY, Guan WL, Lu SX, Wei XL, Shi WJ, Ren C, Li YH, Li SP, Qiu MZ, Wang FH. Optimizing Chemotherapy of Pancreatic Acinar Cell Carcinoma: Our Experiences and Pooled Analysis of Literature. Clin Med Insights Oncol. 2022;16:11795549221090186. doi: 10.1177/11795549221090186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen H, Xu Z, Shen Z, Weng Y, Wang W, Ying X, Wang X, Deng X, Shen B. Clinical characteristics and surgical outcomes of resectable acinar cell carcinoma of the pancreas-propensity score matching analysis with pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2022;48:1062–1067. doi: 10.1016/j.ejso.2021.11.135. [DOI] [PubMed] [Google Scholar]