Abstract
Background:
Massive amounts of patient data are captured daily in electronic medical records (EMR); utilizing the power of such large data may help identify disease associations and generate hypotheses that can lead to a better understanding of disease associations and mechanisms. We aimed to comprehensively identify and validate associations between inflammatory bowel disease (IBD) and concurrent comorbid diagnoses.
Methods:
We performed a cross-sectional study using EMR data collected between 1986 and 2009 at a large tertiary referral center to identify associations with a diagnosis of IBD. The resulting associations were externally validated using the Truven MarketScan database, a large nationwide dataset of private insurance claims.
Results:
6,225 IBD patients and 31,125 non-IBD controls identified using EMR data were used to abstract 41 comorbid diagnoses associated with an IBD diagnosis. The strongest associations included Clostridiodes difficile infection, pyoderma gangrenosum, parametritis, pernicious anemia, erythema nodosum, and cytomegalovirus infection. Two IBD association clusters were found, including diagnoses of nerve conduction abnormalities and nonspecific inflammatory conditions of organs outside the gut. These associations were validated in a national cohort of 80,907 IBD patients and 404,535 age- and sex-matched controls.
Conclusion:
We leveraged a big data approach to identify several associations between IBD and concurrent comorbid diagnoses. EMR and big data provide the opportunity to explore disease associations with large sample sizes. Further studies are warranted to refine the characterization of these associations and evaluate their usefulness for increasing our understanding of disease associations and mechanisms.
Keywords: Electronic medical records, Inflammatory bowel disease, Clostridium difficile infection, disease associations
INTRODUCTION
The rate at which medical data are captured within electronic medical records (EMR) is rising exponentially1,2. It is estimated that a tertiary referral center generates up to 20 terabytes (TB) of data per patient annually from clinical records, laboratory values, and imaging. The National Institute of Health Precision Medicine initiative has proposed the collection of 20 TB of data per individual on 1 million Americans3. This volume of information is a novel medical research resource, and highlights the need to identify methods of utilizing these data in a way that can assist in the diagnosis and treatment of patients4.
One novel approach is to use these data to identify potential relationships between disease states and concurrent comorbid diagnoses. Prior studies have used EMR data to identify relationships between diagnoses that alter a patient’s risk of certain diseases or events5. Mined EMR data has been used to predict mortality6, identify disease-gene associations7, and detect adverse events8. . Work such as this not only provides a novel approach to identifying associations between diagnoses, but may also help generate novel hypotheses about potential disease mechanisms5,9.
Inflammatory bowel disease (IBD) is a chronic autoimmune condition that has been shown to be associated with extra intestinal manifestations including episcleritis, uveitis, arthritis, back pain, oral ulcers, and skin conditions such as erythema nodosum and pyoderma gangrenosum10,11. These conditions may appear prior to or after the diagnosis of IBD10. Understanding such associations may lead to earlier detection and improved clinical outcomes. In addition, such relationships could have implications for generating hypotheses as to disease mechanisms and progression.
As such, utilizing the power of large EMR data, we sought to evaluate the relationship between the diagnosis of IBD and other concurrent comorbid diagnoses in the medical record. These associations were then externally validated in a large nationwide dataset of private insurance claims. The findings presented included well-established associations, which help to confirm the validity of our approach, and other associations that are less commonly known.
METHODS
Data Source
We designed a cross-sectional study to examine associations between IBD and various comorbid diagnoses. We used de-identified data from the Michigan Medicine Data Warehouse to identify all IBD patients seen between 1986 and 2009 at the University of Michigan, as well as matched control patients. Data examined included International Classification of Diseases, Ninth Revision clinical modification (ICD-9-CM) billing codes and coded patient demographics.
We then queried the Truven Health MarketScan database (“MarketScan”) to identify similar IBD patients and age- and sex-matched controls enrolled between 2010 and 2012 in order to externally validate our results. The MarketScan database includes commercial health insurance claims for more than 230 million privately insured enrollees in the United States. Rigorous methods have been employed to ensure that this database is complete, accurate, and reliable 12. Within this database, we examined claims for outpatient visits and inpatient admissions, as well as enrollment tables.
Both the Michigan Medicine and MarketScan datasets are de-identified and have been used to capture healthcare outcomes and relationships across multiple clinical settings 13-16. We obtained approval from the Institutional Review Board (IRB) at Michigan Medicine prior to evaluating each dataset (HUM00073569 and HUM00127665).
Study Sample
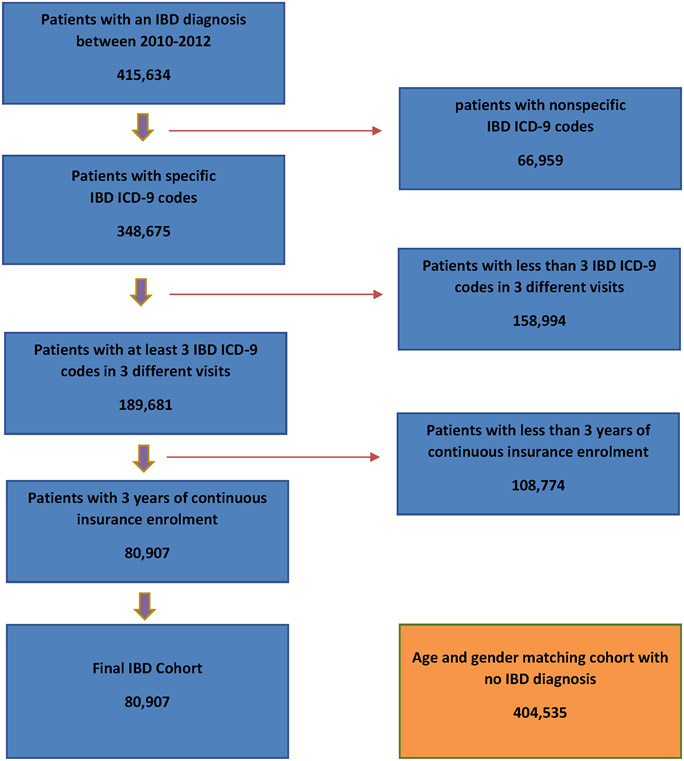
We used ICD-9-CM diagnosis codes to identify patients with a diagnosis of IBD (555.x and 556.x). Patients with nonspecific IBD diagnosis codes (555.9, 556.4, 556.8, 556.9) were excluded from the study. We required the presence of at least three IBD diagnosis codes in three different visits for the case definition of IBD. Within the Michigan Medicine cohort, we randomly selected five controls for each IBD patient, given lack of age and gender variables in the dataset. Within the MarketScan database, we identified five non-IBD patient controls matched by age and sex for every IBD patient identified to maximize the best matches and reduce bias while still maintaining precision17. In order to accurately capture health conditions and comorbidities in both cohorts, we required at least 3 years of continuous follow-up for inclusion. The algorithm used to select the study cohort within the MarketScan database is summarized in Figure 1.
Figure 1:
Patient Selection Algorithm for MarketScan Validation Cohort
Outcomes
Within the Michigan Medicine cohort, we abstracted information on the presence or absence a predetermined list of diagnoses using ICD-9 codes (Table 1). We then estimated the odds of a patient with IBD having each of these comorbid diagnoses, and compared these odds to the odds of this comorbid diagnosis being present in the matched controls. A similar approach was replicated using the MarketScan data. To investigate for possible confounding by increased utilization of healthcare services in IBD patients, we extracted healthcare utilization parameters from the MarketScan cohort. These parameters include numbers of hospitalizations and outpatient claims, in addition to numbers of electrocardiograms (EKG), gastric emptying studies, and rapid strep tests performed.
Table 1:
Univariate analyses for the association between IBD and health morbidities within the Michigan Medicine Cohort
| Variable | IBD N= 6,225 |
Control N= 31,125 |
OR | 95% Lower CI |
95% Upper CI |
Adjusted * p- Value** |
||
|---|---|---|---|---|---|---|---|---|
| N | % | n | % | |||||
| Clostridiodes difficile | 259 | 4.16 | 41 | 0.13 | 32.91 | 23.65 | 45.81 | 0.0001 |
| Pyoderma | 44 | 0.71 | 10 | 0.03 | 22.15 | 11.14 | 44.03 | 0.0001 |
| Parametritis | 70 | 1.12 | 24 | 0.08 | 14.74 | 9.26 | 23.45 | 0.0001 |
| Pernicious anemia | 40 | 0.64 | 19 | 0.06 | 10.59 | 6.13 | 18.29 | 0.0001 |
| Erythema nodosum | 39 | 0.63 | 19 | 0.06 | 10.32 | 5.96 | 17.87 | 0.0001 |
| CMV | 26 | 0.42 | 14 | 0.04 | 9.32 | 4.86 | 17.86 | 0.0001 |
| Klebsiella | 19 | 0.31 | 12 | 0.04 | 7.94 | 3.85 | 16.36 | 0.0001 |
| Autoimmune hemolytic anemia | 22 | 0.35 | 14 | 0.04 | 7.88 | 4.03 | 15.41 | 0.0001 |
| Gastroparesis | 70 | 1.12 | 49 | 0.16 | 7.21 | 5.00 | 10.40 | 0.0001 |
| Acute embolism of lower ext. | 45 | 0.72 | 32 | 0.10 | 7.08 | 4.49 | 11.14 | 0.0001 |
| Escherichia coli | 38 | 0.61 | 27 | 0.09 | 7.07 | 4.32 | 11.59 | 0.0001 |
| IVC Thrombosis | 29 | 0.47 | 23 | 0.07 | 6.33 | 3.66 | 10.95 | 0.0001 |
| Endocarditis | 76 | 1.22 | 78 | 0.25 | 4.92 | 3.58 | 6.76 | 0.0001 |
| Staphylococcus | 73 | 1.17 | 76 | 0.24 | 4.85 | 3.51 | 6.69 | 0.0001 |
| Bartholin gland cyst | 22 | 0.35 | 23 | 0.07 | 4.80 | 2.67 | 8.61 | 0.0001 |
| Genitourinary□ | 138 | 2.22 | 161 | 0.52 | 4.36 | 3.47 | 5.48 | 0.0001 |
| Wegener's | 16 | 0.26 | 19 | 0.06 | 4.22 | 2.17 | 8.21 | 0.0001 |
| Thrombosis-all | 317 | 5.09 | 408 | 1.31 | 4.04 | 3.48 | 4.69 | 0.0001 |
| Mycosis | 22 | 0.35 | 29 | 0.09 | 3.80 | 2.18 | 6.62 | 0.0001 |
| Pulmonary collapse | 290 | 4.66 | 429 | 1.38 | 3.502 | 3.00 | 4.07 | 0.0001 |
| Pseudomonas | 16 | 0.26 | 23 | 0.07 | 3.48 | 1.84 | 6.60 | 0.0001 |
| Mononeuritis | 167 | 2.68 | 279 | 0.90 | 3.05 | 2.51 | 3.70 | 0.0001 |
| Pleurisy | 47 | 0.76 | 87 | 0.28 | 2.71 | 1.90 | 3.87 | 0.0001 |
| Tracheitis | 75 | 1.20 | 151 | 0.49 | 2.50 | 1.89 | 3.30 | 0.0001 |
| Pulmonary fibrosis | 70 | 1.12 | 147 | 0.47 | 2.40 | 1.80 | 3.19 | 0.0001 |
| Pneumonia | 750 | 12.05 | 1595 | 5.12 | 2.54 | 2.31 | 2.78 | 0.0001 |
| Cardiac dysrhythmias | 1535 | 24.66 | 3971 | 12.76 | 2.24 | 2.09 | 2.39 | 0.0001 |
| Orchitis | 33 | 0.53 | 76 | 0.24 | 2.18 | 1.45 | 3.28 | 0.0001 |
| Spontaneous tension pneumothorax | 24 | 0.39 | 57 | 0.18 | 2.12 | 1.31 | 3.40 | 0.0017 |
| Pericarditis | 80 | 1.29 | 203 | 0.65 | 1.98 | 1.53 | 2.57 | 0.0001 |
| Urethritis | 17 | 0.27 | 43 | 0.14 | 1.98 | 1.13 | 3.47 | 0.0152 |
| Raynaud’s | 26 | 0.42 | 66 | 0.21 | 1.97 | 1.25 | 3.11 | 0.0028 |
| RBBB | 49 | 0.79 | 131 | 0.42 | 1.88 | 1.35 | 2.61 | 0.0001 |
| Peripheral vascular disease | 101 | 1.62 | 274 | 0.88 | 1.86 | 1.48 | 2.34 | 0.0001 |
| Emphysema | 97 | 1.56 | 264 | 0.85 | 1.85 | 1.46 | 2.34 | 0.0001 |
| Migraine | 194 | 3.12 | 543 | 1.74 | 1.81 | 1.53 | 2.14 | 0.0001 |
| LBBB | 18 | 0.29 | 60 | 0.19 | 1.50 | 0.89 | 2.54 | 0.1283 |
| Pharyngitis | 507 | 8.14 | 1824 | 5.86 | 1.42 | 1.29 | 1.58 | 0.0001 |
| Parkinson’sdisease | 72 | 1.16 | 267 | 0.86 | 1.35 | 1.04 | 1.76 | 0.0233 |
| Conduction disorders | 129 | 2.07 | 488 | 1.57 | 1.33 | 1.09 | 1.62 | 0.0044 |
| Acute tonsillitis | 20 | 0.32 | 116 | 0.37 | 0.86 | 0.54 | 1.39 | 0.5388 |
adjusted p-values with the False Discovery Rate (FDR) method
Based on the Cochran-Mantel-Haenszel test
Including parametritis, urethritis, orchitis, and Bartholin gland cyst.
IVC: Inferior vena cava; RBBB: Right bundle branch block; LBBB: Left bundle branch block; CMV: cytomegalovirus.
Statistical analysis
We performed univariate analyses to examine the relationship between IBD and each of the predetermined comorbid diagnoses. For categorical matched data, we used the Cochran–Mantel–Haenszel method. This method allows for testing the association between two binary variables while taking into consideration the stratification resulting from matching two cohorts. The method also allows for computing a weighted average for odds ratios across the strata. The Wilcoxon Rank-Sum test was used to test the association between continuous variables of non-normal data. A two sided p-value ≤ 0.05 after false discovery rate adjustment18 (FDR) was used to determine statistical significance. All analyses were performed using SAS software (version 9.4, SAS Institute Inc., Cary, NC, USA).
RESULTS
The Michigan Medicine Data Warehouse contains about 1.62 million unique patients with a total of 14,499 distinct ICD-9 codes. Within this cohort, we identified 15,437 patients with a single IBD diagnosis code, of whom 6,225 had met our inclusion criteria. These 6,225 IBD patients were matched with 31,125 randomly chosen non-IBD controls. In the larger MarketScan database, 415,634 patients with a single IBD diagnosis code were identified, of whom 80,907 met our inclusion criteria. These 80,907 patients were matched with 404,535 age and sex-matched controls. Females represented 53.5% of each cohort. The mean age (±SD) was 44 (±17) years in both cohorts.
In the Michigan Medicine cohort, the majority of the comorbid diagnoses were significantly associated with IBD (Table 1). To externally validate these findings, we abstracted the ICD-9 codes for these diagnoses from the MarketScan database (Table 2) (Appendix 1).
Table 2:
Univariate analyses for the association between IBD and health morbidities within the MarketScan Cohort
| Variable | IBD N=80,907 |
Control§ N=404,535 |
OR | 95% Lower CI |
95% Upper CI |
Adjusted * p- Value** |
||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||
| Clostridiodes difficile | 2888 | 3.6 | 785 | 0.2 | 19.4 | 17.9 | 21 | 0.0001 |
| Erythema nodosum | 479 | 0.6 | 157 | 0 | 15.4 | 12.8 | 18.4 | 0.0001 |
| CMV | 254 | 0.3 | 112 | 0 | 11.4 | 9.1 | 14.2 | 0.0001 |
| Parametritis | 376 | 0.5 | 181 | 0 | 10.5 | 8.8 | 12.5 | 0.0001 |
| Pyoderma | 607 | 0.8 | 492 | 0.1 | 6.2 | 5.5 | 7 | 0.0001 |
| Klebsiella | 209 | 0.3 | 204 | 0.1 | 5.1 | 4.2 | 6.2 | 0.0001 |
| Pernicious anemia | 1893 | 2.3 | 2077 | 0.5 | 4.7 | 4.4 | 5 | 0.0001 |
| IVC Thrombosis | 68 | 0.1 | 76 | 0 | 4.5 | 3.2 | 6.2 | 0.0001 |
| Gastroparesis | 678 | 0.8 | 881 | 0.2 | 3.9 | 3.5 | 4.3 | 0.0001 |
| Autoimmune hemolytic anemia | 79 | 0.1 | 101 | 0 | 3.9 | 2.9 | 5.3 | 0.0001 |
| Endocarditis | 113 | 0.1 | 159 | 0 | 3.6 | 2.8 | 4.5 | 0.0001 |
| Escherichia coli | 289 | 0.4 | 425 | 0.1 | 3.4 | 2.9 | 4 | 0.0001 |
| Pseudomonas | 162 | 0.2 | 244 | 0.1 | 3.3 | 2.7 | 4.1 | 0.0001 |
| Thrombosis-all | 2807 | 3.5 | 4745 | 1.2 | 3.1 | 2.9 | 3.2 | 0.0001 |
| Mycosis | 233 | 0.3 | 379 | 0.1 | 3.1 | 2.6 | 3.6 | 0.0001 |
| Spontaneous tension pneumothorax | 37 | 0.1 | 61 | 0.0 | 3.03 | 2.02 | 4.56 | 0.0001 |
| Acute embolism of lower extremity | 1843 | 2.3 | 3110 | 0.8 | 3 | 2.9 | 3.2 | 0.0001 |
| Wegener's | 42 | 0.1 | 72 | 0 | 2.9 | 2 | 4.3 | 0.0001 |
| Staphylococcus | 309 | 0.4 | 564 | 0.1 | 2.7 | 2.4 | 3.2 | 0.0001 |
| Pulmonary collapse | 3542 | 4.4 | 6904 | 1.7 | 2.7 | 2.6 | 2.8 | 0.0001 |
| Bartholin gland cyst | 184 | 0.2 | 370 | 0.1 | 2.5 | 2.1 | 3 | 0.0001 |
| Pleurisy | 33 | 0 | 75 | 0 | 2.2 | 1.5 | 3.3 | 0.0001 |
| Pericarditis | 471 | 0.6 | 1146 | 0.3 | 2.1 | 1.9 | 2.3 | 0.0001 |
| Pulmonary fibrosis | 931 | 1.2 | 2390 | 0.6 | 2 | 1.8 | 2.1 | 0.0001 |
| Genitourinary □ | 1370 | 1.7 | 3625 | 0.9 | 1.9 | 1.8 | 2 | 0.0001 |
| Raynaud’s | 480 | 0.6 | 1248 | 0.3 | 1.9 | 1.7 | 2.1 | 0.0001 |
| Pneumonia | 5234 | 6.5 | 14159 | 3.5 | 1.9 | 1.9 | 2 | 0.0001 |
| Cardiac dysrhythmias | 9965 | 12.3 | 30701 | 7.6 | 1.8 | 1.8 | 1.8 | 0.0001 |
| Migraine | 4017 | 5 | 12118 | 3 | 1.7 | 1.6 | 1.8 | 0.0001 |
| Emphysema | 958 | 1.2 | 2837 | 0.7 | 1.7 | 1.6 | 1.8 | 0.0001 |
| Parkinson’s disease | 1919 | 2.37 | 5869 | 1.45 | 1.7 | 1.6 | 1.7 | 0.0001 |
| Mononeuritis | 1650 | 2 | 5092 | 1.3 | 1.6 | 1.5 | 1.7 | 0.0001 |
| LBBB | 148 | 0.2 | 457 | 0.1 | 1.6 | 1.3 | 2 | 0.0001 |
| RBBB | 570 | 0.7 | 1881 | 0.5 | 1.5 | 1.4 | 1.7 | 0.0001 |
| Conduction disorders | 1851 | 2.3 | 6296 | 1.6 | 1.5 | 1.4 | 1.6 | 0.0001 |
| Orchitis | 505 | 0.6 | 1857 | 0.5 | 1.4 | 1.2 | 1.5 | 0.0001 |
| Pharyngitis | 15128 | 18.7 | 57635 | 14.2 | 1.4 | 1.4 | 1.4 | 0.0001 |
| Urethritis | 320 | 0.4 | 1266 | 0.3 | 1.3 | 1.1 | 1.4 | 0.0002 |
| Tracheitis | 92 | 0.1 | 341 | 0.1 | 1.3 | 1.1 | 1.7 | 0.0105 |
| Peripheral vascular disease | 2136 | 2.6 | 8201 | 2 | 1.3 | 1.3 | 1.4 | 0.0001 |
| Acute tonsillitis | 1450 | 1.8 | 6535 | 1.6 | 1.1 | 1.1 | 1.2 | 0.0003 |
1-to-5 matching in age and gender.
adjusted p-values with the False Discovery Rate (FDR) method
Based on the Cochran-Mantel-Haenszel test
Including parametritis, urethritis, orchitis, and Bartholin gland cyst.
IVC: Inferior vena cava; RBBB: Right bundle branch block; LBBB: Left bundle branch block; CMV: cytomegalovirus.
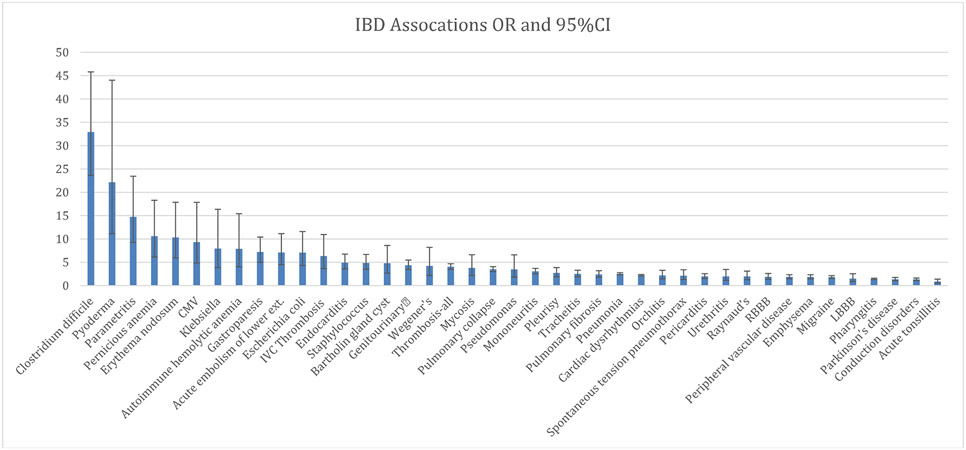
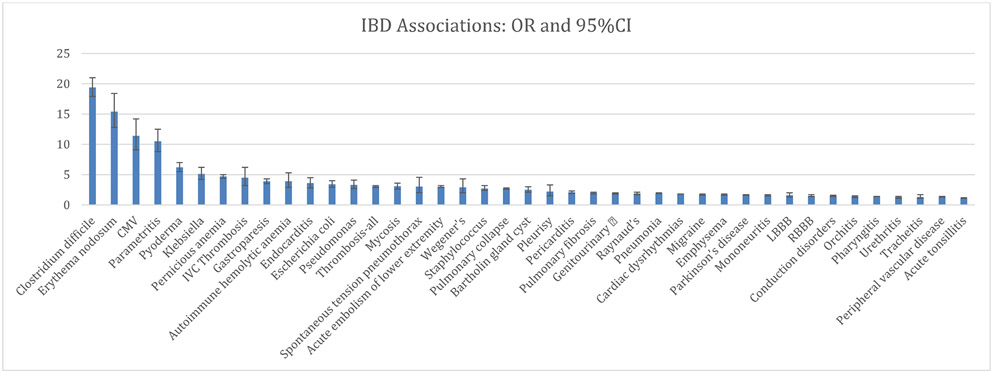
The top ten strongest associations remained consistent, to a large extent, within the two cohorts, although with a slightly different order (Table 1 and 2). Of all tested diagnoses, Clostridiodes difficile infection had the strongest association with IBD, with an odds ratio [OR] of 32.9 (95% CI: 23.6 - 45.8) in the Michigan Medicine population and an OR of 19.4 (95% CI: 17.9 – 21.0) in the MarketScan population. Pyoderma (OR 22.1; 95% CI: 11.1 - 44.0), parametritis (OR 14.7; 95% CI: 9.3 - 23.5), pernicious anemia (OR 10.6; 95% CI: 6.1 - 18.3), erythema nodosum (OR 10.3; 95% CI: 6.0 - 17.9), and CMV (OR 9.3; 95% CI: 4.9 - 17.9) demonstrated the strongest associations with IBD.
Similar results were seen within the MarketScan cohort for erythema nodosum (OR 15.4; 95% CI: 12.8 - 18.4), CMV infection (OR 11.4; 95%CI: 9.1 - 14.2), parametritis (OR 10.5; 95%CI: 8.8 - 12.5), pyoderma (OR 6.2; 95% CI: 5.5 - 7.0), and pernicious anemia (OR 4.7; 95% CI: 4.4 - 5.0). The estimated odds ratios for the rest of the examined comorbid diagnoses are shown for the Michigan Medicine cohort in Table 1 and Figure 2, and for the MarketScan cohort in Table 2 and Figure 3.
Figure 2:
Odds ratios and confidence intervals for University of Michigan cohort
Figure 3:
Odds ratios and confidence intervals for MarketScan Cohort
Examining healthcare utilization parameters with the MarketScan data revealed that the IBD cohort, as expected, had a higher median number of outpatient claims (138 vs 49; p<.0001) over the three-year study period than did the control patients, as well as a higher incidence of EKG testing (47.31% vs 36.13% of the cohort; p<.0001), and rapid strep tests (14.40% vs 11.05; p<.0001) compared with the non-IBD cohort. The ratio of these tests in IBD patients vs. non-IBD patients was less than the ratio of outpatient encounters. In contrast, the ratio for performance of gastric emptying studies (1.02% vs 0.26%; p<.0001), was nearly four-fold (Table 3). Other healthcare utilization data for corresponding groups are provided in Appendix 2.
Table 3:
Healthcare Utilization Parameters in IBD vs non-IBD patients
| Variable | IBD n= 80,907 |
Non IBD n= 404,535 |
p-Value |
|---|---|---|---|
| If ever underwent a gastric emptying study | 824 (1.02%) | 1,034 (0.26%) | <.0001 |
| Number of studies, Median (IQR)* | 2 (1-2) | 2 (1-2) | 0.6692 |
| If ever underwent a rapid strep test | 11,649 (14.4%) | 44,698 (11.05%) | <.0001 |
| Number of rapid strep tests, Median (IQR)* | 1 (1-2) | 1 (1-2) | <.0001 |
| If ever done an EKG study | 38,278 (47.31%) | 146,149 (36.13%) | <.0001 |
| Number of EKG studies, Median (IQR)* | 2 (1-4) | 2 (1-3) | <.0001 |
| Number of outpatient claims, median (IQR) | 138 (80-230) | 49 (21-101) | <.0001 |
| Number of inpatient admissions, median (IQR) | 1 (1-1) | 1 (1-1) | <.0001 |
For those who underwent each respective study
Discussion:
Using big data, we identified multiple comorbid diagnoses strongly associated with IBD in our cohorts, several of which are not traditionally considered IBD-associated conditions. This finding has important implications which could alter the way IBD is diagnosed and treated. First, knowledge of these associations can help clinicians broaden their differential to include IBD when treating these comorbid conditions. For example, physicians are aware of the well-established association between IBD and such conditions as C. difficile, pyoderma gangrenosum, erythema nodosum, CMV, and venous thromboembolism, and are prompted to consider the possibility of undiagnosed IBD when patients present with these diagnoses19. Our work suggests that other diagnoses like parametritis, pernicious anemia, gastroparesis, endocarditis, and certain infections such as Klebsiella, Staphylococcus and fungal infections should perhaps prompt this same consideration. Understanding such associations may lead to earlier detection and improved clinical outcomes.
One of the primary purposes for presenting our results was to help generate hypotheses for further testing and confirmation, which may have implications for better explaining of disease mechanisms and progression. There are some interesting patterns in these associations. While speculative, there are several conditions that can be clustered as nerve conduction issues (including mononeuritis, gastroparesis, and cardiac dysrhythmias) and nonspecific inflammatory conditions (including pharyngitis, tonsillitis, tracheitis, paracarditis, pleurisy, orchitis, urethritis, and parametritis). These could represent previously unsuspected extra-intestinal manifestations of IBD. As expected, there are patterns consistent with known extra-intestinal manifestations of IBD (e.g., pyoderma gangrenosum, erythema nodosum), other autoimmune diseases, infections (likely influenced by IBD itself, and/or by the immunosuppressive medications used to treat IBD), and thromboembolism.
Our finding that parametritis, which is a form of inflammatory pelvic disease20, is closely associated with IBD requires special attention as the symptoms of parametritis can closely mimic those of IBD including lower abdominal pain, tenderness, and discomfort. Parametritis is usually treated with antibiotics rather than IBD-targeted immunosuppressant therapy. Klebsiella pneumoniae has been identified as one of the environmental triggers of both Crohn’s and ulcerative colitis. Repeated subclinical infections increase antibody titers for K. pneumoniae which in turn cross react with intestinal collagen fibers and activate the complement pathway and proinflammatory cascades. This leads to influx of cytokines that causes inflammation. Repeated infections result in a continuous cycle of insult that can eventually lead to IBD21-23. In addition, there is a dysbiotic role of K. pneumoniae that results in the reduction of the lactic acid bacteria population which is known to have a protective role against colonic inflammation24,25
Pernicious anemia, which is characterized by vitamin B12 deficiency attributed to the absence of the intrinsic factor, is an autoimmune disease and can be found in association with other autoimmune disorders26. Our findings suggest that pernicious anemia may be another independent cause of B12 deficiency in patient with IBD which is classically attributed to impaired absorption of B12 in the ileum secondary to the chronic inflammation, bacterial overgrowth, or surgical resection27. Multiple case reports have demonstrated an association between autoimmune hemolytic anemia (AIHA) and IBD especially in ulcerative colitis and to a lesser extent in Crohn’s patients. Many theories have been hypothesized to explain this phenomenon of which an assumption was made that the colon is the source of anti-red blood cells antibody production or induction. This was confirmed by the fact that AIHA resolves in such patients following colectomy28-32.
The relationship between gastroparesis and IBD demonstrated in the present study has been reported before in small case series especially in patients with Crohn’s disease despite the lack of foregut involvement33,34. A proposed explanation for this phenomenon is that the distal motility disturbances caused by ileal or colonic inflammation might indirectly impair gastric emptying. In published case series, gastroparesis was observed even in the lack of radiological signs of obstruction or intestinal inflammation, and was attributed to the potential presence of minor intestinal fibrosis that cannot be detected using standard clinical methods33. The association between neurological disorders and IBD has been previously reported and hypothesized to be related to immune mechanisms and thrombotic states35..
Overall, the control groups of the present study, with five matches for each IBD patient, demonstrated lower prevalence of the comorbid conditions across the board. The higher comorbidity burden in patients with IBD reflects the debilitating nature of IBD as a chronic condition which necessitates care provided by multidisciplinary teams and collaboration between specialists for shared decision making36.
There are several limitations to this study. First, use of administrative databases requires dependence on billing codes for subject identification, and limited availability of descriptive data needed to fully control for confounders such as severity of disease or use of immunosuppressive therapy. Our ability to demonstrate similar relationships between the Michigan Medicine cohort and the significantly larger nationwide Truven-Health MarketScan insurance claims database supports the robustness of these findings, and the generalizability beyond our single center. Second, the difference in healthcare utilization among patients with IBD and controls could also suggest that some of the identified comorbid diagnoses are associated with IBD primarily as a result of increased testing and detection, rather than increased prevalence, which needs to be considered for the associations with lower odds ratios. Third, our results identify associations between IBD and other conditions; however, no causality should be assumed.
Despite limitations, this study, utilizing two large independent databases, demonstrates that data mining of the EMR provides a practical approach to identifying relevant disease associations within the massive amounts of data that health care systems collect with each patient encounter. Future studies are warranted to better define and utilize these associations which may have implications for explaining disease mechanisms and progression.
Supplementary Material
Abbreviations:
- EMR
Electronic medical records
- IBD
Inflammatory bowel disease
- ICD-9-CM
International Classification of Diseases, Ninth Revision clinical modification
- CMV
Cytomegalovirus
- LBBB
Left bundle branch block
- RBBB
right bundle branch block
- EKG
Electrocardiograms
Footnotes
Conflict of Interest and Source of Funding: The authors declare there are no conflicts to disclose. No funding was received for this manuscript.
References
- 1.Dinov ID. Volume and Value of Big Healthcare Data. Journal of medical statistics and informatics. 2016;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghupathi W, Raghupathi V. Big data analytics in healthcare: promise and potential. Health Inf Sci Syst. 2014;2:3–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NLM. What is the Precision Medicine Initiative? . https://ghr.nlm.nih.gov/primer/precisionmedicine/initiative. Published 2018. Accessed 9/24, 2018.
- 4.Jensen PB, Jensen LJ, Brunak S. Mining electronic health records: towards better research applications and clinical care. Nature reviews Genetics. 2012;13(6):395–405. [DOI] [PubMed] [Google Scholar]
- 5.Roque FS, Jensen PB, Schmock H, et al. Using electronic patient records to discover disease correlations and stratify patient cohorts. PLoS computational biology. 2011;7(8):e1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marlin BM, Kale DC, Khemani RG, Wetzel RC. Unsupervised pattern discovery in electronic health care data using probabilistic clustering models. Proceedings of the 2nd ACM SIGHIT International Health Informatics Symposium; 2012; Miami, Florida, USA. [Google Scholar]
- 7.Denny JC, Ritchie MD, Basford MA, et al. PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics. 2010;26(9):1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coloma PM, Schuemie MJ, Trifiro G, et al. Combining electronic healthcare databases in Europe to allow for large-scale drug safety monitoring: the EU-ADR Project. Pharmacoepidemiol Drug Saf. 2011;20(1):1–11. [DOI] [PubMed] [Google Scholar]
- 9.Kost R, Littenberg B, Chen ES. Exploring generalized association rule mining for disease co-occurrences. AMIA Annu Symp Proc. 2012;2012:1284–1293. [PMC free article] [PubMed] [Google Scholar]
- 10.Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterology & hepatology. 2011;7(4):235–241. [PMC free article] [PubMed] [Google Scholar]
- 11.Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflammatory bowel diseases. 2015;21(8):1982–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA surgery. 2017;152(6):e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanauer DA, Ramakrishnan N, Seyfried LS. Describing the relationship between cat bites and human depression using data from an electronic health record. PloS one. 2013;8(8):e70585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramakrishnan N, Hanauer D, Keller B. Mining Electronic Health Records. Computer. 2010;43(10):77–81. [Google Scholar]
- 15.Munson ME, Wrobel JS, Holmes CM, Hanauer DA. Data Mining for Identifying Novel Associations and Temporal Relationships with Charcot Foot. Journal of Diabetes Research. 2014;2014:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noureldin M, Higgins PDR, Govani SM, et al. Incidence and predictors of new persistent opioid use following inflammatory bowel disease flares treated with oral corticosteroids. Alimentary pharmacology & therapeutics. 2019;49(1):74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ming K, Rosenbaum PR. Substantial gains in bias reduction from matching with a variable number of controls. Biometrics. 2000;56(1):118–124. [DOI] [PubMed] [Google Scholar]
- 18.Jafari M, Ansari-Pour N. Why, When and How to Adjust Your P Values? Cell J. 2019;20(4):604–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jose FA, Heyman MB. Extraintestinal Manifestations of Inflammatory Bowel Disease. Journal of Pediatric Gastroenterology and Nutrition. 2008;46(2):124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crossman SH. The challenge of pelvic inflammatory disease. Am Fam Physician. 2006;73(5):859–864. [PubMed] [Google Scholar]
- 21.Tiwana H, Natt RS, Benitez-Brito R, et al. Correlation between the immune responses to collagens type I, III, IV and V and Klebsiella pneumoniae in patients with Crohn's disease and ankylosing spondylitis. Rheumatology (Oxford, England). 2001;40(1):15–23. [DOI] [PubMed] [Google Scholar]
- 22.Rashid T, Ebringer A, Wilson C. The role of Klebsiella in Crohn's disease with a potential for the use of antimicrobial measures. International journal of rheumatology. 2013;2013:610393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebringer A, Rashid T, Tiwana H, Wilson C. A possible link between Crohn's disease and ankylosing spondylitis via Klebsiella infections. Clinical rheumatology. 2007;26(3):289–297. [DOI] [PubMed] [Google Scholar]
- 24.Kaur CP, Vadivelu J, Chandramathi S. Impact of Klebsiella pneumoniae in lower gastrointestinal tract diseases. Journal of digestive diseases. 2018;19(5):262–271. [DOI] [PubMed] [Google Scholar]
- 25.Marcinkiewicz J, Ciszek M, Bobek M, et al. Differential inflammatory mediator response in vitro from murine macrophages to lactobacilli and pathogenic intestinal bacteria. International journal of experimental pathology. 2007;88(3):155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banka S, Ryan K, Thomson W, Newman WG. Pernicious anemia - genetic insights. Autoimmun Rev. 2011;10(8):455–459. [DOI] [PubMed] [Google Scholar]
- 27.Guagnozzi D, Lucendo AJ. Anemia in inflammatory bowel disease: a neglected issue with relevant effects. World J Gastroenterol. 2014;20(13):3542–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plikat K, Rogler G, Scholmerich J. Coombs-positive autoimmune hemolytic anemia in Crohn's disease. European journal of gastroenterology & hepatology. 2005;17(6):661–666. [DOI] [PubMed] [Google Scholar]
- 29.Gumaste V, Greenstein AJ, Meyers R, Sachar DB. Coombs-positive autoimmune hemolytic anemia in ulcerative colitis. Dig Dis Sci. 1989;34(9):1457–1461. [DOI] [PubMed] [Google Scholar]
- 30.Yates P, Macht LM, Williams NA, Elson CJ. Red cell autoantibody production by colonic mononuclear cells from a patient with ulcerative colitis and autoimmune haemolytic anaemia. British journal of haematology. 1992;82(4):753–756. [DOI] [PubMed] [Google Scholar]
- 31.Shashaty GG, Rath CE, Britt EJ. Autoimmune hemolytic anemia associated with ulcerative colitis. American journal of hematology. 1977;3:199–208. [DOI] [PubMed] [Google Scholar]
- 32.Park BS, Park S, Jin K, et al. Coombs negative autoimmune hemolytic anemia in Crohn's disease. The American journal of case reports. 2014;15:550–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kristinsson JO, Hopman WPM, Oyen WJG, Drenth JPH. Gastroparesis in patients with inactive Crohn's disease: a case series. BMC Gastroenterol. 2007;7:11–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keller J, Melle U, Schneider M, et al. Delayed gastric emptying of solids in Crohn's disease and ulcerative colitis. Gastroenterology. 2000;118(4):A1180. [Google Scholar]
- 35.Moris G. Inflammatory bowel disease: an increased risk factor for neurologic complications. World J Gastroenterol. 2014;20(5):1228–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Román ALS, Muñoz F. Comorbidity in inflammatory bowel disease. World J Gastroenterol. 2011;17(22):2723–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.