Keywords: endocannabinoids, hyperalgesia, PF-3845, spared nerve injury, URB597
Abstract
The complexity of neuropathic pain and its associated comorbidities, including dysautonomia, make it difficult to treat. Overlap of anatomical regions and pharmacology of sympathosensory systems in the central nervous system (CNS) provide targets for novel treatment strategies. The dorsal periaqueductal gray (dPAG) is an integral component of both the descending pain modulation system and the acute stress response and is critically involved in both analgesia and the regulation of sympathetic activity. Local manipulation of the endocannabinoid signaling system holds great promise to provide analgesia without excessive adverse effects and also influence autonomic output. Inhibition of fatty acid amide hydrolase (FAAH) increases brain concentrations of the endocannabinoid N-arachidonoylethanolamine (AEA) and reduces pain-related behaviors in neuropathic pain models. Neuropathic hyperalgesia and reduced sympathetic tone are associated with increased FAAH activity in the dPAG, which suggests the hypothesis that inhibition of FAAH in the dPAG will normalize pain sensation and autonomic function in neuropathic pain. To test this hypothesis, the effects of systemic or intra-dPAG FAAH inhibition on hyperalgesia and dysautonomia developed after spared nerve injury (SNI) were assessed in male and female rats. Administration of the FAAH inhibitor PF-3845 into the dPAG reduces hyperalgesia behavior and the decrease in sympathetic tone induced by SNI. Prior administration of the CB1 receptor antagonist AM281, attenuated the antihyperalgesic and sympathetic effects of FAAH inhibition. No sex differences were identified. These data support an integrative role for AEA/CB1 receptor signaling in the dPAG contributing to the regulation of both hyperalgesia behavior and altered sympathetic tone in neuropathic pain.
INTRODUCTION
Chronic neuropathic pain is a debilitating condition caused by peripheral nervous system damage. Hallmarks of neuropathic pain include hyperalgesia, and/or allodynia, exaggerated responses to noxious and normally innocuous stimuli, respectively (1–3). Current treatments are inadequate, providing modest and inconsistent effects, further limited by undesirable side effects including tolerance and dependence (4, 5). Overprescription and overuse of opioids and their synthetic counterparts administered for pain management has contributed to a public health crisis of addiction, highlighting the need for treatment strategies that provide effective analgesia without unwanted side effects (6). An understanding of the central mechanisms underlying neuropathic pain could identify targets for localized delivery of therapeutic agents to achieve analgesia without off-target side effects that are associated with systemic administration. Treatment of chronic pain conditions are further complicated by comorbidities including dysautonomia, or malfunctions of the autonomic nervous system, that underlie cardiovascular disorders (7–9). The central structures controlling pain and autonomic physiology overlap in terms of anatomic location and pharmacology, providing therapeutic targets to mitigate maladaptations of sympathosensory regulation.
There is considerable evidence to suggest that cannabinoids can reduce neuropathic pain at least in part via cannabinoid 1 (CB1) receptors in the central nervous system (CNS) (10, 11). Synaptic concentrations of the CB1 receptor endogenous ligand N-arachidonoylethanolamine (anandamide or AEA) are tightly regulated by the catabolic enzyme fatty acid amide hydrolase (FAAH) (12). Since inhibitors of FAAH act preferentially on active pathways, reduce AEA degradation, and prolong target activation, there is a reduced risk for adverse effects compared with direct agonists (13, 14). FAAH inhibitors administered intraperitoneally increase brain concentrations of AEA (15–17) and attenuates pain behaviors in animal models of neuropathic pain (13, 18–20). Systemic administration effectively reduces thermal and mechanical hyperalgesia and cold and mechanical allodynia after peripheral nerve injury (16, 21, 22) and exposure to chemotherapeutic agents (2, 23, 24), effects mediated by both CB1 receptors and cannabinoid 2 (CB2) receptors (2, 21–23). Regarding the specific central site(s) of cannabinergic analgesia, there is evidence to suggest a role for the periaqueductal gray (PAG) as endocannabinoid-based analgesia is reported from the dorsal and lateral regions (collectively termed the dPAG) (25–27), an area rich in CB1 receptors (28). The dPAG is not only a key component of the pain relief system that influences nociceptive transmission through descending modulation of dorsal horn networks (29), but also functions as an integrative site engaged during a stress response to maintain homeostasis by coordinating analgesia with increased cardiovascular performance (30). Peripheral nerve injury has been shown to result in the opposite, maladaptive effects of hyperalgesia and reduced sympathetic tone (31). We have shown that peripheral nerve injury has been shown to result in the opposite, maladaptive effects of hyperalgesia and reduced sympathetic tone (31). This compromised sympathosensory regulation is associated with increased mRNA for FAAH, increased FAAH activity and reduced AEA content in the dPAG, indicating an altered biochemical phenotype of the dPAG endocannabinoid signaling system. There is some evidence to support this hypothesis as dPAG administration of a cannabinoid agonist is antinociceptive (26), stress-induced analgesia (SIA) is mediated at least in part by AEA, released in the dPAG acting at CB1 receptors (27, 32), and exogenous AEA in the dPAG increases sympathetic nerve activity, via CB1 receptor activation (33).
Specific central sites of action of endocannabinoids in neuropathic pain relief and the role of the endocannabinoid system in integrative functions remain unknown. The present study tests whether the dPAG is a central site for enhanced endocannabinoid signaling coordinating the regulation of neuropathic pain and sympathetic tone, in male and female rats. The primary objective was to assess the dPAG as a site of antinociceptive action of FAAH inhibitors in the spared nerve injury (SNI) rat model of neuropathic pain, and the involvement of CB1 receptors. The secondary objective of this study was to determine the integrative effects of FAAH inhibition on the simultaneous autonomic dysregulation consequent to SNI.
MATERIALS AND METHODS
The protocols for the study were approved by the Animal Care and Use Committees at the Medical College of Wisconsin and the Zablocki Department of Veterans Affairs Medical Center. Male (n = 139; 259–394 g) and female (n = 61; 211–348 g) Sprague-Dawley rats (RRID:RGD_70508) were obtained from Charles River (Wilmington MA) and were maintained and used according to the NIH Guide for the Care and Use of Laboratory Animals, and in compliance with federal, state, and local laws and the ARRIVE guidelines (34). Animals were housed individually for radiotelemetric blood pressure monitoring. Animals were housed and studied undisturbed in a room on a reverse 12 h light/12 h dark cycle from 6 AM/6 PM, maintained at 22 ± 1°C at 35%–45% humidity. Animals had free access to food (Purina laboratory rodent diet 5001) and water, and bedding was Beta Chip (Warrensburg, NY). At the conclusion of the protocol, rats were killed by decapitation under full isoflurane anesthesia.
Instrumentation and Blood Pressure Monitoring
All animals except for one male and three females, and the rats for the long-term evaluation of hyperalgesia behavior (see below) were implanted with PA-S10 or PA-X10 transmitters (Data Sciences International, St. Paul, MN) for radiotelemetric monitoring of arterial blood pressure. Rats were anesthetized with isoflurane (5% induction, 2% maintenance) in O2, and the cannula (0.43 mm outer diameter polyethylene) attached to the transmitter was inserted into the left femoral artery through a small inguinal incision, with care taken to avoid manipulation of the adjacent femoral nerve. The transmitter was fixed in a subcutaneous pocket on the left flank of the rat and the incision was closed with 3-0 silk suture. A redundant loop in the cannula allowed for the growth of the rat. Animals were treated with Carprofen (5 mg/kg sc) at the start of surgery and 24 h postoperatively. After 7 days, animals were again anesthetized for removal of inguinal stitches and for spared nerve injury (SNI) surgery.
Spared Nerve Injury
Under full isoflurane anesthesia (5% induction, 2% maintenance), an incision was made through the skin and the biceps femoris muscle on the lateral surface of the right thigh to expose the sciatic nerve and its three terminal branches (tibial, common peroneal, and sural nerves). The tibial and common peroneal nerves were ligated with 6.0 silk suture and transected, while care was taken to avoid trauma to the sural nerve, which remained intact. All injuries were performed by the same experienced surgeon that has performed this surgery regularly (31). The muscle layers were closed with 5-0 absorbable polyglycolic suture and the skin layer was closed with staples that were removed after 7 days. Carprofen (5 mg/kg sc) was administered at the start of surgery and 24 h post.
Implantation of Bilateral Guide Cannulas
Indwelling guide cannulas were implanted for intra-dPAG microinjections during the same surgery as for SNI. To avoid inhalational anesthesia in conjunction with a stereotaxic frame, rats undergoing both SNI and guide cannula implantation were anesthetized with ketamine (75 mg/kg) and dexmedetomidine (0.25 mg/kg ip). The head of the rat was fixed in a stereotaxic frame (Kopf, Tujunga, CA) with nonpenetrating ear bars and a midline incision made through the skin overlying the skull. Bilateral guide cannulas (23-gauge miniature stainless steel tubing; McMaster–Carr, Elmhurst, IL; 16 mm) were placed through burr-hole craniotomies at stereotaxic coordinates provided by prior studies targeting the dPAG (35). For insertion, the cannulas were mounted in the same lateral plane centered on the midline, in a device designed and fabricated in the Anesthesiology Department machine shop. The cannulas were fixed in place with dental cement (Lang Dental, Wheeling, IL), and stainless-steel wire plugs (A-M Systems, Sequim, WA; 16 mm) were inserted to maintain patency. The incision was closed with 3-0 silk, which was removed in 7–10 days under brief isoflurane anesthesia. Carprofen (5 mg/kg sc) was administered at the start of surgery and 24 h postoperatively. At the conclusion of the surgery, anesthesia was reversed with atipamezole (1 mg/kg sc). On days scheduled for intra-dPAG test agent administration, rats were briefly anesthetized with isoflurane (5% induction, 2% for injections) for drug administration through the guide cannulas.
Behavioral Evaluation of Hyperalgesia
Mechanical hyperalgesia, which is “Increased pain from a stimulus that normally provokes pain” (IASP terminology: https://www.iasp-pain.org/resources/terminology/#hyperalgesia), was evaluated after each resting blood pressure monitoring period. Animals were placed individually in clear plastic enclosures on an elevated 0.25-in wire grid for a 15-min acclimation period that allowed animals to cease exploratory activity. The point of a 22-gauge spinal anesthesia needle was applied to the lateral part of the glabrous plantar surface of the paw with sufficient needle force to indent, but not penetrate, the skin. The behaviors induced by this noxious stimulus were of two types, either a brisk, simple withdrawal with immediate return of the foot the wire floor, which is typical of normal animals, or a hyperalgesia-type response that consisted of sustained elevation of the paw with shaking, licking and grooming (36, 37). Although there are numerous measures of an animal’s pain experience, this test was chosen because hyperalgesia induced by a pin touch is a common finding in clinical pain patients, especially those with peripheral neuropathic pain (38). In addition, we have confirmed that positive responses to this test specifically correlate with conditioned place avoidance in plantar skin testing of nerve-injured rats, thus identifying an aversive experience, whereas other tests such as simple response to calibrated monofilaments to determine withdrawal threshold (von Frey test) are not aversive even at the force that causes withdrawal (39, 40). Therefore, the frequency of hyperalgesia responses during needle testing was used as the most meaningful measure of pain hypersensitivity. One individual assessed the response type for each of 5 applications to each hindpaw, ipsilateral, and contralateral to the injury. Mechanical stimuli were separated by at least 10 s, and repeated after 5 min, for a total of 10 touches to each paw. For each time point, the total number of hyperalgesia responses (a maximum of 10) was converted to percent to provide the hyperalgesia response rate for analysis. In agreement with our previous findings (31), all rats responded with a brisk withdrawal of the paw but no exaggerated response on stimulation of the paw contralateral to injury, so only observations on the paw ipsilateral to injury are reported here. The investigator evaluating behavior was blind to the autonomic findings of the subjects. To monitor development of hyperalgesia, testing was performed before SNI (baseline day −1) and on days 0, 3, 7, 10, 14, and 18 after SNI. Testing on days 21, 22, 23, and 24 followed the protocol outlined below in Drug administration criteria and hyperalgesia behavior testing. Some data for days −1 to 21 have been included in another report (41).
Long-Term Evaluation of Hyperalgesia
To verify sustained hyperalgesia after SNI, additional male (n = 27) and female (n = 13) rats were housed for 12 wk after SNI, without radiotelemetry implants. Hyperalgesia behavior testing was performed before injury, and weekly thereafter for 12 wk.
Heart Rate Variability Determination
In all animals with telemetry devices, blood pressure was monitored before behavioral evaluation of hyperalgesia on days −1, 0, 3, 7, 10, 14, and 18 and continuously on day 21 to day 24. Monitoring took place in the room in which the animals were housed so that they did not need to be moved or require a period of habituation. On days 21–24, heart rate variability (HRV) was derived from blood pressure measurements taken at least 30 min after handling and hyperalgesia testing, when blood pressure was observed to be stable, at 1.5 h/2.5 h after drug administration.
Blood pressure was analyzed for HRV to indicate autonomic changes to the heart, using the Data Sciences International Ponemah 6.4.2 acquisition, analysis, and archive system. Spectral analysis of heart rate was used to determine HRV from blood pressure data as described previously (36). The blood pressure data were used to calculate an interbeat interval (IBI) series from three 5-min segments, each with 5 overlapping (50%) subsegments of 512 points. The IBI was converted into an instantaneous heart rate using the formula: heart rate (beats/min) = 60/IBI (s). The IBI values were then interpolated at 50 Hz (cubic algorithm), detrended, and the mean was suppressed to create the data points equally spaced in time for further analysis. Utilizing a Hanning window, the power was calculated for each data set over the frequency ranges of 0.25–1 Hz (low frequency, LF) and 1–3 Hz (high frequency, HF), with the results from the three segments averaged for the final determination of density within each frequency band. LF and HF powers were normalized and expressed as percentages of total power (LF + HF powers) and the LF-to-HF power ratio was calculated from these values as a representation of sympathetic tone from sympathovagal balance of direct autonomic influence on the heart (42).
Materials
URB597 ([3-(3-carbamoylphenyl)phenyl] N-cyclohexylcarbamate; Tocris Cookson, Minneapolis, MN), PF-3845 (N-3-pyridinyl-4-[[3-[[5-(trifluoromethyl)-2-pyridinyl]oxy]phenyl]methyl]-1-piperidinecarboxamide; Cayman Chemical, Ann Arbor, MI), and AM281 (1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide; Tocris Cookson, Minneapolis, MN) were dissolved in a vehicle consisting of ethanol, cremaphor (Sigma Aldrich, St. Louis, MO), and saline in a ratio of 1:1:18.
The FAAH inhibitors URB597 and PF-3845 (2 mg/kg) or vehicle were injected intraperitoneally at a volume of 1 µL/g body mass in both male and female rats based on previous reports (19, 43). For intra-dPAG administration of PF-3845 (1.75 nmol; 200 nL/injection) or vehicle, in both male and female rats, two bilateral microinjections were made through the indwelling cannulas using a Hamilton syringe (32 gauge, 50 nL; Hamilton Company, Reno, NV). In a group of male rats, the CB1 receptor antagonist AM281 (1 µM) was administered bilaterally (200 nL/injection) intra-dPAG immediately before PF-3845. We have previously established that this dose effectively antagonizes the effects of exogenously administered AEA in the dPAG (35). The synaptic excitant dl-homocysteic acid (DLH, 4 mM; Sigma Chemicals. St. Louis, MO) was diluted in 1% pontamine sky blue dye (VWR International, Radnow, PA) and injected intra-dPAG in the same volume as PF-3845. dl-homocysteic acid is a glutamic acid analog and a receptor agonist of NMDA that reliably evokes reproducible responses by activation of neurons in the PAG (33, 35, 44).
Drug Administration Criteria and Hyperalgesia Behavior Testing
There is expected variability between rats in the development of hyperalgesia following nerve injury (37). At hyperalgesia behavior testing on day 21 after SNI, rats that had developed a hyperalgesia response rate of >20%, a value considered outside the range of random error (31, 36, 41), underwent the drug/vehicle administration protocols (n = 85 male, 31 female). Twenty-seven male and 17 female rats did not meet the criteria for intervention. To avoid loss of sensitivity to mechanical stimuli, hyperalgesia behavior testing was limited to up to 3 time points per day.
The antihyperalgesic effects of systemic administration of URB597 and PF-3845 were initially tested with the time points of 1.5 and 3 h, based on prior reports (13, 15). Although both drugs elicited similar responses, the selectivity of PF-3845 is considered greater (15) and was selected for intra-dPAG administration. Anticipating a shorter time to effect for direct administration of drugs (35), test time points of 0.5 and 1 h were added. These studies were performed in male rats and established 0.5 and 1 h as effective time points, at which times hyperalgesia was subsequently assessed in female rats. Initial analysis indicated that there were no significant sex differences, and therefore, the role of CB1 receptors was subsequently assessed only in male rats. For all test groups, recovery from the effects of FAAH inhibitors was assessed at 24 h after each dose. For intra-dPAG injections, animals were briefly anesthetized to avoid stress and were ambulatory within 5 min after termination of isoflurane. An anesthetic control was employed with hyperalgesia testing at 0.5 and 1 h after anesthesia in a group of animals before euthanasia. In total, 13 groups of animals were used according to the protocols detailed in Table 1.
Table 1.
Hyperalgesia behavior test schedule for all rat groups
| Hyperalgesia Behavior Test (Hours after Intervention on Days 21/22/23 post-SNI) |
|||||||
|---|---|---|---|---|---|---|---|
| Test Agent | Route of Administration | Sex | 0.5 | 1 | 1.5 | 3 | 24 |
| URB597 | Intraperitoneal | Male | X | X | X | ||
| PF-3845 | Intraperitoneal | Male | X | X | X | ||
| PF-3845 | Intraperitoneal | Female | X | X | X | ||
| PF-3845 + AM281 | Intraperitoneal + intra-dPAG | Male | X | X | X | ||
| vehicle | Intraperitoneal | Male | X | X | |||
| vehicle | Intraperitoneal | Female | X | X | |||
| PF-3845 | Intra-dPAG | Male | X | X | X | ||
| PF-3845 | Intra-dPAG | Male | X | X | X | ||
| vehicle | Intra-dPAG | Male | X | X | |||
| PF-3845 | Intra-dPAG | Female | X | X | X | ||
| vehicle | Intra-dPAG | Female | X | X | |||
| AM281 + PF-3845 | Intra-dPAG | Male | X | X | X | ||
| None (anesthetic control) | Male | X | X | ||||
SNI, spared nerve injury.
Hyperalgesia Testing Protocols and HRV Assessment
Hyperalgesia behavior was tested on day 21 before drug/vehicle administration providing the value to which subsequent data were normalized. For each group of rats, drugs/vehicle were administered on sequential days 21, 22, and 23 after SNI. Each rat was retested at the same time points on each of the 3 days as shown in Table 1. Rats were acclimated to the elevated plastic enclosures for 15 min before each test and returned to their home cages after each test period.
Analysis of Injection Sites
After hyperalgesia testing on day 24, rats with indwelling guide cannulas were anesthetized with isoflurane (5% induction, 2% maintenance) and blood pressure was monitored radiotelemetrically. The synaptic excitant DLH, diluted in blue dye, was microinjected through each cannula to identify an increase in blood pressure and/or movement of the limbs and tail that are indicative of activation of neurons in the stress-responsive region of the dPAG (30, 35). Brains were removed postmortem and frozen for subsequent histological analysis of microinjection sites marked by blue dye. Sequential frozen transverse sections through the midbrain were cut (25 μm), stained with neutral red and examined microscopically to visualize sites marked by dye. At least one of the two following criteria was required for verification of drug injection into the dorsal region of the PAG and for inclusion in data analysis: 1) microscopic confirmation of microinjection sites in the dPAG and 2) an increase in arterial blood pressure/limb movement.
Data and Statistical Analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (45). Power analysis was performed using G*Power 3.1 (RRID:SCR_013726). A minimum of 8 animals per group were required; counts were increased to account for technical and experimental attrition.
The percent change in LF/HF power ratio from the baseline value before SNI to that on day 21 before intervention was calculated to confirm a decrease in sympathetic tone, similar to that seen in prior studies (31, 41). For all time points after drug/vehicle intervention, to control for natural variation, hyperalgesia response rate, and LF/HF power ratio was normalized to the value before drug/vehicle administration on day 21, when hyperalgesia is developed.
Analysis was performed using SAS 9.4–14.3. One-way ANOVA was used to ensure animals did not differ statistically before drug administration, and to observe changes in HRV before surgery versus before drug administration. Data were analyzed using repeated measures generalized linear mixed models via PROC MIXED. This method was chosen because of its structural relationship to both repeated measures ANOVA and general linear models; generalized linear mixed models can have multiple independent variables and perform a repeated measures analysis. Line coefficients for each included variable were calculated and their P values indicate confidence in calculated coefficient values. Within each variable, vehicle served as the reference for drug data, male was the reference for sex, and values at 24 h were the reference for time point data. Within each route of administration, hyperalgesia and HRV were separately compared using sex, time after drug administration, day, drug, and the interaction of drug with day. Vehicle and anesthesia controls were longitudinally compared in a similar manner. Visualizations of the results were performed in R 3.6.0., Sigmaplot v14.5 (RRID:SCR_003210), and Adobe Photoshop 22.4.2 (RRID:SCR_014199).
RESULTS
Sustained Hyperalgesia after Nerve Injury
To determine hyperalgesia behavior over an extended period of time, we first examined a group of animals with SNI and no subsequent interventions over 12 wk postinjury. Figure 1 demonstrates that when hyperalgesia in both male and female rats, meets the established criterion of >20% response rate on day 21, this is followed by sustained hyperalgesia over at least 12 wk postinjury. Rats demonstrating hyperalgesia response rates below the 20% range of random error on day 21 plateau around this value.
Figure 1.
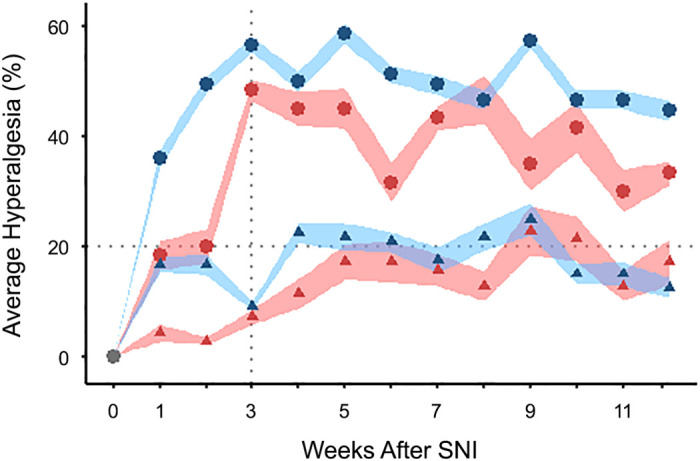
Hyperalgesia is sustained over 12 wk after spared nerve injury (SNI). Rats are categorized by the hyperalgesia response rate at 3 wk after SNI as either >20% (circles) or ≤20% (triangles). Hyperalgesia for all male (blue; >20%, n = 15; ≤ 20%, n = 12) and female (red; >20%, n =6; ≤ 20%, n = 7) rats reaches a plateau around 3 wk that is maintained thereafter for at least 12 wk postinjury. n, Number of rats. Shaded regions show standard errors of the plotted means (points).
Control Data
Vehicle and anesthetic (used for intra-dPAG injections) did not affect hyperalgesia behavior. Specifically, there was no significant effect of time alone (anesthetic control 0.5 vs. 1 h, P = 0.246), treatment (anesthesia alone vs. vehicle, P = 0.388), or route of administration of vehicle (systemic vs. intra-dPAG, P = 0.896).
Hyperalgesia Responses at 21 Days after SNI
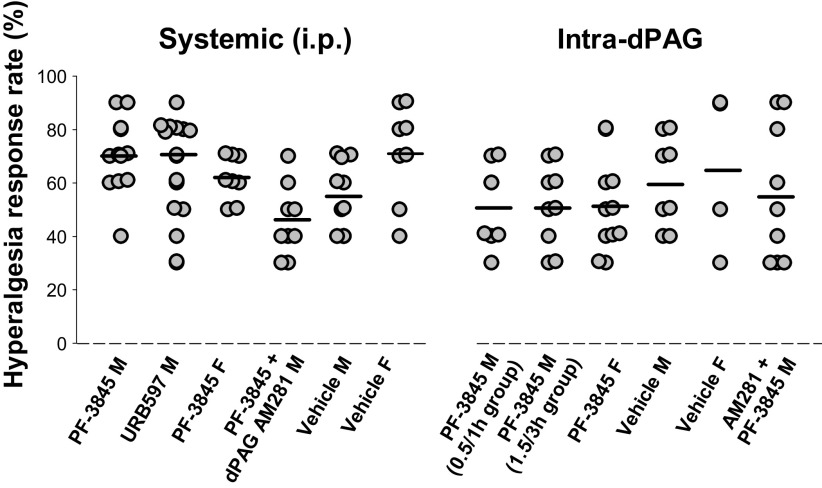
The hyperalgesia response rate for all rats at baseline (preinjury) was zero. For all SNI rats that received interventions, the individual hyperalgesia response rates at 21 days postinjury ranged from 30% to 90% and were not significantly different across the experimental groups (P = 0.204 for systemic, 0.656 for intra-dPAG; Fig. 2).
Figure 2.
Individual values for the hyperalgesia response on day 21 postinjury. Individual hyperalgesia response rates on day 21 after spared nerve injury (SNI) were similar for each experimental group and were the values to which drug intervention data were normalized. Horizontal bar illustrates mean value for each group. F, female; M, male.
Systemic Administration of FAAH Inhibitors
Systemic PF-3845 was tested in male and female rats. There was no effect of sex on the drug-induced attenuation of hyperalgesia (P = 0.365; Fig. 3A); therefore, the sexes were combined for analysis. URB597 was only administered to male rats.
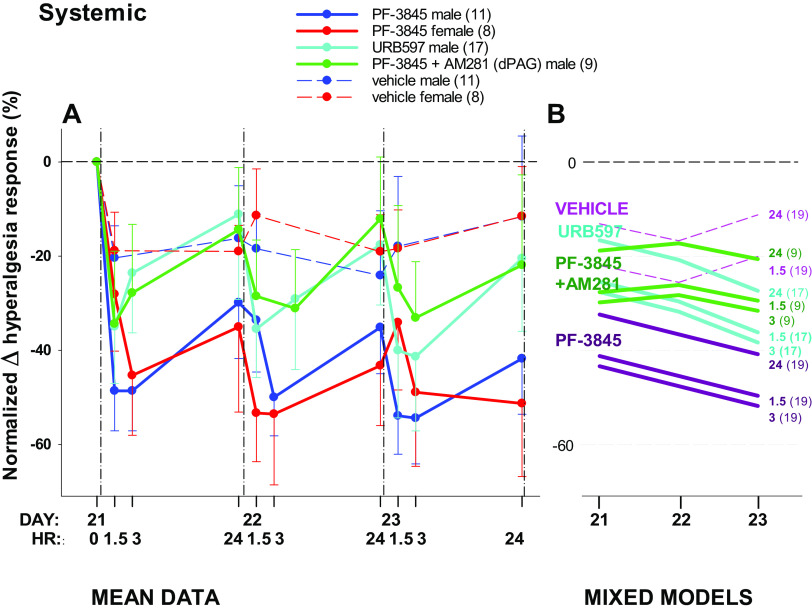
Figure 3.
Effects of FAAH inhibitor administered intraperitoneally systemically on hyperalgesia developed after spared nerve injury (SNI). A: mean data over time for male and female rats. B: repeated measures generalized linear mixed models via PROC MIXED showing a line for each postintervention time point by day. There was no effect of sex, assessed for PF-3845 (A), which was excluded from the mixed model (B). Values after drug/vehicle interventions are normalized to the hyperalgesia response on day 21 after injury (0 in A). Administered daily for 3 days, PF-3845 attenuates hyperalgesia behavior mediated by CB1 receptors in the dPAG, with partial recovery evident at 24 h. The number of animals per group is shown in parentheses; unidirectional standard error bars are shown in A; hours after intervention shown in bold type in B. FAAH, fatty acid amide hydrolase.
Hyperalgesia was attenuated by systemically administered FAAH inhibitors (P = 0.055; Fig. 3). Relative to vehicle, hyperalgesia was significantly attenuated by PF-3845 (line coefficient −19.278, P = 0.053) but not URB597 (line coefficient −3.497, P = 0.728; Fig. 3B). Although the magnitude of responses cannot be directly compared because the same dose for different drugs may have different efficacy, these data demonstrate that the patterns of response for both FAAH inhibitors were similar.
There was no significant interaction of day and drug (P = 0.533) and the FAAH inhibitor-induced attenuation of hyperalgesia was similar at the 1.5- and the 3-h time points (Fig. 3B). There was a significant recovery of hyperalgesia by 24 h after drug administration for both drugs (P = 0.001), although mixed models suggests that there is an accumulation of effect over the 3-day treatment period.
Assessment of an Antihyperalgesic Role for CB1 Receptors in the dPAG
To examine whether the antihyperalgesic effect of systemic FAAH inhibition is mediated by CB1 receptors in the dPAG, the antagonist AM281 was administered intra-dPAG whereas PF-3845 was administered intraperitoneally (Fig. 3, A and B). Hyperalgesia was not attenuated by AM281 (model line coefficient −5.698, P = 0.636; Fig. 3B), identifying the dPAG as a site of hyperalgesic action of PF-3845.
Intra-dPAG Administration of PF-3845
To further evaluate the dPAG as a site of action of FAAH inhibitors in the attenuation of hyperalgesia seen with systemic administration, PF-3845 was administered intra-dPAG. As was found for systemic administration, there was no effect of sex on the change in hyperalgesia response rate when PF-3845 was administered directly into the dPAG (P = 0.638; Fig. 4A). It was therefore excluded from the model (Fig. 4B), and those results are reported here.
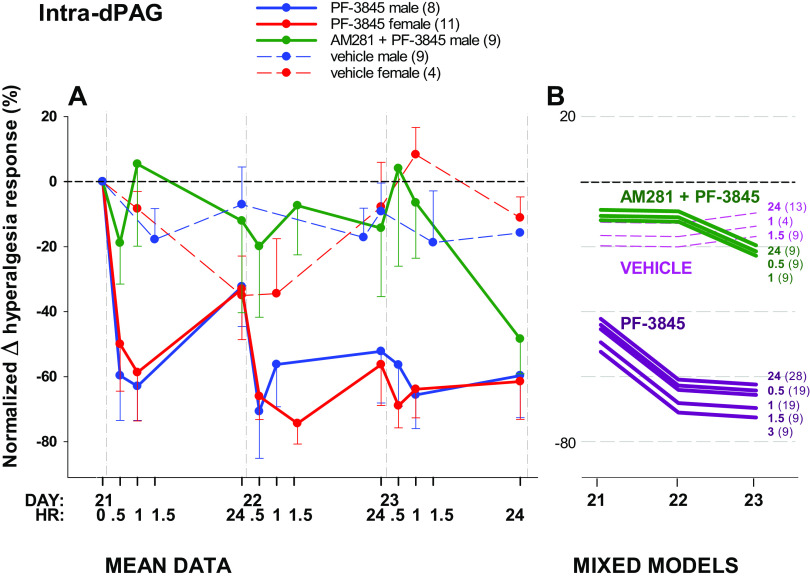
Figure 4.
Effect of PF-3845 administered intra-dPAG on hyperalgesia developed after spared nerve injury (SNI). A: mean data over time in male and female rats. B: repeated measures generalized linear mixed models via PROC MIXED showing a line for each postintervention time point by day. There was no effect of sex (A), which was excluded from the mixed model (B). Values after drug interventions are normalized to the hyperalgesia response rate on day 21 after injury (0 in A). Administered daily for 3 days, PF-3845 significantly attenuates hyperalgesia behavior tested at 0.5, 1, 24 (A and B) and 1.5 and 3 h time points (B). Antihyperalgesic effects of PF-3845 are abolished by prior administration of the CB1 receptor antagonist, AM281 initially. Number of animals per group is shown in parentheses; unidirectional standard error bars are shown in A; hours after intervention is shown in bold type in B. dPAG, dorsal periaqueductal gray.
There were differences in hyperalgesia response rate over time across drug administered intra-dPAG (P < 0.0001). PF-3845 significantly reduced hyperalgesia by 40%–70% (model line coefficient −29.738, P = 0.009). The interaction of drug and day was a significant factor (P = 0.008). Time after microinjection did not affect the change in hyperalgesia (P = 0.686) indicating that, unlike the systemic administration, there was no recovery after 24 h, suggesting a more effective inhibition with local administration of PF-3845 compared with systemic.
Confirmation of an Antihyperalgesic Role for CB1 Receptors in the dPAG
To confirm that the antihyperalgesic effect of FAAH inhibition is mediated by CB1 receptors, the antagonist AM281 was administered intra-dPAG immediately before PF-3845. AM281 abolished the attenuation of hyperalgesia responses elicited by PF-3845 alone (Fig. 4). The line coefficient of 3.792 (P = 0.786) for the drug combination was reduced from −29.738 for PF-2845 alone. However, AM281 was less effective on day 3, when the effect of PF-3845 was at its greatest (Fig. 4B).
Change in LF/HF Power Ratio at 21 Days after SNI
The values for the change in LF/HF power ratio, an indicator of change in sympathetic tone, on day 21 after SNI served as the value to which data from subsequent days was normalized. In agreement with previous studies (31, 41), for all rats, there was a significant decrease (mean= −14.288, 95% CI −9.179 to −19.398) in the LF/HF power ratio by 21 days after injury (P < 0.0001; Fig. 5).
Figure 5.
Individual values for the change in LF/HF power ratio on day 21 postinjury from baseline (preinjury) values for all protocol groups. The changes in LF/HF power ratio, an indicator of sympathetic tone, on day 21 after SNI were similar for each experimental group. Horizontal bar illustrates mean value for each group. F, female; HF, high frequency; LF, low frequency; M, male; SNI, spared nerve injury.
Autonomic Consequences of FAAH Inhibitors
As found for hyperalgesia data, sex was not a significant factor for both routes (dPAG P = 0.304; systemic P = 0.210), so the combined-sex model’s results are reported.
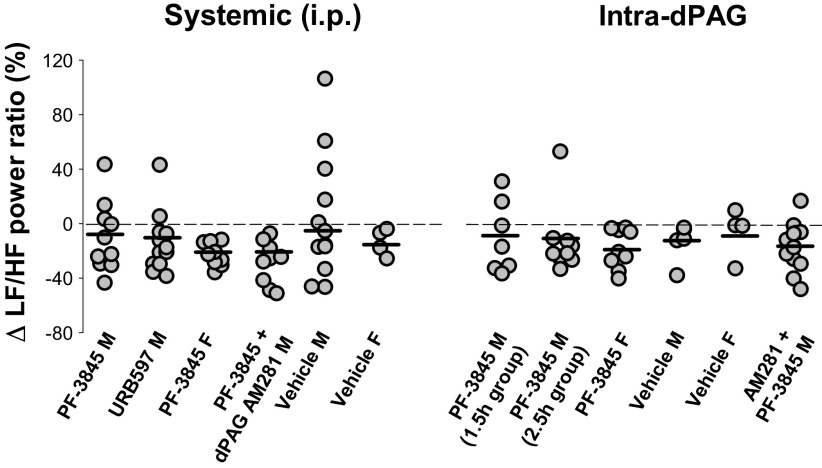
Systemic administration of the FAAH inhibitors URB597 and PF-3845, regardless of administration of intra-dPAG AM281, had no significant effect on the drop in LF/HF power ratio at any time point (P = 0.069), in any group (drug P = 0.115, interaction of drug and day P = 0.931; Fig. 6, A and C). However, when PF-3845 was administered directly into the dPAG, there were differences by drug (P = 0.038) and time (P = 0.038), but not an interaction of day and drug. PF-3845 enhanced the LF/HF power ratio compared with vehicle (model line coefficient 39.769, P = 0.027), indicative of an increase in sympathetic tone, with greater changes at time points closer to drug administration (model line coefficients at 1.5 h 16.714, P = 0.015; at 2.5 h 1.365, P = 0.900; 24 h is the reference; Fig. 6, B and D). Local blockade of CB1 receptors by prior intra-dPAG administration of AM281 attenuated the increase in LF/HF power ratio elicited by administration of PF-3845 (model line coefficient 16.027, P = 0.450).
Figure 6.
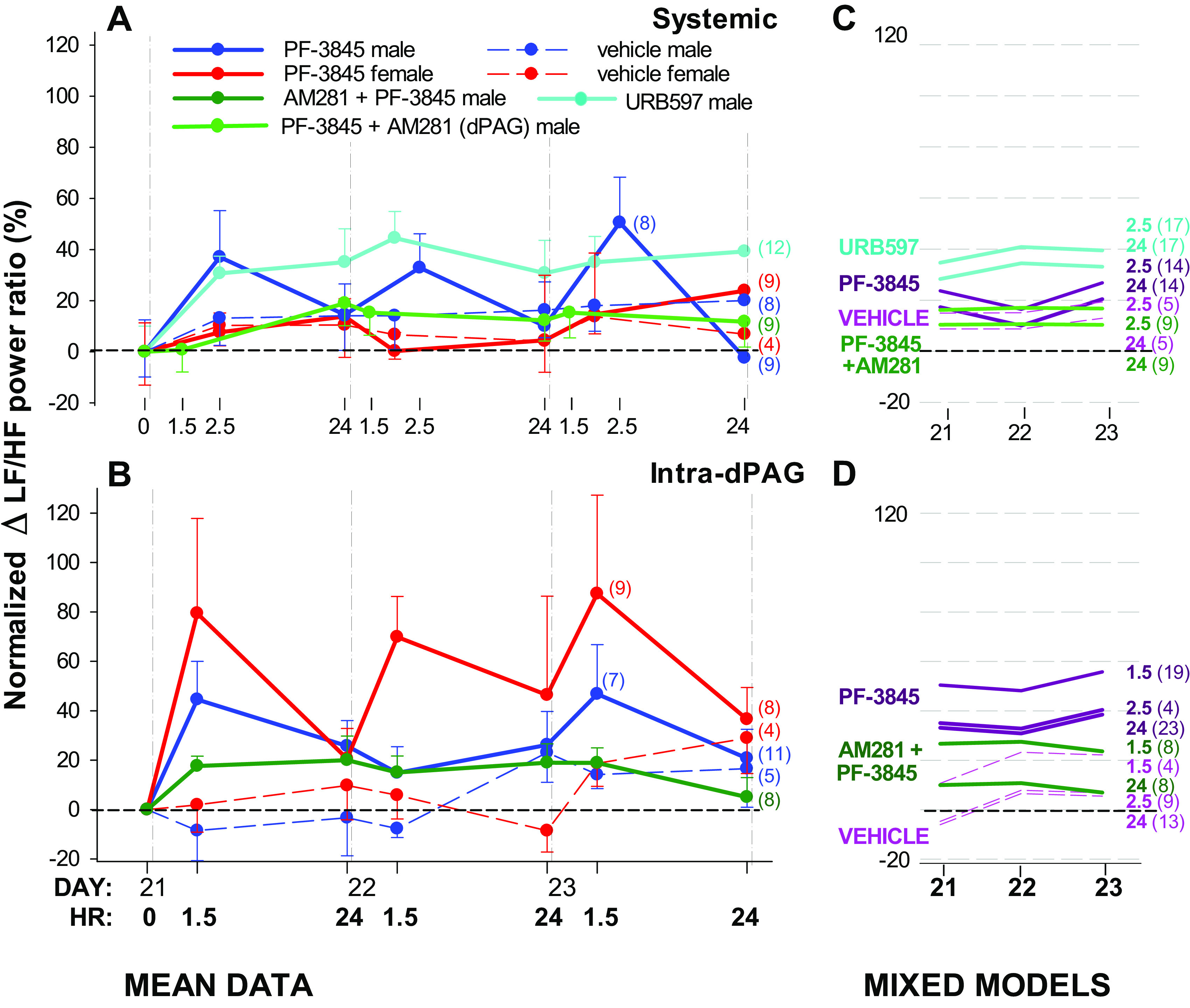
Effects of FAAH inhibitors on the decrease in sympathetic tone after spared nerve injury (SNI), as indicated by LF/HF power ratio. A and B: mean data over time in male and female rats. C and D: repeated measures generalized linear mixed models via PROC MIXED showing a line for each postintervention time point by day. There was no effect of sex (A and B), which was therefore excluded from the models (C and D). Values after drug interventions are normalized to the LF/HF power ratio on day 21 after injury (0 in A and B). No significant changes were identified after systemic administration of either URB597 or PF-3845, but time and drug were significant factors after intra-dPAG administration of PF-3845 with sexes combined. Prior intra-dPAG administration of AM281 attenuated the increase in LF/HF power ratio elicited by administration of PF-3845 (B and D). Number of animals per group is shown in parentheses; unidirectional standard error bars are shown in A; time after intervention is shown in bold type in B. dPAG, dorsal periaqueductal gray; HF, high frequency; LF, low frequency.
Assessment of Injection Sites
For histological confirmation of the dPAG as a site of action for the antihyperalgesic effects of FAAH inhibition, DLH diluted in blue dye was microinjected directly through 54 of the 59 indwelling bilateral guide cannulas, and injection sites were recovered histologically in 51 of those 54 brains. Microscopic evaluation of midbrain tissue showed injection sites to be consistently within the dPAG, primarily the dorsolateral and lateral regions (Fig. 7, A and B). A few were located unilaterally on the lateral border but their contralateral counterpart was within the dPAG. As a secondary indicator of placement, at the majority of injection sites (37/54), DLH elicited increases in blood pressure and/or movement of the limbs and tail, indicative of activation of neurons in the dPAG (Fig. 7C). No blood pressure change or limb movement was noted at the remaining sites, but these animals were included in analysis as histology demonstrated that sites were within the dPAG.
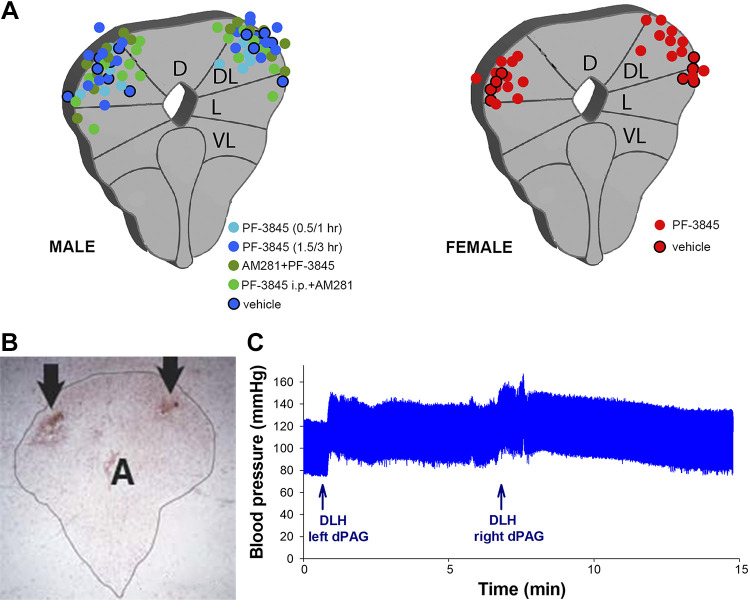
Figure 7.
Assessment of injection sites. A: diagrammatic representations of location of injection sites within the PAG for male (n = 39) and female (n = 15) rats. B: example of a neutral red stained transverse section through the PAG showing injection sites (arrows) bilaterally within the dorsolateral region of the PAG (PAG outlined in black, A aqueduct). C: example of blood pressure increases in response to administration of dl-homocysteic acid (DLH) at the injection sites shown in B, indicating accurate placement in the dPAG. D, dorsal; DL, dorsolateral; dPAG, dorsal periaqueductal gray; L, lateral; PAG, periaqueductal gray; VL, ventrolateral column.
DISCUSSION
The observations from this study identify the dPAG as a central site of sex-insensitive action of the FAAH inhibitor PF-3845 in the attenuation of neuropathic hyperalgesia. Blockade of this effect by a CB1 receptor antagonist indicates that increased endocannabinoid availability consequent to FAAH inhibition drives the suppression of hyperalgesia. Simultaneous mitigation of injury-induced sympathoinhibition is the first evidence that enhanced endocannabinoid signaling in the dPAG has an integrative role in maintaining homeostasis. Reducing catabolism of endogenous cannabinoids in the dPAG is a strategy to enhance local signaling that may restore both sensory and autonomic functions compromised by peripheral neuropathy.
The dPAG is recognized as a site of pain processing that both receives input from the spinal dorsal horn and sends direct and indirect input projections to the dorsal horn to influence sensory afferent input (29). Both endocannabinoids and opioids facilitate analgesia in the PAG, with their receptors distributed throughout the columns (46), although there are important distinctions in pain-related effects. Nonopioid analgesia is associated with the dPAG (26), whereas opioid-based analgesia is preferentially associated with the ventrolateral region (47). The dPAG is implicated as a site of cannabinoid antinociception after local microinjection of synthetic cannabinoid agonists (26), whereas electrical stimulation in the dPAG produces analgesia that is blocked by intracerebroventricular administration of a CB1 receptor antagonist and is accompanied by local release of AEA (25). In a physiological context, SIA in rats is accompanied by endocannabinoid release in the dorsal midbrain (27). Endocannabinoid-dependent SIA is mediated by CB1 receptors as it is attenuated by administration of a CB1 receptor antagonist to the dPAG, but unchanged by systemic administration of a TRPV1 antagonist (27, 32). Furthermore, intra-dPAG FAAH inhibition enhances CB1 receptor-dependent SIA (27, 32). Similarly, neuropathic hyperalgesia is associated with altered endocannabinoid signaling in the dPAG, specifically increased FAAH transcript and activity and reduced AEA content in the dPAG, without change in 2-AG content (31). In accord with these findings, the present study demonstrates that inhibition of FAAH in this pain modulatory region reduces neuropathic hyperalgesia at least in part via a CB1 receptor mechanism. Convincing evidence implicates AEA as the primary ligand because both URB597 and PF-3845 are reported to rapidly inactivate brain FAAH, elevate brain levels of AEA, and alleviate a range of neuropathic pain phenotypes systemically (2, 15, 17, 19, 20, 24), without altering 2-AG levels in rodents (13, 18, 19, 27). Of the two irreversible inhibitors of FAAH assessed here, PF-3845 is more recently developed and considered to have increased FAAH selectivity and a longer duration of activity in vivo of at least 24 h (15). Our data are in agreement with reports that URB597 may be less effective than PF-3845 in relief of neuropathic pain (48) and that brain AEA levels in rats treated with PF-3845 showed a greater increase compared with those treated with the same dose and route of URB597 (48). Although higher doses of URB 597 could be more potent (2, 22), there is no gain in N-acylethanolamine (NAE) levels in the CNS (49); therefore. individual pharmacokinetics may make PF-3845 more efficacious than URB597. It is also possible that the pain phenotype may play a role as the same dose of URB597 is antinociceptive in peripheral inflammatory pain but not in sciatic neuropathy or spinal cord injury (22, 48).
It is well established that the somatic sensory system and cardiovascular function are closely linked, as acute noxious stimuli increase sympathetic nerve activity and blood pressure, activating a feedback loop associated with elevated pain thresholds (50–52). The dPAG supports homeostatic sympathosensory integration in stress and the normal, acute function of this circuitry is to elevate sympathetic nerve activity and blood pressure and trigger analgesia (30, 35, 53), both of which can be achieved by endocannabinoids (26, 27, 33, 35). After nerve injury, normal functioning of the endocannabinoid signaling system in the dPAG is compromised, leading to elevated FAAH transcript and activity and reduced AEA content that is associated with hyperalgesia and decreased sympathetic tone (31, 41). The onset of hyperalgesia and the reduction in sympathetic tone occur simultaneously further supporting a sympathosensory correlation (41). Here, we demonstrate that FAAH inhibition in the dPAG can reverse the effects of nerve injury by reducing hyperalgesia and increasing sympathetic tone. These data suggest that enhancing dPAG endocannabinoid signaling could contribute to the restoration of sympathosensory function that is impaired by nerve injury, thereby having an integrative role in maintaining homeostasis. SIA and neuropathic pain and autonomic dysfunction could be interconnected through the acute stress circuitry. SIA engages an endocannabinoid-mediated dPAG response (27, 32) whose altered biochemical foundation after nerve injury could contribute to maladaptations in sensory and sympathetic outflow (31), that can be improved by direct administration of a FAAH inhibitor as shown here for sympathosensory regulation and by Suplita et al. for SIA (32).
At the cellular level, exogenous AEA in the dPAG acts at CB1 receptors to inhibit GABA neurotransmission and increase sympathetic nerve activity (33, 35). This mechanism has been identified in lateral PAG neurons in vitro, and considered consistent with analgesia (54), but without definitive physiological function could also be attributed to sympathetic neurons. Although the analgesic and autonomic effects are both mediated in part through CB1 receptors, the biochemical processes may differ. Although FAAH expression is significantly correlated with that of CB1 receptors (55), FAAH inhibitor substrates include amidated lipids other than AEA. FAAH also hydrolyzes the non-endocannabinoid lipid amides oleoylethanolamide (OEA) and palmitoylethanolamide (PEA), both of which possess affinity for the peroxisome proliferator-activated receptor PPAR-α. PEA also shows affinity for the GPR55 receptor, as does AEA (13, 56, 57). Although our present study demonstrates a contribution of CB1 receptors in the dPAG in the mediation of the antihyperalgesic effects of PF-3845, it does not preclude involvement of PEA and OEA at receptors other than CB1. It is relevant to acknowledge that targets other than the CB1 receptor could be engaged by AEA, which is known to modulate PPARs and transient receptor potential (TRP) channels. In addition, GPR55 (56), GPR18 (58), and serotonin receptors (59) are influenced by endocannabinoids, and interactions between cannabinoid, opioid and TRP receptors in pain modulation (60) highlight the complexity of the system.
Although the present study focuses on the role of the dPAG, these data also indicate that additional site/s of action may contribute to the observed effects. The responses to systemic administration of FAAH inhibitors and consequent receptor activation reflects the sum of peripheral and central effects, although their relative contributions to both neuropathic hyperalgesia and dysautonomia remain unclear, and may depend on the underlying pathogenesis. Several brainstem regions contain CB1 receptors that could contribute to analgesic effects including the ventrolateral (vl) PAG, a site at which FAAH inhibition elevates AEA locally and produces CB1 receptor mediated antinociception that is mediated through the rostral ventromedial medulla (61). Several lines of evidence indicate a peripheral component to the analgesic effects of peripheral CB1 receptors. Systemic and oral administration of a brain-impairment FAAH inhibitor was found to be antihyperalgesic in mouse models of neuropathy, an effect prevented by CB1 receptor antagonism (62, 63). Furthermore, SNI-induced hypersensitivity is enhanced, and analgesic effects of a systemic CB1 receptor agonist reduced, in mice with selective deletion of CB1 receptor in peripheral nociceptive neurons, suggesting a site of action in these peripheral neurons (64).
The complexity of global CB1 receptor effects extends to autonomic regulation, can at times be contradictory, and may not be represented by effects elicited in the dPAG alone. The ubiquity of CB1 receptors may impose site-specific and compensatory effects on autonomic tone, impacting the magnitude and the sum total of response elicited by systemic FAAH inhibitors. Differences in distribution or density of receptors may influence the relative contribution of central and peripheral receptors. Typically, cannabinoids administered systemically in anesthetized animals evoke a depressor and bradycardic response, attributed at least in part to activation of peripheral CB1 receptors on autonomic nerves innervating the heart and on sympathetic nerves innervating vascular smooth muscle (65–69). In line with these data centrally, CB1 receptor activation in the nucleus tractus solitarius enhances baroreflex sympathoinhibition (70), and in the vlPAG, attenuates sympathoexcitatory reflexes (71), although without effect on baseline sympathetic activity. These effects could be counteracted by activation of central CB1 receptors in the rostroventrolateral medulla (RVLM) and the dPAG that produce sympathoexcitation (33, 72, 73). FAAH inhibition that elevates brain AEA has little effect in normotensive rats but reduces sympathetic tone in hypertensive rats, suggesting that tonic levels of endocannabinoids may influence responses (74). It should be noted that conflicting outcomes of the effects of global CB1 receptors may be heavily influenced by anesthesia that may mask autonomic effects. In contrast to the bradycardia seen in anesthetized rats and dogs, l-trans-Δ9-tetrahydrocannabinol (THC) and CB1 receptor agonists evoke tachycardia in humans and conscious animals, considered to be mediated by sympathetic activation and parasympathetic inhibition (68, 75–78).
There are limitations inherent to this study that could impact the data interpretation. Although each drug was shown to be effective over 24 h regardless of route of administration, different times of onset and times of maximal response may have influenced the responses. The selected testing times, established in the single drug protocols, may not be optimal for the protocol with a combination of routes of administration. In addition, the intra-dPAG injection likely does not encompass all target CB1 receptors, but increasing volume of injection would compromise the objective of limiting injectate to the dPAG. These caveats could lead to a reduced magnitude of effect. When administering both drugs intra-dPAG, the injectate would reach similar fields to produce almost total blockade as is shown here.
In the absence of techniques to successfully and consistently record autonomic nerve activity directly over extended periods of time in conscious rats (79), this study utilizes HRV as an indicator of sympathetic changes. The classical concept that LF/HF power ratio reflects sympathovagal balance is often challenged, primarily because the relationship between LF power and sympathetic nerve activity is loose and it is based on the assumption that physiological interventions always elicit reciprocal changes in parasympathetic and sympathetic nerve activity (80). Under some conditions, changes in one branch of the autonomic nervous system can occur independent of changes in the other but data supporting this finding is heavily focused on vagal contributions and generally from studies utilizing indirect measurements (81–83). Despite its limitations, HRV is considered to provide a qualitative index of cardiac autonomic activity but changes in LF/HF power should be interpreted cautiously. Supporting the use of HRV in this study is our current finding that dPAG CB1 receptor activation increases the LF/HF power ratio which aligns with our previous data that directly demonstrated an increase in sympathetic nerve activity elicited by dPAG CB1 receptors (33). Although this route of administration would lack a contribution from peripheral CB1 receptors which could act in opposition, we did observe a similar increase in LF/HF power ratio after systemic administration of FAAH inhibitors in conscious rats.
Several preclinical studies in rodents found no sex differences in the extent of hyperalgesia or allodynia after nerve injury (84–86). However, we have demonstrated that there are sex dimorphisms in the pace at which neuropathic pain develops after SNI, with the peak magnitude of hyperalgesia reached in females being similar to, or higher, than that of males. (41) Yet, when data for all females were combined, the hyperalgesia at 21 days postinjury was the same as combined male data, as was observed in the present study (Fig. 1) in which the sample size did not allow for separation of groups with distinct hyperalgesia trajectories. The hyperalgesia response rates at 21 days after SNI were similar for both female and male rats and no sex differences were identified in the antihyperalgesic effects of the FAAH inhibitors, regardless of route of administration. However, if nerve injury alters endocannabinoid signaling in the dPAG, as this study would suggest, then differences in the time course of dysregulation might contribute to the sexual dimorphisms identified previously in the time course of the development of neuropathic pain (41).
Targeting the endogenous cannabinoid system is an advantageous analgesic strategy and selective administration of a FAAH inhibitor into the dPAG for pain relief is additionally beneficial by avoiding unwanted effects mediated at other brain sites. Anatomically focused drug delivery is a treatment modality that is gaining momentum (87, 88) and will become a priority for achieving optimum therapeutic potential since the ability to limit drug exposure to a known site of action could optimize outcomes in the absence of undesirable systemic side effects. The concept of targeted delivery is not new and approaches to provide local bioavailability have shown initial success in treatments, mainly for cancer (89, 90). Nanotechnology approaches have used liposomes, nanoparticles and other molecular platforms as customized carriers that solubilize, stabilize and provide controlled release of therapeutic agents for targeted delivery (87–90). Active targeting strategies utilize a variety of ligands, including antibodies, receptor ligands, proteins and peptides attached to nanocarriers that bind to specific targets for effective drug delivery (89, 90).
Although the antinociceptive efficacy of FAAH inhibition is evident in preclinical studies (3, 16, 20–24), analgesia has yet to be achieved in patients. Two clinical trials of a FAAH inhibitor in peripheral neuropathic pain and chronic osteoarthritic pain showed increased NAEs, including AEA, and no adverse cannabinoid-related effects, but failed to demonstrate analgesia (91, 92). However, joint pain has a significant dependence on CB2Rs (93), which are weakly responsive to AEA (94) suggesting that receptors other than CB1 may contribute to osteoarthritic pain. A resistant pain phenotype, drug tolerance or maximal elevation of AEA levels may contribute to the lack of analgesic effects of chronic FAAH inhibition (91, 94, 95). The scope of these clinical trials was narrow and since pain phenotype and sensory stimulus modality may influence nociceptive and antinociceptive outcomes, a better understanding of underlying mechanisms will improve clinical translation. Furthermore, the trajectories of development of pain behavior after a uniform neuropathy can be different (41), suggesting that personalized treatments may be appropriate, and the exploration of contributing mechanisms or biomarkers for diagnostic use will be necessary.
Nerve injury reduces endocannabinoid signaling in the dPAG causing hyperalgesia and sympathoinhibition, both maladaptations of the acute stress response. dPAG is a compelling target not only for the treatment of neuropathic hyperalgesia, but also for the improvement of sympathosensory regulation in the context of peripheral neuropathy. As endocannabinoid concentrations are tightly regulated by their catabolic enzymes, the benefits of targeted manipulation of CB1 receptors, indirectly by FAAH inhibition, could be to limit unwanted and off-target effects but further study is required for confirmation. In addition, exploration of strategies for directed administration of cannabinergic drugs will be essential for developing novel therapies for neuropathic pain.
Perspectives and Significance
Analgesia and sympathoexcitation are protective efforts coordinated in the acute stress response from the dPAG (30) where activation of local CB1 receptors have been shown to elicit both responses (26, 35). This study assessed an integrative role for endogenous cannabinoid signaling in the dPAG in alleviating sympathosensory dysfunction developed after nerve injury. Inhibition of FAAH reduced neuropathic hyperalgesia, mediated at least in part through CB1 receptors in the dPAG in both male and female rats. When administered locally, PF3845 was also effective in reducing the sympathetic effects of neuropathy indicated by HRV. These data highlight the importance of restoring altered cannabinoid signaling in chronic neuropathic pain and comorbid dysautonomia. Targeting aspects of endogenous signaling combined with local administration of compounds is a potentially valuable strategy to recover compromised sensory and autonomic effects associated with neuropathy, potentially limiting off-target side effects.
GRANTS
This study was funded by US Department of Veterans Affairs Rehabilitation, Research and Development Program Merit Review Award RX002747 from the (to C.D. and Q.H.H.).
DISCLAIMERS
The contents do not represent the views of the US Department of Veterans Affairs or the US Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.J.H., Q.H.H., and C.D. conceived and designed research; V.W. and C.D. performed experiments; V.W., K.S., C.J.H., F.A.H., and C.D. analyzed data; K.S., C.J.H., Q.H.H., and C.D. interpreted results of experiments; K.S. and C.D. prepared figures; C.D. drafted manuscript; V.W., K.S., C.J.H., F.A.H., Q.H.H., and C.D. edited and revised manuscript; V.W., K.S., C.J.H., F.A.H., Q.H.H., and C.D. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors express their appreciation to Dr. Rodney Sparapani (Medical College of Wisconsin) for deeply thoughtful and illustrative discussions and advice on statistical considerations.
REFERENCES
- 1. Woolf C. Somatic pain–pathogenesis and prevention. Br J Anaesth 75: 169–176, 1995. doi: 10.1093/bja/75.2.169. [DOI] [PubMed] [Google Scholar]
- 2. Kinsey S, Long J, O'Neal S, Abdullah R, Poklis J, Boger D, Cravatt B, Lichtman A. Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J Pharmacol Exp Ther 330: 902–910, 2009. doi: 10.1124/jpet.109.155465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kiso T, Watabiki T, Sekizawa T. ASP8477, a fatty acid amide hydrolase inhibitor, exerts analgesic effects in rat models of neuropathic and dysfunctional pain. Eur J Pharmacol 881: 173194, 2020. doi: 10.1016/j.ejphar.2020.173194. [DOI] [PubMed] [Google Scholar]
- 4. McQuay H, Tramèr M, Nye B, Carroll D, Wiffen P, Moore R. A systematic review of antidepressants in neuropathic pain. Pain 68: 217–227, 1996. doi: 10.1016/s0304-3959(96)03140-5. [DOI] [PubMed] [Google Scholar]
- 5. Dellemijn P. Are opioids effective in relieving neuropathic pain? Pain 80: 453–462, 1999. doi: 10.1016/S0304-3959(98)00256-5. [DOI] [PubMed] [Google Scholar]
- 6. Kolodny A, Courtwright D, Hwang C, Kreiner P, Eadie J, Clark T, Alexander G. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Ann Rev Public Health 36: 559–574, 2015. doi: 10.1146/annurev-publhealth-031914-122957. [DOI] [PubMed] [Google Scholar]
- 7. Verrotti A, Prezioso G, Scattoni R, Chiarelli F. Autonomic neuropathy in diabetes mellitus. Front Endocrinol (Lausanne) 5: 205, 2014. doi: 10.3389/fendo.2014.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bruehl S, Chung O, Jirjis J, Biridepalli S. Prevalence of clinical hypertension in patients with chronic pain compared with nonpain general medical patients. Clin J Pain 21: 147–153, 2005. doi: 10.1097/00002508-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 9. Chao CC, Tseng MT, Hsieh PC, Lin CH, Juang SL, Hseih ST, Chiang MC. Brain mechanisms of pain and dysautonomia in diabetic neuropathy: connectivity changes in thalamus and hypothalamus. J Clin Endocrinol Metabolism 107: e1167–e1180, 2022. doi: 10.1210/clinem/dgab754. [DOI] [PubMed] [Google Scholar]
- 10. Sim-Selley L. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol 15: 91–119, 2003. doi: 10.1615/critrevneurobiol.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- 11. Rahn E, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics 6: 713–737, 2009. doi: 10.1016/j.nurt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cravatt B, Giang D, Mayfield S, Boger D, Lerner R, Gilula N. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384: 83–87, 1996. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 13. Piomelli D, Tarzia G, Duranti A, Tontini A, Mor M, Compton T, Dasse O, Monaghan E, Parrott J, Putman D. Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597). CNS Drug Rev 12: 21–38, 2006. doi: 10.1111/j.1527-3458.2006.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deutsch D. A personal retrospective: elevating anandamide (AEA) by targeting fatty acid amide hydrolase (FAAH) and the fatty acid binding proteins (FABPs). Front Pharm/ 7: 370, 2016. doi: 10.3389/fphar.2016.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Weerapana E, Sadagopan N, Liimatta M, Smith SE, Lazerwith S, Stiff C, Kamtekar S, Bhattacharya K, Zhang Y, Swaney S, Van Becelaere K, Stevens RC, Cravatt BF. Discovery and characterization of a highly selective FAAH inhibitor that reduces imflammatory pain. Chem Biol 16: 411–420, 2009. doi: 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang L, Luo L, Palmer J, Sutton S, Wilson S, Barbier A, Breitenbucher J, Chaplan S, Webb M. Inhibition of fatty acid amide hydrolase produces analgesia by multiple mechanisms. Br J Pharmacol 148: 102–113, 2006. [Erratum in Br J Pharmacol 148: 114, 2006]. doi: 10.1038/sj.bjp.0706699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Booker L, Kinsey S, Abdullah R, Blankman J, Long J, Ezzili C, Boger D, Cravatt B, Lichtman A. The fatty acid amide hydrolase (FAAH) inhibitor PF-3845 acts in the nervous system to reverse LPS-induced tactile allodynia in mice. Br J Pharmacol 165: 2485–2496, 2012. doi: 10.1111/j.1476-5381.2011.01445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghosh S, Kinsey S, Liu Q-S, Hruba L, McMahon L, Grim T, Merritt C, WIse L, Abdullah R, Selley D, Sim-Selley L, Cravatt B, Lichtman A. Full fatty acid amide hydrolase inhibition combined with partial monoacylglycerol lipase inhibition: augmented and sustained antinociceptive effects with reduced cannabimimetic side effects in mice. J Pharmacol Exp Ther 354: 111–120, 2015. doi: 10.1124/jpet.115.222851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grim T, Ghosh S, Hsu K, Cravatt B, Kinsey S, Lichtman A. Combined inhibition of FAAH and COX produces enhanced anti-allodynic effects in mouse neuropathic and inflammatory pain models. Pharmacol Biochem Behav 124: 405–411, 2014. doi: 10.1016/j.pbb.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kinsey S, Long JZ, Cravatt BF, Lichtman AH. Fatty acid amide hydrolase and monacylglycerol lipase inhibitors produce anti-allodynic effects through distinct cannabinoid receptor mechanisms. J Pain 11: 1420–1428, 2010. doi: 10.1016/j.jpain.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guindon J, Lai Y, Takacs S, Bradshaw H, Hohmann AG. Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment. Pharmacol Res 67: 94–109, 2013. doi: 10.1016/j.phrs.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Russo R, LoVerme J, La Rana G, Compton T, Parrott J, Duranti A, Tontini A, Mor M, Tarzia G, Calignano A, Piomelli D. The fatty acid amide hydrolase inhibitor URB597 (cyclohexylcarbamic acid 3′-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice. J Pharmacol Exp Ther 322: 236–242, 2007. doi: 10.1124/jpet.107.119941. [DOI] [PubMed] [Google Scholar]
- 23. Slivicki RA, Saberi SA, Iyer V, Vemuri VK, Makriyannis A, Hohmann AG. Brain-permeant and -impermeant Inhibitors of fatty acid amide hydrolase synergize with the opioid analgesic morphine to suppress chemotherapy-induced neuropathic nociception without enhancing effects of morphine on gastrointestinal transit. J Pharmacol Exp Ther 367: 551–563, 2018. doi: 10.1124/jpet.118.252288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Slivicki R, Xu Z, Mali S, Hohmann AG. Brain permeant and impermeant inhibitors of fatty-acid amide hydrolase suppress the development and maintenance of paclitaxel-induced neuropathic pain without producing tolerance or physical dependence in vivo and synergize with paclitaxel to reduce tumor cell line viability in vitro. Pharmacol Res 142: 267–282, 2019. doi: 10.1016/j.phrs.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walker J, Huang S, Strangman N, Tsou K, Sañudo-Peña M. Pain modulation by release of the endogenous cannabinoid anandamide. Proc Natl Acad Sci USA 96: 12198–12203, 1999. doi: 10.1073/pnas.96.21.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martin WJ, Patrick SL, Coffin PO, Tsou K, Walker JM. An examination of the central sites of action of cannabinoid-induced antinocoception in the rat. Life Sci 56: 2103–2109, 1995. doi: 10.1016/0024-3205(95)00195-C. [DOI] [PubMed] [Google Scholar]
- 27. Hohmann AG, Suplita R, Bolton N, Neely M, Fegley D, Mangieri R, Krey J, Walker J, Holmes P, Crystal J, Durant IA, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature 435: 1108–1112, 2005. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- 28. Tsou K, Brown S, Sañudo-Peña M, Mackie K, Walker J. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83: 393–411, 1998. doi: 10.1016/S0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 29. Millan M. Descending control of pain. Prog Neurobiol 66: 355–474, 2002. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 30. Keay K, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev 25: 669–678, 2001. doi: 10.1016/S0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 31. Dean C, Hillard CJ, Seagard JL, Hopp FA, Hogan QH. Upregulation of fatty acid amide hydrolase in the dorsal periaqueductal gray is associated with neuropathic pain and reduced heart rate in rats. Am J Physiol Regul Integr Comp Physiol 312: R585–R596, 2017. doi: 10.1152/ajpregu.00481.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Suplita RL, Farthing JN, Gutierrez T, Hohmann AG. Inhibition of fatty-acid hydrolase enhances cannabinoid stress-induced analgesia: sites of action in the dorsolateral periaqueductal gray and rostral ventromedial medulla. Neuropharm 49: 1201–1209, 2005. doi: 10.1016/j.neuropharm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 33. Dean C. Cannabinoid and GABA modulation of sympathetic nerve activity and blood pressure in the dorsal periaqueductal gray of the rat. Am J Physiol Regul Integr Comp Physiol 301: R1765–R1772, 2011. doi: 10.1152/ajpregu.00398.2011. [DOI] [PubMed] [Google Scholar]
- 34. Percie Du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Würbel H. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. Exp Physiol 105: 1459–1466, 2020. doi: 10.1113/EP088870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dean C. Endocannabinoid modulation of sympathetic and cardiovascular responses to acute stress in the periaqueductal gray of the rat. Am J Physiol Regul Integr Comp Physiol 300: R771–R779, 2011. doi: 10.1152/ajpregu.00391.2010. [DOI] [PubMed] [Google Scholar]
- 36. Gemes G, Rigaud M, Dean C, Hopp F, Hogan Q, Seagard J. Baroreceptor reflex is suppressed in rats that develop hyperalgesia behavior after nerve injury. Pain 146: 293–300, 2009. doi: 10.1016/j.pain.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hogan Q, Sapunar D, Modric-Jednacak K, McCallum J. Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology 101: 476–487, 2004. doi: 10.1097/00000542-200408000-00030. [DOI] [PubMed] [Google Scholar]
- 38. Bennett M. The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain 92: 147–157, 2001. doi: 10.1016/s0304-3959(00)00482-6. [DOI] [PubMed] [Google Scholar]
- 39. Wu H-E, Gemes G, Zoga V, Kawano T, Hogan QH. Learned avoidance from noxious mechanical stimulation but not threshold Semmes Weinstein filament stimulation after nerve injury in rats. J Pain 11: 280–286, 2010. doi: 10.1016/j.jpain.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scholz J, Mannion R, Hord D, Griffin R, Rawal B, Zheng H, Scoffings D, Phillips A, Gu J, Laing R, Abdi S, Decosterd I, Woolf C. A novel tool for the assessment of pain: Validation in low back pain. PLoS Med 6: e1000047, 2009. doi: 10.1371/journal.pmed.1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sherman K, Woyach V, Eisenach J, Hopp FA, Cao F, Hogan QH, Dean C. Heterogeneity in patterns of pain development after nerve injury in rats and the influence of sex. Neurobiol Pain 10: 100069, 2021. doi: 10.1016/j.ynpai.2021.100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eckberg DL. Sympathovagal balance: a critical appraisal. Circulation 96: 3224–3232, 1997. doi: 10.1161/01.cir.96.9.3224. [DOI] [PubMed] [Google Scholar]
- 43. Bambico F, Cassano T, Dominguez-Lopez S, Katz N, Walker C, Piomelli D, Gobbi G. Genetic deletion of fatty acid amide hydrolase alters emotional behavior and serotonergic transmission in the dorsal raphe, prefrontal cortex, and hippocampus. Neuropsychopharmacology 35: 2083–2100, 2010. doi: 10.1038/npp.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bago M, Dean C. Sympathoinhibition from ventrolateral periaqueductal gray mediated by 5-HT1A receptors in the RVLM. Am J Physiol Regul Integr Comp Physiol 280: R976–R984, 2001. doi: 10.1152/ajpregu.2001.280.4.R976. [DOI] [PubMed] [Google Scholar]
- 45. Curtis M, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA, Hoyer D, Insel PA, Izzo AA, Ji Y, MacEwan DJ, Sobey CG, Stanford SC, Texeira MM, Wonnacott S, Ahluwalia A. Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Br J Pharmacol 175: 985–1108, 2018. doi: 10.1111/bph.14153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wilson-Poe A, Morgan M, Aicher S, Hegarty D. Distribution of CB1 cannabinoid receptors and their relationship with mu-opioid receptors in the rat periaqueductal gray. Neuroscience 213: 191–200, 2012. doi: 10.1016/j.neuroscience.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sims-Williams H, Matthews JC, Talbot P, Love-Jones S, Brooks J, Patel N, Pickering A. Deep brain stimulation of the periaqueductal gray releases endogenous opioids in humans. NeuroImage 146: 833–842, 2017. doi: 10.1016/j.neuroimage.2016.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hama A, Germano P, Varghese M, Cravatt B, Milne G, Pearson J, Sagen J. Fatty acid amide hydrolase (FAAH) inhibitors exert pharmacological effects, but lack antinociceptive efficacy in rats with neuropathic spinal cord injury pain. PloS One 9: e96396, 2014. doi: 10.1371/journal.pone.0096396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Karbarz M, Luo L, Chang L, Tham C, Ja P, Wilson S, Wennerholm M, Brown S, Scott B, Apodaca R, Keith J, Wu J, Breitenbucher J, Chaplan S, Webb M. Biochemical and biological properties of 4-(3-phenyl-[1,2,4] thiadiazol-5-yl)-piperazine-1- carboxylic acid phenylamide, a mechanism-based inhibitor of fatty acid amide hydrolase. Anesth Analg 108: 316–329, 2009. doi: 10.1213/ane.0b013e31818c7cbd. [DOI] [PubMed] [Google Scholar]
- 50. Bruehl S, Chung O. Interactions between the cardiovascular and pain regulatory systems: an updated review of mechanisms and possible alterations in chronic pain. Neurosci Biobehav Rev 28: 395–414, 2004. doi: 10.1016/j.neubiorev.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 51. Nordin M, Fagius J. Effect of noxious stimulation on sympathetic vasoconstrictor outflow to human muscles. J Physiol 489: 885–894, 1995. doi: 10.1113/jphysiol.1995.sp021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sheps DS, Bragdon EE, Gray TF 3rd, Ballenger M, Usedom JE, Maixner W. Relation between systemic hypertension and pain perception. Am J Cardiol 70: 3F–5F, 1992. doi: 10.1016/0002-9149(92)90181-w. [DOI] [PubMed] [Google Scholar]
- 53. Lovick T. Selective modulation of the cardiovascular response but not the antinociception evoked from the dorsal PAG, by 5-HT in the ventrolateral medulla. Pflugers Arch 416: 222–224, 1990. doi: 10.1007/BF00370249. [DOI] [PubMed] [Google Scholar]
- 54. Vaughan C, Connor M, Bagley E, Christie M. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol Pharmacol 57: 288–295, 2000. [PubMed] [Google Scholar]
- 55. Egertová M, Giang D, Cravatt B, Elphick M. A new perspective on cannabinoid signalling: complementary localization of fatty acid amide hydrolase and the CB1 receptor in rat brain. Proc Biol Sci 265: 2081–2085, 1998. doi: 10.1098/rspb.1998.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Staton PC, Hatcher JP, Walker DJ, Morrison AD, Shapland EM, Hughes JP, Chong E, Mander PK, Green PJ, Billinton A, Fulleylove M, Lancaster HC, Smith JC, Bailey LT, Wise A, Brown AJ, Richardson JC, Chessell IP. The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain 139: 225–236, 2008. doi: 10.1016/j.pain.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 57. Beggiato S, Tomasini M, Ferraro L. Palmitoylethanolamide (PEA) as a potential therapeutic agent in Alzheimer’s Disease. Front Pharmacol 10: 821, 2019. doi: 10.3389/fphar.2019.00821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huang S, Bisogno T, Petros T, Chang S, Zavitsanos P, Zipkin R, Sivakumar R, Coop A, Maeda D, De Petrocellis L, Burstein S, di Marzo V, Walker J. Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain. J Biol Chem 276: 42639–42644, 2001. doi: 10.1074/jbc.M107351200. [DOI] [PubMed] [Google Scholar]
- 59. Russo E, Burnett A, Hall B, Parker K. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res 30: 1037–1043, 2005. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- 60. Pertwee RG, Howlett A, Abood M, Alexander S, Di Marzo V, Elphick M, Greasley P, Hansen H, Kunos G, Mackie K, Mechoulam R, Ross R. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev 62: 588–631, 2010. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Maione S, Bisogno T, de Novellis V, Palazzo E, Cristino L, Valenti M, Petrosino S, Guglielmotti V, Rossi F, di Marzo V. Elevation of endocannabinoid levels in the ventrolateral periaqueductal grey through inhibition of fatty acid amide hydrolase affects descending nociceptive pathways via both cannabinoid receptor type 1 and transient receptor potential vanilloid type-1 receptors. J Pharmacol Exp Ther 316: 969–982, 2006. doi: 10.1124/jpet.105.093286. [DOI] [PubMed] [Google Scholar]
- 62. Clapper J, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, Tontini A, Sanchini S, Sciolino S, Spradley J, Hohmann AG, Calignano A, Mor M, Tarzia G, Piomelli D. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci 13: 1265–1270, 2010. doi: 10.1038/nn.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sasso O, Bertorelli R, Bandiera T, Scarpelli R, Colombano G, Armirotti A, Moreno-Sanz G, Reggiani A, Piomelli D. Peripheral FAAH inhibition causes profound antinociception and protects against indomethacin-induced gastric lesions. Pharmacol Res 65: 553–563, 2012. doi: 10.1016/j.phrs.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, Kuner R. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci 10: 870–879, 2007. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lake K, Martin B, Kunos G, Varga K. Cardiovascular effects of anandamide in anesthetized and conscious normotensive and hypertensive rats. Hypertension 29: 1204–1210, 1997. doi: 10.1161/01.hyp.29.5.1204. [DOI] [PubMed] [Google Scholar]
- 66. Varga K, Lake K, Huangfu D, Guyenet P, Kunos G. Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension 28: 682–686, 1996. doi: 10.1161/01.hyp.28.4.682. [DOI] [PubMed] [Google Scholar]
- 67. Malinowska B, Kwolek G, Göthert M. Anandamide and methanand- amide induce both vanilloid VR1-and cannabinoid CB1 receptor-mediated changes in heart rate and blood pressure in anaesthetized rats. Naunyn Schmiedebergs Arch Pharmacol 364: 562–569, 2001. doi: 10.1007/s00210-001-0498-6. [DOI] [PubMed] [Google Scholar]
- 68. Krylatov A, Maslov L, Nam I, Bushov Y. Cannabinergic regulation of the functional state of the heart. The role of the autonomic nervous system. Neurosci Behav Physi 49: 331–340, 2019. doi: 10.1007/s11055-019-00736-w. [DOI] [Google Scholar]
- 69. Niederhoffer N, Schmid K, Szabo B. The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn Schmiedebergs Arch Pharmacol 367: 434–443, 2003. doi: 10.1007/s00210-003-0755-y. [DOI] [PubMed] [Google Scholar]
- 70. Brozoski D, Dean C, Hopp FA, Hillard CJ, Seagard JL. Differential endocannabinoid regulation of baroreflex-evoked sympathoinhibition in normotensive versus hypertensive rats. Auton Neurosci 150: 82–93, 2009. doi: 10.1016/j.autneu.2009.05.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tjen-A-Looi S, Li P, Longhurst J. Processing cardiovascular information in the vlPAG during electroacupuncture in rats: roles of endocannabinoids and GABA. J Appl Physiol (1985) 106: 1793–1799, 2009. doi: 10.1152/japplphysiol.00142.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Padley JR, Li Q, Pilowsky PM, Goodchild AK. Cannabinoid receptor activation in the rostral ventrolateral medulla oblongata evokes cardiorespiratory effects in anesthetized rats. Br J Pharm 140: 384–394, 2003. doi: 10.1038/sj.bjp.0705422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ibrahim B, Abdel-Rahman A. Cannabinoid receptor 1 signaling in cardiovascular regulating nuclei in the brainstem: A review. J Adv Res 5: 137–145, 2014. doi: 10.1016/j.jare.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Godlewski G, Alapafuja S, Bátkai S, Nikas S, Cinar R, Offertáler L, Osei-Hyiaman D, Li J, Mukhopadhyay B, Harvey-White J, Tam J, Pacak K, Blankman J, Cravatt B, Makriyannis A, Kunos G. Inhibitor of fatty acid amide hydrolase normalizes cardiovascular function in hypertension without adverse metabolic effects. Chem Biol 17: 1256–1266, 2010. doi: 10.1016/j.chembiol.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Niederhoffer N, Szabo B. Effect of the cannabinoid receptor agonist WIN55212-2 on sympathetic cardiovascular regulation. Br J Pharmacol 126: 457–466, 1999. doi: 10.1038/sj.bjp.0702337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Benowitz N, Jones R. Cardiovascular and metabolic considerations in prolonged cannabinoid administration in man. J Clin Pharmacol 21: 214S–223S, 1981. doi: 10.1002/j.1552-4604.1981.tb02598.x. [DOI] [PubMed] [Google Scholar]
- 77. Benowitz N, Rosenberg J, Rogers W, Pharm D, Bachman J, Jones R. Cardiovascular effects of intravenous delta-9-tetrahydrocannabinol: autonomic nervous mechanisms. Clin Pharm Ther 25: 440–446, 1979. doi: 10.1002/cpt1979254440. [DOI] [PubMed] [Google Scholar]
- 78. Huestis M, Georelick D, Heishman S, Preston K, Nelson R, Moolchan E, Frank R. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psych 58: 322–388, 2001. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- 79. Stocker SD, Muntzel MS. Recording sympathetic nerve activity chronically in rats: surgery techniques, assessment of nerve activity, and quantification. Am J Physiol Heart Circ Physiol 305: H1407–H1416, 2013. doi: 10.1152/ajpheart.00173.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Billman G. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Phsyiol 4: 26, 2013. doi: 10.3389/fphys.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Billman G, Kukielka M. Effect of endurance exercise training on heart rate onset and heart rate recovery responses to submaximal exercise in animals susceptible to ventricular fibrillation. J Appl Physiol (1985) 102: 231–240, 2007. doi: 10.1152/japplphysiol.00793.2006. [DOI] [PubMed] [Google Scholar]
- 82. Smith LL, Kukielka M, Billman GE. Heart rate recovery after exercise: a predictor of ventricular fibrillation susceptibility after myocardial infarction. Am J Physiol Heart Circ Physiol 288: H1763–H1769, 2005. doi: 10.1152/ajpheart.00785.2004. [DOI] [PubMed] [Google Scholar]
- 83. Eckberg DL, Mohanty S, Raczkowska M. Trigeminal-baroreceptor reflex interactions modulate human cardiac vagal efferent activity. J Physiol 347: 75–83, 1984. doi: 10.1113/jphysiol.1984.sp015054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Severino A, Chen R, Hayashida K, Aschenbrenner C, Sun H, Peters C, Gutierrez S, Pan B, Eisenach J. Plasticity and function of spinal oxytocin and vasopressin signaling during recovery from surgery with nerve injury. Anesthesiology 129: 544–556, 2018. doi: 10.1097/ALN.0000000000002290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dominguez C, Kouya P, Wu W, Hao J, Xu X, Wiesenfeld-Hallin Z. Sex differences in the development of localized and spread mechanical hypersensitivity in rats after injury to the infraorbital or sciatic nerves to create a model for neuropathic pain. Gender Med 6: 225–234, 2009. doi: 10.1016/j.genm.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 86. Bourquin A, Süveges M, Pertin M, Gilliard N, Sardy S, Davison A, Spahn D, Decosterd I. Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain 122: 14.e1–14.e14, 2006. doi: 10.1016/j.pain.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 87. Chung Y, Cai H, Steinmetz N. Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Adv Drug Deliv Rev 156: 214–235, 2020. doi: 10.1016/j.addr.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Vatta L, Sanderson R, Koch K. Magnetic nanoparticles: properties and potential applications. Pure Appl Chem 78: 1793–1801, 2006. doi: 10.1351/pac200678091793. [DOI] [Google Scholar]
- 89. Alavi M, Hamidi M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab Pers Ther 34: 20180032, 2019. doi: 10.1515/dmpt-2018-0032. [DOI] [PubMed] [Google Scholar]
- 90. Bahrami B, Hojjat-Farsangi M, Mohammadi H, Anvari E, Ghalamfarsa G, Yousefi M, Jadidi-Niaragh F. Nanoparticles and targeted drug delivery in cancer therapy. Immunol Lett 190: 64–83, 2017. doi: 10.1016/j.imlet.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 91. Huggins J, Smart T, Langman S, Taylor L, Young T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain 153: 1837–1846, 2012. doi: 10.1016/j.pain.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 92. Bradford D, Stirling A, Ernault E, Liosatos M, Tracy K, Moseley J, Blahunka P, Smith M. The MOBILE Study—A Phase IIa enriched enrollment randomized withdrawal trial to assess the analgesic efficacy and safety of ASP8477, a fatty acid amide hydrolase inhibitor, in patients with peripheral neuropathic pain. Pain Med 18: 2388–2400, 2017. doi: 10.1093/pm/pnx046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. La Porta C, Bura SA, Aracil-Fernández A, Manzanares J, Maldonado R. Role of CB1 and CB2 cannabinoid receptors in the development of joint pain induced by monosodium iodoacetate. Pain 154: 160–174, 2013. doi: 10.1016/j.pain.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 94. Hillard CJ. The endocannabinoid signaling system in the CNS: a primer. Int Review Neurobiology 125: 1–47, 2015. doi: 10.1016/bs.irn.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Woodhams S, Chapman V, Finn D, Hohmann AG, Neugebauer V. The cannabinoid system and pain. Neuropharm 124: 105–120, 2017. doi: 10.1016/j.neuropharm.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]