Abstract
Background
Postmarketing evaluations have linked myocarditis to COVID-19 mRNA vaccines. However, few population-based analyses have directly compared the safety of the 2 mRNA COVID-19 vaccines.
Objectives
This study aimed to compare the risk of myocarditis, pericarditis, and myopericarditis between BNT162b2 and mRNA-1273.
Methods
We used data from the British Columbia COVID-19 Cohort (BCC19C), a population-based cohort study. The exposure was the second dose of an mRNA vaccine. The outcome was diagnosis of myocarditis, pericarditis, or myopericarditis during a hospitalization or an emergency department visit within 21 days of the second vaccination dose. We performed multivariable logistic regression to assess the association between vaccine product and the outcomes of interest.
Results
The rates of myocarditis and pericarditis per million second doses were higher for mRNA-1273 (n = 31, rate 35.6; 95% CI: 24.1-50.5; and n = 20, rate 22.9; 95% CI: 14.0-35.4, respectively) than BNT162b2 (n = 28, rate 12.6; 95% CI: 8.4-18.2 and n = 21, rate 9.4; 95% CI: 5.8-14.4, respectively). mRNA-1273 vs BNT162b2 had significantly higher odds of myocarditis (adjusted OR [aOR]: 2.78; 95% CI: 1.67-4.62), pericarditis (aOR: 2.42; 95% CI: 1.31-4.46) and myopericarditis (aOR: 2.63; 95% CI: 1.76-3.93). The association between mRNA-1273 and myocarditis was stronger for men (aOR: 3.21; 95% CI: 1.77-5.83) and younger age group (18-39 years; aOR: 5.09; 95% CI: 2.68-9.66).
Conclusions
Myocarditis/pericarditis following mRNA COVID-19 vaccines is rare, but we observed a 2- to 3-fold higher odds among individuals who received mRNA-1273 vs BNT162b2. The rate of myocarditis following mRNA-1273 receipt is highest among younger men (age 18-39 years) and does not seem to be present at older ages. Our findings may have policy implications regarding the choice of vaccine offered.
Key Words: BNT162b2, COVID-19, mRNA-1273, myocarditis, pericarditis, vaccine safety
Abbreviations and Acronyms: ICD, International Classification of Disease; mRNA, messenger ribonucleic acid
Central Illustration
In December 2020, 2 messenger ribonucleic acid (mRNA) vaccines, Pfizer BioNTech (BNT162b2) and Moderna Spikevax (mRNA-1273) obtained market authorization in Canada under the Interim Order respecting the importation, sale, and advertising of drugs for use in relation to COVID-19.1 , 2 As of March 20, 2022, more than 52 million doses of BNT162b2 and 22 million doses of mRNA-1273 had been administered in Canada. Specifically, in the province of British Columbia, 38% of the population has received BNT162b2, and 15% have been vaccinated with mRNA-1273 (at least 2 doses of the same product).3
Prelicensure randomized phase 3 clinical trials evaluating the mRNA COVID-19 vaccines demonstrated an acceptable safety profile,4 , 5 and postauthorization monitoring in large heterogeneous populations has found most adverse events to be mild and short in duration.6 However, certain rare but potentially severe effects such as myopericarditis have been observed. As of March 18, 2022, a total of 1,933 postvaccination cases of myocarditis/pericarditis had been reported in Canada.7 Studies suggest that the rate of myocarditis/pericarditis following mRNA vaccination is higher than expected, particularly among younger men following a second dose of an mRNA vaccine.8, 9, 10, 11, 12, 13 Although this finding has been observed for both mRNA vaccines, there appears to be a higher risk following a second dose of mRNA-1273.13
Few population-based analyses have been conducted to directly compare the safety of the 2 mRNA COVID-19 vaccines, which differ in important ways that could impact safety (eg, dose). Instead, the majority of evidence originates from indirect comparisons within epidemiological studies, which can be confounded by factors such as differential uptake of the vaccines. Comparative analyses are needed to inform decisions related to vaccine rollout and future booster doses. The primary aim of this study was to compare the risk of myocarditis, pericarditis, and myopericarditis between the BNT162b2 and mRNA-1273.
Methods
Data source
We conducted a population-based cohort study using data from the British Columbia COVID-19 Cohort (BCC19C). The BCC19C integrates COVID-19 data sets (laboratory testing, case surveillance, provincial immunization registry, hospitalizations) with administrative health data holdings for the British Columbia population (medical visits, hospital admissions, emergency department visits, chronic conditions, dispensations, mortality, among others) (Supplemental Table 1). The BCC19C was established as a public health surveillance system under the British Columbia Centre for Disease Control's public health mandate. This study was reviewed and approved by the Behavioural Research Ethics Board at the University of British Columbia (approval # H20-02097).
Study population
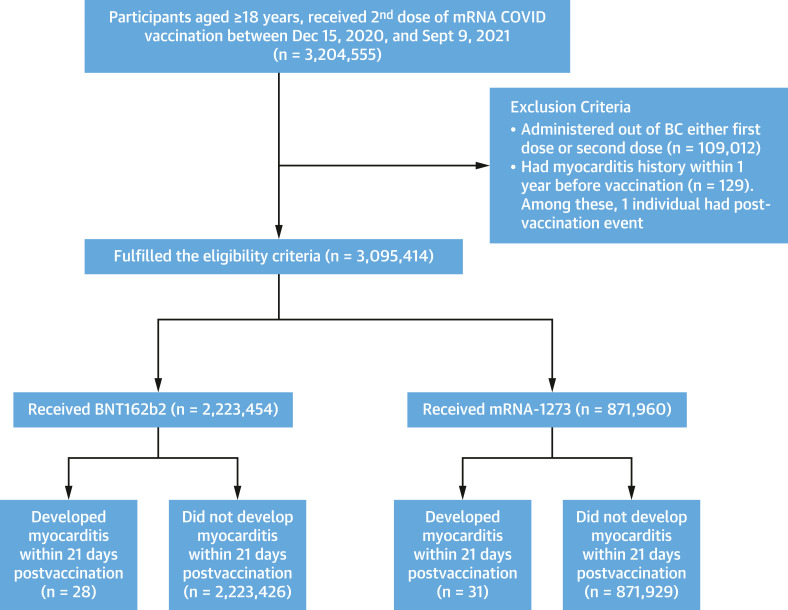
We studied individuals aged ≥18 years with a record of a second mRNA vaccination dose (BNT162b2 or mRNA-1273) following receipt of the first dose of an mRNA vaccine in the PIR (Provincial Immunization Registry) between January 1, 2021, and September 9, 2021. PIR is integrated into the BCC19C and includes records of all COVID-19 vaccines administered in British Columbia and some immunizations administered outside of British Columbia. We excluded individuals whose first or second vaccine dose was administered outside of British Columbia, as well as those with a history of myocarditis or pericarditis (depending on outcome assessed) within 1 year before the second dose.
SARS-CoV-2 vaccination
The primary exposure was a second dose of an mRNA vaccine (BNT162b2 or mRNA-1273).
Myocarditis and pericarditis
The outcomes of interest were diagnosis of myocarditis, pericarditis, and myopericarditis during a hospitalization or emergency department visit within 21 days following the second dose of an mRNA vaccine. Outcomes were assessed using International Classification of Diseases-10th Revision (ICD-10) codes. Myocarditis codes included I40.1 (isolated myocarditis), I40.8 (other acute myocarditis), I40.9 (acute myocarditis, unspecified), and I51.4 (myocarditis unspecified), and pericarditis codes included I30.0 (acute nonspecific idiopathic pericarditis), I30.8 (other forms of acute pericarditis), and I30.9 (acute pericarditis, unspecified).14 Myopericarditis was defined as any myocarditis or pericarditis ICD-10 code. For the cases identified through ICD-10 codes, a crosscheck was conducted using the Provincial Laboratory Information Solution data to determine if they were tested for at least 1 type of troponin within 30 days after their relevant vaccination dose. To be more specific with the outcome definition, a sensitivity analysis was conducted using a 7-days postvaccination risk period.
Covariates
Covariates ascertained for participants included sex, age, the interval between the 2 mRNA vaccine doses (categorized as ≤30, 31-55, and ≥56 days), calendar time of vaccine dose (month), history of SARS-CoV-2 infection, and a range of comorbidities. Given the small number of events, age was categorized as 18-39 and ≥40 years for modeling. Comorbidities were assessed separately for descriptive purposes and combined into an aggregate binary variable indicating any history of any comorbidity for modeling. More information on covariate measurement can be found in Supplemental Table 2.
Statistical methods
Chi-square/Fisher exact or 2-sample Student's t-tests were applied to test baseline differences between individuals by exposure (type of mRNA vaccine) and outcome (with and without myocarditis/pericarditis). A cumulative incidence curve for myocarditis events in 21 days following the receipt of the second mRNA vaccine dose was generated through the Kaplan-Meier method.
We conducted multivariable logistic regression models to assess the association between vaccine product and our outcomes of interest (myocarditis, pericarditis, myopericarditis). Separate models were run for each outcome. Akaike Information Criterion was used to select covariates. The final model was adjusted for sex, age, and history of comorbidities. Based on findings from the previous studies, history of SARS-CoV-2 infection and calendar month/year of vaccination were also added to the multivariable analyses in a stepwise approach. However, both variables were excluded from the final model because they had no effect on the direction or strength of the measure of association for the primary exposure variable.15 , 16
In addition to an overall model, we also ran adjusted models with interaction terms between primary exposure (type of mRNA vaccine) and covariates, including age and sex, to assess the association of interest at different levels of the covariates. A separate model was conducted for each interaction term. Furthermore, in a subanalysis, we used logistic regression to examine the association between any dose of the mRNA vaccine and myocarditis/pericarditis/myopericarditis.
SAS software version 9.4 (SAS Institute) and R statistical software version 3.6.2 (R Foundation for Statistical Computing) were used to perform the analyses.
Results
Study participants
A total of 3,204,555 individuals received 2 doses of an mRNA vaccine (Figure 1 ). Of these, 109,012 (3.4%) received 1 or more of their doses outside of British Columbia and were excluded. A total of 129, 369, and 488 individuals were excluded from the myocarditis (Figure 1), pericarditis (Supplemental Figure 1), and myopericarditis (Supplemental Figure 2) analyses, respectively, because the respective outcome was observed in the year before vaccination. In all analyses, the majority of second doses were BNT162b2 (75%), and the remainder were mRNA-1273 (25%).
Figure 1.
Myocarditis Study Population and Participant Enrollment Flowchart
Individuals aged ≥18 years with a record of a second messenger RNA (mRNA) vaccination dose (BNT162b2 or mRNA-1273) following receipt of the first dose of an mRNA vaccine in the Provincial Immunization Registry (PIR) between January 1, 2021, and September 9, 2021, were eligible for participation. Individuals with any vaccine dose administered outside of British Columbia (BC) and with a history of myocarditis within 1 year before the second dose were excluded. An occurrence of myocarditis within 7 and 21 days after second dose of vaccine was recorded as an event.
Characteristics of the participants by the second dose of mRNA vaccine for myocarditis analysis are presented in Table 1 . The characteristics of the participants by the second dose of mRNA for pericarditis and myopericarditis analysis are given in Supplemental Tables 3 and 4. The median age of the 2 groups was similar at 50 years (IQR: 34-66 years) for BNT162b2 and 51 years (IQR: 34-67 years) for mRNA-1273. The median time between administration of the first and second dose was also similar: 64 days (IQR: 57-76 days) for BNT162b2 and 62 days (IQR: 54-73 days) for mRNA-1273. A slightly higher percentage of BNT162b2 recipients were women (53.2%) compared with mRNA-1273 recipients (49.5%). The distribution of comorbidities was similar by mRNA vaccine type.
Table 1.
Baseline Characteristics of Participants for Myocarditis Analyses by Vaccine Product
| BNT162b2 (n = 2,223,454) | mRNA-1273 (n = 871,960) | Standardized Mean Difference | |
|---|---|---|---|
| Dose interval, da | 64.0 (57.0, 76.0) | 62.0 (54.0, 73.0) | 0.160 |
| Age, y | 50.0 (34.0, 67.0) | 51.0 (34.0, 66.0) | 0.002 |
| Age category, y | 0.035 | ||
| 18-29 | 397,586 (17.8) | 147,176 (16.8) | |
| 30-39 | 370,445 (16.6) | 146,034 (16.7) | |
| 40-49 | 318,242 (14.3) | 119,955 (13.7) | |
| ≥50 | 1,137,181 (51.1) | 458,795 (52.6) | |
| Sex | 0.058 | ||
| Male | 1,028,494 (46.3) | 428,691 (49.2) | |
| Female | 1,194,960 (53.7) | 443,269 (50.8) | |
| Comorbidities | |||
| Alzheimer’s/dementia | 24,966 (1.1) | 9,351 (1.1) | 0.005 |
| Anxiety | 758,386 (34.1) | 294,790 (33.8) | 0.006 |
| Schizophrenia | 20,843 (0.9) | 8,288 (0.9) | 0.001 |
| Depression | 619,867 (27.9) | 242,067 (27.8) | 0.003 |
| Ischemic heart disease | 173,739 (7.8) | 64,565 (7.4) | 0.015 |
| Heart failure | 54,328 (2.4) | 20,051 (2.3) | 0.009 |
| Hypertension | 552,464 (24.8) | 211,275 (24.2) | 0.014 |
| Asthma | 271,995 (12.2) | 104,078 (11.9) | 0.009 |
| Chronic obstructive pulmonary disease | 86,349 (3.9) | 35,126 (4.0) | 0.007 |
| Chronic kidney disease | 89,936 (4.0) | 32,018 (3.7) | 0.019 |
| Diabetes | 247,959 (11.1) | 96,763 (11.1) | 0.002 |
| Gout | 70,297 (3.2) | 27,153 (3.1) | 0.003 |
| Hemorrhagic stroke | 4,183 (0.2) | 1,669 (0.2) | 0.001 |
| Ischemic stroke | 14,799 (0.7) | 5,633 (0.6) | 0.002 |
| Epilepsy | 19,853 (0.9) | 8,187 (0.9) | 0.005 |
| Multiple sclerosis | 5,701 (0.3) | 2,400 (0.3) | 0.004 |
| Osteoarthritis | 274,923 (12.4) | 107,843 (12.4) | <0.001 |
| Osteoporosis | 113,350 (5.1) | 36,725 (4.2) | 0.042 |
| Rheumatoid arthritis | 35,451 (1.6) | 13,799 (1.6) | 0.001 |
| Injection drug use | 59,031 (2.6) | 24,569 (2.8) | 0.010 |
| Alcohol misuse | 73,168 (3.3) | 33,705 (3.9) | 0.031 |
| Cirrhosis | 10,287 (0.5) | 4,575 (0.5) | 0.009 |
| Cancer | 370,406 (16.7) | 135,703 (15.6) | 0.030 |
| Immunosuppression | 46,003 (2.1) | 16,271 (1.9) | 0.015 |
| Any comorbidity | 1,401,266 (63.0) | 543,098 (62.3) | 0.015 |
| Prevaccination SARS-CoV-2 infection | 61,392 (2.8) | 23,414 (2.7) | 0.005 |
Values are median (Q1, Q3) or n (%).
Interval between 2 vaccine doses in days.
Myocarditis, pericarditis, and myopericarditis
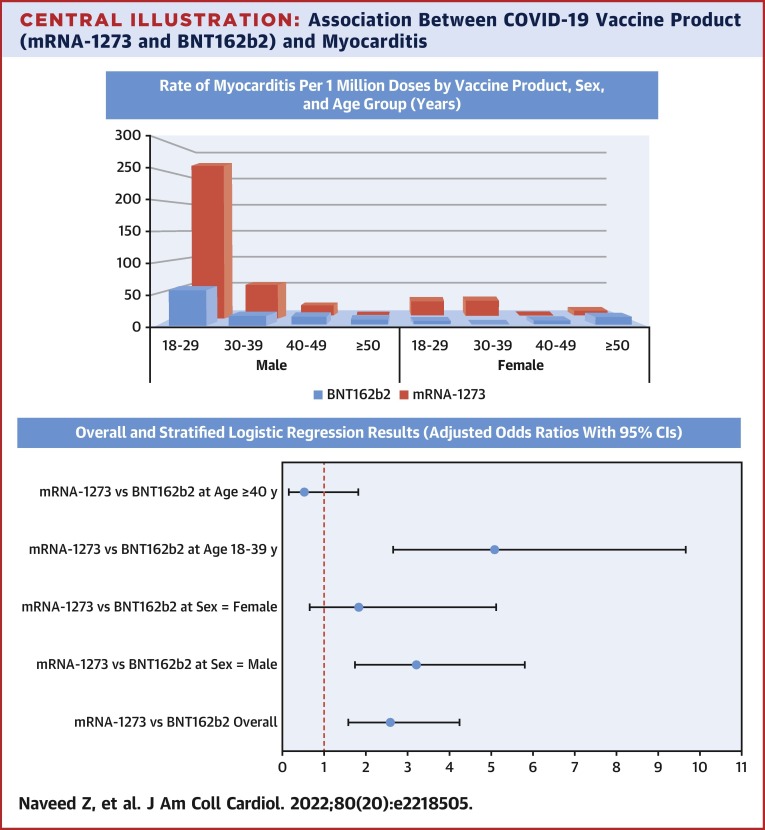
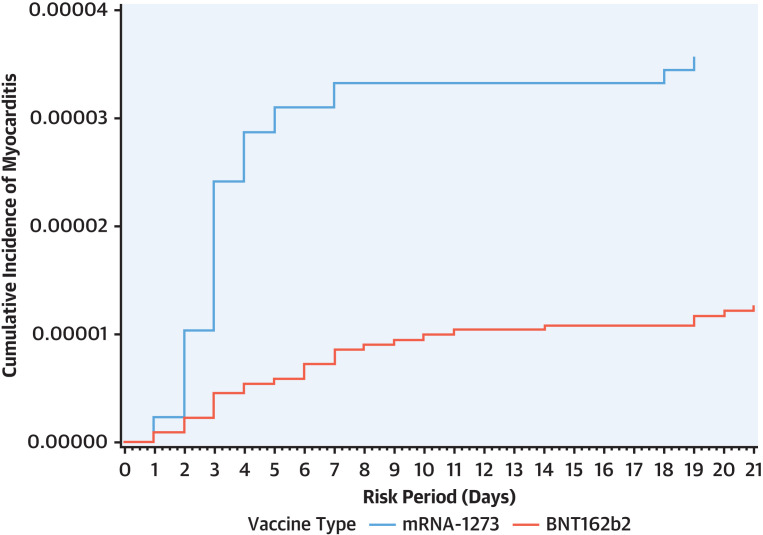
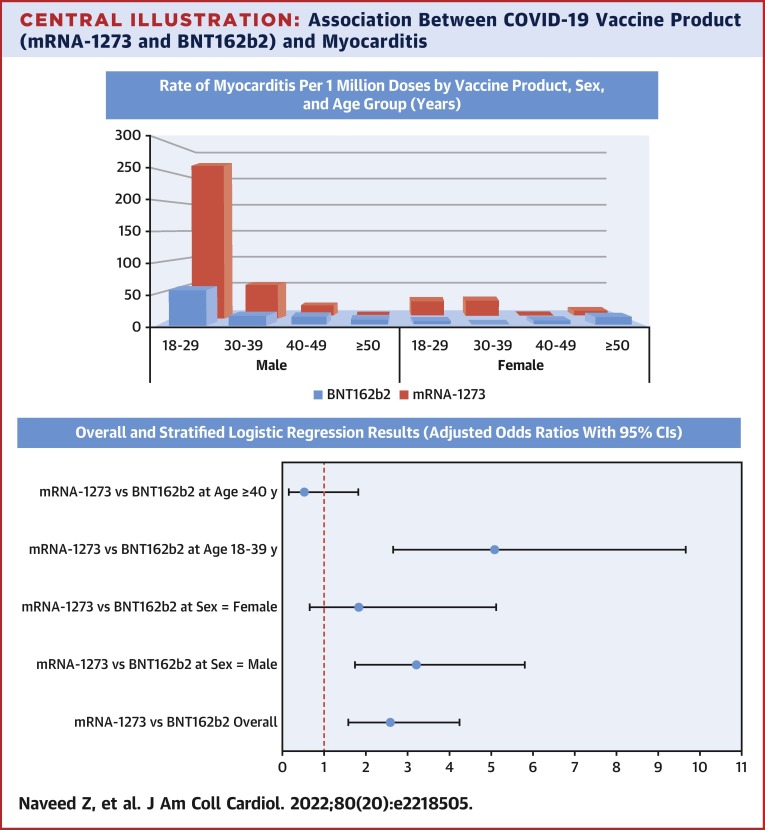
In the 21 days following the second dose of mRNA vaccine, a total of 59 myocarditis (28 BNT162b2, 31 mRNA-1273) and 41 pericarditis (21 BNT162b2, 20 mRNA-1273) events were observed. A total of 2,223,454 second BNT162b2 doses and 871,960 second mRNA-1273 doses were administered over the study period. Although numbers of myocarditis and pericarditis events were 31 and 20, respectively, for mRNA-1273 and 28 and 21, respectively, for BNT162b2 recipients, the rate per million doses was higher for mRNA-1273 (35.6; 95% CI: 24.1-50.5 and 22.9; 95% CI: 14.0-35.4, respectively) than BNT162b2 (12.6; 95% CI: 8.4-18.2 and 9.4; 95% CI: 5.8-14.4, respectively) (Table 2 and Supplemental Tables 5 and 6). The cumulative incident plot (Figure 2 ) shows a rapid rise in incident myocarditis within the first 7 to 10 days after second vaccination dose for both mRNA vaccines with a comparatively much higher rate for mRNA-1273. Rates of outcomes for both vaccine types were higher for men compared with women and were highest in the age 18 to 29 years category. Rates were higher for mRNA-1273 (compared with BNT162b2) for both men and women and in the age 18 to 29 and 30 to 39 years categories. The highest rates per million doses were observed among men aged 18 to 29 years following a second dose of mRNA-1273 compared with BNT162b2 (269.6 vs 58.1).
Table 2.
Myocarditis Events 21 Days After Second Dose and Rate/Million Doses by Vaccine Product, Sex, and Age
| Pfizer |
Moderna |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total |
Men |
Women |
Total |
Men |
Women |
|||||||
| N | Rate (95% CI) | n | Rate (95% CI) | n | Rate (95% CI) | N | Rate (95% CI) | n | Rate (95% CI) | n | Rate (95% CI) | |
| Total | 28 | 12.59 (8.37-18.20) | 19 | 18.47 (11.12-28.85) | 9 | 7.53 (3.44-14.29) | 31 | 35.55 (24.15-50.46) | 25 | 58.32 (37.74-86.08) | 6 | 13.53 (4.96-29.46) |
| Age, y | ||||||||||||
| 18-29 | 12 | 30.18 (15.59-52.72) | 11 | 58.05 (28.98-103.87) | 1 | 4.80 (0.12-26.77) | 22 | 149.48 (93.67-226.31) | 20 | 269.57 (164.66-416.33) | 2 | 27.40 (3.32-98.98) |
| 30-39 | 2 | 5.39 (0.65-19.50) | 2 | 11.39 (1.38-41.15) | 0 | 0 (—) | 6 | 41.08 (15.07-89.42) | 4 | 53.90 (14.68-138.01) | 2 | 27.84 (3.37-100.58) |
| 40-49 | 3 | 9.43 (1.94-27.55) | 2 | 13.79 (1.67-49.83) | 1 | 5.77 (1.38-41.15) | 1 | 8.33 (0.21-46.44) | 1 | 16.84 (0.42-93.82) | 0 | 0 (—) |
| ≥50 | 11 | 9.67 (4.83-17.31) | 4 | 7.71 (2.10-19.75) | 7 | 11.31 (4.55-23.31) | 2 | 4.36 (0.52-15.74) | 0 | 0 (—) | 2 | 8.41 (1.02-30.37) |
Figure 2.
Cumulative Incidence Plots for Myocarditis 21 Days Following COVID-19 mRNA Vaccines
Cumulative incidence plots for myocarditis 21 days after second dose of COVID-19 messenger RNA (mRNA) vaccine stratified by the type of vaccine, created using the Kaplan-Meier approach. The plot shows a rapid rise in incident myocarditis within the first 7-10 days after second dose for both mRNA vaccines. Comparatively, the cumulative incidence is higher and rises rapidly among mRNA-1273 recipients than BNT162b2 recipients.
In the adjusted multivariable logistic regression models (Table 3 ), compared with BNT162b2, mRNA-1273 was associated with more than 2-fold higher odds of myocarditis (adjusted OR [aOR]: 2.78; 95% CI: 1.67-4.62), pericarditis (aOR: 2.42; 95% CI: 1.31-4.46), and myopericarditis (aOR: 2.63; 95% CI: 1.76-3.93). The models were adjusted for age, sex, and any comorbidity. Based on Akaike Information Criterion, the variable dose interval was removed from the final model. History of SARS-CoV-2 infection and calendar month/year of vaccination were also excluded from the final model because they had no effect on the direction or strength of the measure of association for the primary exposure variable.
Table 3.
Odds of Myocarditis/Pericarditis 21 Days After Second Dose of COVID-19 mRNA Vaccination (mRNA-1273 vs BNT162b2)
| Myocarditis |
Pericarditis |
Myopericarditis |
||||
|---|---|---|---|---|---|---|
| Crude OR (95% CI) | Adjusted OR (95% CI)a | Crude OR (95% CI) | Adjusted OR (95% CI) | Crude OR (95% CI) | Adjusted OR (95% CI) | |
| Overall | 2.82 (1.69-4.71) | 2.78 (1.67-4.62) | 2.42 (1.32-4.48) | 2.42 (1.31-4.46) | 2.66 (1.78-3.97) | 2.63 (1.76-3.93) |
| Sex | ||||||
| Men | 3.16 (1.74-5.73) | 3.21 (1.77-5.83) | 2.08 (0.99-4.37) | 2.12 (1.01-4.46) | 2.70 (1.67-4.34) | 2.77 (1.72-4.48) |
| Women | 1.79 (0.64-5.05) | 1.82 (0.65-5.12) | 3.14 (1.06-9.36) | 3.21 (1.07-9.54) | 2.34 (1.11-4.91) | 2.40 (1.14-5.05) |
| Age, y | ||||||
| 18-39 | 5.24 (2.76-9.95) | 5.09 (2.68-9.66) | 2.24 (1.04-4.85) | 2.21 (1.02-4.78) | 3.83 (2.32-6.30) | 3.74 (2.27-6.16) |
| ≥40 | 0.54 (0.15-1.87) | 0.52 (0.15-1.82) | 2.87 (1.04-7.93) | 2.83 (1.02-7.79) | 1.32 (0.63-2.73) | 1.29 (0.62-2.67) |
Each OR represents the odds of the outcome among mRNA-1273 relative to BNT162b2, and therefore, an OR >1.00 indicates a higher odds of the outcome among mRNA-1273. P values for interactions: age = 0.014, sex = 0.612.
Overall analysis adjusted for sex, age, and history of any comorbidity, while the stratified analyses are adjusted for all variables except for the stratified variable.
In the separate models with interaction terms (Table 3) between vaccine types and covariates to assess if the effect differs across categories of these variables, the association between mRNA-1273 and myocarditis was stronger for those aged 18-39 years (aOR: 5.09; 95% CI: 2.68-9.66), but was not present for those aged ≥40 years (aOR: 0.52; 95% CI: 0.15-1.82) or among women (aOR: 1.82; 95% CI: 0.65-5.12), although the number of outcomes and power to detect the effect was very small. In contrast, the association between mRNA-1273 and pericarditis was present for both age groups and sexes. Results from sensitivity analyses using a 7-day window to ascertain outcomes (as opposed to the 21 days used in primary analysis) were similar (Supplemental Table 7). Considering both first and second doses, although the overall results and interpretation remained the same, the overall and stratified strength of associations was reduced (Supplemental Table 8c, Supplemental Figure 3).
Discussion
In our large population-based analysis of individuals who received an mRNA vaccine as a first and second dose, we observed 2- to 3-fold higher odds of myocarditis and pericarditis among individuals who received mRNA-1273 compared with BNT162b2. Compared with the background rates of myocarditis calculated for 2018 from BCC19C (overall = 2.01 per million, aged 18-39 years = 1.79 per million, and aged ≥40 years = 2.20 per million), both Moderna and Pfizer had higher overall as well as age-specific rates. However, overall rates of outcomes per million second doses were still very low for both vaccine products (myocarditis: 35.6 for mRNA-1273 and 12.6 for BNT162b2; pericarditis: 22.9 and 9.4), highlighting their favorable safety profile. The postvaccination rates were higher for men and in younger age groups for both vaccine products—with the highest rate observed in men aged 18 to 29 years following a second dose of mRNA-1273 (269.6 events per million doses), as also observed in other studies of mRNA vaccines.17 , 18 Notably, the increased odds of myocarditis with mRNA-1273 (vs BNT162b2) were not present at older ages (≥40 years), although odds of pericarditis were higher with mRNA-1273 for both younger and older individuals (Central Illustration ).
Central Illustration.
Association Between COVID-19 Vaccine Product (mRNA-1273 and BNT162b2) and Myocarditis
(Top) Rate of myocarditis per 1 million doses by vaccine product, sex, and age group (years). The highest rate of myocarditis was observed among those aged 18-29 years, following the second dose of mRNA-1273. (Bottom) Overall and stratified logistic regression results (adjusted odds ratios with 95% CI).
Our analysis builds upon phase 3 clinical trials (which were not powered to detect rare adverse events) and supports findings from other passive surveillance systems identifying an association between mRNA vaccine products and myocarditis/pericarditis. However, most prior analyses on this topic assessed the safety of these mRNA vaccines separately and indirectly compared their safety. Our findings have implications for strategizing the rollout of mRNA vaccines, which should also consider the self-limiting and mild nature of most myocarditis events,17 , 18 benefits provided by vaccination, higher effectiveness of mRNA-1273 (vs BNT162b2) against infection and hospitalization, and the apparent higher risk of myocarditis following SARS-COV-2 infection than with mRNA vaccination.19
Our results generally align with findings from studies that allow for indirect comparison of COVID-19 mRNA vaccine product safety. In an analysis of passive safety surveillance data from Ontario, Canada, rates of myocarditis after second vaccination dose were much higher for mRNA-1273 than BNT162b2 among individuals 18-39 years of age, with a rate per million doses of 195.5 vs 44.3 among individuals aged 18 to 24 years.20 In a self-controlled case series study, the risk of myocarditis was much higher following mRNA-1273 (vs self-controlled period before mRNA-1273; IRR: 9.84; 95% CI: 2.69-36.03) compared with BNT162b2 (vs self-controlled period before BNT162b2; IRR: 1.30; 95% CI: 0.98-1.72), although the number of events following mRNA-1273 was small, leading to wide CIs.13 In this study, the risk of myocarditis was higher in individuals 18-39 years of age. In contrast, a descriptive study conducted by Oster et al21 observed only slightly higher rates of myocarditis 7 days post–mRNA-1273 vaccine than BNT162b2 across multiple age and sex strata (eg, among men aged 18 to 24 years, the rate per million second doses of myocarditis post–mRNA-1273 was 56.3 compared with 52.4 post–BNT162b2). Also, a disproportionality analysis conducted by Li et al,12 found that BNT162b2 had a higher overall risk of myocarditis (reporting OR: 5.37; 95% CI: 4.10-7.04) than mRNA-1273 (reporting OR: 2.91; 95% CI: 2.21-3.83). However, these contrasting results may be attributed to the inclusion of a younger age group (12-17 years) among the BNT162b2 group. This age group did not receive the mRNA-1273 vaccine, and among BNT162b2 recipients, it had the highest risk of myocarditis (reporting OR: 8.19; 95% CI: 4.37-15.36) compared with other age groups. The contrasting findings may highlight the importance of direct comparison studies to adjust for potential differences between individuals who received these different vaccine products.12
Our results are also similar to the few safety studies that have directly compared vaccine products and adjusted for differences between populations. Two population-based studies have been conducted in which study participants could contribute time as unvaccinated individuals. In a Danish study, the adjusted hazard of myocarditis was 1.34 (95% CI: 0.90-2.00) for BNT162b2 and 3.92 (95% CI: 2.30-6.68) for mRNA-1273, compared with being unvaccinated.22 Similar to our study, no association with myocarditis was identified in individuals aged ≥40 years. In a meta-analysis of data from 4 Nordic countries (Denmark, Finland, Norway, and Sweden), adjusted incident rate ratios for the second mRNA dose were 1.75 (95% CI: 1.43-2.14) for BNT162b2 and 6.57 (95% CI: 4.64-9.28) for mRNA-1273. Although the association was still present in individuals ≥40 years of age, it was greatly attenuated compared with younger age groups.23 In the only study directly comparable to ours (ie, it did not include an unvaccinated comparator), an unpublished presentation (N.P. Klein, October 21, 2021, ACIP meeting COVID-19 Vaccines) using electronic health record data from the U.S. Vaccine Safety Datalink project found the rate of myocarditis among individuals aged 18 to 39 years to be 2.2 (95% CI: 0.98-4.97) times higher for mRNA-1273 compared with BNT162b2 after adjusting for age, sex, race/ethnicity, geography, and calendar date.24
Study Strengths and limitations
The major strength of this study is the comprehensive population-based capture of immunization data, as well as the data sets used to identify myocarditis/pericarditis and ascertain potential confounders. Unlike many other studies, we directly compared vaccine products and adjusted for these potential confounders in multivariable analysis. Due to the exclusion of any individual with a history of myopericarditis within the year preceding the date of second vaccination dose, the likelihood of previous history of myocarditis guiding the postvaccination occurrence was eliminated. Limitations include the study's observational nature, which limits the ability to infer causality. Indeed, we were unable to determine whether myocarditis/pericarditis events were related to vaccination, although temporality was ensured in the study design. Importantly, the use of a shorter time window (7 days) for ascertainment of safety events led to very similar results as the 21-day window in our primary analysis. Despite adjustment for potential confounders, residual confounding may contribute to the differences in the rate of outcomes across vaccine products. Furthermore, because of the rarity of events, we were unable to control for additional potential confounders such as geography. We also relied on hospital and emergency department visit data and, therefore, may have missed less severe myocarditis/pericarditis events that did not require medical attention. Furthermore, as the outcome definition was based on diagnostic codes with no chart reviews to include symptomatology or results of laboratory tests or diagnostic imaging, our outcomes may be subjected to misclassification. However, because this analysis compared 2 vaccine types, any bias resulting from this classification would be nondifferential because the 2 vaccine groups had the same case ascertainment procedure. Furthermore, a crosscheck using Provincial Laboratory Information Solution data found that of the 59 identified myocarditis cases, 57 were subjected to at least 1 type of troponin test within 30 days after the second vaccination dose. The troponin levels were higher than normal for 51 of the 57 (89.5%) participants with a troponin test. An overall limitation of analyses with rare outcomes is the small number of outcomes and its impact on statistical power. Therefore, the lack of an association between vaccine products and in some strata (eg, older ages and women) may be caused by a lack of power.
Conclusions
Myocarditis/pericarditis following mRNA COVID-19 vaccines is rare, but we observed a 2- to 3-fold higher odds among individuals who received mRNA-1273 recipients relative to BNT162b2. The higher rate of myocarditis following mRNA-1273 receipt is highest among younger men (18-39 years of age) and is not present at older ages. Our findings may have policy implications regarding the choice of vaccine offered.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: The Moderna Spikevax (mRNA-1273) vaccine is associated with a higher risk of myocarditis and pericarditis then the Pfizer BioNTech (BNT162b2) vaccine in young male recipients.
TRANSLATIONAL OUTLOOK: Further studies are needed to assess the risks of myocarditis and pericarditis with booster doses and lower-dose formulations of the Moderna Spikevax (mRNA-1273) vaccine.
Funding Support and Author Disclosures
This work was supported by the British Columbia Centre for Disease Control and the Canadian Immunization Research Network (CIRN) through a grant from the Public Health Agency of Canada and the Canadian Institutes of Health Research (CNF 151944). This project was also supported by funding from the Public Health Agency of Canada through the Vaccine Surveillance Reference Group and the COVID-19 Immunity Task Force. All inferences, opinions, and conclusions drawn in this paper are those of the authors and do not reflect the opinions or policies of the Data Steward(s). Dr Kwong is supported by a Clinician-Scientist Award from the University of Toronto Department of Family and Community Medicine. Dr Janjua has participated in advisory boards and has spoken for AbbVie, not related to the current work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors are grateful for the assistance of the Provincial Health Services Authority, British Columbia Ministry of Health, and Regional Health Authority staff involved in data access, procurement, and management, as well as the residents of British Columbia whose data are integrated in the British Columbia COVID-19 Cohort (BCC19C).
Footnotes
Listen to this manuscript's audio summary by Editor-in-Chief Dr Valentin Fuster onwww.jacc.org/journal/jacc.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Government of Canada Regulatory decision summary - Moderna COVID-19 vaccine - health Canada. Updated 2020. https://covid-vaccine.canada.ca/info/regulatory-decision-summary-detailTwo.html?linkID=RDS00736
- 2.Government of Canada Regulatory decision summary - Pfizer-BioNTech COVID-19 vaccine- health Canada. Updated 2020. https://covid-vaccine.canada.ca/info/regulatory-decision-summary-detailTwo.html?linkID=RDS00730
- 3.Government of Canada Covid-19 vaccination in Canada. Updated 2022. https://health-infobase.canada.ca/covid-19/vaccination-coverage/#a6
- 4.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenblum H.G., Gee J., Liu R., et al. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: an observational study of reports to the vaccine adverse event reporting system and v-safe. Lancet Infect Dis. 2022;22(6):802–812. doi: 10.1016/S1473-3099(22)00054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Government of Canada Reported side effects following COVID-19 vaccination in Canada. Updated 2022. https://health-infobase.canada.ca/covid-19/vaccine-safety/#a3
- 8.Lee A.S., Iswaree D., Balakrishnan O., et al. Myocarditis following COVID-19 vaccination: a systematic review (October 2020–October 2021) Heart Lung Circ. 2022;31(6):757–765. doi: 10.1016/j.hlc.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goyal M, Ray I, Mascarenhas D, Kunal S, Sachdeva RA, Ish P. Myocarditis post SARS-CoV-2 vaccination: a systematic review. QJM. Published online March 3, 2022. https://doi.org/10.1093/qjmed/hcac064. [DOI] [PMC free article] [PubMed]
- 10.Luk A., Clarke B., Dahdah N., et al. Myocarditis and pericarditis after covid-19 mRNA vaccination: practical considerations for care providers. Can J Cardiol. 2021;37(10):1629–1634. doi: 10.1016/j.cjca.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yap J., Tham M.Y., Poh J., et al. Pericarditis and myocarditis after COVID-19 mRNA vaccination in a nationwide setting. Ann Acad Med Singap. 2022;51(2):96–100. [PubMed] [Google Scholar]
- 12.Li M., Yuan J., Lv G., Brown J., Jiang X., Lu Z.K. Myocarditis and pericarditis following COVID-19 vaccination: Inequalities in age and vaccine types. J Pers Med. 2021;11(11):1106. doi: 10.3390/jpm11111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patone M., Mei X.W., Handunnetthi L., et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28(2):410–422. doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cates J., Lucero-Obusan C., Dahl R.M., et al. Risk for in-hospital complications associated with COVID-19 and influenza—Veterans Health Administration, United States, October 1, 2018–May 31, 2020. Morb Mortal Weekly Rep. 2020;69(42):1528–1534. doi: 10.15585/mmwr.mm6942e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozierański K., Tymińska A., Chabior A., et al. Sex differences in incidence, management, and outcomes in adult patients aged over 20 years with clinically diagnosed myocarditis in the last ten years: Data from the MYO-PL nationwide database. J Clin Med. 2021;10(23):5502. doi: 10.3390/jcm10235502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraser M., Agdamag A.C.C., Maharaj V.R., et al. COVID-19-associated myocarditis: an evolving concern in cardiology and beyond. Biology. 2022;11(4):520. doi: 10.3390/biology11040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mevorach D., Anis E., Cedar N., et al. Myocarditis after BNT162b2 mRNA vaccine against covid-19 in Israel. N Engl J Med. 2021;385(23):2140–2149. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witberg G., Barda N., Hoss S., et al. Myocarditis after covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barda N., Dagan N., Ben-Shlomo Y., et al. Safety of the BNT162b2 mRNA covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchan S.A., Seo C.Y., Johnson C., et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval. JAMA Netw Open. 2022;5(6) doi: 10.1001/jamanetworkopen.2022.18505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oster M.E., Shay D.K., Su J.R., et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331–340. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Husby A., Hansen J.V., Fosbøl E., et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ. 2021;375 doi: 10.1136/bmj-2021-068665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karlstad Ø., Hovi P., Husby A., et al. SARS-CoV-2 vaccination and myocarditis in a Nordic cohort study of 23 million residents. JAMA Cardiol. 2022;7(6):600–612. doi: 10.1001/jamacardio.2022.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein N.P. Myocarditis analyses in the vaccine safety datalink: rapid cycle analyses and “head-to-head” product comparisons. ACIP meeting COVID-19 Vaccines 2021. https://stacks.cdc.gov/view/cdc/110921
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.