Abstract
Background
Solid cancer is an independent prognostic factor for poor outcome with COVID-19. As guidelines for patient management in that setting depend on retrospective efforts, we here present the first analyses of a nationwide database of patients with cancer hospitalized with COVID-19 in Belgium, with a focus on changes in anticancer treatment plans at the time of SARS-CoV-2 infection.
Methods
Nineteen Belgian hospitals identified all patients with a history of solid cancer hospitalized with COVID-19 between March 2020 and February 2021. Demographic, cancer-specific and COVID-specific data were pseudonymously entered into a central Belgian Society of Medical Oncology (BSMO)-COVID database. The association between survival and primary cancer type was analyzed through multivariate multinomial logistic regression. Group comparisons for categorical variables were carried out through a Chi-square test.
Results
A total of 928 patients were registered in the database; most of them were aged ≥70 years (61.0%) and with poor performance scores [57.2% Eastern Cooperative Oncology Group (ECOG) ≥2]. Thirty-day COVID-related mortality was 19.8%. In multivariate analysis, a trend was seen for higher mortality in patients with lung cancer (27.6% versus 20.8%, P = 0.062) and lower mortality for patients with breast cancer (13.0% versus 23.3%, P = 0.052) compared with other tumour types. Non-curative treatment was associated with higher 30-day COVID-related mortality rates compared with curative or no active treatment (25.8% versus 14.3% versus 21.9%, respectively, P < 0.001). In 33% of patients under active treatment, the therapeutic plan was changed due to COVID-19 diagnosis, most frequently involving delays/interruptions in systemic treatments (18.6%). Thirty-day COVID-related mortality was not significantly different between patients with and without treatment modifications (21.4% versus 20.5%).
Conclusion
Interruption in anticancer treatments at the time of SARS-CoV-2 infection was not associated with a reduction in COVID-related mortality in our cohort of patients with solid cancer, highlighting that treatment continuation should be strived for, especially in the curative setting.
Key words: COVID-19, cancer, treatment changes, prognosis, solid tumours
Introduction
The COVID-19 pandemic has caused unprecedented strains on health care systems worldwide. Early medical responses and policy making relied on incomplete knowledge about the disease and its risk factors. Scientific efforts amidst this crisis, however, have since helped to improve insight so that policies can and could be adapted accordingly. Specifically in the field of oncology, it is now known that the pandemic impacted screening programs as well as diagnostic and therapeutic care planning, resulting in delayed care for many patients.1,2 On an individual patient level, current or previous history of cancer increases the risk of SARS-CoV-2 infection, hospitalization with COVID-19 and even subsequent mortality, as recently reviewed extensively elsewhere.3 Some factors that have been identified as poorly prognostic in the general population also apply to patients with solid cancer, such as male sex, older age, history of smoking, lower overall performance status and higher number of comorbidities.4, 5, 6, 7 Many risk factors, however, distinguish oncology patients from the general population as well. Patients with lung cancer, and in some reports patients with lung metastases, have been found to have higher mortality rates from COVID-19 compared with patients with other primary solid tumour types.4,5,8,9 This might reflect how their disease and/or risk factors (e.g. smoking) negatively affect respiratory capacity. Likewise, systemic cancer treatments such as chemotherapy may disrupt the immune response to infectious diseases.3 The role of cancer immunotherapy is less clear. On top of this, seroconversion after vaccination for SARS-CoV-2 appears to be lower in cancer patients, and even more so in those with metastatic disease and those treated with chemotherapy, steroids or cyclin-dependent kinase 4/6 (CDK4/6) inhibitors.10
Nationwide and even worldwide registries focusing on patients with cancer and COVID-19 specifically, such as the COVID-19 and Cancer Consortium (CCC19) database, TERAVOLT and ESMO CoCare initiatives are essential to fill the remaining knowledge gaps. As data collection on outcomes in patients hospitalized with COVID-19 in Belgium was streamlined through the national health institution Sciensano, the Belgian Society of Medical Oncology (BSMO) decided to contribute to that growing knowledge by setting up a population-based nationwide study on the interplay between cancer and COVID-19. The first results of that effort focused on the comparison between patients with and without solid cancer and highlighted an important increase in in-hospital mortality among patients with solid cancer (31.7% versus 20.0%, respectively; adjusted odds ratio 1.34; 95% confidence interval 1.13-1.58).11 Here we present the results of the second part of the study, where additional information was collected on patients with cancer and COVID-19 hospitalized in Belgium. Our aim was to investigate possible differences in outcomes between patients with different primary tumour types, between patients under active treatment versus those without, according to treatment received, and according to changes in the oncological treatment plans due to COVID-19.
Material and methods
Patient identification
Adults (individuals aged ≥18 years) hospitalized with COVID-19 in Belgium between 1 March 2020 and 1 February 2021 registered in the Sciensano COVID-19 database and identified by each participating institution as having a prior or current solid cancer were eligible for inclusion in the study. Patients with a haematological malignancy, but without a solid tumour and patients with only non-melanoma skin cancer were not included. Diagnosis of COVID-19 was based on a molecular test (PCR) and/or chest computed tomography imaging.
Data collection
A central electronic case report form (eCRF) was created using REDCap®. Data on patient demographics, comorbidities, primary cancer diagnosis, anticancer treatments previously received, anticancer treatment changes due to COVID-19 and survival outcome were retrospectively registered by the participating institutions in this central database. Standardized data reported by the hospitals on COVID-19 baseline characteristics (signs, symptoms, laboratory values) and COVID-19 disease evolution, treatment and outcomes during hospitalization were sent back by Sciensano directly to each reporting hospital from their central database.12 Within each hospital, data were pseudonymized by the participating investigators, and then merged with the REDCap® eCRF.
Statistical analysis
Descriptive analyses of baseline patient, cancer and treatment characteristics were carried out and are provided as frequencies with percentages for categorical variables or mean with standard deviation/median with interquartile range (IQR) for continuous variables. Survival outcomes defined for analysis were overall mortality at 30 days after COVID-19 diagnosis (30-day mortality) and overall mortality at 3 months after COVID-19 diagnosis (3-month mortality), and analogously defined COVID-19-related and non-COVID-19-related 30-day and 3-month mortality. For subgroup analyses, an ‘active cancer’ subgroup was defined including patients undergoing active anticancer treatment at or within 90 days before diagnosis of COVID-19, as well as patients under palliative care only (without specific anticancer treatment). Active anticancer treatment was defined as any form of locoregional or systemic treatment given with the purpose of treating cancer and/or preventing cancer relapse. Subgroup analysis was also carried out for the first and second wave of the COVID-19 pandemic in Europe separately, with a cut-off on 30 June 2020.
Group comparisons with regards to categorical variables were carried out by means of a Chi-square test. The association between (non-)COVID-19-related death with cancer type, correcting for possible confounders was analyzed by means of multivariate multinomial logistic regression models including age, gender, Eastern Cooperative Oncology Group (ECOG) performance status, body mass index, Charlson comorbidity index (which includes the presence or absence of metastatic cancer disease at COVID-19 diagnosis), haematological malignancies and ICU admission as covariates. All P values reported are two-sided, and P values <0.05 were considered to reflect statistical significance. All analyses were carried out using SAS software (SAS Institute, Cary, NC) (version 9.4 of the SAS System for Windows).
Results
A total of 19 Belgian hospitals involved in cancer care received approval for the study by their local ethics committee and subsequently participated in patient registration. In total, 928 patients with solid cancer hospitalized with COVID-19 during the predefined study period were registered and available for subsequent analysis upon database lock on 26 April 2022. A descriptive analysis of baseline patient characteristics can be found in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100610. Median age at COVID-19 diagnosis was 73 years (IQR 64-81 years) and most of the patients were of male sex (57.1%). ECOG performance status was ≥2 in 57.2% of patients, and median Charlson comorbidity index was 7 (IQR 6-9). Almost half of the patients (n = 457, 49.3%) had received any type of anticancer treatment within 3 months of COVID-19 diagnosis. This included chemotherapy in 43.1%, endocrine treatment in 23.0%, immunotherapy in 17.1% and targeted treatment in 11.4%. Most of these patients (62.3%) received treatment in a non-curative setting. The main reason for hospitalization was the clinical status of the patient in 68.0% (n = 631), whereas 27.6% (n = 256) of patients were hospitalized for another reason but concomitantly got diagnosed with COVID-19 and 3.1% (n = 29) were hospitalized out of precaution.
Thirty-day overall and COVID-19-related mortality in all patients assessable for survival analysis (n = 895) were 26.8% and 19.8%, respectively (Table 1). COVID-19-related 30-day mortality was numerically higher in the first compared with the second wave of the pandemic, though this observed difference did not reach statistical significance (22.1% versus 17.1%, P = 0.13). In multivariate analysis, primary tumour type did not influence COVID-19-related mortality (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100610). However, a trend for lower mortality in patients with breast cancer compared with patients with other solid cancers (13.0% versus 23.3%, P = 0.058), and higher mortality for patients with lung cancer (27.6% versus 20.8%, P = 0.074) were seen. Patients under active treatment in the non-curative setting had higher 30-day COVID-19-related mortality rates in comparison to patients not receiving active treatment or those patients receiving active cancer treatment in the curative setting (25.8% versus 21.9% versus 14.3%, respectively, for each comparison, P < 0.001) (Figure 1, Table 2).
Table 1.
All-cause and COVID-related mortality at 30 days and 3 months overall and according to time of COVID-19 diagnosis
|
Variable |
Total n/N (%) | Wave 1 n/N (%) | Wave 2 n/N (%) | P value |
|---|---|---|---|---|
| 30-Day mortality | ||||
| Alive | 655/895 (73.2) | 345/485 (71.1) | 310/410 (75.6) | 0.175 |
| COVID-related death | 177/895 (19.8) | 107/485 (22.1) | 70/410 (17.1) | |
| Non-COVID-related death | 63/895 (7.0) | 33/485 (6.8) | 30/410 (7.3) | |
| 3-Month mortality | ||||
| Alive | 571/875 (65.3) | 308/475 (64.8) | 263/400 (65.8) | 0.600 |
| COVID-related death | 191/875 (21.8) | 109/475 (23.0) | 82/400 (20.5) | |
| Non-COVID-related death | 113/875 (12.9) | 58/475 (12.2) | 55/400 (13.8) |
Variables were analyzed using a Chi-square test. The reported P values are for the comparison of wave 1 versus wave 2 and are two-sided.
Figure 1.
Mortality in function of oncological treatment setting at time of COVID-19 diagnosis. (A) 3-Month mortality according to oncological treatment setting at time of COVID-19 diagnosis. (B) Kaplan–Meier survival estimates for COVID-19-related survival according to oncological treatment setting at time of COVID-19 diagnosis. <3 months: ‘in the 3 months before COVID-19 diagnosis’. P values (Chi-square test) for % COVID-19-related survival at 3 months: no active treatment <3 months versus curative treatment P = 0.117; no active treatment versus non-curative treatment P < 0.001; curative treatment versus non-curative treatment P < 0.001.
Table 2.
All-cause and COVID-related mortality at 3 months according to treatment setting at time of COVID-19 diagnosis
|
Variable |
No active treatment (A) n/N (%) | Non-curative treatment (B) n/N (%) | Curative treatment (C) n/N (%) | P value | |||
|---|---|---|---|---|---|---|---|
| 3-Month mortality | Overall | A versus B | A versus C | B versus C | |||
| Alive | 307/439 (69.9) | 140/275 (50.9) | 115/147 (78.2) | <0.001 | <0.001 | 0.117 | <0.001 |
| COVID-related death | 96/439 (21.9) | 71/275 (25.8) | 21/147 (14.3) | ||||
| Non-COVID-related death | 36/439 (8.2) | 64/275 (23.3) | 11/147 (7.5) | ||||
Active treatment defined as having received any type of anticancer treatment in the 3 months before COVID-19 diagnosis. Variables were analyzed using a Chi-square test. The reported P values are two-sided.
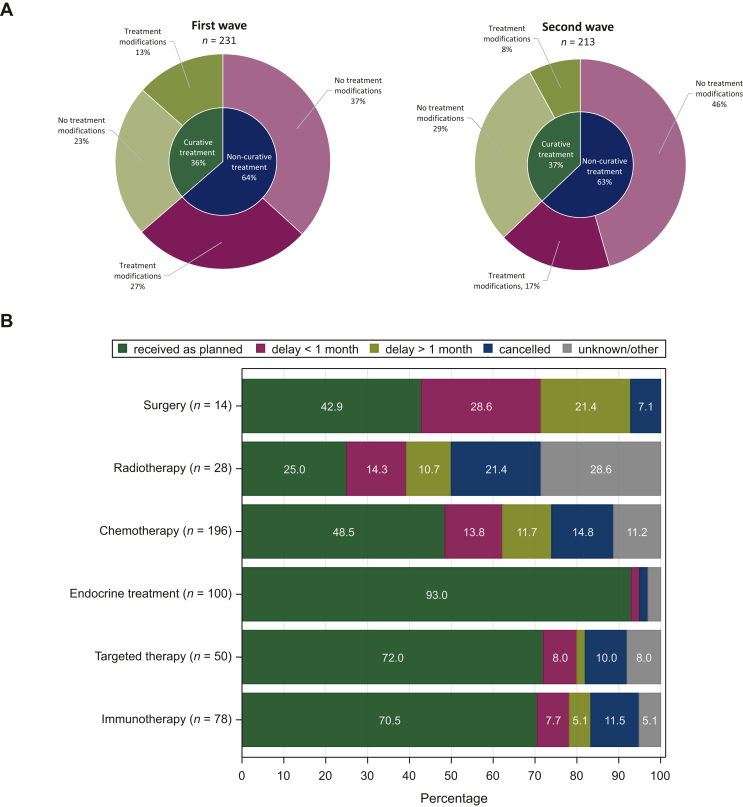
Of patients under active anticancer treatment, the anticancer treatment plan was modified due to COVID-19 diagnosis in about one-third of patients, both in the non-curative [34.9% (99/284)] and the curative [29.5% (48/163)] treatment setting (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100610). The number of treatment changes was higher in the first wave compared with the second [39.7% (93/234) versus 25.4% (54/213), P < 0.001] (Figure 2). Most frequent treatment changes were delay/interruption (20.1% and 16.0% for non-curative and curative setting, respectively) and full cancellation (10.6% and 6.1% for non-curative and curative setting, respectively) of systemic treatment. Within the subgroup of patients receiving chemotherapy at the time of COVID-19 diagnosis, almost half of them experienced changes in their chemotherapy treatment plan (93/195, 47.7%). This involved mainly delays/interruptions due to COVID-19 diagnosis (n = 62), with interruptions for ≥1 month in almost half of all patients where chemotherapy delay time was known, and even full cancellation in 29 patients. For patients already under systemic treatment in the curative setting at the time of COVID-19 diagnosis, cancellation of systemic treatment was observed in only 9.3% (10/108). Fourteen patients under active treatment were planned to undergo surgery, whereas only six received it as planned. Seventy-eight patients were receiving immunotherapy at the time of COVID-19 diagnosis, and this treatment was interrupted in 10 and cancelled in 9. The main reasons for changes in treatment plan were COVID-19-related complications, followed by fear for/existence of anticancer treatment-related toxicity such as neutropenia (in 77.6% and 14.3% of patients with treatment changes, respectively). Neither 30-day COVID-19-related mortality nor 3-month non-COVID-19-related mortality were significantly different between patients with and without treatment modifications (21.4% versus 20.5%, P = 0.18 and 18.8% versus 17.0%, P = 0.89, respectively) (Table 3).
Figure 2.
Treatment modificationsaccording to timing of COVID-19 diagnosis, treatment setting and type of treatment planned. (A) Treatment modifications according to timing of COVID-19 diagnosis. First wave: 1 March 2020-30 June 2020; second wave: 1 July 2020-1 February 2021. Three patients from the first wave with unknown treatment modification status were excluded from this graph. (B) Treatment modifications according to anticancer treatment type for patients under active anticancer treatment (any type of anticancer treatment received in the 3 months before COVID-19 diagnosis).
Table 3.
Comparative analysis of all-cause and COVID-related mortality according to anticancer treatment modifications at time of COVID-19 diagnosis
|
Variable |
No treatment modifications n/N (%) | Any treatment modifications n/N (%) | P value |
|---|---|---|---|
| 30-Day mortality | |||
| Alive | 194/283 (68.6) | 106/145 (73.1) | 0.180 |
| COVID-related death | 58/283 (20.5) | 31/145 (21.4) | |
| Non-COVID-related death | 31/283 (11.0) | 8/145 (5.5) | |
| 3-Month mortality | |||
| Alive | 169/276 (61.2) | 85/144 (59.0) | 0.885 |
| COVID-related death | 60/276 (21.7) | 32/144 (22.2) | |
| Non-COVID-related death | 47/276 (17.0) | 27/144 (18.8) |
Variables were analyzed using a Chi-square test. The reported P values are two-sided.
Discussion
The impact of COVID-19 on patients with cancer and their treatments has been ubiquitous and unprecedented. Knowledge on how the disease course is influenced by the patients’ oncological setting is needed to guide treatment decisions; however, patients with malignancies have largely been excluded from prospective clinical trials in this setting. We thus rely on retrospective and real-world data analysis to learn for the future. The BSMO-COVID database is a large (∼1000 patients) nationwide database of patients with solid cancer hospitalized with COVID-19, set up to help answer some of the remaining questions on this topic. To enhance the leverage of our database, our data were transferred to the ESMO CoCare initiative on 12 April 2022. While prognostic analyses presented in this paper are thus limited, the results from analyses on that merged dataset will be reported separately.
The European OnCovid and American Society of Clinical Oncology (ASCO) registries, including patients with a history of solid or haematological cancer and a diagnosis of SARS-CoV-2 infection, already reported major differences in mortality rates between what we here defined as the first and second waves of the pandemic.13,14 The same trend was seen in our hospitalized population, likely reflecting an improvement in diagnosis and management of patients with COVID-19, thanks to the increasing knowledge on the disease. Our database only covered patients hospitalized up until February 2021, expected to be infected mainly with alpha and delta variants of the SARS-CoV-2 virus. We assume mortality rates will have continued to evolve favourably for patients with cancer, as the omicron variant has become dominant later and vaccines have become available to most of our patients (primary course vaccination campaign in Belgium started in the first quarter of 2021, with coverage in adults now being 89%).15 Of note, our database included mostly patients with poor ECOG performance scores and many of older age, as would be expected in a population hospitalized mainly because of their clinical status upon infection, so mortality rates should not be generalized to ambulatory and fitter patients.
Many early reports and guidelines have focused on delivering cancer care in challenging pandemic times.16, 17, 18, 19, 20, 21, 22 The goal of these guidelines was to maintain high-quality oncological management while mitigating the risk of hospital-acquired SARS-CoV-2 infection for both patients and caregivers. Our focus was different, as for patients under active anticancer treatment who do have COVID-19, no clear guidelines for treatment modifications exist, but mostly expert recommendations. Although many patients experienced delays or even cancellations of their anticancer treatment plans, no significant difference in 30-day COVID-19-related mortality was observed between those with and without treatment changes in the population included in our study. This is in line with the observations in the OnCovid dataset, where only permanent treatment discontinuation resulted in increased risk of medium-term death, but regimen adjustments did not.23 In contrast with our database, OnCovid also included ambulatory patients and haematological patients, and did not look at COVID-19-related mortality separately. Although treatment delays thus did not reduce COVID-19 mortality in our study, it is expected and modelled by others that these delays could result in a late increase in cancer-related mortality.24 Our findings are therefore reassuring for the future, highlighting that treatment discontinuations upon COVID-19 diagnosis should be avoided where possible. This is of utmost relevance for the subsets of patients where cancer diagnosis influences their long-term prognosis, but not necessarily their COVID-19-related mortality. Factors to take into account in assessing that risk balance are the following: firstly, for most primary solid tumour types excluding lung cancer, no association has been proven with increased COVID-19-related mortality.3 Secondly, some non-immune-modifying treatment regimens, such as endocrine treatment, have not been shown to influence COVID-19 outcomes.3 Thirdly, seroconversion rates after SARS-CoV-2 vaccination tend to be lower in patients with solid cancer compared with the general population.10 Lower vaccine efficacy is thought, however, to be restricted to only some subgroups of oncology patients. Although these subgroups are not yet well defined, rapidly advancing knowledge might teach us which patients are as protected against severe COVID-19 disease course as patients without a malignancy after full vaccination. And lastly, for patients in the curative setting long treatment discontinuation or even cancellation might jeopardize their chances of cure and impact long-term outcomes.
Conclusion
Our large nationwide database of patients with solid cancer hospitalized with COVID-19 during the first two waves of the pandemic in Belgium highlights that discontinuation of anticancer treatment at the time of COVID-19 diagnosis is not associated with reduced COVID-related mortality rates. Continuation of anticancer therapy while infected by SARS-CoV-2 should thus be strived for in patients not severely ill from and not likely to die of COVID-19, in order not to compromise long-term oncological outcomes.
Acknowledgements
The authors acknowledge all participating institutions and their ethics committees; all health care professionals who completed the information in the central electronic case report form; the Belgian Society of Medical Oncology Executive Board for their full support of this research; and the Belgian National Health Institution Sciensano for providing the required data back to the institutions. This study was an investigator-initiated trial conducted by the Belgian Society of Medical Oncology as legal sponsor, independently of the funding sources.
Funding
This work was supported by Pfizer, Bristol Myers SquibbCompany (BMS), Roche, Amgen, Astellas Pharma, Eli Lilly and Company, Novartis, Pierre Fabre and Leo Pharma (no grant numbers).
Role of the funder
The funders of the study had no role in the design and conduct of the study, data collection, data management, statistical analysis and interpretation of the data, the preparation, review or approval of the manuscript and the decision to submit the manuscript for publication.
Disclosure
MB: speaker honoraria and travel grants from Roche/GNE; research grants to her institution from Roche/GNE, AstraZeneca, GlaxoSmithKline (GSK)/Novartis and Servier. JC: advisory board and lectures from Amgen, Servier, Bayer, Novartis, Pfizer, Celgen, Ipsen (paid to institution); travel grants from Roche, Pfizer, Amgen, Novartis. CVM: consulting fees from Chrysalis Biomed (paid to institution); support for attending meetings and/or travel from Pfizer, Roche, Merck; participation of institution on advisory board for Eli Lilly and AstraZeneca. WD: payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Merck, Pfizer. CF: support for attending meetings and/or travel from Eli Lilly, Gilead, PharmaMar, Pfizer, Roche; participation on advisory board for Eli Lilly, GSK. SA: consulting fees from Galapagos; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Merck Sharp & Dohme (MSD), Sanofi, Roche, Bristol Myers Squibb (BMS); support for attending meetings and/or travel from MSD, Sanofi, Roche, BMS, Pfizer; participation on advisory board from MSD, Sanofi, Roche, BMS; receipt of equipment, materials, drugs, medical writing, gifts or other services from BMS. HVDB: participation on advisory board from AstraZeneca. MI: grants from Roche, Pfizer, Natera Inc. (paid to institution); consulting fees from Novartis; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis; travel grant from Roche; participation on advisory board from Seattle Genetics. KP: speaker fees and/or honoraria for consultancy or advisory functions from AstraZeneca, Eli Lilly, Medscape/Exact Sciences, Focus Patient, Gilead Sciences, MSD, Novartis, Roche and Seagen; travel support from AstraZeneca, Novartis, Pfizer, PharmaMar, Roche; speaker fees and/or honoraria for consultancy or advisory functions from AstraZeneca, Eli Lilly, Gilead Sciences, Medscape/Genomic Health, MSD, Mundipharma, Novartis, Pfizer, Pierre Fabre, Roche, Seagen, Teva and Vifor Pharma (paid to institution); research funding from Sanofi and MSD (paid to institution). EdA: honoraria and/or advisory board from Roche/GNE, Novartis, Seagen, Zodiac, Libbs, Pierre Fabre, Lilly, AstraZeneca; travel grants from Roche/GNE and AstraZeneca; research grant from Roche/GNE, AstraZeneca, GSK/Novartis and Servier (paid to institution). All other authors have declared no conflicts of interest.
Patient consent for publication
As only retrospective data from the patients’ medical files were collected by the principal investigator from the site where the patient was hospitalized, with no direct impact on patients’ care or well-being and as the data were pseudoanonymized, the investigators of the present study received a waiver for the need to obtain informed consent from individual patients from the Independent Ethical Review Committee/Institutional Review Board of each participating institution.
Ethics approval
This study was approved by the Independent Ethical Review Committee/Institutional Review Board of each participating institution.
Supplementary data
References
- 1.Alom S., Chiu C.M., Jha A., et al. The effects of COVID-19 on cancer care provision: a systematic review. Cancer Control. 2021;28 doi: 10.1177/1073274821997425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maringe C., Spicer J., Morris M., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elkrief A., Wu J.T., Jani C., et al. Learning through a pandemic: the current state of knowledge on COVID-19 and cancer. Cancer Discov. 2022;12:303–330. doi: 10.1158/2159-8290.CD-21-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grivas P., Khaki A.R., Wise-Draper T.M., et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann Oncol. 2021;32:787–800. doi: 10.1016/j.annonc.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lièvre A., Turpin A., Ray-Coquard I., et al. Risk factors for Coronavirus Disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: A French nationwide cohort study (GCO-002 CACOVID-19) Eur J Cancer. 2020;141:62–81. doi: 10.1016/j.ejca.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.TERAVOLT investigators, Garassino M.C., Whisenant J.G., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Joode K., Dumoulin D.W., Tol J., et al. Dutch oncology COVID-19 consortium: outcome of COVID-19 in patients with cancer in a nationwide cohort study. Eur J Cancer. 2020;141:171–184. doi: 10.1016/j.ejca.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nader Marta G., Bonadio R.C., Sejas O.N.E., et al. Outcomes and prognostic factors in a large cohort of hospitalized cancer patients with COVID-19. JCO Glob Oncol. 2021;7:1084–1092. doi: 10.1200/GO.21.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fendler A., de Vries E.G.E., GeurtsvanKessel C.H., et al. COVID-19 vaccines in patients with cancer: immunogenicity, efficacy and safety. Nat Rev Clin Oncol. 2022;19:385–401. doi: 10.1038/s41571-022-00610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Azambuja E., Brandão M., Wildiers H., et al. Impact of solid cancer on in-hospital mortality overall and among different subgroups of patients with COVID-19: a nationwide, population-based analysis. ESMO Open. 2020;5 doi: 10.1136/esmoopen-2020-000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Goethem N., Vilain A., Wyndham-Thomas C., et al. Rapid establishment of a national surveillance of COVID-19 hospitalizations in Belgium. Arch Public Heal. 2020;78:1–10. doi: 10.1186/s13690-020-00505-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.OnCovid Study Group. Pinato D.J., Patel M., et al. Time-dependent COVID-19 mortality in patients with cancer: an updated analysis of the OnCovid registry. JAMA Oncol. 2022;8:114–122. doi: 10.1001/jamaoncol.2021.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mileham K.F., Bruinooge S.S., Aggarwal C., et al. Changes over time in COVID-19 severity and mortality in patients undergoing cancer treatment in the United States: initial report from the ASCO registry. JCO Oncol Pract. 2022;18:e426–e441. doi: 10.1200/OP.21.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epistat – COVID-19 Monitoring. https://epistat.sciensano.be/covid/ Available at.
- 16.Ferro A., Cristofolini P., Garcia-Etienne C.A., et al. Learning from organisational changes in the management of breast cancer patients during the COVID-19 pandemic: preparing for a second wave at a breast unit in northern Italy. Int J Health Plann Manage. 2021;36:1030–1037. doi: 10.1002/hpm.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yashavantha Rao H.C., Siddeeqh S., Taqui S.N. Caring for cancer patients during COVID-19 global pandemic: risk factors and precautionary principle. Int J Clin Pract. 2021;75 doi: 10.1111/ijcp.14141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liontos M., Kastritis E., Markellos C., et al. Continuing cancer therapy through the pandemic while protecting our patients: results of the implementation of preventive strategies in a referral oncology unit. Cancers (Basel) 2021;13:1–10. doi: 10.3390/cancers13040763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chazan G., Franchini F., Alexander M., et al. Impact of COVID-19 on cancer service delivery: results from an international survey of oncology clinicians. ESMO Open. 2020;5 doi: 10.1136/esmoopen-2020-001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crusz S.M., Hall P.E., Earwicker K., et al. Providing an acute oncology service during the COVID-19 pandemic. Clin Med. 2021;21:E548–E551. doi: 10.7861/clinmed.2020-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark J.J., Dwyer D., Pinwill N., Clark P., Johnson P., Hackshaw A. The effect of clinical decision making for initiation of systemic anticancer treatments in response to the COVID-19 pandemic in England: a retrospective analysis. Lancet Oncol. 2021;22:66–73. doi: 10.1016/S1470-2045(20)30619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Azambuja E., Trapani D., Loibl S., et al. ESMO Management and treatment adapted recommendations in the COVID-19 era: breast cancer. ESMO Open. 2020;5 doi: 10.1136/esmoopen-2020-000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinato D.J., Tabernero J., Bower M., et al. Prevalence and impact of COVID-19 sequelae on treatment and survival of patients with cancer who recovered from SARS-CoV-2 infection: evidence from the OnCovid retrospective, multicentre registry study. Lancet Oncol. 2021;22:1669–1680. doi: 10.1016/S1470-2045(21)00573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bardet A., Fraslin A.M., Marghadi J., et al. Impact of COVID-19 on healthcare organisation and cancer outcomes. Eur J Cancer. 2021;153:123–132. doi: 10.1016/j.ejca.2021.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.