Abstract
Background:
COVID-19 is responsible for the latest pandemic. Dipeptidyl peptidase-4 (DPP-4) is one of the cellular receptors of interest for coronavirus. The aim of this study was to assess the roles of DPP-4 inhibitors in prognosis of COVID-19 infection in patients with type 2 diabetes mellitus.
Materials and Methods:
retrospective cohort study was performed in 2020 in military medical centers affiliated to AJA University of Medical Sciences in Tehran on 220 patients with type 2 diabetes mellitus who were admitted in medical centers with COVID-19 infection. We collected demographic data of patients including age, gender, drug history, usage of DPP-4 inhibitors, clinical presentations at the time of the first visit, and the disease outcome including hospitalization duration and need for respiratory assist.
Results:
The study population consisted of 133 males (60.5%) and 87 females (39.5%), with a mean age of 66.13 ± 12.3 years. Forty-four patients (20%) consumed DPP-4 inhibitors (sitagliptin and linagliptin). Patients who were treated with DPP-4 inhibitors required less oxygen (O2) therapies compared to other cases (76.7% vs. 88.6%, P = 0.04). Patients who were treated with DPP-4 inhibitors had significantly lower hospitalization duration compared to other cases (6.57 ± 2.3 days vs. 8.03 ± 4.4 days, respectively, P = 0.01). There were no significant differences between the two groups of patients regarding survival rates (P = 0.55). Age was a predictive factor for survival (odds ratio, 1.13; 95% confidence interval, 1.04–1.23; P = 0.004).
Conclusion:
DPP-4 inhibitors could significantly decrease hospitalization days in patients with type 2 diabetes mellitus who were hospitalized for COVID-19. However, DPP-4 inhibitor usage showed no statistically significant impact on survival. Age was the important prognostic factor.
Keywords: COVID-19, dipeptidyl peptidase IV inhibitors, prognosis
INTRODUCTION
COVID-19 is a disease currently known as the coronavirus causing respiratory distress syndrome 2 (SARS-CoV-2).[1] The virus was first discovered in Wuhan, China, and soon led to an epidemic around the world.[2] The incubation period of the disease is reported to be between 2 and 14 days. So far, a wide range of clinical symptoms have been seen in people infected with the virus from mild upper respiratory symptoms to severe respiratory distress, respiratory failure, and death.[3] Most of these viruses are benign pathogens, but some deadly members of this family had discovered in the past which caused serious mortality and morbidities in human beings.[4] These viruses have some superficial proteins called spikes that allow them to attach to cellular receptors and enter the cell for multiplication afterward. Angiotensin convertase enzyme 2 receptor (ACE2R) is one of the well-known cellular receptors used by SARS-CoV-2 for attachment.[5] The distribution of these communicating receptors in the different body organs determines the resulting disease's clinical presentation. ACE2R, for instance, has considerable expression in alveolar cells, and it is well known that COVID-19 causes serious lower respiratory tract presentations.[6]
Dipeptidyl peptidase-4 (DPP-4) which is also known as CD-26 is one of the cellular receptors of interest for MERS coronavirus.[7,8,9] This receptor is also expressed considerably in alveoli, and this expression could explain the respiratory consequences of MERS disease. SARS-CoV-2's Spike protein in S1 section potentially could form an attachment with DPP-4 protein, and this attachment could play an important role in the pathophysiology of COVID-19. DPP-4 is expressed in myeloid lineage, myocardium, endothelium, respiratory tract, thymocytes, and many other tissues.[10,11]
DPP-4 inhibitors are a class of prescription medicines that are used to control high blood sugar in adults with type 2 diabetes mellitus (DM-II). Sitagliptin is a well-known member of this pharmacological family.[12,13,14] These agents control the patient's blood glucose level via decreasing the breakdown of glucagon-like peptide 1 (GLP-1) in the periphery. This increased lifetime of GLP-1, in turn, stimulates insulin secretion and suppresses the secretion of glucagon. This process ultimately causes blood glucose control in the healthy range for DM-II patients.[15,16]
In a study reported by Kifle et al. in 2021, it was stated that DPP-4 inhibitors could have a potential therapeutic effect in COVID-19 infection. It was suggested that more data should be provided in this regard.[17] Furthermore, in a meta-analysis published in 2021, it was suggested that DPP-4 inhibitors may improve the mortality of coronavirus disease, however, the low sample size and the unknown types of DPP-4is in the included studies were the limitations of meta-analysis. They suggested that more clinical studies need to be performed to confirm the relationship between the use of DPP-4is and the outcomes of COVID-19.[18] Whether DPP-4 inhibitors may affect the activity of COVID-19 and have beneficial effects may need more evidence to demonstrate.[19]
People with diabetes are more likely to have serious complications from COVID-19 and have more severe symptoms and complications when infected with any virus.[20,21] Considering the importance and prevalence of COVID-19 infection and possible preventive roles of DPP-4 inhibition, we aimed to assess the roles of DPP-4 inhibitors in prognosis of COVID-19 infection in patients with DM-II in a retrospective cohort study.
MATERIALS AND METHODS
This was a retrospective cohort study that was performed in 2020–2021 in military medical centers affiliated to AJA University of Medical Sciences in Tehran. The current study was conducted on patients with DM-II who were admitted in medical centers with COVID-19 infection. The study protocol was approved by the Research Committee of AJA University of Medical Sciences, and the Ethics Committee has confirmed it (ethics code: IR.AJAUMS.REC.1399.200).
The inclusion criteria were age over 18 years, previously diagnosed DM-II for more than 5 years, hospitalization due to COVID-19 in either intensive care unit (ICU) or general ward from February 2020 until November 2020, and signing the written informed consent to participate in this study. Diagnosis of COVID-19 was performed via positive polymerase chain reaction (PCR) test, clinical presentations, and low-dose high-resolution computed tomography scan findings related to the infection. The exclusion criteria were incomplete medical documents, possible infections with other respiratory, and patient's will to exit the study.
Patients were recruited based on the inclusion criteria. We collected demographic data of patients including age, gender. Other collected data were drug history, usage of DPP 4 inhibitors, duration of DPP 4 inhibitor usage, clinical presentations at the time of the first visit, blood oxygen saturation (O2 Sat), and temperature in the first visit. All data were extracted from the medical record of patients. Laboratory data of each patient at the time of admission were also requested. If any of the patients’ clinical data was missed, the authors called patients and completed the data, and in the case of no response, patients’ data were excluded from the study. Patients were divided into two groups: user of DPP-4 inhibitors (for more than 1 year) and not user of DPP-4 inhibitors, and different variables including demographic, clinical manifestations, lab data, and outcomes were compared between two groups.
The obtained data were entered into the Statistical Package for the Social Sciences (SPSS) (version 24, SPSS Inc., Chicago, IL, USA). Quantitative data were reported as mean ± standard deviation and qualitative data as frequency distribution (percentage). Independent t-test and Chi-square test were used to analyze the data. P < 0.05 was considered a significance threshold.
RESULTS
In the present study, we evaluated data of 1233 patients with COVID-19 infection who were referred to our medical center during the study period. Among them, 220 patients with DM-II were included in the study. The study population consisted of 133 males (60.5%) and 87 females (39.5%) with a mean age of 66.13 ± 12.3 years. Primary analysis of patients’ data indicated that 129 patients (58.6%) had no past medical diseases and the most common past medical diseases in other patients were cardiovascular (23.6%), renal (5%), and pulmonary (4.1%) diseases, respectively. One hundred and fifty-two patients (69.1%) had positive PCR results for COVID-19. The average symptom duration was 7.81 days.
Evaluation of initial vital signs and distribution of gastrointestinal symptoms and dyspnea at the admission time showed no significant differences between two groups of patients treated with DPP-4 inhibitors and other cases (P > 0.05) [Table 1].
Table 1.
Comparison of initial vital signs and clinical manifestations of patients at the admission time
| Variable | Mean±SD | ||
|---|---|---|---|
|
| |||
| DPP-4 inhibitor users | Non-DPP-4 inhibitor users | P | |
| Heart rate (beats/min) | 87.23±17.49 | 87.31±16.25 | 0.08 |
| Respiratory rate (min) | 18.77±2.22 | 19.52±4.58 | 0.75 |
| Oral body temperature (°C) | 37.08±0.71 | 37.61±4.66 | 0.97 |
| Capillary oxygen saturation | 89.11±7.53 | 86.77±9.11 | 0.12 |
| Gastrointestinal symptoms, n (%) | 6 (14) | 30 (19) | 0.69 |
| Dyspnea, n (%) | 33 (76.7) | 122 (77.2) | 0.97 |
DPP-4=Dipeptidyl peptidase-4; SD=Standard deviation
One hundred and ninety-one patients (86.8%) required oxygen (O2) therapy, 12 patients (5.5%) required noninvasive ventilation (NIV), and 38 patients (17.3%) required endotracheal intubation during hospitalization.
Evaluation of antidiabetic treatments indicated that 146 patients (66.4%) consumed metformin that was the most common drug in patients. Forty-four patients (20%) consumed DPP-4 inhibitors (sitagliptin and linagliptin). We evaluated the prognosis of all cases. Based on our findings, 174 patients (79.1%) were eventually discharged from the hospital and 46 patients (20.9%) died. One hundred and eighty-one cases (82.2%) were admitted in the general ward, in which 164 (74.5%) of them stayed there until their discharge from the hospital and 17 patients (7.7%) were transferred to ICU because of clinical deterioration. Thirty-nine patients (17.7%) also were hospitalized in the ICU ward directly after admission. We also compared laboratory data at the time of admission between patients with DPP-4 inhibitors use and others. Based on the results presented in Table 2, we observed no significant differences between the two groups regarding the laboratory findings at admission time (P > 0.05).
Table 2.
Comparison of laboratory data between patients at the admission time
| Variable | Mean±SD | ||
|---|---|---|---|
|
| |||
| DPP-4 inhibitor users | Non-DPP-4 inhibitor users | P | |
| White blood cell (cells/m3) | 7.78±4.06 | 7.86±4.46 | 0.43 |
| Lymphocytes (cells/L) | 1327.46±578.02 | 1380.68±913.60 | 0.10 |
| Platelet (109/L) | 226.60±80.80 | 207.53±88.73 | 0.20 |
| Creatinine (mg/dL) | 1.33±0.88 | 1.43±0.65 | 0.39 |
| ALT (IU/L) | 38.28±51.72 | 48.74±136.15 | 0.65 |
| AST (IU/L) | 52.55±54.56 | 60.74±128.07 | 0.71 |
| ESR (mm/h) | 51.08±32.83 | 50.80±26.42 | 0.96 |
ALT=Alanine aminotransferase; ESR=Erythrocyte sedimentation rate; SD=Standard deviation; DPP-4=Dipeptidyl peptidase-4; AST=Aspartate aminotransferase
Comparison of different data between patients who consumed DPP-4 inhibitors and other cases showed that the patients who were previously treated with DPP-4 inhibitors had significantly lower hospitalization duration (6.57 ± 2.3 vs. 8.03 ± 4.4, respectively, P = 0.01) [Figure 1].
Figure 1.
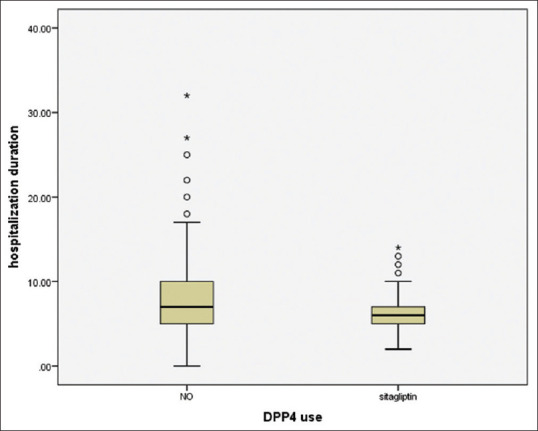
Comparison of hospitalization duration between dipeptidyl peptidase-4 inhibitor users and other cases
Our data also demonstrated that patients who were treated with DPP-4 inhibitors required less O2 therapies (76.7% vs. 88.6%, P = 0.046). There were no significant differences between the two groups of patients regarding survival rates (P = 0.55). These data are summarized in Table 3.
Table 3.
Comparison of hospitalization duration, O2 therapy requirement, and survival rates between dipeptidyl peptidase-4 inhibitor users and other patients
| Variable | DPP-4 inhibitor users | Non-DPP-4 inhibitor users | P |
|---|---|---|---|
| Hospitalization duration (days), mean±SD | 6.57±2.3 | 8.03±4.4 | 0.01 |
| Oxygen therapy, n (%) | 33 (76.7) | 140 (88.6) | 0.04 |
| Survival rate, n (%) | 35 (81.4) | 122 (77.2) | 0.55 |
DPP-4 = Dipeptidyl peptidase-4; SD = Standard deviation
We have also assessed several factors including age, sex, body mass index, past medical history, and metformin/DPP-4 use of the patients for survival risk assessment. The resulting data are presented in Table 4. Metformin usage significantly increased patients’ chance of survival (odds ratio [OR] = 11.96, P = 0.018) while DPP-4 inhibitor usage showed no statistically significant impact on survival. Older patients were in increased risk for mortality because of COVID-19 (OR = 1.13, P = 0.004). Other comparisons showed no statistically significant difference.
Table 4.
Risk assessment for patients’ survival
| Variable | P | Adjusted OR | 95% CI for EXP (B) | |
|---|---|---|---|---|
|
| ||||
| Lower | Upper | |||
| Age | 0.004 | 1.13 | 1.04 | 1.23 |
| Gender (female) | 0.72 | 0.74 | 0.15 | 3.75 |
| BMI | 0.37 | 0.93 | 0.81 | 1.08 |
| PMH | 0.67 | 0.71 | 0.15 | 3.40 |
| HTN | 0.50 | 1.83 | 0.30 | 10.87 |
| DPP-4 use | 0.76 | 0.76 | 0.13 | 4.41 |
| Metformin | 0.01 | 11.96 | 1.54 | 92.88 |
BMI=Body mass index; PMH=Past medical history; HTN=Hypertension; DPP-4=Dipeptidyl peptidase-4; CI=Confidence interval; OR=Odds ratio
DISCUSSION
In the present study, we evaluated data of patients with DM-II who were hospitalized due to COVID-19. Based on the findings of our study, patients who were previously treated with DPP-4 inhibitors for DM-II had significantly lower hospitalization duration and requiring O2 therapies. However, no changes in patients’ survival rates were observed. By assessing different risk factors for patients’ survival rates, we demonstrated that metformin usage was associated with increased survival rates while higher age was associated with decreased survival rates.
Among COVID-19 patients, patients with chronic underlying disease are at an increased risk for presenting severe and life-threatening complications after SARS-CoV-2 infection.[22] Type II diabetes mellitus is one of the most prevalent noncommunicable diseases worldwide which harms patients’ health status. Studies suggest that patients with diabetes are in increased danger for involving in severe COVID-19 complications.[23,24]
There have been previous studies that assess the prognosis of COVID-19 infection in patients with DM-II. In 2020, a study was conducted by Lakhani et al. that assessed the data of 13 patients with COVID-19 and acute respiratory distress syndrome. Based on the findings of this study, patients who were previously treated with DPP-4 inhibitors had significantly lower hospitalization duration.[25,26] Du et al. also conducted a study on the roles of DPP-4 inhibitors in COVID-19 infection. It was mentioned that DPP-4 is a ubiquitous glycoprotein which could act both as a cell membrane-bound protein and a soluble enzymatic protein after cleavage and release into the circulation. It was reported that DPP-4 inhibitors could alter the prognosis of DM patients with COVID-19 infection and their requirements for O2 therapies through its effects on cardiovascular system.[27,28] These results were in line with the findings of our study that demonstrated significantly reduced hospitalization duration and O2 therapy requirement in patients treated with DPP-4 inhibitors.
Another study was reported by Noh et al. in 2021. This cohort study assessed data of 586 patients with DM-II and COVID-19 infection. Based on the findings of this study, the usage of DPP-4 inhibitors was not associated with changes in the survival of patients, but decreased hospitalization duration was observed among them.[29] Similar results were demonstrated in the current study.
Another finding of our study was that our participants showed COVID-19 clinical symptoms for 7.81 days. Based on the literature and clinical observations, different symptoms of COVID-19 differ in presentation duration. We considered the overall hesitance of COVID-19 as our clinical course duration which was reported to be 7.81 days on average. Based on prior researches, some COVID-19 symptoms like dyspnea can last for months in some patients.[30,31]
We observed that most of the participants did not need respiratory support by extra O2 therapy or intubation/NIV. Respiratory support in the case of patients’ needs is a key part of COVID-19 patients’ hospital management.[32] Low oxygen saturation of COVID-19 patients and respiratory failure is one of the most important causes of death among these patients.[33] We should note that all participants who needed respiratory support received proper management. We demonstrated that cardiovascular disease was the most frequent medical illness found in the participants. Junior Rika Matangila et al. reported hypertension and diabetes as the most frequent underlying disease of hospitalized COVID-19 patients.[34] In a local study by Nasser Malekpour Alamdari et al. in Iran, several chronic diseases including diabetes were found to be more inpatient that died due to COVID-19.[35] It is evident that the accumulative effect of several diseases in patients could be worth his/her health status and leads the patient to more severe outcomes.
The mentioned data were consistent with the findings of our study. Another important finding of our study was reduced risks of mortality in patients consuming metformin. Similar data have been reported earlier.[36,37] These data show that metformin could possibly be useful in patients with COVID-19 and further research should be conducted in this regard. However, the large OR (11.96, 95% OR [1.54–92.88]) may reduce the strength of this association in clinical practice.
Our data show that DPP-4 inhibitors could contribute to lower hospitalization duration and lower need for O2 therapies and as a result might be useful in the treatment of COVID-19. However, DPP-4 inhibitor usage showed no statistically significant impact on survival which may be due to small sample size.
Limitations of the study
No data was available regarding the other drugs such as antihypertensive and statins that may have potential effects on coronavirus, which could be a limitation of the study. The other limitations of this study may be the small sample size and nature of our study compared with prospective studies. Furthermore, we could not evaluate other prescribed drugs for comorbid disorders or complications of DM which may be the confounding factors.
CONCLUSION
DPP-4 inhibitors could significantly decrease hospitalization days in diabetic patients who were hospitalized for COVID-19. However, DPP-4 inhibitor usage showed no statistically significant impact on survival. Lower age was a factor that decreases the odds of death due to COVID-19 complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We should appreciate the chairman and the staff of AJA University of Medical Sciences in Tehran for their contribution to data gathering during this study. The ethical code of the current study is IR.AJAUMS.REC.1399.200 provided by the Ethics Committee of AJA University of Medical Sciences.
REFERENCES
- 1.Fauci AS, Lane HC, Redfield RR. COVID-19 – Navigating the uncharted. Mass Med Soc. 2020;382:1268–9. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Velavan TP, Meyer CG. The COVID-19 epidemic. Trop Med Int Health. 2020;25:278. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lei S, Jiang F, Su W, Chen C, Chen J, Mei W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;21:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sattar Y, Ullah W, Rauf H, Virk HU, Yadav S, Chowdhury M, et al. COVID-19 cardiovascular epidemiology, cellular pathogenesis, clinical manifestations and management. Int J Cardiol Heart Vasc. 2020;29:100589. doi: 10.1016/j.ijcha.2020.100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lalchhandama K. The chronicles of coronaviruses: The electron microscope, the doughnut, and the spike. Sci Vis. 2020;20:78–92. [Google Scholar]
- 6.Harrison AG, Lin T, Wang P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol. 2020;41:1100–15. doi: 10.1016/j.it.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang N, Shi X, Jiang L, Zhang S, Wang D, Tong P, et al. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013;23:986–93. doi: 10.1038/cr.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol. 2020;215:108427. doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andalib A, Etemadifar MR, Rafiee Zadeh A, Moshkdar P. Treatment of pilon fractures with low profile plates. Int J Burns Trauma. 2021;11:486–93. [PMC free article] [PubMed] [Google Scholar]
- 10.Pitocco D, Tartaglione L, Viti L, Di Leo M, Pontecorvi A, Caputo S. SARS-CoV-2 and DPP4 inhibition: Is it time to pray for Janus Bifrons? Diabetes Res Clin Pract. 2020;163:108162. doi: 10.1016/j.diabres.2020.108162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strollo R, Pozzilli P. DPP4 inhibition: Preventing SARS-CoV-2 infection and/or progression of COVID-19? Diabetes Metab Res Rev. 2020;36:e3330. doi: 10.1002/dmrr.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller S, Krumins T, Zhou H, Huyck S, Johnson J, Golm G, et al. Ertugliflozin and sitagliptin co-initiation in patients with type 2 diabetes: The VERTIS SITA randomized study. Diabetes Ther. 2018;9:253–68. doi: 10.1007/s13300-017-0358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zadeh AR, Askari M, Azadani NN, Ataei A, Ghadimi K, Tavoosi N, et al. Mechanism and adverse effects of multiple sclerosis drugs: A review article. Part 1. Int J Physiol Pathophysiol Pharmacol. 2019;11:95. [PMC free article] [PubMed] [Google Scholar]
- 14.Zadeh AR, Ghadimi K, Ataei A, Askari M, Sheikhinia N, Tavoosi N, et al. Mechanism and adverse effects of multiple sclerosis drugs: A review article. Part 2. Int J Physiol Pathophysiol Pharmacol. 2019;11:105. [PMC free article] [PubMed] [Google Scholar]
- 15.Thornberry NA, Gallwitz B. Mechanism of action of inhibitors of dipeptidyl-peptidase-4 (DPP-4) Best Pract Res Clin Endocrinol Metab. 2009;23:479–86. doi: 10.1016/j.beem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014;35:992–1019. doi: 10.1210/er.2014-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rakhmat II, Kusmala YY, Handayani DR, Juliastuti H, Nawangsih EN, Wibowo A, et al. Dipeptidyl peptidase-4 (DPP-4) inhibitor and mortality in coronavirus disease 2019 (COVID-19) – A systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2021;15:777–82. doi: 10.1016/j.dsx.2021.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Cai Z, Zhang J. DPP-4 inhibitors may improve the mortality of coronavirus disease 2019: A meta-analysis. PLoS One. 2021;16:e0251916. doi: 10.1371/journal.pone.0251916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CF, Chien CH, Yang YP, Chou SJ, Wang ML, Huo TI, et al. Role of dipeptidyl peptidase-4 inhibitors in patients with diabetes infected with coronavirus-19. J Chin Med Assoc. 2020;83:710–1. doi: 10.1097/JCMA.0000000000000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roussel R, Darmon P, Pichelin M, Goronflot T, Abouleka Y, Ait Bachir L, et al. Use of dipeptidyl peptidase-4 inhibitors and prognosis of COVID-19 in hospitalized patients with type 2 diabetes: A propensity score analysis from the CORONADO study. Diabetes Obes Metabol. 2021;23:1162–72. doi: 10.1111/dom.14324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rafiee Zadeh A, Ghadimi K, Mohammadi B, Hatamian H, Naghibi SN, Danaeiniya A. Effects of estrogen and progesterone on different immune cells related to multiple sclerosis. Caspian J Neurol Sci. 2018;4:83–90. [Google Scholar]
- 22.Hernández-Galdamez DR, González-Block MÁ, Romo-Dueñas DK, Lima-Morales R, Hernández-Vicente IA, Lumbreras-Guzmán M, et al. Increased risk of hospitalization and death in patients with COVID-19 and Pre-existing noncommunicable diseases and modifiable risk factors in Mexico. Arch Med Res. 2020;51:683–9. doi: 10.1016/j.arcmed.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gazzaz ZJ. Diabetes and COVID-19. Open Life Sci. 2021;16:297–302. doi: 10.1515/biol-2021-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zadeh AR, Farrokhi M, Etemadifar M, Beni AA. Prevalence of benign tumors among patients with multiple sclerosis. Am J Exp Clin Res. 2015;2:127–32. [Google Scholar]
- 25.Lakhani JD, Pandya H, Jain A, Ghadiya S. Continuous blood glucose monitoring to determine the glycemic variability in patients having SARS CoV-2 infection with ARDS and its bearing on the severity of the disease. J Adv Med Med Res. 2021;116 [Google Scholar]
- 26.Rafiee Zadeh A, Falahatian M, Alsahebfosoul F. Serum levels of histamine and diamine oxidase in multiple sclerosis. Am J Clin Exp Immunol. 2018;7:100–5. [PMC free article] [PubMed] [Google Scholar]
- 27.Du H, Wang DW, Chen C. The potential effects of DPP-4 inhibitors on cardiovascular system in COVID-19 patients. J Cell Mol Med. 2020;24:10274–8. doi: 10.1111/jcmm.15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babak A, Rouzbahani R, Khalili Nejad R, Rafiee Zadeh A. Comparison of nutritional behaviors and physical activities between overweight/obese and normal-weight adults. Adv Biomed Res. 2019;8:62. doi: 10.4103/abr.abr_134_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noh Y, Oh IS, Jeong HE, Filion KB, Yu OHY, Shin JY. Association between DPP-4 inhibitors and COVID-19-related outcomes among patients with type 2 diabetes. Diabetes Care. 2021;44:e64–6. doi: 10.2337/dc20-1824. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, Villapol S. More than 50 Long-term effects of COVID-19: a systematic review and meta-analysis. medRxiv [Preprint] 2021 Jan 30; doi: 10.1038/s41598-021-95565-8. 2021.01.27.21250617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lerum TV, Aaløkken TM, Brønstad E, Aarli B, Ikdahl E, Lund KM, et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J. 2021;57:2003448. doi: 10.1183/13993003.03448-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shang Y, Pan C, Yang X, Zhong M, Shang X, Wu Z, et al. Management of critically ill patients with COVID-19 in ICU: Statement from front-line intensive care experts in Wuhan, China. Ann Intensive Care. 2020;10:73. doi: 10.1186/s13613-020-00689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mejía F, Medina C, Cornejo E, Morello E, Vásquez S, Alave J, et al. Oxygen saturation as a predictor of mortality in hospitalized adult patients with COVID-19 in a public hospital in Lima, Peru. PLoS One. 2020;15:e0244171. doi: 10.1371/journal.pone.0244171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matangila JR, Nyembu RK, Telo GM, Ngoy CD, Sakobo TM, Massolo JM, et al. Clinical characteristics of COVID-19 patients hospitalized at Clinique Ngaliema, a public hospital in Kinshasa, in the Democratic Republic of Congo: A retrospective cohort study. PLoS One. 2020;15:e0244272. doi: 10.1371/journal.pone.0244272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alamdari NM, Afaghi S, Rahimi FS, Tarki FE, Tavana S, Zali A, et al. Mortality risk factors among hospitalized COVID-19 patients in a major referral center in Iran. Tohoku J Exp Med. 2020;252:73–84. doi: 10.1620/tjem.252.73. [DOI] [PubMed] [Google Scholar]
- 36.Sharma S, Ray A, Sadasivam B. Metformin in COVID-19: A possible role beyond diabetes. Diabetes Res Clin Pract. 2020;164:108183. doi: 10.1016/j.diabres.2020.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Arabey AA, Abdalla M. Metformin and COVID-19: A novel deal of an old drug. J Med Virol. 2020;92:2293–4. doi: 10.1002/jmv.25958. [DOI] [PMC free article] [PubMed] [Google Scholar]


