Keywords: enterocyte, intermicrovillar adhesion complex (IMAC), intestine, microvilli, myosin
Abstract
Intestinal enterocytes have an elaborate apical membrane of actin-rich protrusions known as microvilli. The organization of microvilli is orchestrated by the intermicrovillar adhesion complex (IMAC), which connects the distal tips of adjacent microvilli. The IMAC is composed of CDHR2 and CDHR5 as well as the scaffolding proteins USH1C, ANKS4B, and Myosin 7b (MYO7B). To create an IMAC, cells must transport the proteins to the apical membrane. Myosin 5b (MYO5B) is a molecular motor that traffics ion transporters to the apical membrane of enterocytes, and we hypothesized that MYO5B may also be responsible for the localization of IMAC proteins. To address this question, we used two different mouse models: 1) neonatal germline MYO5B knockout (MYO5B KO) mice and 2) adult intestinal-specific tamoxifen-inducible VillinCreERT2;MYO5Bflox/flox mice. In control mice, immunostaining revealed that CDHR2, CDHR5, USH1C, and MYO7B were highly enriched at the tips of the microvilli. In contrast, neonatal germline and adult MYO5B-deficient mice showed loss of apical CDHR2, CDHR5, and MYO7B in the brush border and accumulation in a subapical compartment. Colocalization analysis revealed decreased Mander’s coefficients in adult inducible MYO5B-deficient mice compared with control mice for CDHR2, CDHR5, USH1C, and MYO7B. Scanning electron microscopy images further demonstrated aberrant microvilli packing in adult inducible MYO5B-deficient mouse small intestine. These data indicate that MYO5B is responsible for the delivery of IMAC components to the apical membrane.
NEW & NOTEWORTHY The intestinal epithelium absorbs nutrients and water through an elaborate apical membrane of highly organized microvilli. Microvilli organization is regulated by the intermicrovillar adhesion complexes, which create links between neighboring microvilli and control microvilli packing and density. In this study, we report a new trafficking partner of the IMAC, Myosin 5b. Loss of Myosin 5b results in a disorganized brush border and failure of IMAC proteins to reach the distal tips of microvilli.
INTRODUCTION
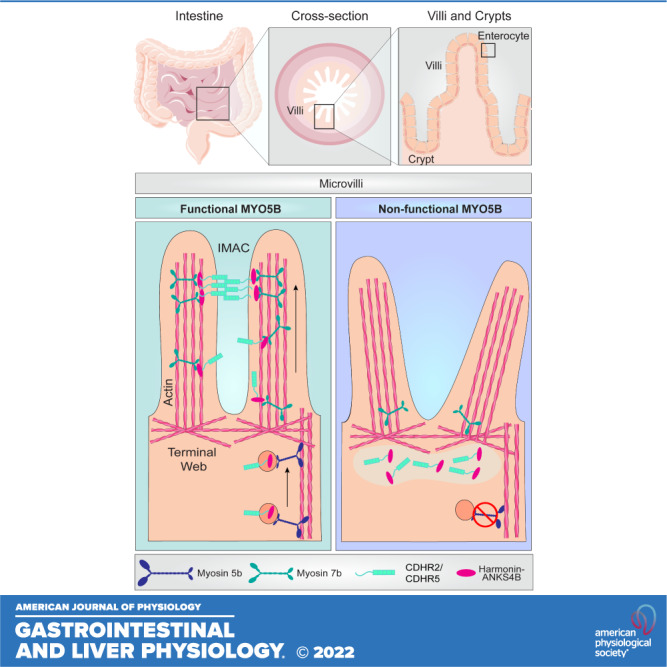
The gastrointestinal tract is essential for the absorption of nutrients and water. These important functions are facilitated at a macroscopic level by fingerlike projections of the small intestine known as villi and at a microscopic level by actin-rich fingerlike projections known as microvilli (1). Enterocytes in the small intestine harbor ∼1,000 microvilli per cell. These microvilli are rooted in the terminal web; an area of the apical membrane that is rich in actin filaments. The microvilli and terminal web together make up what is known as the “brush border.” This brush border dramatically increases the absorptive surface area of the intestine and contains the enzymes and transporters required for the breakdown and uptake of dietary components. To optimally function, microvilli must be properly packaged, aligned and connected to each other. The dense, tightly packed microvilli structure is achieved by links between neighboring microvilli known as the intermicrovillar adhesion complex (IMAC). IMACs consist of heterophilic links between adjacent microvilli made up of brush-border-specific protocadherin 24 (CDHR2) and mucin like protocadherin (CDHR5 or µ-protocadherin) (2). The protocadherins interact with the scaffolding proteins, ankyrin repeat protein and sterile a motif domain containing 4B (ANKS4B) and Harmonin [Usher syndrome 1C (USH1C)] (2–5). In addition to these IMAC components, another component has recently been identified: calmodulin-like protein 4 (CALML4). CALML4 functions as a light chain for the molecular motor Myosin 7b (MYO7B) (6). MYO7B transports IMAC cargo to the distal tips of microvilli and is necessary for proper brush-border assembly (3). Perturbations in the localization of components of the IMAC result in changes in microvilli assembly and packing that are postulated to impact intestinal permeability and susceptibility to injury.
The importance of the IMAC in intestinal health is highlighted by the fact that infection and inflammation alter IMAC components. Previously, In et al. (7) reported that the pathogenic bacteria enterohemorrhagic Escherichia coli (EHEC) targets CDHR2 during early infection in the colon disrupting intermicrovillar links. This dysregulation was speculated to promote a malabsorptive diarrhea. VanDussen et al. (8) also reported that IMAC gene expression was decreased in the ileum of patients with Crohn’s disease compared with noninflammatory bowel disease controls. Interestingly, the authors found that decreased IMAC mRNA corresponded with decreased brush-border height in Crohn’s disease tissue samples (8). These findings support a crucial role for proper IMAC localization and enrichment in the distal brush border to maintain proper homeostatic conditions.
One of the key questions is how the IMAC proteins are initially transported to the apical membrane of enterocytes. A potential trafficking partner for the IMAC components is Myosin 5b (MYO5B). MYO5B is a molecular motor that is highly expressed in the gastrointestinal tract and localizes to the base of the brush border. Loss of MYO5B in humans and animal models results in a severe diarrheal disorder known as microvillus inclusion disease (MVID) (9–15). Animal models lacking functional MYO5B exhibit many intestinal abnormalities including intracellular inclusions, fused villi, villous blunting, and disruption of cellular differentiation (13, 16). Another apical defect present in MYO5B-deficient mice is decreased apical localization of key transporters such as NHE3, SGLT1, AQP7, and DRA (17, 18). Although many cellular and intestinal defects are known to result from loss of MYO5B, alterations in brush-border assembly and organization have not been explored. We sought to determine whether IMAC proteins were trafficked to the brush border by MYO5B using a neonatal and adult mouse model of MYO5B deficiency. We observed decreased apical localization of CDHR2, CDHR5, USH1C, and MYO7B in the brush border of germline MYO5B knockout mice (MYO5B KO) and adult intestinal-specific tamoxifen-inducible VillinCreERT2;MYO5Bflox/flox mice. We also found that adult mice lacking MYO5B have disorganized microvilli with improper packing. Our results suggest that functional MYO5B is responsible for trafficking of CDHR2 and CDHR5 to the base of the brush-border where MYO7B would then deliver these proteins to the distal tips of microvilli. Loss of MYO5B was also found to impact the localization of MYO7B and USH1C. Collectively, our data point to a new role for MYO5B in regulating brush-border organization and IMAC trafficking to the brush border.
MATERIALS AND METHODS
Animal and Tissue Collection
Animal care, maintenance, and treatment were all conducted with approval by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and Vanderbilt University Medical Center. Germline MYO5B KO mice and intestinal-specific tamoxifen-inducible VillinCreERT2;MYO5Bflox/flox mice were used as previously described (13). For all experiments, male and female mice were used to account for any differences in sex that may contribute to brush-border assembly. Briefly, intestinal tissue from neonatal littermate control and MYO5B KO mice was collected 3–5 days after birth. 8- to 12-wk-old VillinCreERT2;MYO5Bflox/flox and littermate MYO5Bflox/flox or VillinCreERT2 (control) mice were all administered 2 mg of tamoxifen intraperitoneally. Intestinal tissue was collected 4 days posttamoxifen treatment. All intestinal tissue was fixed in either 4% paraformaldehyde or 10% neutral-buffered formalin overnight at 4°C before processing and paraffin embedding.
Immunofluorescence Staining
Paraffin tissue sections of 5 μm were heated for 15 min at 60°C to facilitate the adhesion of intestinal tissue to the glass slides. Slides were then deparaffinized using Histoclear (HS-200; National Diagnostics) and rehydrated in a series of ethanol. Antigen retrieval was performed in a pressure cooker using either citrate (pH 6) or Tris-EDTA (pH 9) epitope retrieval solution. After antigen retrieval slides were cooled on ice and washed before blocking using Dako serum-free protein block (X0909; Dako) for 1.5 h at room temperature in a humidified chamber. For all unconjugated mouse antibodies, mouse on mouse block (MKB-2213; Vector Laboratories) was added to tissue sections for 15 min after blocking. Primary antibodies were diluted in Dako antibody diluent with background-reducing components (S3022; Dako). Slides were incubated with primary antibodies overnight at 4°C in a humidified chamber. Tissue sections were washed three times in PBS and secondary antibodies diluted 1:200 in antibody diluent (S0809; Dako) were applied to slides for 1 h at room temperature. Hoechst (62249, 12.3 mg/mL; Thermo Fisher Scientific) diluted 1:1,000 in PBS was then added to each tissue section for 5 min followed by three washes in PBS. Slides were coverslipped using Prolong Gold Antifade (P36934; Thermo Fisher Scientific) and allowed to dry before imaging.
Primary and Secondary Antibodies
Primary antibodies and dilutions used in this study are as follows: mouse γ-actin 1:100 (sc-65638; Santa Cruz Biotechnology); rabbit CDHR2 1:100 (Prestige Antibodies HPA1010569; Sigma); mouse microprotocadherin 1:100 (sc-16953; Santa Cruz Biotechnology); rabbit MYO7B 1:100 (Prestige Antibodies HPA039131; Sigma); rabbit USH1C 1:200 (Prestige Antibodies HPA02739; Sigma); rabbit α-actinin-4 1:250 (ab108198; Abcam); mouse Villin 1:50 (sc-58897; Santa Cruz Biotechnology); and rat Lamp1 1:100 (sc-19992; Santa Cruz Biotechnology). Secondary antibodies conjugated to Alexa Fluor 488, Cy3, Alexa Fluor 594, Alexa Fluor 647, or Cy5, and diluted 1:200.
Imaging
A Zeiss Axio Imager.M2 microscope and a Leica SP8 microscope were used to acquire micrographs. The ×10 and ×20 objective was used for imaging on the Zeiss Axio. For Leica SP8 confocal images, a ×63 oil immersion objective was used. Zen and Leica software were used to export single channel tiff images and grayscale images were merged into red, green, and blue using Adobe Photoshop.
Scanning Electron Microscopy
For analysis of microvilli packing and organization, scanning electron microscopy (SEM) was performed as previously described (19). Briefly, ∼5-mm-sized pieces of the proximal small intestine of control and VillinCreERT2;MYO5Bflox/flox mice were fixed in 2.5% glutaraldehyde in 0.1 mol/L cacodylate buffer at room temperature for 1 h. Samples were then placed at 4°C for further fixation. Samples were processed sequentially in 1% tannic acid, 1% OsO4, and 1% uranyl acetate before dehydration through graded ethanol. Samples were critical point dried and mounted onto carbon tabs, a part of each sample’s villi were fractured with a scalpel to permit cross-sectional views. Samples were sputter-coated with platinum. Images were acquired on a Quanta 250 FEG scanning electron microscope at 5 kV (FEI).
Intestinal Organoids
Intestinal organoids were generated from crypts harvested from the proximal small intestine of adult uninduced VillinCreERT2;MYO5Bflox/flox mice. For in vitro induction of Cre recombinase to deplete MYO5B, intestinal organoids were treated with 10 µM 4-hydroxy-tamoxifen (4-OHT) overnight after differentiation as previously described (12). Alternatively, VillinCreERT2;MYO5Bflox/flox organoids were treated with an equal volume of ethanol overnight as a vehicle control. Intestinal organoids were fixed with 4% paraformaldehyde for 30 min at room temperature and embedded in paraffin for sectioning.
Quantification and Statistical Analysis
Small intestinal tissue from adult intestinal-specific tamoxifen-treated VillinCreERT2;MYO5Bflox/flox mice and littermate control mice was stained for components of the IMAC and the brush-border marker γ-actin or villin. Immunostained tissue sections were also analyzed for IMAC proteins and Lamp1-positive lysosomes. Confocal images were taken of the jejunal epithelium and the degree of colocalization of an IMAC protein and a brush-border marker or lysosomal marker was measured using ImageJ (National Institutes of Health) software and the just another colocalization plugin (JACoP) (20). Thresholds were set for each image to reduce autofluorescence and nonspecific background. Three to four representative images per mouse were analyzed and the results were averaged. Each data point represents the averaged JACoP analysis per mouse. For microvilli height and terminal web measurements, well-oriented cells were imaged (at least three images were acquired per mouse) and microvilli, terminal, and total brush-border height were measured using the measurement tool in ImageJ software. Mean fluorescence intensity was measured in ImageJ software using the measure tool on individual grayscale images. Significance was determined by an unpaired t test using GraphPad Prism software.
RESULTS
Germline MYO5B KO Mice Have Decreased Apical CDHR2, CDHR5, and MYO7B
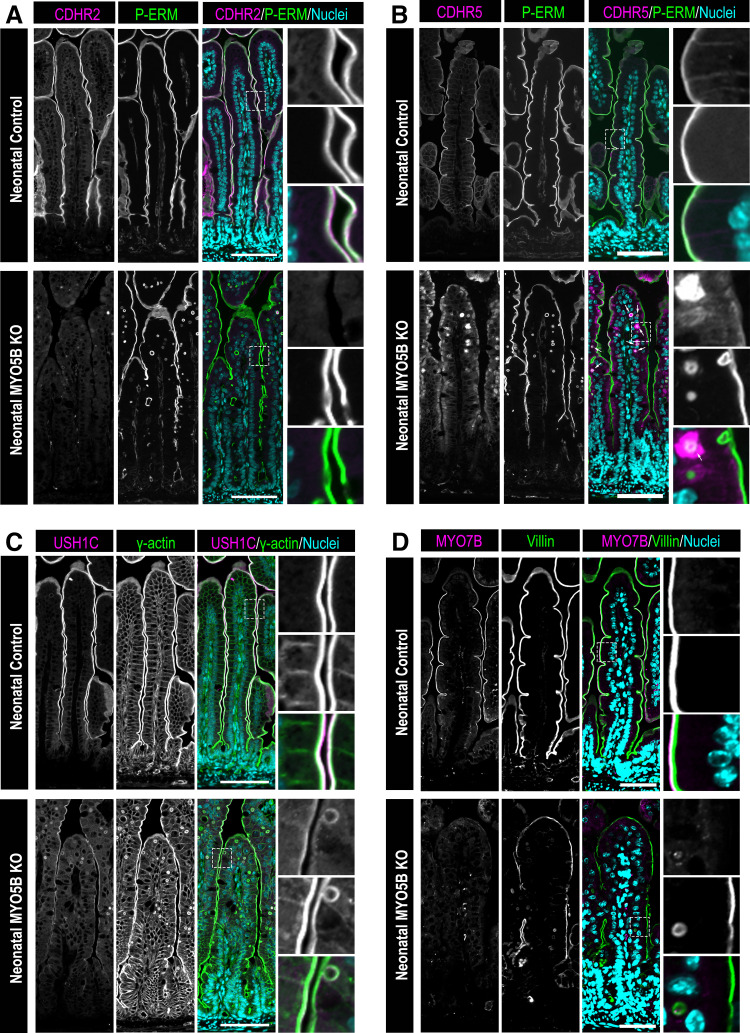
To determine whether MYO5B is responsible for trafficking IMAC components to the brush border, we immunostained small intestinal tissue from neonatal control and germline MYO5B KO mice. Staining for the protocadherin linker CDHR2 and the microvilli cross-linker phosphorylated Ezrin-Radixin-Moesin (P-ERM) showed considerable overlap in small intestinal enterocytes of control mice (Fig. 1A). CDHR2 was observed exclusively in the brush border of enterocytes in neonatal control mice. Littermate MYO5B KO mice exhibited apical P-ERM, but CDHR2 was absent from the brush border (Fig. 1A). Immunostaining for CDHR5 in neonatal control mice showed similar localization as CDHR2, with strictly apical CDHR5 localization that colocalized with P-ERM. In contrast, neonatal MYO5B KO mice had cytoplasmic CDHR5 that dispersed below the apical membrane. Interestingly, CDHR5 was especially evident in or surrounding several inclusions in the small intestine of MYO5B KO mice (Fig. 1B, inclusions containing CDHR5 indicated by arrows).
Figure 1.
Neonatal germline MYO5B KO mice exhibit altered IMAC gene expression and protein localization in the small intestine. Immunostaining of the small intestine for: the IMAC components (A) CDHR2 (magenta) and (B) CDHR5 (magenta), apical membrane marker phosphorylated-ezrin-radixin-moesin (P-ERM; green) and nuclei (blue) in neonatal control mice and germline MYO5B KO mice. Arrows indicate inclusions containing CDHR5 in the merged image. C: small intestinal tissue stained for the IMAC protein USH1C (magenta), the apical membrane protein γ actin (green) and nuclei (blue) in neonatal control and germline MYO5B KO mice. D: immunofluorescence images of the IMAC protein MYO7B (magenta), the apical membrane marker Villin (green) and nuclei (blue) in control and MYO5B KO mice. n = 4–6 mice/group. Scale bars = 50 µm. IMAC, intermicrovillar adhesion complex; KO, knockout.
We next stained for the scaffolding protein USH1C and γ-actin, which labels the actin-rich microvilli core. In neonatal control mice, USH1C was observed at the distal tips of microvilli and had an overlapping expression with γ-actin. MYO5B KO mice maintained apical USH1C; however, we observed decreased levels of USH1C compared with littermate control mice (Fig. 1C). Finally, we stained neonatal control and germline MYO5B KO mice for the molecular motor MYO7B. Neonatal control mice exhibited MYO7B localized to the apical domain of microvilli that were labeled by villin, an actin-bundling protein highly expressed in microvilli. In neonatal germline MYO5B KO mice, MYO7B was faintly detected in the apical membrane; indicating that MYO5B KO mice have decreased overall levels of MYO7B compared with littermate controls (Fig. 1D). Our results focused on alterations in the IMAC proteins in mice lacking MYO5B but excluded analysis of ANKS4B, an essential scaffolding protein within the IMAC. Currently, there are no mouse-specific ANKS4B antibodies that are commercially available, which limited our ability to determine the localization of ANKS4B in the small intestine of mice lacking MYO5B.
Adult inducible intestinal-specific MYO5B-deficient mice exhibit alterations in the localization of IMAC proteins.
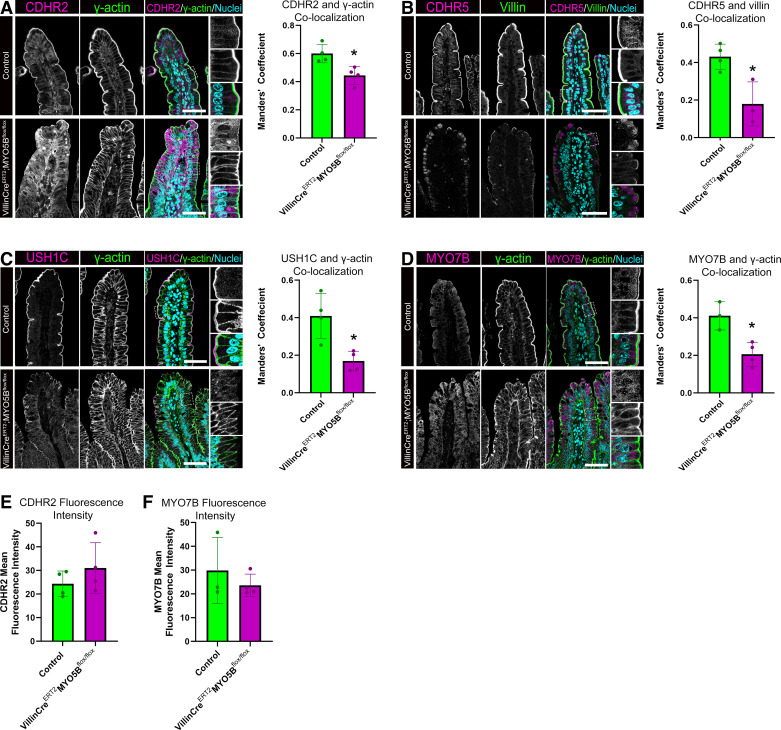
Germline MYO5B KO mice represent an important neonatal model of loss of MYO5B. However, because of their early demise resulting from dehydration, these mice do not have fully developed intestines. To determine the impact of loss of MYO5B in fully mature enterocytes, we used VillinCreERT2;MYO5Bflox/flox mice treated with tamoxifen to deplete MYO5B in the adult intestinal epithelium. We analyzed the localization of IMAC components in tamoxifen-treated littermate control and VillinCreERT2;MYO5Bflox/flox mice using immunofluorescence staining. CDHR2 staining in control mice showed CDHR2 concentrated in the brush border identified by γ-actin staining (Fig. 2A). In contrast, VillinCreERT2;MYO5Bflox/flox mice were observed to have a large amount of cytoplasmic CDHR2 and decreased CDHR2 in the brush border. Colocalization analysis of overlapping expression of CDHR2 with γ actin demonstrated a significant decrease in apical CDHR2 localization in VillinCreERT2;MYO5Bflox/flox mice compared with control mice (Fig. 2A).
Figure 2.
Adult tamoxifen-induced VillinCreERT2;MYO5Bflox/flox mice have aberrant localization of IMAC proteins. Small intestinal tissue stained for (A) CDHR2 (magenta), γ-actin (green) and nuclei (blue) in littermate control and tamoxifen-induced VillinCreERT2;MYO5Bflox/flox mice. Colocalization analysis of confocal micrographs for CDHR2 and γ-actin overlap. B: immunostaining of small intestinal tissue showing CDHR5 (magenta), Villin (green) and nuclei (blue) in control and VillinCreERT2;MYO5Bflox/flox mice. Graph shows colocalization analysis of CDHR5 and Villin in control and VillinCreERT2;MYO5Bflox/flox mice. C: staining for USH1C (magenta), γ-actin (green), and nuclei (blue) in control and VillinCreERT2;MYO5Bflox/flox mice along with colocalization analysis of USH1C and γ-actin. D: immunostaining for the IMAC protein MYO7B (magenta), γ-actin (green), and nuclei in the small intestine of control and VillinCreERT2;MYO5Bflox/flox mice. Mean fluorescence intensity measurements from immunofluorescence staining of adult control and VillinCreERT2;MYO5Bflox/flox mice for CDHR2 (E) and MYO7B (F). Colocalization analysis of MYO7B and γ-actin coexpression. *P < 0.05, n = 3 or 4 mice/group. Scale bars = 50 µm. IMAC, intermicrovillar adhesion complex.
CDHR5 immunostaining of control and VillinCreERT2;MYO5Bflox/flox mice was similar to the pattern observed in neonatal control and MYO5B KO mice. VillinCreERT2;MYO5Bflox/flox mice had a cytoplasmic distribution of CDHR5, whereas control mice had CDHR5 localized to the tips of the brush border. Colocalization analysis confirmed a decrease in apically oriented CDHR5 in VillinCreERT2;MYO5Bflox/flox mice compared with littermate control mice (Fig. 2B). In adult control mice treated with tamoxifen, USH1C was present in the distal tips of microvilli. Similar to germline MYO5B KO mice, USH1C staining showed maintenance of some apical USH1C; however, the level of USH1C in the brush border was significantly decreased in VillinCreERT2;MYO5Bflox/flox mice compared with control mice as demonstrated by colocalization analysis (Fig. 2C). MYO7B staining in control mice exhibited enrichment of MYO7B at the tips of microvilli, whereas VillinCreERT2;MYO5Bflox/flox mice exhibited MYO7B in the cytoplasm. Colocalization analysis further confirmed that VillinCreERT2;MYO5Bflox/flox mice had decreased levels of MYO7B in the same cellular compartment as γ-actin (Fig. 2D). Measurement of CDHR2 (Fig. 2E) and MYO7B (Fig. 2F) mean fluorescence intensity showed no significant differences between control and VillinCreERT2;MYO5Bflox/flox mice. Interestingly, RNA sequencing results from control and VillinCreERT2;MYO5Bflox/flox mice in the online GEO database GSE139302 showed significantly decreased CDHR2 expression in VillinCreERT2;MYO5Bflox/flox mice compared with control mice but MYO7B was unchanged (CDHR2 log twofold change = −1.1785; P-adjusted value = 0.039723 and MYO7B log twofold change = −0.74596; P-adjusted value = 0.47799) (18).
IMAC Protocadherin Proteins Are Increased in the Lysosomal Compartment in the Absence of MYO5B
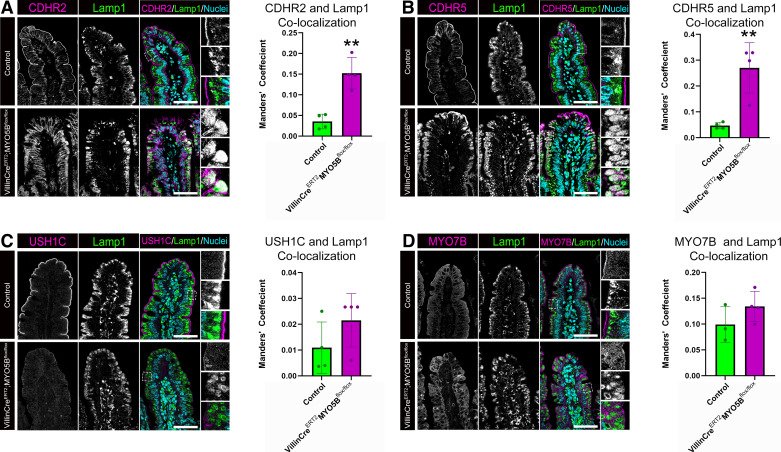
The subapical localization of IMAC components in VillinCreERT2;MYO5Bflox/flox mice suggested that an improper targeting of these proteins to the apical membrane may result in lysosomal degradation. To address this hypothesis, we performed immunostaining for IMAC proteins and Lamp1 which labels lysosomes. Control mice displayed predominantly apical CDHR2 and Lamp1-positive lysosomes aligned below the apical membrane of enterocytes (Fig. 3A). VillinCreERT2;MYO5Bflox/flox mice exhibited cytoplasmic CDHR2 and expanded Lamp1 lysosomes. Image J analysis demonstrated a significant increase in Lamp1 lysosomes containing CDHR2 in VillinCreERT2; MYO5Bflox/flox mice compared with control mice (Fig. 3A). Staining for CDHR5 and Lamp1 revealed similar findings as CDHR2, with VillinCreERT2;MYO5Bflox/flox mice having a significant increase in the overlap of Lamp1 lysosomes that are positive for CDHR5 (Fig. 3B). Interestingly, staining for USH1C or MYO7B and Lamp1 did not show any increase in staining in the lysosomal compartment in VillinCreERT2;MYO5Bflox/flox mice (Fig. 3, C and D). These data suggest that in the absence of intestinal epithelial MYO5B, only the protocadherin components of the IMAC are mistargeted to the lysosome.
Figure 3.
Loss of MYO5B results in increased localization of CDHR2 and CDHR5 to Lamp1+ lysosomes. Adult control and VillinCreERT2;MYO5Bflox/flox mice were immunostained for the IMAC components (A) CDHR2 (magenta), (B) CDHR5 (magenta), (C) USH1C (magenta), (D) MYO7B (magenta), and Lamp1 (green) to identify lysosomes. Colocalization analysis of the degree of overlap between IMAC protein and Lamp1+ lysosomes was performed and plotted. **P < 0.01, n = 3 or 4 mice/group. Scale bars = 50 µm. IMAC, intermicrovillar adhesion complex.
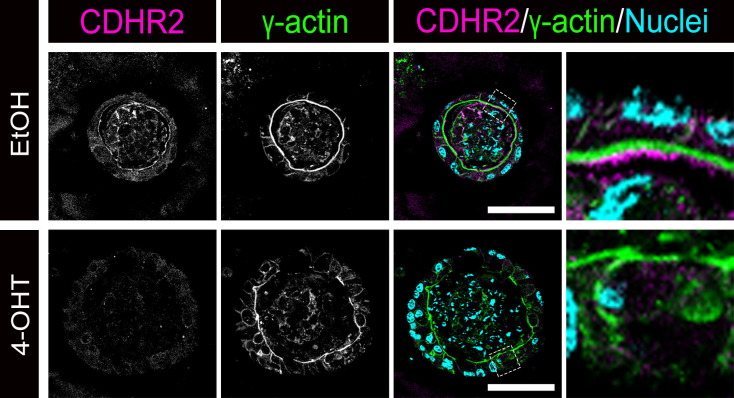
In Vitro Deletion of MYO5B Results in Loss of Apical CDHR2 in Intestinal Organoids
To determine the impact of loss of MYO5B on differentiated intestinal epithelial cells apart from other cell types (i.e., immune cells, enteric neurons, fibroblasts, etc.), we used intestinal organoids generated from untreated VillinCreERT2;MYO5Bflox/flox mice. VillinCreERT2;MYO5Bflox/flox intestinal organoids were grown in 3-D in Matrigel and differentiated, then organoids were treated with either ethanol as a vehicle control or 10 µM 4-hydroxytamoxifen to delete MYO5B. Immunostaining of ethanol-treated (control) organoids showed CDHR2 localized above the brush border as marked by γ-actin (Fig. 4). Intestinal organoids treated with 4-hydoxytamoxifen to knockout MYO5B in culture showed a decrease in CDHR2 and no CDHR2 was observed on the apical membrane of enterocytes (Fig. 4). These findings confirm our in vivo results and indicate that loss of MYO5B in the intestinal epithelium is sufficient to disorganize IMAC localization.
Figure 4.
Loss of MYO5B in vitro impacts the expression and localization of CDHR2. Intestinal organoids derived from uninduced VillinCreERT2;MYO5Bflox/flox mice and treated with either ethanol (control) or 4-hydroxytamoxifen (4-OHT) to deplete MYO5B in vitro. Differentiated intestinal organoids were immunostained for CDHR2 (magenta), γ actin (green), and nuclei (blue). Three uninduced VillinCreERT2;MYO5Bflox/flox mice were used to generate organoids and multiple wells of each treatment group (i.e., ethanol or 4-OHT) were pooled for paraffin embedding and staining. Scale bars = 50 µm. IMAC, intermicrovillar adhesion complex.
Loss of MYO5B Results in a Disorganized Brush Border
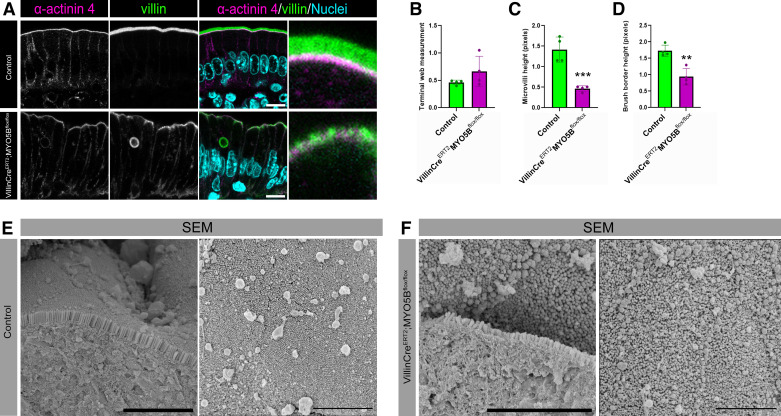
Previous work has demonstrated shortened microvilli in VillinCreERT2;MYO5Bflox/flox mice compared with control mice (13). However, this previous work did not specifically examine the terminal web, which anchors the microvilli in the enterocytes. To determine if the terminal web was altered in VillinCreERT2;MYO5Bflox/flox mice, we performed immunostaining to measure microvilli using a Villin antibody and the terminal web using an α-actinin 4 antibody (Fig. 5A). Terminal web height remained the same between control and VillinCreERT2;MYO5Bflox/flox mice (Fig. 5B). Measurement of microvilli confirmed a significant reduction in microvilli height in VillinCreERT2;MYO5Bflox/flox mice compared with littermate control mice (Fig. 5C). Measurement of the whole brush border revealed a significant decrease in brush-border size in VillinCreERT2;MYO5Bflox/flox mice (Fig. 5D).
Figure 5.
Adult mice lacking MYO5B have decreased brush-border height and altered microvilli packing. A: confocal microscopy of small intestinal enterocytes from littermate control and VillinCreERT2;MYO5Bflox/flox mice stained for the terminal web marker α-actinin 4 (magenta), the microvilli label villin (green), and nuclei (blue). Scale bars = 10 µm. Graphs showing measurements of (B) α-actinin 4, (C) villin, and (D) total brush-border height in control and VillinCreERT2;MYO5Bflox/flox mice. **P < 0.01, ***P < 0.001, n = 4 mice/group. Scanning electron micrographs of the apical membrane of (E) control and (F) VillinCreERT2;MYO5Bflox/flox mice. Scale bars = 5 µm.
Finally, we sought to determine whether the alterations in the localization of IMAC proteins in VillinCreERT2;MYO5Bflox/flox mice impacted microvilli packing using SEM. Visualization of microvilli with SEM supported a decrease in microvilli height in VillinCreERT2;MYO5Bflox/flox mice (Fig. 5, E and F). SEM also showed dense packing of microvilli in control mice. In contrast VillinCreERT2;MYO5Bflox/flox mice had disorganized microvilli that were not packed in the typical hexagonal pattern and demonstrated a loss of ordered microvilli packing compared with control mice.
DISCUSSION
In this study, we identified that IMAC components were mislocalized in the absence of MYO5B. Loss of MYO5B in germline and adult intestinal-specific tamoxifen-inducible MYO5B mice resulted in a redistribution of CDHR2 and CDHR5 from their normal apical localization to the subapical compartment of enterocytes. USH1C and MYO7B exhibited decreased apical expression in mouse models lacking MYO5B compared with control mice. In addition, immunostaining for IMAC proteins and the lysosomal marker, Lamp1, demonstrated that mice lacking MYO5B had significantly more lysosomes that were positive for CDHR2 and CDHR5, suggesting increased degradation of these proteins. Analysis of microvilli height and terminal web measurement showed a marked decrease in microvilli height, but no alteration in terminal web and SEM revealed disrupted microvilli packing. The findings presented herein indicate that MYO5B is necessary for the proper distribution of IMAC proteins in the apical membrane of enterocytes. Based on our findings, we postulate that MYO5B delivers cargo, including CDHR2 and CDHR5, from the Golgi to the terminal web and that other motors, such as MYO7B, then traffic these protocadherins to the distal tips of microvilli. In the absence of functional MYO5B, we also propose that the proper localization of USH1C and MYO7B is impacted. Our data indicate that MYO5B is critical for IMAC delivery and thus microvilli assembly in small intestinal enterocytes.
The localization of MYO5B in small intestinal enterocytes to the terminal web region suggests that MYO5B delivers its cargo from the Golgi to the base of microvilli and that other motors are responsible for microvilli tip targeting. MYO7B is well characterized as the molecular motor that delivers IMAC proteins to the tips of microvilli and is enriched in the distal tips of microvilli. Although MYO5B is necessary for intestinal alkaline phosphatase, AQP7, NHE3, and SGLT1, localization in the small intestine (17), other myosins are likely responsible for the delivery of these important brush-border components from the terminal web to the microvilli. Our data demonstrate that in the absence of MYO5B, the localization of MYO7B in the small intestine is impacted. In support of this, Tyska et al. (21) reported that the brush border of Myosin 1a knockout mice exhibited decreased Myosin 6 expression as well as reduced Myosin 1e levels. Similarly, we predict that loss of MYO5B impacts the localization and expression levels of multiple brush-border myosins including MYO7B. We speculate that alterations in these other myosins may prevent the proper delivery of multiple microvilli components. Future studies will likely delineate the trafficking patterns and cargo of individual myosins that are highly expressed in microvilli within the gastrointestinal tract.
One interesting finding of this study is that germline neonatal MYO5B KO did not exhibit the same levels of MYO7B and CDHR2 as adult inducible mice (VillinCreERT2;MYO5Bflox/flox). We speculate that the differences between protein levels in neonatal and adult inducible MYO5B KO mice arise from the differences in the developmental stage. During early life development, small-intestinal enterocytes are highly endocytic, and the apical endocytic complex plays a critical role in the uptake of luminal contents (22–27). This process is known as “bulk endocytosis” (28). Neonatal MYO5B mice exhibit multiple intracellular inclusions in enterocytes, and it has been theorized that these inclusions are the result of loss of apical membrane recycling after large endocytic events (28). Interestingly, these inclusions are not as prevalent in adult-induced VillinCreERT2;MYO5Bflox/flox mice as observed in neonatal germline MYO5B mice (13); suggesting that bulk endocytosis largely occurs during early life development.
In addition to functional differences, it is also possible that neonatal mice have an immature brush border compared with adult mice. Work in rats has shown that the brush-border enzyme activity increases during aging (29). As a result, we speculate that the brush border is not fully developed in our neonatal mouse model. Consistent with this hypothesis, we have previously shown that the localization of other apical transporters was also dependent on mouse age (17). We previously found that the sodium transporters NHE3 and SGLT1 were located subapically in neonatal germline MYO5B KO, but largely decreased in inducible adult mice (VillinCreERT2;MYO5Bflox/flox) (17). In this study, we found that total CDHR2 was decreased in neonatal mice but still present and diffuse in adult inducible VillinCreERT2;MYO5Bflox/flox mice. Interestingly, we found that VillinCreERT2;MYO5Bflox/flox-derived organoids treated with 4-hydroxytamoxifen had decreased CDHR2. These results are consistent with our staining of neonatal germline MYO5B KO mice but differs from our VillinCreERT2;MYO5Bflox/flox mice. We postulate that loss of CDHR2 in organoids and neonatal mice is due to an immature brush border. Mouse intestinal organoids even when fully differentiated in 3-D may not possess a mature brush border and as a result, organoids may more closely align with neonatal intestinal cells rather than fully mature enterocytes (30).
Intestinal IMAC links closely resemble extracellular links that are present at the tips of stereocilia of the inner ear (2). Stereocilia are similar to microvilli in that they are highly organized actin-rich protrusions. Stereocilia are required for hearing and balance and are organized into distinct rows that increase in height in a staircase-like pattern (31). Shorter rows of stereocilia are connected to the adjacent taller stereocilia by protocadherin 15 (PCDH15) and cadherin 23 (CDH23) links (32). Stereocilia length, linkages, and anchoring are highly regulated by myosin motors including Myosin 3a, Myosin 3b, Myosin 6, Myosin 7a, and Myosin 15a (33). Microvilli also harbor high levels of Myosin 6 and 7 (34) and exhibit uniformity in length and width, similar to the remarkable uniformity of stereocilia rows. Another common feature between stereocilia and intestinal microvilli is Harmonin (USH1C), which serves to anchor both the IMAC and stereocilia links to the actin core. The importance of the intestinal IMAC is underscored by Usher syndrome (type 1), a disorder caused by mutations in the USH1C gene. Usher syndrome causes deafness, blindness and is frequently associated with severe gastrointestinal symptoms including diarrhea (35). A mouse model of Usher syndrome (Harmonin knockout mice) exhibits irregular and shortened intestinal microvilli that lack the characteristic tight microvilli packing present in wild-type mice (2). MYO5B has been previously linked to deafness; however, the mechanism is unknown (36). Based on these findings, we would predict that loss of MYO5B could alter the tip link assembly between stereocilia, thereby impacting hearing. This work will be interesting to explore in the future.
In summary, this study finds that two different mouse models lacking MYO5B have altered localization of CDHR2, CDHR5, USH1C, and MYO7B. Collectively, these data support the conclusion that functional MYO5B is required for IMAC targeting to the tips of microvilli to facilitate proper microvilli packing. These findings suggest that MYO5B is a critical component of the trafficking pathway that is involved in the delivery as well as the proper distribution of IMAC proteins. Alterations in microvilli height and packing may represent a major defect in epithelial barrier function, contributing to intestinal epithelial vulnerability to insults as well as aberrant immune responses.
GRANTS
This study was supported by the National Institutes of Health (NIH) Grants K01 DK121869 (to A.C.E.), F32 DK130288 (to K.A.E.), and RO1 DK128190 (to I.K.). This work was supported by startup funds from the Medical University of South Carolina (MUSC; to A.C.E.) and MUSC Grants P30 DK123704 and P20 GM120475. The MUSC Digestive Disease Research Core Center imaging core also provided support.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.A.D., K.A.E., and A.C.E. conceived and designed research; S.A.D., K.A.E., J.D., R.S., I.K., E.K., and A.C.E. performed experiments; S.A.D., K.A.E., J.D., R.S., I.K., E.K., and A.C.E. analyzed data; S.A.D., K.A.E., and A.C.E. interpreted results of experiments; S.A.D. and A.C.E. prepared figures; S.A.D. and A.C.E. drafted manuscript; S.A.D., K.A.E., R.S., I.K., E.K., and A.C.E. edited and revised manuscript; S.A.D., K.A.E., J.D., R.S., I.K., E.K., and A.C.E. approved final version of manuscript.
REFERENCES
- 1. Crawley SW, Mooseker MS, Tyska MJ. Shaping the intestinal brush border. J Cell Biol 207: 441–451, 2014. doi: 10.1083/jcb.201407015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crawley SW, Shifrin DA Jr, Grega-Larson NE, McConnell RE, Benesh AE, Mao S, Zheng Y, Zheng QY, Nam KT, Millis BA, Kachar B, Tyska MJ. Intestinal brush border assembly driven by protocadherin-based intermicrovillar adhesion. Cell 157: 433–446, 2014. doi: 10.1016/j.cell.2014.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weck ML, Crawley SW, Stone CR, Tyska MJ. Myosin-7b promotes distal tip localization of the intermicrovillar adhesion complex. Curr Biol 26: 2717–2728, 2016. doi: 10.1016/j.cub.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crawley SW, Weck ML, Grega-Larson NE, Shifrin DA Jr, Tyska MJ. ANKS4B is essential for intermicrovillar adhesion complex formation. Dev Cell 36: 190–200, 2016. doi: 10.1016/j.devcel.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li J, He Y, Lu Q, Zhang M. Mechanistic basis of organization of the Harmonin/USH1C-mediated brush border microvilli tip-link complex. Dev Cell 36: 179–189, 2016. doi: 10.1016/j.devcel.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 6. Choi MS, Graves MJ, Matoo S, Storad ZA, El Sheikh Idris RA, Weck ML, Smith ZB, Tyska MJ, Crawley SW. The small EF-hand protein CALML4 functions as a critical myosin light chain within the intermicrovillar adhesion complex. J Biol Chem 295: 9281–9296, 2020. doi: 10.1074/jbc.RA120.012820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. In J, Foulke-Abel J, Zachos NC, Hansen AM, Kaper JB, Bernstein HD, Halushka M, Blutt S, Estes MK, Donowitz M, Kovbasnjuk O. Enterohemorrhagic Escherichia coli reduce mucus and intermicrovillar bridges in human stem cell-derived colonoids. Cell Mol Gastroenterol Hepatol 2: 48–62.e3, 2016. doi: 10.1016/j.jcmgh.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. VanDussen KL, Stojmirović A, Li K, Liu TC, Kimes PK, Muegge BD, Simpson KF, Ciorba MA, Perrigoue JG, Friedman JR, Towne JE, Head RD, Stappenbeck TS. Abnormal small intestinal epithelial microvilli in patients with Crohn's disease. Gastroenterology 155: 815–828, 2018. doi: 10.1053/j.gastro.2018.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davidson GP, Cutz E, Hamilton JR, Gall DG. Familial enteropathy: a syndrome of protracted diarrhea from birth, failure to thrive, and hypoplastic villus atrophy. Gastroenterology 75: 783–790, 1978. doi: 10.1016/0016-5085(78)90458-4. [DOI] [PubMed] [Google Scholar]
- 10. Müller T, Hess MW, Schiefermeier N, Pfaller K, Ebner HL, Heinz-Erian P, Ponstingl H, Partsch J, Röllinghoff B, Köhler H, Berger T, Lenhartz H, Schlenck B, Houwen RJ, Taylor CJ, Zoller H, Lechner S, Goulet O, Utermann G, Ruemmele FM, Huber LA, Janecke AR. MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nat Genet 40: 1163–1165, 2008. doi: 10.1038/ng.225. [DOI] [PubMed] [Google Scholar]
- 11. Ruemmele FM, Müller T, Schiefermeier N, Ebner HL, Lechner S, Pfaller K, Thöni CE, Goulet O, Lacaille F, Schmitz J, Colomb V, Sauvat F, Revillon Y, Canioni D, Brousse N, de Saint-Basile G, Lefebvre J, Heinz-Erian P, Enninger A, Utermann G, Hess MW, Janecke AR, Huber LA. Loss-of-function of MYO5B is the main cause of microvillus inclusion disease: 15 novel mutations and a CaCo-2 RNAi cell model. Hum Mutat 31: 544–551, 2010. doi: 10.1002/humu.21224. [DOI] [PubMed] [Google Scholar]
- 12. Schneeberger K, Vogel GF, Teunissen H, van Ommen DD, Begthel H, El Bouazzaoui L, van Vugt AH, Beekman JM, Klumperman J, Müller T, Janecke A, Gerner P, Huber LA, Hess MW, Clevers H, van Es JH, Nieuwenhuis EE, Middendorp S. An inducible mouse model for microvillus inclusion disease reveals a role for myosin Vb in apical and basolateral trafficking. Proc Natl Acad Sci USA 112: 12408–12413, 2015. doi: 10.1073/pnas.1516672112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weis VG, Knowles BC, Choi E, Goldstein AE, Williams JA, Manning EH, Roland JT, Lapierre LA, Goldenring JR. Loss of MYO5B in mice recapitulates microvillus inclusion disease and reveals an apical trafficking pathway distinct to neonatal duodenum. Cell Mol Gastroenterol Hepatol 2: 131–157, 2016. doi: 10.1016/j.jcmgh.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cartón-García F, Overeem AW, Nieto R, Bazzocco S, Dopeso H, Macaya I, Bilic J, Landolfi S, Hernandez-Losa J, Schwartz S Jr, Ramon y Cajal S, van Ijzendoorn SC, Arango D. Myo5b knockout mice as a model of microvillus inclusion disease. Sci Rep 5: 12312, 2015. doi: 10.1038/srep12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Engevik AC, Coutts AW, Kaji I, Rodriguez P, Ongaratto F, Saqui-Salces M, Medida RL, Meyer AR, Kolobova E, Engevik MA, Williams JA, Shub MD, Carlson DF, Melkamu T, Goldenring JR. Editing myosin VB gene to create porcine model of microvillus inclusion disease, with microvillus-lined inclusions and alterations in sodium transporters. Gastroenterology 158: 2236–2249.e9, 2020. doi: 10.1053/j.gastro.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaji I, Roland JT, Rathan-Kumar S, Engevik AC, Burman A, Goldstein AE, Watanabe M, Goldenring JR. Cell differentiation is disrupted by MYO5B loss through Wnt/Notch imbalance. JCI Insight 6: e150416, 2021. doi: 10.1172/jci.insight.150416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Engevik AC, Kaji I, Engevik MA, Meyer AR, Weis VG, Goldstein A, Hess MW, Müller T, Koepsell H, Dudeja PK, Tyska M, Huber LA, Shub MD, Ameen N, Goldenring JR. Loss of MYO5B leads to reductions in Na+ absorption with maintenance of CFTR-dependent Cl- secretion in enterocytes. Gastroenterology 155: 1883–1897.e10, 2018. doi: 10.1053/j.gastro.2018.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaji I, Roland JT, Watanabe M, Engevik AC, Goldstein AE, Hodges CA, Goldenring JR. Lysophosphatidic acid increases maturation of brush borders and SGLT1 activity in MYO5B-deficient mice, a model of microvillus inclusion disease. Gastroenterology 159: 1390–1405.e20, 2020. doi: 10.1053/j.gastro.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Engevik AC, Krystofiak ES, Kaji I, Meyer AR, Weis VG, Goldstein A, Coutts AW, Melkamu T, Saqui-Salces M, Goldenring JR. Recruitment of polarity complexes and tight junction proteins to the site of apical bulk endocytosis. Cell Mol Gastroenterol Hepatol 12: 59–80, 2021. doi: 10.1016/j.jcmgh.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tyska MJ, Mackey AT, Huang JD, Copeland NG, Jenkins NA, Mooseker MS. Myosin-1a is critical for normal brush border structure and composition. Mol Biol Cell 16: 2443–2457, 2005. doi: 10.1091/mbc.e04-12-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gonnella PA, Neutra MR. Membrane-bound and fluid-phase macromolecules enter separate prelysosomal compartments in absorptive cells of suckling rat ileum. J Cell Biol 99: 909–917, 1984. doi: 10.1083/jcb.99.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gonnella PA, Neutra MR. Glycoconjugate distribution and mobility on apical membranes of absorptive cells of suckling rat ileum in vivo. Anat Rec 213: 520–528, 1985. doi: 10.1002/ar.1092130408. [DOI] [PubMed] [Google Scholar]
- 24. Siminoski K, Gonnella P, Bernanke J, Owen L, Neutra M, Murphy RA. Uptake and transepithelial transport of nerve growth factor in suckling rat ileum. J Cell Biol 103: 1979–1990, 1986. doi: 10.1083/jcb.103.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Knutton S, Limbrick AR, Robertson JD. Regular structures in membranes. I. Membranes in the endocytic complex of ileal epithelial cells. J Cell Biol 62: 679–694, 1974. doi: 10.1083/jcb.62.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arévalo Sureda E, Weström B, Pierzynowski SG, Prykhodko O. Maturation of the intestinal epithelial barrier in neonatal rats coincides with decreased FcRn expression, replacement of vacuolated enterocytes and changed Blimp-1 expression. PLoS One 11: e0164775, 2016. [Erratum in PLoS One 12: e0169724, 2017]. doi: 10.1371/journal.pone.0164775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilson JM, Whitney JA, Neutra MR. Identification of an endosomal antigen specific to absorptive cells of suckling rat ileum. J Cell Biol 105: 691–703, 1987. doi: 10.1083/jcb.105.2.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Engevik AC, Kaji I, Postema MM, Faust JJ, Meyer AR, Williams JA, Fitz GN, Tyska MJ, Wilson JM, Goldenring JR. Loss of myosin Vb promotes apical bulk endocytosis in neonatal enterocytes. J Cell Biol 218: 3647–3662, 2019. doi: 10.1083/jcb.201902063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Raul F, Gosse F, Doffoel M, Darmenton P, Wessely JY. Age related increase of brush border enzyme activities along the small intestine. Gut 29: 1557–1563, 1988. doi: 10.1136/gut.29.11.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hofer M, Lutolf MP. Engineering organoids. Nat Rev Mater 6: 402–420, 2021. doi: 10.1038/s41578-021-00279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ebrahim S, Avenarius MR, Grati M, Krey JF, Windsor AM, Sousa AD, Ballesteros A, Cui R, Millis BA, Salles FT, Baird MA, Davidson MW, Jones SM, Choi D, Dong L, Raval MH, Yengo CM, Barr-Gillespie PG, Kachar B. Stereocilia-staircase spacing is influenced by myosin III motors and their cargos espin-1 and espin-like. Nat Commun 7: 10833, 2016. [Erratum in Nat Commun 8: 16133, 2017]. doi: 10.1038/ncomms10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Müller U, Kachar B. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 449: 87–91, 2007. doi: 10.1038/nature06091. [DOI] [PubMed] [Google Scholar]
- 33. Houdusse A, Titus MA. The many roles of myosins in filopodia, microvilli and stereocilia. Curr Biol 31: R586–R602, 2021. doi: 10.1016/j.cub.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Engevik MA, Engevik AC. Myosins and membrane trafficking in intestinal brush border assembly. Curr Opin Cell Biol 77: 102117, 2022. doi: 10.1016/j.ceb.2022.102117. [DOI] [PubMed] [Google Scholar]
- 35. Bitner-Glindzicz M, Lindley KJ, Rutland P, Blaydon D, Smith VV, Milla PJ, Hussain K, Furth-Lavi J, Cosgrove KE, Shepherd RM, Barnes PD, O'Brien RE, Farndon PA, Sowden J, Liu XZ, Scanlan MJ, Malcolm S, Dunne MJ, Aynsley-Green A, Glaser B. A recessive contiguous gene deletion causing infantile hyperinsulinism, enteropathy and deafness identifies the Usher type 1C gene. Nat Genet 26: 56–60, 2000. doi: 10.1038/79178. [DOI] [PubMed] [Google Scholar]
- 36. García-Cazorla A, Oyarzábal A, Saudubray JM, Martinelli D, Dionisi-Vici C. Genetic disorders of cellular trafficking. Trends Genet 38: 724–751, 2022. doi: 10.1016/j.tig.2022.02.012. [DOI] [PubMed] [Google Scholar]