Abstract
Background:
Paraquat (PQ) poisoning is a serious public health concern, especially in developing countries, due to its easy access and lack of awareness of potential harms. No effective treatment has been reported yet. Conventional hemodialysis (HD) is still used in many centers for excreting PQ or reducing acute kidney injury, but there is no consensus on its efficacy. Therefore, we aimed to review the HD efficacy in PQ poisoning mortality.
Materials and Methods:
We searched Web of Science, PubMed, Excerpta Medical Database, Google Scholar, Scopus, Cochrane, Web of Knowledge, Pro-Quest, ScienceDirect, Springer, Clinical Key, Scientific Information Database, Magiran, and Iran-doc, in publications before January 1, 2020. We compared patients who underwent HD (Group 1) with those who did not (Group 2). The outcome was considered mortality/survival. The data were analyzed by Comprehensive Meta-analysis Software.
Results:
This systematic review and meta-analysis included five studies with a combined total of 203 patients. The patients in the Group 1 had higher mortality than Group 2 (odds ratio, 2.84; 95% confidence interval: 1.22–6.64; P = 0.02). There was no evidence of publication bias (P value for Egger's test = 0.833).
Conclusion:
Although HD did not affect the survival of patients, other variables such as the amount of ingested PQ, poisoning severity, the time between PQ ingestion and the start of HD, duration, and times of HD sessions may influence the results regarding mortality.
Keywords: Meta-analysis, mortality, paraquat, poisoning, survival, systematic review
INTRODUCTION
Paraquat (PQ) is an effective herbicide with a high mortality rate in acute poisoning.[1,2] Exposure occurs accidentally or intentionally as a suicide attempt. PQ poisoning is becoming a serious public health concern, especially in developing countries, due to its easy access and lack of awareness of potential harms. Multiple organ failure, including lung, kidney, and liver failure, occurs in severe poisoning. The most common cause of death is respiratory failure.[2,3,4,5]
There is still no standard and effective treatment.
Decreased absorption by gastric lavage and oral activated charcoal; elimination through different techniques (conventional hemodialysis [HD], hemoperfusion [HP], continuous venovenous hemofiltration [CVVH], and continuous renal replacement therapy [CRRT]); decreased inflammatory response by the immunosuppressants (corticosteroids and cyclophosphamide); antioxidants (N-acetyl cysteine [NAC], Vitamin C, and Vitamin E); and a combination of different therapies have been used to treat patients with PQ intoxication with different outcomes for survival.[6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29] In addition, prognostic factors have been reported in some studies concerning different treatments.[30,31]
Conventional HD is used in many developing countries for patients with PQ intoxication. Some studies have shown that HD reduces mortality, while others have reported that HD has little effect on patient recovery.[4,9,10,29,32,33,34]
Although the effect of HP, CVVH, CRRT, immunosuppressants, and antioxidants has been reported in PQ poisoning in systematic review studies,[35,36,37,38,39,40,41,42,43] the impact of HD on the outcome of patients with PQ poisoning has not been evaluated systematically yet. Due to the high rate of PQ patients, a systematic review and meta-analysis study was conducted on the HD effect on the outcome of the patients (survival/death).
MATERIALS AND METHODS
This study was a systematic review, and a meta-analysis was carried out according to the PRISMA guideline.[44] The Institutional Ethics Committee of Isfahan University of Medical Sciences approved the project.
Search strategy and study selection
Three specialists of the research team searched the international reference databases: Web of Science, PubMed, Excerpta Medical Database, Google Scholar, Scopus, Cochrane, Web of knowledge, Pro-Quest, Science Direct, Springer, Clinical Key, Scientific Information Database, Magiran, Iran-doc, Ministry of Health (MOH) Thesis, and MOH articles, in publications before January 1, 2020. We selected all studies with the following keywords: Paraquat, hemodialysis, dialysis, poisoning, overdose, intoxication, mortality, survival, fatality. Our search strategy was as the following patterns: ((hemodialysis OR dialysis or “Renal Dialysis” OR hemodialysis) AND (paraquat OR Gramoxone) AND (poisoning OR intoxication OR overdose OR toxicity) AND (survival OR death OR mortality OR fatality)) in TITLE, as well as ((haemodialysis OR dialysis or “Renal Dialysis” OR hemodialysis) AND (paraquat OR Gramoxone) AND (poisoning OR intoxication OR overdose OR toxicity) AND (survival OR death OR mortality OR fatality)) in KEYWORD/MESH/SUBJECT [Supplementary Table 1]. Three researchers independently conducted the screening process by PICO search tool: P (population): Patients with PQ poisoning either accidentally or intentionally as a suicide attempt. I (interventions): Performing HD with or without other treatment modalities such as gastrointestinal decontamination, immunosuppressant (dexamethasone, methylprednisolone, and cyclophosphamide), and antioxidants (NAC, Vitamin C, Vitamin E, and deferoxamine). C (comparison): Comparison between patients who received HD (Group 1) with those who did not (Group 2). The other standard treatments in both groups were similar. O (outcome): Assessment of death or survival. Three researchers evaluated the full texts of included studies independently. Finally, in a consensus meeting, the research team, according to inclusion/exclusion criteria, decided whether the study should be accepted for quality and quantity analysis or not.
We did not apply any limitation on article types and searched all interventional (randomized clinical trial), noninterventional, and observational studies that included all historical cohorts, cross-sectional, and case–control. Furthermore, one researcher reviewed all articles’ references to find more relevant studies in the second round of the project (hand searching).
The inclusion criteria were: (1) articles published in English/non-English and Persian (with English abstract) before January 1, 2020; (2) the studied population was PQ poisoning patients in all age ranges; (3) some patients were treated with HD (Group 1), and others were not (Group 2); (4) other treatment modalities such as immunosuppressants and antioxidants were similar in both groups; (5) the outcome had been reported as death or survival; and (6) the full text of the articles was available. In the case of articles whose full text was not available, the necessary data could be obtained from the summary of the article we included. Otherwise, we e-mailed the corresponding author to access the full text. If we did not receive a response, that article was excluded. To find gray sources, we performed a particular search in related databases such as clinical trial records (e.g., http://www.irct.ir/, http://www.trialscentral.com/, https://www.proquest.com/, and http://www.gateway.com/worldwide/). The exclusion criteria were as follows: (1) all animal studies, case reports, and narrative review articles, and (2) route of exposure was through the skin, eye, or inhalation.
Data extraction
Two researchers independently extracted information from each article and recorded them in an excel table. These data included bibliographic details (name of the first author, year of publication, country, type of study), participant's number in each group, characteristics (gender, age), PQ-ingested dose, co-ingestion of PQ with other substances, performing sodium dithionite test, urine or blood PQ level, time from ingestion of PQ to admission in the hospital, time to performing HD, duration and number of HD times, other treatment modalities, presence or absence of underlying disease, mortality/survival, and study ethical approval/or trial registration. The observed differences were evaluated by the third researcher in a consensus meeting.
Quality assessment
We evaluated the quality of the included studies using the Joanna Bridges Institute (JBI) for Integrated Information Management, Evaluation, and Review system.[45,46] Any approved article is eligible for design as an expert consensus. The first quality assessment tool used was the JBI checklist for cross-sectional analytical studies. This checklist was applied to the four cross-sectional studies in this study. The checklist included eight items that enable the critical evaluation of studies for possible biases. The second JBI tool used was the cross-sectional checklist for two specific studies in our review and two cross-sectional studies with a series of four and six patients. This checklist includes ten items for evaluating these studies’ quality assessment in the following aspects: erosion selection, performance, diagnosis, and bias, as well as pilot designs. In addition, the randomized trial JBI checklist assessed the following items for one RCT in our review; selection, performance, detection, attrition bias, and trial designs. The four answers indicate the degree to which the article meets the criteria; yes, no, unclear, and not applicable. Finally, these studies were scored based on Yes cases.[47] Two researchers scored articles by relevant checklist independently. Finally, we resolved the disagreement on the assessment through discussion sessions until we reached a consensus.
Statistical analysis
We pooled the relative association measures of outcome from included studies. We analyzed the data using the fixed-effects model if there was homogeneity and the random fixed-effect model if there was heterogeneity described by DerSimonian and Laird.[48] Heterogeneity in results across studies was determined using the I2 statistic, which assesses the proportion of variation in results across studies. An I2 of 50% or more indicates a considerable inconsistency between studies. Meta-analysis was completed using Comprehensive Meta-analysis version 2.2 (Biostat Inc., Englewood, NJ, USA). Publication bias was estimated using Egger's regression test. In this regression, the treatment effect size and bias are captured by the slope of the regression line and the intercept, respectively.[49] The odds ratio (OR) and 95% confidence interval (CI) were estimated and included in a forest plot. The significance level that defined the existence of study heterogeneity was set as <0.05.
Dealing with unclearly presented data
We sent an e-mail to the corresponding/first authors of articles regarding unclear information.[4,9,29]
RESULTS
Figure 1 shows study selection (PRISMA flowchart). We could not find any studies that conclusively compared the outcome (survival/mortality) of patients treated with HD compared with those not treated with HD considering inclusion criteria. However, among all the studies, we found six studies, with some patients being treated with HD and others not receiving HD. Then, we extracted mortality and survival rates from these articles and considered them for systematic review.
Figure 1.
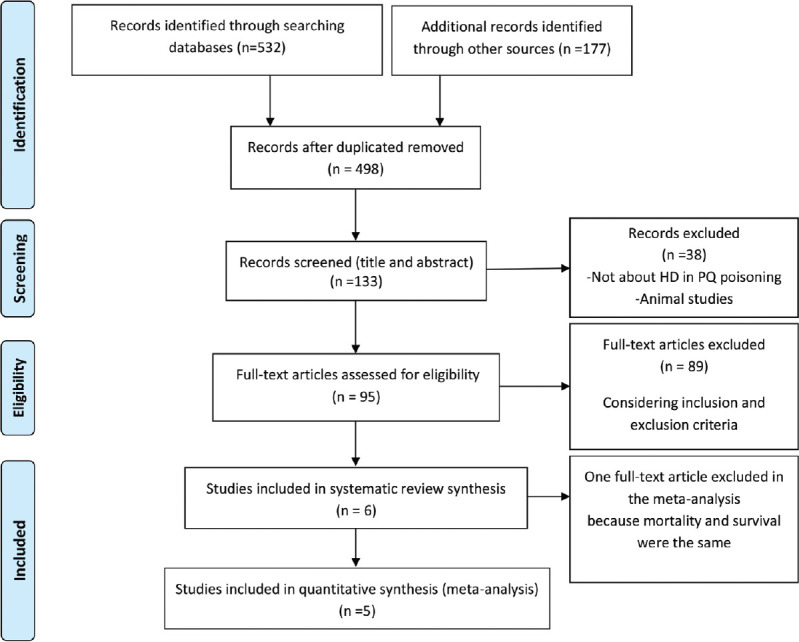
A flowchart of literature searches in this meta-analysis
Studies’ characteristics
We found one randomized trial study,[34] three cross-sectional studies,[4,9,29] and two cross-sectional studies consisting of four and six patients.[10,33] Tables 1 and 2 summarize all studies’ characteristics. Following are some of the results of individual studies.
Table 1.
Different variables of included studies in the systematic review
| Author/year | Study design/area | Sample size (HD/NHD) | Group 1 (HD) | Group 2 (NHD) | PQ blood levels | PQ urine level | DT | Time to HD (h) | Duration/times of HD | In hospital mortality (%) | JBI scoreb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cooke et al., 1973 [10] | Cross-sectional (case series) | 4 (2/2)a | 1 case: HD + lung transplant 1 case: HD + Con. (forced diuresis) |
1 case: Assisted ventilation 1 case: Con. (forced diuresis) + lung transplantation |
+ (1 case) | + | NM | Different in each patients Minimum: 3 days |
Different in each patients | Group 1: 100% Group 2: 100% |
7/10 |
| Ponce et al., 1986[34] | Randomized trial/Portugal (Lisbon) | 17 (7/10) | HD+Con. (GL, Fuller’s earth), methylprednisolone | Plasmapheresis + Con. (GL, Fuller’s earth), methylprednisolone | NM | NM | + | NM | Minimum: 12 h/5 times | Total mortality: 64% Group 1: 71% Group 2: 54% |
10/13 |
| Proudfoot et al., 1987[9]η | Cross-sectional (retrospective)/UK | 35 (9/26) | HD | NM | + | + | + | Mentioned for 4 cases: 0.5/2.5/4.3/10 h Mean: 4.32 |
Mentioned for 4 cases 2 cases: 8 h 2 cases: 6 h Mean: 7 |
Group 1: 32.07% Group 2: 33.33% |
6/8 |
| Pavan, 2013[33] | Cross-sectional (case series) l/India | 6 (5/1) | 3 cases: HD + methylprednisolone 1 case: HD + Con. (AC), methylprednisolone 1 case: HD + Con. (AC GL), furosemide IV, methylprednisolone |
Con. (AC, GL, mannitol and normal saline), IV furosemide, methylprednisolone | NM | NM | + | NM | NM | Group 1: 80% Group 2: 0% |
7/10 |
| Delirrad et al., 2015[29] | Cross-sectional (retrospective)/Iran (West Azerbaijan) | 41 (38/3) | HD + Con. (GL, AC, sorbitol), immunosuppression/corticosteroid/NAC | Con. (GL, AC and sorbitol), immunosuppression/corticosteroid/NAC | NM | NM | + | 6.73±12.7 | NM/1.46±0.84 times | Total mortality: 46.4% Group 1:50% Group 2: 0% |
5/8 |
| Kavousi-Gharbi et al., 2017[4] | Cross-sectional (retrospective)/Iran (Shiraz) | 100 (86/14) | HD + Con. (GL, AC, Fuller’s earth), corticosteroid/cyclophosphamide/NAC, Vitamin E, Vitamin C | Con. (GL, AC, Fuller’s earth), corticosteroid/cyclophosphamide/NAC, Vitamin E, Vitamin C | NM | NM | NM | Minimum: 6 | NM | Total mortality: 43.3% Group 1: 48.8% Group 2: 21.4% |
6/8 |
a Two cases excluded because they received both peritoneal dialysis and hemodialysis, b Maximum JBI score: Randomized trial=13, cross-sectional study=8 and case series study=10, ηThe total number of patients in the study was 52. 17 patients received PD or both HD/PD, therefore excluded from analysis. DT=Dithionate test; HD=Hemodialysis; NHD=Not received HD; PQ=Paraquat; NM=Not mentioned; AC=Activated charcoal; Con.=Conventional therapy; GL=Gastric lavage; NAC=N-acetyl cysteine; PD=Peritoneal dialysis
Table 2.
The comparison of different variables in include studies in the systematic review with respect to toxico-demographic factors
| Author/year | Age (HD), year | Age (NHD), year | Time to admission (HD) | Time to admission (NHD) | Amount of PQ (HD) | Amount of PQ (NHD) | Time to death (HD) | Time to death (NHD) | HD indication | ARF/ARF start day (HD) | ARF/ARF start day (NHD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cooke et al., 1973[10]^ | 1 case: 18 years 1 case: 46 years |
1 case: 28 years 1 case: 15 years |
1 case: 3 days 1 case: 4 days |
1 case: 4 days 1 case: NM |
1 case: Mouthful 1 case: Mouthful PQ 20% |
1 case: 20 g granules PQ 5% 1 case: Mouthful PQ 20% |
2 cases: 12 days | 1 case: 26 days 1 case: 19 days |
2 cases: ARF | 2 cases/4th day | 2 cases/12th day and 6th day No requiring dialysis |
| Ponce et al., 1986[34] | Mean: 42.5 years (13–72 years)* | Death in first 24 h: 6–72 h (mean: 21.2 h) Death after 24 h: 6–48 h (mean: 16.5 h) Survived: Mean 21 h* |
Death in first 24 h: 50–250 ml (mean: 110 ml) Death after 24 h: 15–50 ml (mean: 32.5 ml) Survived: Mean 52 ml* |
NM | NM | PQ decline | None of these patients had ARF* | ||||
| Proudfoot et al., 1987[9]η | NM | NM | NM | NM | NMa | NM | NM | PQ decline | NM | NM | |
| Pavan, 2013[33] | Mean: 33.6 (28–37) | 28 years | 1 case: 1 day 1 case: 2 days 2 cases: 3 days 1 case: 5 days |
1 case: 5 h | 1 case: 100 ml 1 case: 50 ml 1 case: A large cup (250 ml) 1 case: A half spoonful (15 ml) |
A few drops (1 ml) | 2 cases: 4 days 1 case: 6 days 1 case: 7 days 1 case: Survived |
Survived | 4 cases: AKI 1 case: PQ decline |
5 cases/NM | 1 case/NM |
| Delirrad et al., 2015[29] | Mean±SD: 31.9±17.5 (16–75) | Mean±SD: 28±2 (26–28) | <6 h: 65.8% 6–24 h: 23.7% >24 h: 10.5% |
<6 h: 33.3% >24 h: 66.7% |
160.9±232.1 ml | 21.67±17.56 ml | NM | NM | NM | Total ARF: 63.2% ARF at 1st day: 31.6% ARF at 2nd day: 26.3% ARF at 3rd day: 2.6% |
33.3% (1 case)/1st day |
| Kavousi-Gharbi et al., 2017[4] | Female mean age: 22.81±9.87 years Male mean age: 27.21±11.06 years* |
NM | NM | Total: 34.61±55.36 ml (1.5–300 ml) Not survived patients: 66.63±72.61 ml Survived patients: 10.18±5.77 ml* |
4.8±4.62 days* | PQ decline | 54% of the patients were hemodialyzed again due to increase in renal biomarkers after the first day | Expired due to the severity of poisoning during the early h of admission and before HD | |||
*=Data for HD group and non-HD group not mentioned separately. η=The total number of patients in the study was 52. 17 patients received PD or both HD/PD, therefore excluded from analysis; PD=Peritoneal dialysis; PQ=Paraquat; SD=Standard deviation. ^=Two cases excluded because they received both peritoneal dialysis and hemodialysis
Study # 1
52 adults were assigned to one of the four groups based on the risk of mortality (high, borderline, low, or unknown) in the study of Proudfoot et al.[9] PQ intoxication had been confirmed by measuring plasma PQ concentration or urine quality test. Their results showed that dialysis could not reduce the final concentration of PQ; however, the mortality of patients in the high-risk and borderline group who receive HD was lower than that of patients who do not receive HD. Furthermore, patients with low or marginal toxicity, who had undergone HD, might recover. We included 35 patients in this study in the meta-analysis and excluded 17 patients (one patient was treated with peritoneal dialysis, and 16 patients were treated with both peritoneal and HD). Twenty-three took formulations containing 20% PQ (13 cases in high-risk, 5 cases in borderline, and four instances in low-risk groups, and one patient was uncertain of the amount of ingestion). Twenty-eight patients drank solutions of Weedol (2.5% w/w PQ) (3 patients in high, one patient in borderline, and 24 patients in low-risk groups). One of the remaining two ingested a solution containing 8.8% (w/v) PQ (low), and the concentration in the other was never discovered.
Study # 2
Pavan[33] reported six cases of PQ poisoning. Mortality was 66% (80% in the HD group). Acute kidney damage occurred in all cases. Respiratory and multiple organ failure were the leading causes of death. They concluded that dialysis could not remove PQ, and HD is supportive care for kidney failure. Of the six patients, one patient was hospitalized 5 h after accidentally swallowing a few drops of PQ and did not receive HD. He survived without HD. The other five patients who arrived at the hospital between 1 and 5 days late received HD due to acute kidney damage. One patient who ingested a mouthful of PQ in the HD group survived with a relative improvement in kidney function. The amount of PQ consumed in other patients was up to 100 ml. In this study, patients were categorized based on the PQ ingestion amount; less than 20 ml as mild, 20–100 ml as moderate, and more than 100 ml as severe. Three patients had severe poisoning.
Study # 3
A cross-sectional study performed by Delirrad et al. on 41 patients suggested that HD had no significant association with clinical outcomes.[29] In-hospital mortality in their center was 46.4%. HD was performed in 92.7% of their patients. The amount of PQ ingestion significantly affected mortality (149.3 ± 225.4 ml in 36 cases), and it was higher in the patients who received HD. HD started in the 6.73 ± 12.7 h after hospital admission with an average frequency of 1.46 ± 0.84 times. They found no relationship between HD, its frequency, starting time, and outcome of the patients.
Study # 4
Another cross-sectional study by Kavousi-Gharbi et al. on 104 PQ-poisoned patients recommended HD, which was immediately performed on these patients.[4] Eighty-eight patients received HD (two patients were discharged after HD with their consent without follow-up, 44 patients survived, and 42 patients died). Sixteen patients did not receive HD (two patients were discharged with their own consent, 11 patients survived, and three died early before performing HD). Therefore, we excluded four patients from the meta-analysis (those discharged with their own consent). There was a significant difference between outcome and performing HD. The amount of ingested PQ in patients was 34.61 ± 55.36 ml. Seventy-six patients had vomited before admission, which may reduce the toxicity. Time to admission was not available. Three cases had died due to the severity of poisoning during the early hours of admission before performing HD; nonetheless, initiating HD was delayed in all cases, at least for 6 h, mainly due to the delay in receiving the results of viral marker status. Moreover, 54% of the patients received HD again due to the increased renal biomarkers. Based on receiver operating characteristic curve analysis, they suggested that consuming more than 22.5 ml of 20% PQ can lead to a poor prognosis in the patients.
Study # 5
Cook et al.[10] presented serial lung function studies in six cases of PQ intoxication. Two patients were excluded from the meta-analysis because they were treated with both peritoneal and HD methods. Two patients got HD and died. The other two patients did not undergo HD; one was treated with forced diuresis and eventually underwent a lung transplant. He was 15 years old when he ingested a 20% PQ and died 19 days later. Another patient was admitted with hypoxia and was treated with adjuvant ventilation because of the lack of a suitable donor for lung transplantation. He presented to the hospital 4 days after swallowing and died 26 days later. The result of this study was not shown in the meta-analysis because the final result (mortality) of the meta-analysis was the same for both groups.
Study # 6
In the study of Ponce et al.,[34] 17 patients with PQ poisoning were assigned prospectively in two modalities: HD (7 patients) and plasmapheresis (10 patients). Both groups also received standard pulse therapy and methylprednisolone for 3 days. Total mortality was 64% (71% in the HD group and 54% in the plasmapheresis group). Plasmapheresis showed promising results in this study. This study was included in the systematic review; however, we exclude it from the meta-analysis, as one group received plasmapheresis.
The results regarding the quality of studies are presented in [Supplementary Tables 234].
First meta-analysis result
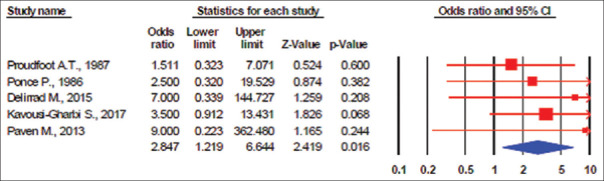
The first meta-analysis includes five studies with a total of 203 patients. We did not show the study of Cook et al. in the meta-analysis figure because mortality and survival were the same.[10] The results showed no evidence of publication bias (I2 = 0%, P value for heterogeneity = 0.386; Q = 1.146, τ2 = 0.00, and P value for Egger's test = 0.833). The patients in the Group 1 had a higher risk of mortality than patients in Group 2 (OR, 2.84; 95% CI: 1.22–6.64; P = 0.02) [Figure 2]. The tau and Q values are added to the figures. Because the heterogeneity was between studies according to the study design, the random effect models were used for combining the results. Calculating the τ2 = 0.00 in both meta-analyses, the prediction interval was the same as the CIs.
Figure 2.
Random effects meta-analysis (death events) (I2 = 0%, P value for heterogeneity was 0.386; Q = 1.146, τ2 = 0.00; P value for Egger's test = 0.833)
Second meta-analysis result
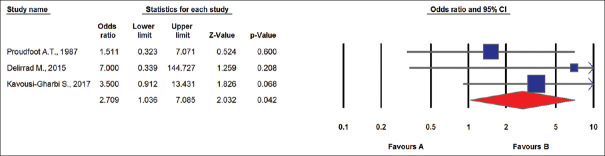
In the second meta-analysis, we included the data regarding those studies that HD performed for PQ elimination before acute kidney injury (AKI). Those studies that patients admitted late and HD was performed for AKI were excluded. Figure 3 presents the results of the second analysis, including three studies. The patients in the Group 1 had a higher risk of mortality than patients in Group 2 (I2 = 2%, P value for heterogeneity = 0.587; Q = 1.067, τ2 = 0.00, and P value for Egger's test = 0.698).
Figure 3.
Random effects meta-analysis (death events) (I2 = 2%, P value for heterogeneity = 0.587; Q = 1.067, τ2 = 0.00, P value for Egger's test = 0.698)
The quality assessment checklist of these studies showed that all of these studies had a good-quality score (more than 7).
Potential biases
Publication bias is an issue in every systematic review. We conducted this review based on the predefined inclusion criteria and methodology to select and appraise eligible studies. The search for studies was extensive and was conducted on English and Persian databases. Although there were only six studies included in this systematic review, we believe that due to the extent of the search considering inclusion and exclusion criteria, these were the only studies addressing this research question at the time of the search.
DISCUSSION
Overall applicability of evidence
We could not find RCT or case–control studies that definitively established the role of HD in PQ poisoning outcomes (mortality/survival). Our results were according to data extracted from the cross-sectional and case series studies. Indeed, the qualities of clinical trial studies are not the same as observational studies. Therefore, their comparison may not be very accurate. However, performing an RCT study with allocation concealment has ethical concerns. Therefore, this review finding should be interpreted with caution until data from RCT or case–control studies become available.
Implications for practice
Based on the findings, HD did not reduce the mortality in PQ poisoning cases. However, it should be mentioned that other variables, such as the amount of PQ ingested, vomiting after ingestion, time of ingestion to hospital admission, performing early gastric lavage or administration of activated charcoal, the severity of PQ intoxication based on serum concentration, the interval of time to the start of HD, and duration of HD sessions, are crucial in the outcome (survival/mortality) and should be considered for the decision to perform HD.
The time interval between PQ ingestion and the start of HD is an important issue. Previous studies demonstrated that the initial hours after PQ ingestion was considered the optimal period to use extracorporeal elimination techniques such as HD.[17,50] Five patients in the study of Pavan[33] presented to the hospital within 1–5 days after PQ ingestion and underwent HD because of AKI. HD was performed at least 6 h after admission in Kavousi-Gharbi's study.[4] Moreover, the time to HD was 6.73 h in Delirrad et al.'s study.[29] This lag of time to HD may cause HD efficacy to decrease. PQ can reach a plasma concentration peak in 1 h due to rapid absorption[51] and accumulate in targeted tissues.
Moreover, there is a chance of re-distribution from tissues to plasma. PQ distribution half-life is around 5 h in humans, and about 6 h after its consumption, it reaches the maximum tissue concentration in the lungs.[52] Clinical features of PQ poisoning and many organs’ cellular damage are primarily due to intracellular effects of PQ, such as generating reactive oxygen species, lipid peroxidation, activation of NF-kB, mitochondrial damage, and apoptosis.[53] Therefore, late admission to the hospital and delay in performing HD might have the reason for a worse outcome.
The amount of PQ ingested is another critical factor. In Delirrad et al.'s study,[29] the amount of PQ ingested in patients treated with HD was higher than those not receiving HD. Furthermore, the dose of PQ ingested in the nonsurvivors was higher significantly from the survivor (290 ± 266 vs. 23.4 ± 22.3). Furthermore, Kavousi-Gharbi et al.[4] reported that the amount of ingested PQ was higher in the patients who died than those who survived. 91.1% of the patients who had consumed more than 20 ml of PQ died. They suggested that consuming more than 22.5 ml of 20% PQ can lead to a poor prognosis in patients with PQ poisoning. This is in accordance with other studies.[29,54] Buckley[55] and Afzali and Gholyaf[23] also showed that consuming around 10-20 cc PQ could lead to fatal complications.
Only in one study, the PQ intoxication severity and PQ serum concentration based on nomogram and severity index of PQ poisoning were considered in the selection of patients for performing HD.[9,56,57] In the other studies, organ involvement might determine the severity of poisoning. The presence of esophageal and stomach ulceration within 24 h after ingestion of PQ shows severe toxicity.[29,58] In Delirrad et al.'s study,[29] five patients have undergone endoscopy, and all had high degrees of ulcerative lesions along the upper gastrointestinal tract. These patients died. In Kavousi-Gharbi's study,[4] pulmonary fibrosis was observed in more than 55% of the patients who died. 14.8% of the patients who survived in the hospital had found lung fibrosis. However, the data regarding the follow-up of the other patients have not been reported to determine possible lung fibrosis and death. In Delirrad et al.'s study,[29] 16 patients from 19 dead patients had respiratory failure. In Pavan's study,[33] two patients survived. One patient ingested a half spoonful of PQ as suicide and the time of ingestion to admission was 1 day. He received HD and progressed to respiratory, renal, and hepatic involvement. He survived, and his kidney function stabilized at serum creatinine of 2 mg/dL. The other case accidentally ingested a few drops of PQ and was admitted to the hospital 5 h after ingestion. He did not receive HD and survived. He also developed respiratory and hepatic involvement. His kidney functions gradually improved to normal. He received a hypoxic breathing mixture as a therapeutic modality. Furthermore, in this study, the follow-up of the survived patients for evaluating lung fibrosis or death has not been reported.
Limitations of the study
The results regarding the follow-up of the survived patients for pulmonary fibrosis and death had not been reported in the studies included in our meta-analysis. It has been shown that pulmonary fibrosis occurs within 14 days[59]
We could not analyze data based on the time from admission to start HD and the number of HD sessions as the data were unavailable in some evaluated studies. Patients who presented later than 4 h may not benefit from HD.
Although the main reason for early HD is the excretion of PQ from the body, this objective was not obvious in all studies
The problem is an objective method of assessing risk in PQ poisoning is the measurement of the plasma concentration concerning the time from ingestion. However, plasma PQ assays are not available in all centers. In addition, once a patient has been selected for HD, there is a further delay before the procedure commences because some laboratory tests are needed for HD. Moreover, we now know that in patients who present early after ingestion, plasma PQ concentrations decline extremely rapidly because of distribution to tissues. It has been reported that HD is used only as a supportive treatment for patients with AKI
All important variables of outcome prediction such as the amount of ingested PQ, time from admission to start HD, and plasma PQ concentration had not been reported in all studies included in the meta-analysis. Therefore, we could not perform a subgroup meta-analysis according to these variables.
CONCLUSION
In conclusion, according to our meta-analysis, sufficient evidence did not exist from all included studies indicating HD's efficacy on the survival of patients with PQ poisoning. Given the importance of this intoxication, especially in a developing country, meticulous and robust preventive strategies are suggested to be applied. Although HD did not affect the survival of patients, other variables such as the amount of ingested PQ, poisoning severity, the time between PQ ingestion and the start of HD, and duration and times of HD sessions may affect the outcome of the patients with PQ poisoning.
Implications for research
More research is needed to determine the effectiveness of HD in patients with PQ poisoning.
Implications for clinician
It may be practical to educate public health professionals and the general population about the fatal consequences of exposure to this toxic agent. It is also suggested to replace the conventional HD with other extracorporeal interventions that may have beneficial effects.
Financial support and sponsorship
This study was supported by the Ethical Committee of Isfahan University of Medical Sciences (IR.MUI.MED.REC.1399.512).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Prof. A. T. Proudfoot, Prof. M. Delirrad, and Dr. S. M. Marashi for their responses to our questions concerning their studies.[4,9,29]
Supplementary Table 1.
Specify the exact keywords searched in each database
| Database | Title search | Subject search |
|---|---|---|
| WOS | TITLE: ((haemodialysis OR dialysis or “Renal Dialysis” OR hemodialysis) AND (paraquat OR gramoxone) AND (poisoning OR intoxication OR overdose OR toxicity) AND (survival OR death OR mortality OR fatality)) | TOPIC: ((haemodialysis OR dialysis or “Renal Dialysis” OR hemodialysis) AND (paraquat OR gramoxone) AND (poisoning OR intoxication OR overdose OR toxicity) AND (survival OR death OR mortality OR fatality)) |
| Scopus | TITLE ((haemodialysis OR dialysis OR “Renal Dialysis” OR hemodialysis) AND (paraquat OR gramoxone) AND (poisoning OR intoxication OR overdose OR toxicity) AND (survival OR death OR mortality OR fatality)) | KEY ((haemodialysis OR dialysis OR “Renal Dialysis” OR hemodialysis) AND (paraquat OR gramoxone) AND (poisoning OR intoxication OR overdose OR toxicity) AND (survival OR death OR mortality OR fatality)) |
| Proquest | Ti ((haemodialysis OR dialysis OR “Renal Dialysis” OR hemodialysis) AND (paraquat OR gramoxone) AND (poisoning OR intoxication OR overdose OR toxicity) AND (survival OR death OR mortality OR fatality))) | Su ((haemodialysis OR dialysis OR “Renal Dialysis” OR hemodialysis) AND (paraquat OR gramoxone) AND (poisoning OR intoxication OR overdose OR toxicity) AND (survival OR death OR mortality OR fatality)) |
| Science direct | Title: ((haemodialysis OR dialysis OR “Renal Dialysis” OR hemodialysis) AND (paraquat OR gramoxone) AND (poisoning OR intoxication OR overdose OR toxicity) AND (survival OR death OR mortality OR fatality)) | Title, abstract, keyords: ((haemodialysis OR dialysis OR “Renal Dialysis” OR hemodialysis) AND (paraquat OR gramoxone) AND (poisoning OR intoxication OR overdose OR toxicity) AND (survival OR death OR mortality OR fatality)) |
| Springer | Title: ((haemodialysis OR dialysis OR “Renal Dialysis” OR hemodialysis) AND (paraquat OR gramoxone) AND (poisoning OR intoxication OR overdose OR toxicity) AND (survival OR death OR mortality OR fatality)) | With all of the words: ((haemodialysis OR dialysis OR “Renal Dialysis” OR hemodialysis) AND (paraquat OR gramoxone) AND (poisoning OR intoxication OR overdose OR toxicity) AND (survival OR death OR mortality OR fatality)) |
| PubMed | (haemodialysis[Title] OR dialysis[Title] OR “Renal Dialysis”[Title] OR hemodialysis[Title]) AND (paraquat[Title] OR gramoxone[Title]) AND (poisoning[Title] OR intoxication[Title] OR overdose[Title] OR toxicity[Title]) AND (survival[Title] OR death[Title] OR mortality[Title] OR fatality[Title]) | (haemodialysis OR dialysis or “Renal Dialysis” OR hemodialysis) AND (paraquat OR gramoxone) AND (poisoning OR intoxication OR overdose OR toxicity) AND (survival OR death OR mortality OR fatality) [MeSH terms] |
| Embase | Title: ((haemodialysis OR dialysis OR “Renal Dialysis” OR hemodialysis) AND (paraquat OR gramoxone) AND (poisoning OR intoxication OR overdose OR toxicity) AND (survival OR death OR mortality OR fatality)) | EMTree: ((haemodialysis OR dialysis OR “Renal Dialysis” OR hemodialysis) AND (paraquat OR gramoxone) AND (poisoning OR intoxication OR overdose OR toxicity) AND (survival OR death OR mortality OR fatality)) |
| Cochrane library | (haemodialysis OR dialysis OR “Renal Dialysis” OR hemodialysis) AND (paraquat OR gramoxone) AND (poisoning OR intoxication OR overdose OR toxicity) AND (survival OR death OR mortality OR fatality) in Record Title | (haemodialysis OR dialysis OR “Renal Dialysis” OR hemodialysis) AND (paraquat OR gramoxone) AND (poisoning OR intoxication OR overdose OR toxicity) AND (survival OR death OR mortality OR fatality) in keyword |
Supplementary Table 2.
Quality assessment of cross-sectional studies based on the Joanna Briggs Institute critical appraisal checklist
| Studies | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Score |
|---|---|---|---|---|---|---|---|---|---|
| Proudfoot A.T.[9] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | 6/8 |
| Delirrad M.[29] | Yes | Yes | No | Yes | No | No | Yes | Yes | 5/8 |
| Kavousi-Gharbi S.[4] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | 6/8 |
Q1. Were the criteria for inclusion in the sample clearly defined?; Q2. Were the study subjects and the setting described in detail?; Q3. Was the exposure measured in a valid and reliable way?; Q4. Were objective, standard criteria used for measurement of the condition?; Q5. Were confounding factors identified?; Q6. Were strategies to deal with confounding factors stated?; Q7. Were the outcomes measured in a valid and reliable way?; Q8. Was appropriate statistical analysis used?
Supplementary Table 3.
Quality assessment of randomized trial studies based on the Joanna Briggs Institute critical appraisal checklist
| Studies | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ponce P.[34] | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 10/13 |
Q1. Was true randomization used for assignment of participants to treatment groups?; Q2. Was allocation to treatment groups concealed?; Q3. Were treatment groups similar at the baseline?; Q4. Were participants blind to treatment assignment?; Q5. Were those delivering treatment blind to treatment assignment?; Q6. Were outcomes assessors blind to treatment assignment?; Q7. Were treatment groups treated identically other than the intervention of interest?; Q8. Was follow up complete and if not, were differences between groups in terms of their follow up adequately described and analyzed?; Q9. Were participants analyzed in the groups to which they were randomized?; Q10. Were outcomes measured in the same way for treatment groups?; Q11. Were outcomes measured in a reliable way?; Q12. Was appropriate statistical analysis used?; Q13. Was the trial design appropriate, and any deviations from the standard RCT design (individual randomization, parallel groups) accounted for in the conduct and analysis of the trial?. RCT=Randomized clinical trial
Supplementary Table 4.
Quality assessment of case series studies based on the Joanna Briggs Institute critical appraisal checklist
| Studies | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Paven M.[33] | Yes | Yes | Yes | No | Yes | No | Yes | Yes | No | Yes | 7/10 |
| Cook NJ.[10] | Yes | Yes | Yes | No | Yes | No | Yes | Yes | No | Yes | 7/10 |
Q1. Were there clear criteria for inclusion in the case series?; Q2. Was the condition measured in a standard, reliable way for all participants included in the case series?; Q3. Were valid methods used for identification of the condition for all participants included in the case series?; Q4. Did the case series have consecutive inclusion of participants?; Q5. Did the case series have complete inclusion of participants?; Q6. Was there clear reporting of the demographics of the participants in the study?; Q7. Was there clear reporting of clinical information of the participants?; Q8. Were the outcomes or follow up results of cases clearly reported?; Q9. Was there clear reporting of the presenting site (s)/clinic (s) demographic information?; Q10. Was statistical analysis appropriate?
REFERENCES
- 1.Sabzghabaee AM, Eizadi-Mood N, Montazeri K, Yaraghi A, Golabi M. Fatality in paraquat poisoning. Singapore Med J. 2010;51:496–500. [PubMed] [Google Scholar]
- 2.Gunnell D, Eddleston M, Phillips MR, Konradsen F. The global distribution of fatal pesticide self-poisoning: Systematic review. BMC Public Health. 2007;7:357. doi: 10.1186/1471-2458-7-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandhu J, Dhiman A, Mahajan R, Sandhu P. Outcome of paraquat poisoning – A five year study. Indian J Nephrol. 2003;13:64–8. [Google Scholar]
- 4.Kavousi-Gharbi S, Jalli R, Rasekhi-Kazerouni A, Habibagahi Z, Marashi SM. Discernment scheme for paraquat poisoning: A five-year experience in Shiraz, Iran. World J Exp Med. 2017;7:31–9. doi: 10.5493/wjem.v7.i1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinis-Oliveira RJ, Duarte JA, Sánchez-Navarro A, Remião F, Bastos ML, Carvalho F. Paraquat poisonings: Mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol. 2008;38:13–71. doi: 10.1080/10408440701669959. [DOI] [PubMed] [Google Scholar]
- 6.Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. Br J Clin Pharmacol. 2011;72:745–57. doi: 10.1111/j.1365-2125.2011.04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherukuri H, Pramoda K, Rohini D, Thunga G, Vijaynarayana K, Sreedharan N, et al. Demographics, clinical characteristics and management of herbicide poisoning in tertiary care hospital. Toxicol Int. 2014;21:209–13. doi: 10.4103/0971-6580.139813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okonek S, Hofmann A, Henningsen B. Efficacy of gut lavage, hemodialysis, and hemoperfusion in the therapy of paraquat or diquat intoxication. Arch Toxicol. 1976;36:43–51. doi: 10.1007/BF00277562. [DOI] [PubMed] [Google Scholar]
- 9.Proudfoot AT, Prescott LF, Jarvie DR. Haemodialysis for paraquat poisoning. Hum Toxicol. 1987;6:69–74. doi: 10.1177/096032718700600111. [DOI] [PubMed] [Google Scholar]
- 10.Cooke NJ, Flenley DC, Matthew H. Paraquat poisoning. Serial studies of lung function. Q J Med. 1973;42:683–92. [PubMed] [Google Scholar]
- 11.Mydlík M, Derzsiová K, Frank K. Renal replacement therapy in acute poisonings-one center experience. Przegl Lek. 2013;70:381–5. [PubMed] [Google Scholar]
- 12.Kang MS, Gil HW, Yang JO, Lee EY, Hong SY. Comparison between kidney and hemoperfusion for paraquat elimination. J Korean Med Sci. 2009;24(Suppl):S156–60. doi: 10.3346/jkms.2009.24.S1.S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Chen Y, Mao L, Zhao G, Hong G, Li M, et al. Effects of hemoperfusion and continuous renal replacement therapy on patient survival following paraquat poisoning. PLoS One. 2017;12:e0181207. doi: 10.1371/journal.pone.0181207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koo JR, Kim JC, Yoon JW, Kim GH, Jeon RW, Kim HJ, et al. Failure of continuous venovenous hemofiltration to prevent death in paraquat poisoning. Am J Kidney Dis. 2002;39:55–9. doi: 10.1053/ajkd.2002.29880. [DOI] [PubMed] [Google Scholar]
- 15.Van de Vyver FL, Giuliano RA, Paulus GJ, Verpooten GA, Franke JP, De Zeeuw RA, et al. Hemoperfusion-hemodialysis ineffective for paraquat removal in life-threatening poisoning? J Toxicol Clin Toxicol. 1985;23:117–31. doi: 10.3109/15563658508990622. [DOI] [PubMed] [Google Scholar]
- 16.Hampson EC, Pond SM. Failure of haemoperfusion and haemodialysis to prevent death in paraquat poisoning. A retrospective review of 42 patients. Med Toxicol Adverse Drug Exp. 1988;3:64–71. doi: 10.1007/BF03259932. [DOI] [PubMed] [Google Scholar]
- 17.Park S, Lee S, Park S, Gil H, Lee E, Yang J, et al. Concurrent hemoperfusion and hemodialysis in patients with acute pesticide intoxication. Blood Purif. 2016;42:329–36. doi: 10.1159/000451051. [DOI] [PubMed] [Google Scholar]
- 18.Lee CJ, Hsu HW, Chang YL. Performance characteristics of combined haemodialysis/haemoperfusion system for removal of blood toxins. Med Eng Phys. 1997;19:658–67. doi: 10.1016/s1350-4533(96)00040-9. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Hu D, Xue W, Li X, Wang Z, Ai Z, et al. Treatment outcome of combined continuous venovenous hemofiltration and hemoperfusion in acute paraquat poisoning: A prospective controlled trial. Crit Care Med. 2018;46:100–7. doi: 10.1097/CCM.0000000000002826. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman S, Jedeikin R, Korzets Z, Shapiro AL, Kaplan R, Bernheim J. Successful management of severe paraquat poisoning. Chest. 1983;84:107–9. doi: 10.1378/chest.84.1.107. [DOI] [PubMed] [Google Scholar]
- 21.Suntres ZE. Role of antioxidants in paraquat toxicity. Toxicology. 2002;180:65–77. doi: 10.1016/s0300-483x(02)00382-7. [DOI] [PubMed] [Google Scholar]
- 22.Lin JL, Leu ML, Liu YC, Chen GH. A prospective clinical trial of pulse therapy with glucocorticoid and cyclophosphamide in moderate to severe paraquat-poisoned patients. Am J Respir Crit Care Med. 1999;159:357–60. doi: 10.1164/ajrccm.159.2.9803089. [DOI] [PubMed] [Google Scholar]
- 23.Afzali S, Gholyaf M. The effectiveness of combined treatment with methylprednisolone and cyclophosphamide in oral paraquat poisoning. Arch Iran Med. 2008;11:387–91. [PubMed] [Google Scholar]
- 24.Drault JN, Baelen E, Mehdaoui H, Delord JM, Flament F. Massive paraquat poisoning. Favorable course after treatment with n-acetylcysteine and early hemodialysis. Ann Fr Anesth Reanim. 1999;18:534–7. doi: 10.1016/s0750-7658(99)80127-0. [DOI] [PubMed] [Google Scholar]
- 25.Hong SY, Hwang KY, Lee EY, Eun SW, Cho SR, Han CS, et al. Effect of vitamin C on plasma total antioxidant status in patients with paraquat intoxication. Toxicol Lett. 2002;126:51–9. doi: 10.1016/s0378-4274(01)00431-3. [DOI] [PubMed] [Google Scholar]
- 26.Dorooshi G, Zolfaghari S, Eizadi-Mood N, Gheshlaghi F. A new treatment approach for acute paraquat poisoning. J Res Pharm Pract. 2018;7:115–6. doi: 10.4103/jrpp.JRPP_18_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eizadi-Mood N, Sabzghabaee AM, Yaraghi A, Montazeri K, Golabi M, Sharifian A, et al. Effect of antioxidants on the outcome of therapy in paraquat-intoxicated patients. Trop J Pharm Res. 2011;10:27–31. [Google Scholar]
- 28.Hedaiaty M, Sabzghabaee AM, Gheshlaghi F, Mood NE. Paraquat poisoning management in Iran (isfahan): Devising a protocol. J Adv Med Med Res. 2016;10:1–10. [Google Scholar]
- 29.Delirrad M, Majidi M, Boushehri B. Clinical features and prognosis of paraquat poisoning: A review of 41 cases. Int J Clin Exp Med. 2015;8:8122–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Weng CH, Chen HH, Hu CC, Huang WH, Hsu CW, Fu JF, et al. Predictors of acute kidney injury after paraquat intoxication. Oncotarget. 2017;8:51345–54. doi: 10.18632/oncotarget.17975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mood NE, Sabzghabaee AM, Ghodousi A, Yaraghi A, Mousavi A, Massoum G, et al. Histo-pathological findings and their relationship with age, gender and toxin amounts in paraquat intoxication. Pak J Med Sci. 2013;29:403–4082013. [Google Scholar]
- 32.Lheureux P, Leduc D, Vanbinst R, Askenasi R. Survival in a case of massive paraquat ingestion. Chest. 1995;107:285–9. doi: 10.1378/chest.107.1.285. [DOI] [PubMed] [Google Scholar]
- 33.Pavan M. Acute kidney injury following Paraquat poisoning in India. Iran J Kidney Dis. 2013;7:64–6. [PubMed] [Google Scholar]
- 34.Ponce P, Lobos AV, Bordalo J, Moreira J. Treatment of paraquat poisoning. Plasmapheresis versus hemodialysis. Acta Med Port. 1986;7:193–6. [PubMed] [Google Scholar]
- 35.Li H, Song L, Jian X. Efficacy of hemoperfusion therapy in patients with paraquat poisoning: A meta-analysis. Int J Clin Exp Med. 2017;10:11371–81. [Google Scholar]
- 36.Nasr Isfahani S, Farajzadegan Z, Sabzghabaee AM, Rahimi A, Samasamshariat S, Eizadi-Mood N. Does hemoperfusion in combination with other treatments reduce the mortality of patients with paraquat poisoning more than hemoperfusion alone: A systematic review with meta-analysis. J Res Med Sci. 2019;24:2. doi: 10.4103/jrms.JRMS_478_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu YG, Lu YQ. Systematic review and meta-analysis of the efficacy and safety of immunosuppressive pulse therapy in the treatment of paraquat poisoning. J Zhejiang Univ Sci B. 2019;20:588–97. doi: 10.1631/jzus.B1800640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eddleston M, Wilks MF, Buckley NA. Prospects for treatment of paraquat-induced lung fibrosis with immunosuppressive drugs and the need for better prediction of outcome: A systematic review. QJM. 2003;96:809–24. doi: 10.1093/qjmed/hcg137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agarwal R, Srinivas R, Aggarwal A, Gupta D. Immunosuppressive Therapy in Lung Injury Due to Paraquat Poisoning: A Meta-Analysis. Database of Abstracts of Reviews of Effects (DARE): Quality-Assessed Reviews: Centre for Reviews and Dissemination (UK) 2007 [PubMed] [Google Scholar]
- 40.Li LR, Chaudhary B, You C, Dennis JA, Wakeford H. Glucocorticoid with cyclophosphamide for oral paraquat poisoning. Cochrane Database Syst Rev. 2021;6:CD008084. doi: 10.1002/14651858.CD008084.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He F, Xu P, Zhang J, Zhang Q, Gu S, Liu Y, et al. Efficacy and safety of pulse immunosuppressive therapy with glucocorticoid and cyclophosphamide in patients with paraquat poisoning: A meta-analysis. Int Immunopharmacol. 2015;27:1–7. doi: 10.1016/j.intimp.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 42.Lin G, Long J, Luo Y, Wang Y, Zewu Q. Continuous venovenous hemofiltration in the management of paraquat poisoning: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96:e6875. doi: 10.1097/MD.0000000000006875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lan C, Lyu Q, Pei H, Meng X, Liu Q, Jia X, et al. Effect of hemoperfusion combined with continuous veno-venous hemofiltration on acute paraquat poisoning: A Meta-analysis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018;30:783–9. doi: 10.3760/cma.j.issn.2095-4352.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 44.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jordan Z, Lockwood C, Munn Z, Aromataris E. The updated Joanna Briggs institute model of evidence-based healthcare. Int J Evid Based Healthc. 2019;17:58–71. doi: 10.1097/XEB.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 46.Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil Med Res. 2020;7:7. doi: 10.1186/s40779-020-00238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al., editors. In: Joanna Briggs Institute Reviewer's Manual. Ch. 7. The Joanna Briggs Institute; 2017. Systematic reviews of etiology and risk; p. 5. [Google Scholar]
- 48.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 49.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gil HW, Hong JR, Jang SH, Hong SY. Diagnostic and therapeutic approach for acute paraquat intoxication. J Korean Med Sci. 2014;29:1441–9. doi: 10.3346/jkms.2014.29.11.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conning DM, Fletcher K, Swan AA. Paraquat and related bipyridyls. Br Med Bull. 1969;25:245–9. doi: 10.1093/oxfordjournals.bmb.a070712. [DOI] [PubMed] [Google Scholar]
- 52.Bismuth C, Scherrmann JM, Garnier R, Baud FJ, Pontal PG. Elimination of paraquat. Hum Toxicol. 1987;6:63–7. doi: 10.1177/096032718700600110. [DOI] [PubMed] [Google Scholar]
- 53.Gawarammana I, Buckley NA, Mohamed F, Naser K, Jeganathan K, Ariyananada PL, et al. High-dose immunosuppression to prevent death after paraquat self-poisoning – A randomised controlled trial. Clin Toxicol (Phila) 2018;56:633–9. doi: 10.1080/15563650.2017.1394465. [DOI] [PubMed] [Google Scholar]
- 54.Senarathna L, Eddleston M, Wilks MF, Woollen BH, Tomenson JA, Roberts DM, et al. Prediction of outcome after paraquat poisoning by measurement of the plasma paraquat concentration. QJM. 2009;102:251–9. doi: 10.1093/qjmed/hcp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buckley NA. Pulse corticosteroids and cyclophosphamide in paraquat poisoning. Am J Respir Crit Care Med. 2001;163:585. doi: 10.1164/ajrccm.163.2.16310a. [DOI] [PubMed] [Google Scholar]
- 56.Bismuth C, Garnier R, Baud FJ, Muszynski J, Keyes C. Paraquat poisoning. An overview of the current status. Drug Saf. 1990;5:243–51. doi: 10.2165/00002018-199005040-00002. [DOI] [PubMed] [Google Scholar]
- 57.Wunnapuk K, Mohammed F, Gawarammana I, Liu X, Verbeeck RK, Buckley NA, et al. Prediction of paraquat exposure and toxicity in clinically ill poisoned patients: A model based approach. Br J Clin Pharmacol. 2014;78:855–66. doi: 10.1111/bcp.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bismuth C, Garnier R, Dally S, Fournier PE, Scherrmann JM. Prognosis and treatment of paraquat poisoning: A review of 28 cases. J Toxicol Clin Toxicol. 1982;19:461–74. doi: 10.3109/15563658208992501. [DOI] [PubMed] [Google Scholar]
- 59.Li S, Li H, Zheng Z, Dong H. Pathologic characteristics of diffuse alveolar damage induced by paraquat in rat lungs. Hua Xi Yi Ke Da Xue Xue Bao. 1994;25:337–40. [PubMed] [Google Scholar]