Abstract
Background
Switching to dolutegravir/lamivudine (DTG/3TC) was noninferior to continuing tenofovir alafenamide (TAF)–based regimens for maintaining virologic suppression at week 48 of the TANGO study. Here we present week 144 outcomes (efficacy, safety, weight, and biomarkers).
Methods
TANGO is a randomized (1:1, stratified by baseline third agent class), open-label, noninferiority phase 3 study. Virologically suppressed (>6 months) adults with human immunodeficiency virus type 1 (HIV-1) switched to once-daily DTG/3TC or continued TAF-based regimens.
Results
A total of 741 participants received study treatment (DTG/3TC, n = 369; TAF-based regimen, n = 372). At week 144, the proportion of participants with an HIV-1 RNA level ≥50 copies/mL (primary end point, Snapshot; intention-to-treat–exposed population) after switching to DTG/3TC was 0.3% (1 of 369) versus 1.3% (5 of 372) for those continuing TAF-based regimens, demonstrating noninferiority (adjusted treatment difference, −1.1 [95% confidence interval, −2.4 to .2), with DTG/3TC favored in the per-protocol analysis (adjusted treatment difference, −1.1 [−2.3 to −.0]; P = .04). Few participants met confirmed virologic withdrawal criteria (none in the DTG/3TC and 3 in the TAF-based regimen group), with no resistance observed. Drug-related adverse events were more frequent with DTG/3TC (15%; leading to discontinuation in 4%) than TAF-based regimens (5%; leading to discontinuation in 1%) through week 144, but rates were comparable after week 48 (4%; leading to discontinuation in 1% in both groups). Changes from baseline in lipid values generally favored DTG/3TC; no clinical impact on renal function and comparable changes in inflammatory and bone biomarkers across groups were observed.
Conclusions
Switching to DTG/3TC demonstrated noninferior and durable efficacy compared with continuing TAF-based regimens in treatment-experienced adults with HIV-1, with good safety and tolerability, and no resistance through 144 weeks.
Keywords: durable, 2-drug regimen, dolutegravir/lamivudine, integrase strand transfer inhibitor, treatment-experienced
Switching to dolutegravir/lamivudine (DTG/3TC), versus continuing tenofovir alafenamide–based regimens, showed durable efficacy and safety through 144 weeks, with no virologic failure or resistance detected, supporting the use of DTG/3TC in virologically suppressed individuals with human immunodeficiency virus type 1.
Because individuals with human immunodeficiency virus (HIV) require lifelong therapy, a need for effective treatments with limited long-term toxicity remains [1]. Two-drug regimens reduce the number of antiretroviral agents in a regimen and may reduce treatment-associated toxicity, drug-drug interactions, and costs [1].
International guidelines, including the 2020 International Antiretroviral Society–USA, 2021 Department of Health and Human Services, and 2021 European AIDS Clinical Society guidelines, recommend the 2-drug regimen dolutegravir/lamivudine (DTG/3TC) as an initial treatment regimen based on results from phase 3 GEMINI-1/GEMINI-2 studies [2–7]. DTG/3TC is also recommended in switch settings [2], supported by 48-week results from the phase 3 SALSA and TANGO studies [8, 9]. In SALSA, the proportion of participants with HIV type 1 (HIV-1) RNA levels ≥50 copies/mL (primary end point, US Food and Drug Administration [FDA] Snapshot algorithm) switching to single-tablet fixed-dose combination DTG/3TC was noninferior to continuing the current antiretroviral regimen in virologically suppressed individuals [8]. In TANGO, switching to DTG/3TC was noninferior to continuing tenofovir alafenamide (TAF)–based 3- or 4-drug regimens, with <1% of participants in both groups having HIV-1 RNA levels ≥50 copies/mL at week 48 [9]. In both groups, 93% of participants had HIV-1 RNA levels <50 copies/mL (Snapshot), with no confirmed virologic withdrawals (CVWs) observed in the DTG/3TC group [9]. Safety profiles were comparable between groups, with lipid profile generally favoring DTG/3TC [9]. Here we report longer-term results from the planned secondary 96-week and 144-week analyses of TANGO.
METHODS
Study Design
TANGO (ClinicalTrials.gov; NCT03446573) is an ongoing, phase 3, randomized, open-label, noninferiority study of the efficacy and safety of switching to DTG/3TC versus continuing TAF-based regimens in virologically suppressed adults with HIV-1. Before the first participant reached the 48-week visit, the protocol was amended to extend the randomized phase through week 148, with additional predefined secondary end points at weeks 96 and 144. Methods have been published elsewhere and are briefly described below (Supplementary Methods) [9]. Adults with virologic suppression (HIV-1 RNA <50 copies/mL) on a first-line TAF-based regimen for >6 months were eligible. Switching from tenofovir disoproxil fumarate to TAF ≥3 months before study entry was allowed, as well as switching pharmacokinetic enhancers ritonavir and cobicistat. Participants were ineligible if they had evidence of major nucleoside reverse-transcriptase inhibitor or any integrase strand transfer inhibitor resistance–associated mutations in historical genotypic results, as provided by investigators (any locally validated commercial or noncommercial assay, including sites’ local laboratory home-brewed assays, were considered); any plasma HIV-1 RNA measurement ≥50 copies/mL within 6 months of screening; ≥2 measurements ≥50 copies/mL or any measurement >200 copies/mL within 6 and 12 months of screening; or a prior regimen switch for virologic failure (HIV-1 RNA ≥400 copies/mL).
Procedures
After a ≤28-day screening period, eligible participants were randomized 1:1 to switch to a once-daily DTG (50 mg)/3TC (300 mg) fixed-dose combination or continue their TAF-based regimen. Randomization was stratified by baseline third agent class. No regimen modifications were allowed, except switching between ritonavir and cobicistat. Study visits were planned at baseline; at weeks 4, 8, and 12; and every 12 weeks thereafter until week 144. Plasma for HIV-1 RNA quantification was collected at each visit and at study withdrawal and analyzed using the Abbott RealTime HIV-1 assay (lower detection limit, 40 copies/mL). For measurements of plasma HIV-1 RNA <40 copies/mL, qualitatively observable results were denoted as “target detected,” and measurements not qualitatively observable as “target not detected.” Safety outcomes were assessed at each study visit through the last participant’s week 144 data cutoff (Supplementary Methods).
Outcomes
The primary end point was the proportion of participants with HIV-1 RNA ≥50 copies/mL at week 48, using the FDA Snapshot algorithm in the intention-to-treat–exposed (ITT-E) population. Secondary analyses through weeks 96 and 144 included the proportion of participants with HIV-1 RNA levels ≥50 and <50 copies/mL (FDA Snapshot; ITT-E) and the incidence of genotypic or phenotypic resistance in participants meeting CVW criteria. We also assessed the following through week 144: immune effects; safety; CVW incidence; weight; weight intervention event (based on source medical notes with affirmative response to “Was this patient advised/referred for dietetic counseling/weight management program including exercise?”); renal, bone, and inflammatory biomarker values; and lipid values.
Statistical Analysis
All randomized participants who received ≥1 dose of treatment were included in the ITT-E population and used for efficacy and safety analyses unless incorrect treatment or no study treatment was received. The per-protocol population included participants with no significant protocol deviations. The week 96 and 144 efficacy evaluable populations excluded participants without Snapshot data because of the coronavirus disease 2019 (COVID-19) pandemic (ie, those who discontinued or were unable to attend a site visit because of the pandemic).
The statistical analysis plan prespecified testing for noninferiority (and superiority only if noninferiority was demonstrated for the end point of HIV-1 RNA levels ≥50 copies/mL) at predefined secondary analysis time points (weeks 96 and 144). The proportions of participants with HIV-1 RNA ≥50 and <50 copies/mL (Snapshot) were analyzed using a Cochran-Mantel-Haenszel test, with adjustment for baseline third agent class. The noninferiority margin for the adjusted difference in proportion of participants with HIV-1 RNA levels ≥50 copies/mL was 4% at both time points. Sensitivity analyses assessing proportions of participants in the per-protocol and efficacy evaluable populations with HIV-1 RNA levels ≥50 copies/mL (Snapshot) were performed. The incidence and severity of adverse events (AEs) were summarized descriptively. The median changes in CD4+ cell count and CD4+/CD8+ cell count ratio at week 144 were assessed. In each group, the adjusted mean change from baseline (or geometric mean ratios of study week to baseline for log-transformed end points) was estimated for the following: weight; body mass index (BMI); renal, bone, and inflammatory biomarkers; homeostasis model of assessment–insulin resistance (HOMA-IR); and fasting lipid values; estimates were done using mixed-model repeated measures (Supplementary Methods).
RESULTS
Participants
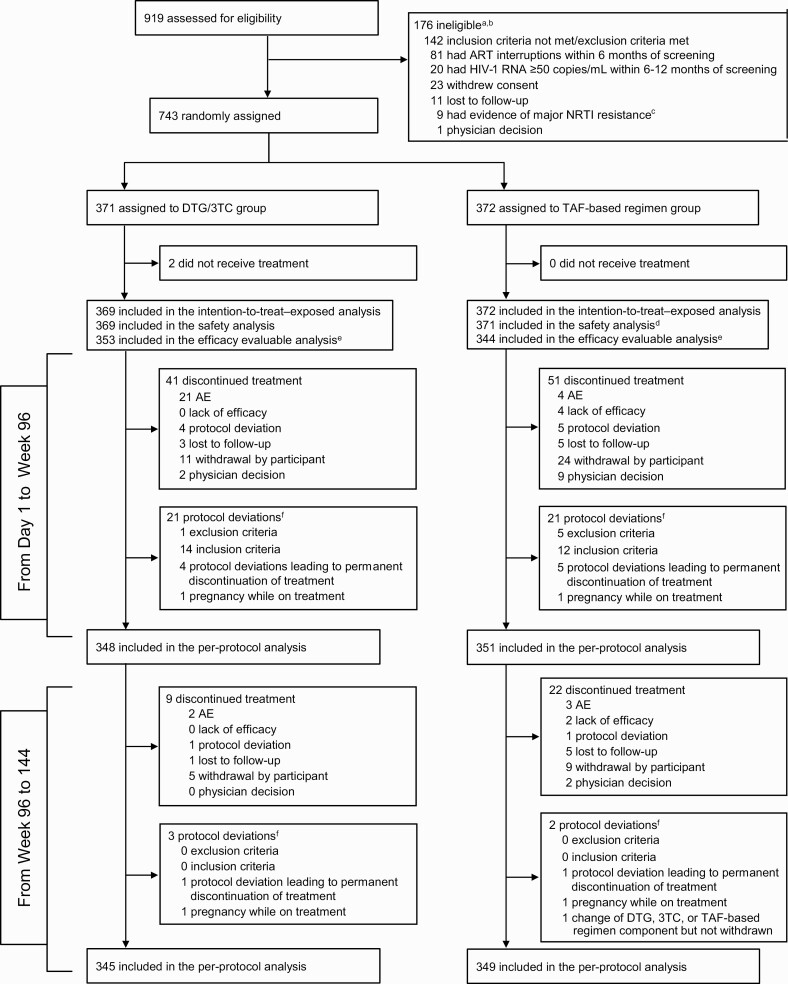
Participant screening began on 1 January 2018. The last-participant-last-visit date for the week 144 analysis was 19 April 2021. Of 919 participants screened, 743 were randomized to switch to DTG/3TC (n = 371) or continue TAF-based regimens (n = 372) (Figure 1); 741 received study treatment (ITT-E). Baseline and disease characteristics were balanced between groups in the ITT-E population (Table 1) [9].
Figure 1.
Trial profile. aParticipants could have multiple reasons for ineligibility. bThe most common reasons for not meeting inclusion criteria or meeting exclusion criteria are listed. All other reasons occurred in <1% of participants. cOf 919 screened participants, 543 (59%) had historical genotypic reports, 9 (2%) of whom were excluded because of major nucleoside reverse-transcriptase inhibitor (NRTI) resistance: 1 had M41L and D67N, 2 had M41L, and the remaining 6 each had a single mutation identified as M184I, K65R, K219E, K219Q, D67N, or L210W (post hoc analysis). dOne participant in the tenofovir alafenamide (TAF)–based regimen group was found to be taking a tenofovir disoproxil fumarate–based regimen at baseline and therefore was excluded from the safety population. eForty-four participants had missing week 96 human immunodeficiency virus type 1 (HIV-1) RNA data owing to the coronavirus disease 2019 pandemic (ie, discontinued or unable to attend week 96 site visit because of the pandemic). fProtocol deviations leading to exclusion from the per-protocol population; participants could have had ≥1 reason. Abbreviations: AEs, adverse events; ART, antiretroviral therapy; DTG/3TC, dolutegravir/lamivudine.
Table 1.
Baseline Demographics and Clinical Characteristics in the Intention-to-Treat–Exposed Populationa
| Demographic/Characteristic | DTG/3TC (n = 369) | TAF-Based Regimen (n = 372) |
|---|---|---|
| Age | ||
| Median (range), y | 40.0 (20–74) | 39.0 (18–73) |
| <50 y, no. (%) | 290 (79) | 280 (75) |
| Sex, no. (%) | ||
| Female | 25 (7) | 33 (9) |
| Male | 344 (93) | 339 (91) |
| Race, no. (%) | ||
| White | 297 (80) | 289 (78) |
| Black or African American | 50 (14) | 58 (16) |
| Asian | 13 (4) | 13 (3) |
| Other | 9 (2) | 12 (3) |
| Ethnicity, no. (%) | ||
| Hispanic or Latino | 69 (19) | 66 (18) |
| Not Hispanic or Latino | 300 (81) | 306 (82) |
| CD4+ cell count, median (range), cells/µL | 682 (133–1904) | 720 (119–1810) |
| CD4+ cell count, no. (%) | ||
| <500/µL | 98 (27) | 74 (20) |
| ≥500/µL | 271 (73) | 298 (80) |
| Baseline third agent class, no. (%) | ||
| INSTI | 289 (78) | 296 (80) |
| Elvitegravir/cobicistat | 243 (66) | 249 (67) |
| NNRTI | 51 (14) | 48 (13) |
| Rilpivirine | 43 (12) | 45 (12) |
| PI | 29 (8) | 28 (8) |
| Boosted darunavir | 25 (7) | 27 (7) |
| Historical genotypic resistance results available at screening, no (%)b | 221 (60) | 243 (65) |
| Duration of treatment before d 1, median (range), mo | ||
| ART | 33.8 (7.1–201.2) | 35.1 (7.0–160.8) |
| TAF-based regimen | 17.7 (3.6–73.7) | 18.2 (3.9–71.2) |
Abbreviations: ART, antiretroviral therapy; DTG/3TC, dolutegravir/lamivudine; INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; TAF, tenofovir alafenamide.
See van Wyk et al [9] for details.
Historical resistance results (post hoc analysis) provided at screening were not recorded in the electronic case report form nor were they part of the locked database but are data on file that have been source verified and archived in the study trial master file.
Efficacy
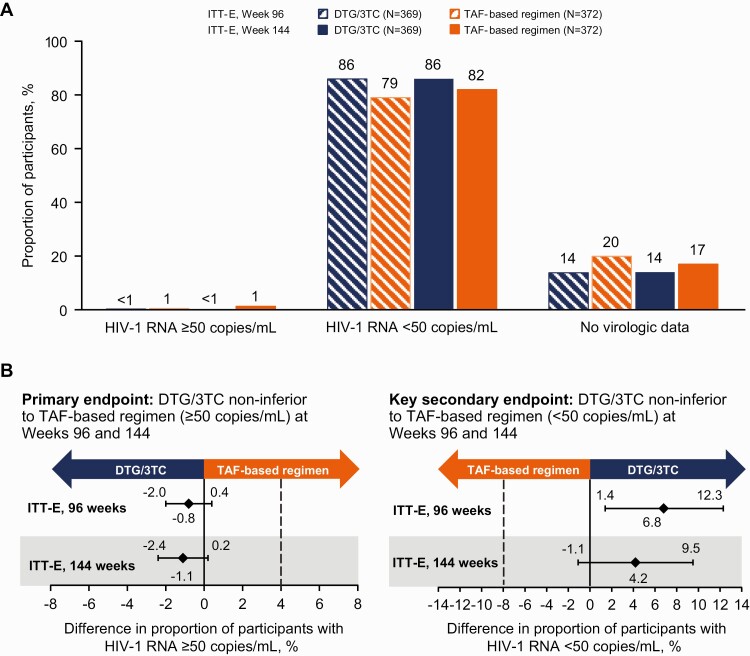
At week 144, 1 (0.3%) participant in the DTG/3TC group and 5 (1.3%) in the TAF-based regimen group had HIV-1 RNA levels ≥50 copies/mL (Snapshot; ITT-E), demonstrating continued noninferior efficacy through 144 weeks (adjusted treatment difference, −1.1 [95% confidence interval (CI), −2.4 to .2]) (Table 2 and Figure 2). The proportions of participants with HIV-1 RNA levels ≥50 copies/mL (Snapshot; ITT-E) were similar at week 96 (DTG/3TC, 1 of 369 [0.3%]; TAF-based regimen, 4 of 372 [1.1%]; adjusted treatment difference, −0.8 [95% CI, −2.0 to .4]). In the per-protocol population, no participants in the DTG/3TC group and 4 (1.1%) in the TAF-based regimen group had HIV-1 RNA levels ≥50 copies/mL at week 144, demonstrating superiority of DTG/3TC compared with TAF-based regimens (adjusted treatment difference, −1.1 [95% CI, −2.3 to −.03085]; P = .04). Superiority was also demonstrated in the per-protocol population at week 96 (DTG/3TC, 0 of 348; TAF-based regimen, 4 of 351 [1%]; adjusted treatment difference, −1.1 [95% CI, −2.3 to −.0298]; P = .04).
Table 2.
Summary of Study Outcomes by Human Immunodeficiency Virus Type 1 RNA Level (≥50 or <50 Copies/mL) at Weeks 96 and 144: Snapshot Analysis of Intention-to-Treat–Exposed and Evaluable Efficacy Populations
| Outcome | Participants at wk 96, No. (%) | Participants at wk 144, No. (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| ITT-E | Efficacy Data Evaluablea | ITT-E | Efficacy Data Evaluableb | |||||
| DTG/3TC (n = 369) | TAF-Based Regimen (n = 372) | DTG/3TC (n = 353) | TAF-Based Regimen (n = 344) | DTG/3TC (n = 369) | TAF-Based Regimen (n = 372) | DTG/3TC (n = 364) | TAF-Based Regimen (n = 370) | |
| HIV-1 RNA <50 copies/mL | 317 (86) | 294 (79) | 317 (90) | 294 (85) | 317 (86) | 304 (82) | 317 (87) | 304 (82) |
| HIV-1 RNA ≥50 copies/mL | 1 (<1) | 4 (1) | 1 (<1) | 4 (1) | 1 (<1) | 5 (1) | 1 (<1) | 5 (1) |
| Data available during window | 0 | 1 (<1) | 0 | 1 (<1) | 0 | 0 | 0 | 0 |
| Discontinued for lack of efficacy | 0 | 3 (<1) | 0 | 3 (<1) | 0 | 4 (1) | 0 | 4 (1) |
| Discontinued for other reason | 1 (<1) | 0 | 1 (<1) | 0 | 1 (<1) | 0 | 1 (<1) | 0 |
| Change in ART | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (<1) |
| No virologic data | 51 (14) | 74 (20) | 35 (10) | 46 (13) | 51 (14) | 63 (17) | 46 (13) | 61 (16) |
| Non–COVID-19 related | 35 (9) | 46 (12) | 35 (10) | 46 (13) | 46 (12) | 61 (16) | 46 (13) | 61 (16) |
| Discontinued because of AE or deathc | 17 (5) | 4 (1) | 17 (5) | 4 (1) | 23 (6) | 6 (2) | 23 (6) | 6 (2) |
| Discontinued for other reasonsd | 18 (5) | 40 (11) | 18 (5) | 40 (12) | 22 (6) | 55 (15) | 22 (6) | 55 (15) |
| Data missing during window but on study | 0 | 2 (<1) | 0 | 2 (<1) | 1 (<1) | 0 | 1 (<1) | 0 |
| COVID-19 related | 16 (4) | 28 (8) | NA | NA | 5 (1) | 2 (<1) | NA | NA |
| Discontinued because of AE or death | 0 | 0 | NA | NA | 0 | 0 | NA | NA |
| Discontinued for other reasonsd | 0 | 0 | NA | NA | 2 (<1) | 2 (<1) | NA | NA |
| Data missing during window but on study | 16 (4) | 28 (8) | NA | NA | 3 (<1) | 0 | NA | NA |
Abbreviations: AE, adverse event; ART, antiretroviral therapy; COVID-19, coronavirus disease 2019; DTG/3TC, dolutegravir/lamivudine; HIV-1, human immunodeficiency virus type 1; ITT-E, intention-to-treat–exposed; NA, not applicable; TAF, tenofovir alafenamide.
Sensitivity analysis excluded 16 participants in the DTG/3TC and 28 in the TAF-based regimen group because of missing week 96 HIV-1 RNA data, owing to effects of the COVID-19 pandemic.
Sensitivity analysis excluded 5 participants in the DTG/3TC and 2 in the TAF-based regimen group because of missing week 144 HIV-1 RNA data, owing to effects of the COVID-19 pandemic.
Three fatal AEs unrelated to study treatment occurred in the DTG/3TC group, 2 (gunshot wound [homicide] and substance abuse [acute intoxication]) by week 96 and the third (ischemic hepatitis) by week 144.
Other reasons for discontinuation through week 144 included protocol deviation, loss to follow-up, physician decision, withdrawal by participant, and lack of efficacy (in 2 participants in the TAF-based regimen group).
Figure 2.
A, Virologic outcomes at weeks 96 and 144 in the intention-to-treat–exposed (ITT-E) population by the US Food and Drug Administration Snapshot algorithm. B, Adjusted treatment differences (dolutegravir/lamivudine [DTG/3TC] group value − tenofovir alafenamide [TAF] group value), based on Cochran-Mantel-Haenszel stratified analysis, with adjustment for baseline third agent class. Abbreviation: HIV-1, human immunodeficiency virus type 1.
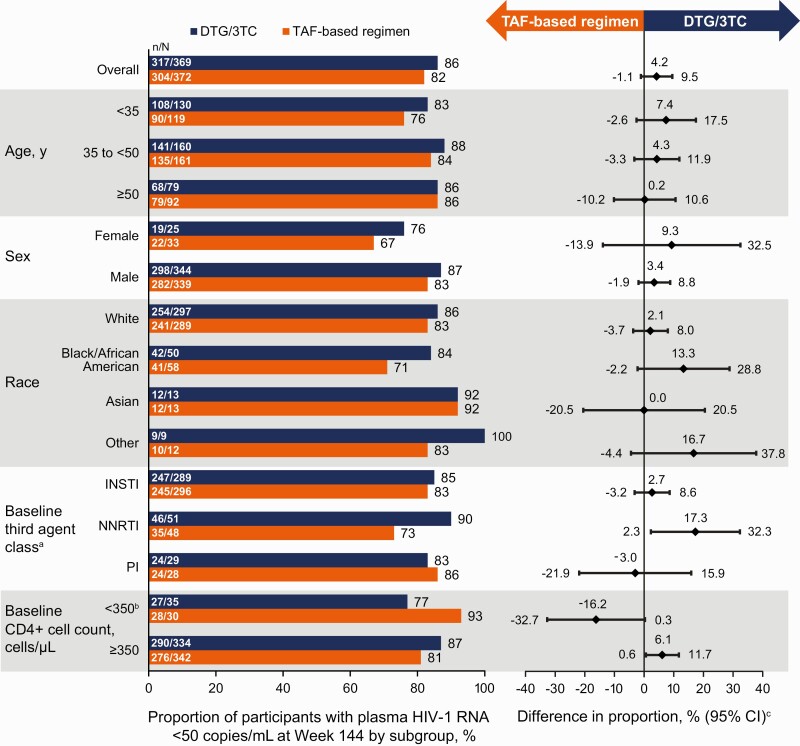
The proportions of participants with Snapshot HIV-1 RNA levels <50 copies/mL were 86% (317 of 369) in the DTG/3TC and 82% (304 of 372) in the TAF-based regimen group (ITT-E population; adjusted treatment difference, 4.2 [95% CI, −1.1 to 9.5]) at week 144. Results from the efficacy evaluable population at weeks 96 and 144 were consistent with the main ITT-E analyses. The proportions of participants with “target not detected” status at week 144 were 76% (279 of 369) in the DTG/3TC group and 72% (267 of 372) in the TAF-based regimen group (ITT-E population; adjusted treatment difference, 3.9 [95% CI, −2.5 to 10.2]) (Supplementary Table 1). The proportions of participants with HIV-1 RNA levels <50 copies/mL (Snapshot; ITT-E) across subgroups at week 144 were generally consistent with overall results (Figure 3).
Figure 3.
Proportion of participants with human immunodeficiency virus type 1 (HIV-1) RNA levels <50 copies/mL by subgroup in the intention-to-treat–exposed population by US Food and Drug Administration Snapshot algorithm at week 144. aThe study population was stratified by baseline third agent class (protease inhibitor [PI], integrase strand transfer inhibitor [INSTI], or nonnucleoside reverse-transcriptase inhibitor [NNRTI]). bIn all 8 Snapshot nonresponders receiving dolutegravir/lamivudine (DTG/3TC) with a baseline CD4+ cell count <350/µL, Snapshot nonresponse occurred for nonvirologic reasons. cAdjusted difference for overall population (DTG/3TC − tenofovir alafenamide [TAF]–based regimen) and 95% confidence intervals (CIs) are based on a stratified analysis (adjusting for baseline third agent class) using Cochran-Mantel-Haenszel weights (meeting noninferiority based on −8% margin); unadjusted differences for subgroups represent proportion on DTG/3TC − proportion on TAF-based regimen.
At week 144, the median change in CD4+ cell count from baseline was 36.0/µL (interquartile range, −64.0/µL to 154.0/µL) in the DTG/3TC group and 35.0/µL (−60.0/µL to 134.0/µL) in the TAF-based regimen group, and the median change in CD4+/CD8+ cell count ratio from baseline was 0.06 (−0.06 to 0.20) in the DTG/3TC group and 0.10 (−0.02 to 0.24) in the TAF-based regimen group.
In the DTG/3TC group, no participants met CVW criteria through week 144. In the TAF-based regimen group, 3 participants (2 since week 48) met CVW criteria through week 144; no resistance mutations were observed. Proviral DNA genotyping was conducted in a post hoc, retrospective analysis based on baseline whole-blood samples (Monogram Biosciences; GenoSure Archive). All 7 participants with archived M184V/I (all detected as mixtures with wild type) at baseline (4 in the DTG/3TC and 3 in the TAF-based regimen group) had postbaseline HIV-1 RNA levels <50 copies/mL at all time points through the last on-study viral load measurement.
Safety
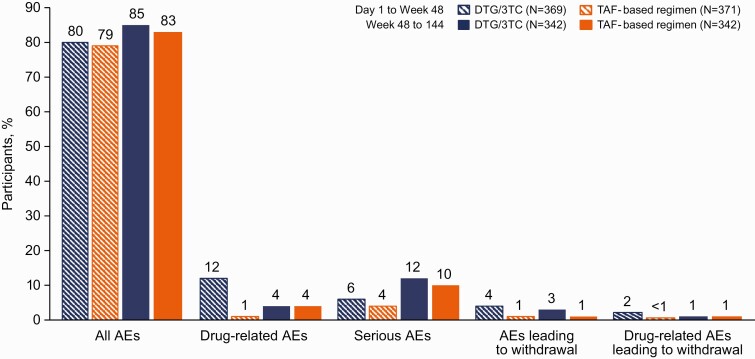
Through week 144, the incidences of ≥1 AE were similar in the 2 groups (DTG/3TC, 91%; TAF-based regimen, 90%) (Table 3). The most frequent AEs were nasopharyngitis, upper respiratory tract infection, diarrhea, and back pain. The incidences of specific AEs (including AEs related to metabolism and nutrition disorders) and serious AEs (SAEs) were comparable between groups; as observed at week 48, the cumulative incidence of drug-related AEs was higher in the DTG/3TC group than in the TAF-based regimen group at week 96 (14% vs 3%, respectively) and week 144 (15% vs 5%, respectively). In the post–week 48 analysis of AEs, rates of all AEs, drug-related AEs, SAEs, and AEs leading to discontinuation were similar between groups (Figure 4).
Table 3.
Summary of Adverse Events in the Safety Population
| Type of AE | Participants at wk 48, No. (%) | Participants at wk 96, No. (%) | Participants at wk 144, No. (%) | |||
|---|---|---|---|---|---|---|
| DTG/3TC (n = 369) | TAF-Based Regimen (n = 371a) | DTG/3TC (n = 369) | TAF-Based Regimen (n = 371a) | DTG/3TC (n = 369) | TAF-Based Regimen (n = 371a) | |
| Any AE | 295 (80) | 292 (79) | 324 (88) | 325 (88) | 336 (91) | 335 (90) |
| AEs occurring in ≥10% of either group | ||||||
| Nasopharyngitis | 43 (12) | 41 (11) | 62 (17) | 61 (16) | 63 (17) | 64 (17) |
| URTI | 31 (8) | 32 (9) | 45 (12) | 43 (12) | 50 (14) | 42 (11) |
| Diarrhea | 30 (8) | 26 (7) | 45 (12) | 38 (10) | 50 (14) | 44 (12) |
| Back pain | 21 (6) | 28 (8) | 32 (9) | 40 (11) | 43 (12) | 49 (13) |
| Syphilis | 24 (7) | 13 (4) | 34 (9) | 26 (7) | 39 (11) | 33 (9) |
| Arthralgia | 12 (3) | 13 (4) | 21 (6) | 22 (6) | 31 (8) | 39 (11) |
| Drug-related AEsb | 45 (12) | 5 (1) | 51 (14) | 12 (3) | 55 (15) | 18 (5) |
| Grade 2–5 | ||||||
| Any | 17 (5) | 3 (<1) | 21 (6) | 7 (2) | 21 (6) | 13 (4) |
| Occurring in ≥0.5% of either group | ||||||
| Depression | 0 | 1 (<1) | 3 (<1) | 1 (<1) | 2 (<1) | 1 (<1) |
| Insomnia | 4 (1) | 0 | 4 (1) | 0 | 4 (1) | 0 |
| Constipation | 2 (<1) | 1 (<1) | 2 (<1) | 1 (<1) | 2 (<1) | 1 (<1) |
| Weight increase | 1 (<1) | 0 | 2 (<1) | 1 (<1) | 3 (<1) | 3 (<1) |
| Flatulence | 2 (<1) | 0 | 2 (<1) | 0 | 2 (<1) | 0 |
| Nausea | 0 | 1 (<1) | 0 | 1 (<1) | 0 | 2 (<1) |
| Metabolism and nutrition disorders | ||||||
| Weight increase | 3 (<1) | 6 (2) | 5 (1) | 7 (2) | 17 (5) | 19 (5) |
| Hyperlipidemia | 3 (<1) | 1 (<1) | 4 (1) | 3 (<1) | 7 (2) | 7 (2) |
| Weight decrease | 1 (<1) | 1 (<1) | 2 (<1) | 1 (<1) | 3 (<1) | 4 (1) |
| Impaired glucose tolerance | 0 | 1 (<1) | 1 (<1) | 2 (<1) | 1 (<1) | 2 (<1) |
| Type 1 diabetes | 1 (<1) | 0 | 1 (<1) | 0 | 1 (<1) | 0 |
| Type 2 diabetes | 2 (<1) | 0 | 2 (<1) | 1 (<1) | 2 (<1) | 1 (<1) |
| AEs leading to study withdrawalc | ||||||
| Any | 13 (4) | 2 (<1) | 21 (6) | 4 (1) | 23 (6) | 7 (2) |
| Drug related | 9 (2) | 1 (<1) | 14 (4) | 3 (<1) | 13 (4) | 5 (1) |
| Any SAEd | 21 (6) | 16 (4) | 42 (11) | 35 (9) | 57 (15) | 44 (12) |
Abbreviations: AE, adverse event; DTG/3TC, dolutegravir/lamivudine; SAE, serious AE; TAF, tenofovir alafenamide; URTI, upper respiratory tract infection.
One participant was found to be taking a tenofovir disoproxil fumarate–based regimen and was excluded from the safety population.
All drug-related AEs through week 96 were grade 1 or 2; most drug-related AEs through week 144 were grade 1 or 2, except for 2 grade 3 events (suicidal ideation and increased transaminases in the DTG/3TC group) and 1 grade 4 event (angioedema in the TAF-based regimen group).
For summary of AEs leading to withdrawal from the study, see Supplementary Table 3.
Two drug-related SAEs occurred through week 144 (increased transaminases in the DTG/3TC and angioedema in the TAF-based regimen group). Three fatal AEs, unrelated to study treatment, occurred in the DTG/3TC group, 2 (gunshot wound [homicide] and substance abuse [acute intoxication]) through week 96 and a third (ischemic hepatitis) through week 144.
Figure 4.
Summary of adverse events (AEs) after week 48. Abbreviations: DTG/3TC, dolutegravir/lamivudine; TAF, tenofovir alafenamide.
An AE of increased weight was observed in 5% of participants in each group, and an AE of increased BMI was observed only in the TAF-based regimen group (<1%); all instances were grade 1 or 2 in severity. Three participants (2 receiving DTG/3TC and 1 the TAF-based regimen) experienced an AE of increased weight, leading to study discontinuation. The adjusted mean changes from baseline in weight in the DTG/3TC and TAF-based regimen groups were 2.2 and 1.7 kg, respectively (treatment difference, 0.49 kg [95% CI, −.46 to 1.44 kg]; P = .31). Changes in weight from baseline to week 144 were similar in the DTG/3TC and TAF-based regimen groups overall and across baseline BMI categories: underweight/normal (2.2 vs 2.1 kg, respectively; adjusted treatment difference, 0.08 kg [95% CI, −1.36 to 1.51 kg]; P = .92), overweight (2.3 vs 1.9 kg; 0.44 kg [−1.16 to 2.03 kg]; P = .59), and obese (2.2 vs 0.6 kg; 1.68 kg [95% CI, −.56 to 3.91 kg]; P = .14).
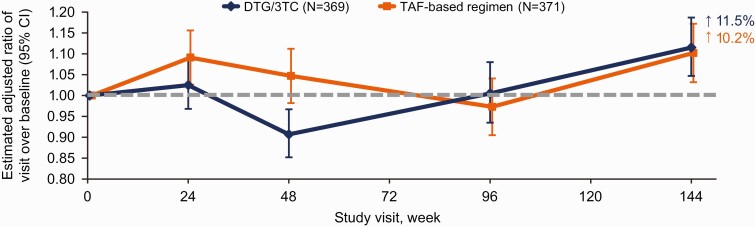
Among participants with obesity at baseline in the TAF-based regimen group, weight that was lost between weeks 48 and 96 was regained by week 144 (Supplementary Figure 1). A post hoc analysis revealed that among participants with obesity at baseline, a higher proportion was referred for dietetic counseling/weight management programs in the TAF-based regimen group than in the DTG/3TC group throughout the study (Supplementary Table 2). The proportions of participants with ≥10% weight increase from baseline were similar in the DTG/3TC (13% [42 of 316]) and TAF-based regimen (12% [37 of 303]) groups. One instance of type 1 diabetes mellitus was observed after baseline (TAF-based regimen group). Adjusted changes from baseline to week 144 in insulin resistance were small and similar in the 2 groups (Figure 5). At baseline, 72% of participants in both groups had HOMA-IR ≥2. At week 144, 78% of participants in the DTG/3TC and 82% in the TAF-based regimen group had insulin resistance, defined as HOMA-IR ≥2 (adjusted odds ratio, 0.78 [95% CI, .49–1.24]; P = .30).
Figure 5.
Change from baseline in homeostasis model of assessment–insulin resistance (HOMA-IR) in the safety population through week 144. The change from baseline was calculated using mixed-model repeated measures adjusting for treatment, visit, baseline third agent class, CD4+ cell count (continuous), age (continuous), sex, race, body mass index (continuous), presence of hypertension, loge-transformed baseline HOMA-IR (continuous), treatment-by-visit interaction, and baseline value-by-visit interaction, with visit as the repeated factor. Abbreviations: CI, confidence interval; DTG/3TC, dolutegravir/lamivudine; TAF, tenofovir alafenamide.
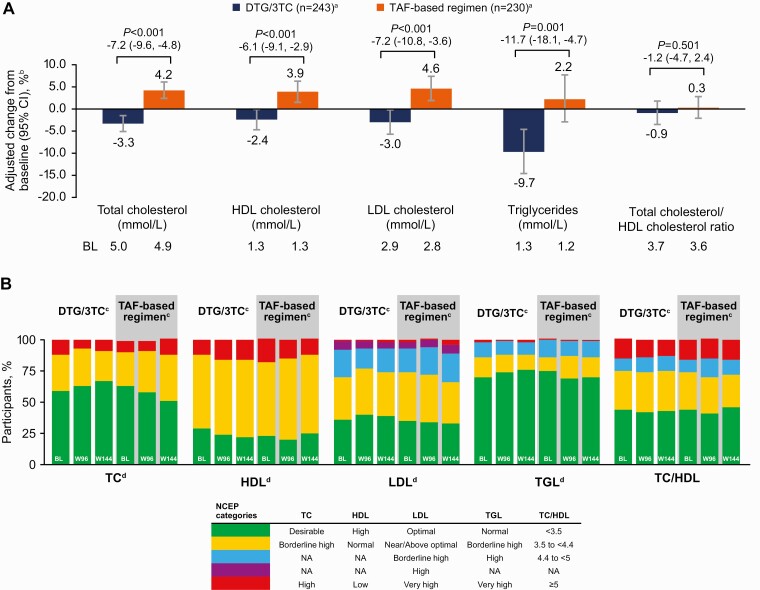
Overall, similar and minimal changes in inflammatory biomarkers were observed through week 144 in both groups (Supplementary Figure 2). For renal biomarkers, small and similar changes between groups were observed at week 144 (Supplementary Figure 3). Changes from baseline in total cholesterol, low-density lipoprotein cholesterol, and triglyceride levels favored DTG/3TC; changes in high-density lipoprotein cholesterol levels favored TAF-based regimens (Figure 6A). No differences in total cholesterol–high-density lipoprotein cholesterol ratio changes were observed between groups. Rates of initiation of lipid-modifying agents since baseline were comparable in the 2 groups (DTG/3TC, 45 of 369 [12%]; TAF-based regimen, 42 of 372 [11%]). In the week 96 and 144 analyses of lipids based on National Cholesterol Education Program categories, proportions of participants with desirable or optimal levels generally favored the DTG/3TC group, particularly with respect to total cholesterol, low-density lipoprotein cholesterol, and triglycerides (Figure 6B). Minimal changes were observed in bone biomarkers (Supplementary Figure 4).
Figure 6.
Change from baseline in fasting lipids at week 144 (A) and at weeks 96 and 144 (B) by National Cholesterol Education Program (NCEP) category. aNumber of participants with nonmissing fasting lipid data at baseline and week 144, excluding those with lipid-modifying agent administered at baseline (lipid data collected after initiation of a lipid-modifying agent were censored and a last observation carried forward method was applied). Use of lipid-modifying agents at baseline was similar between treatment groups (dolutegravir/lamivudine [DTG/3TC], 13%; tenofovir alafenamide [TAF]–based regimen, 15%). bPercentage change from baseline based on adjusted ratio (week 144 to baseline) in each group calculated from mixed-model repeated measures applied to change from baseline in loge-transformed data adjusting for treatment, visit, baseline third agent class, age (continuous), body mass index (continuous), race, CD4+ cell count (continuous), loge-transformed baseline value (continuous), treatment-by-visit interaction, and baseline value-by-visit interaction, with visit as the repeated factor. cNumbers of participants with nonmissing fasting lipid data at baseline and study week (week 96: DTG/3TC, n = 238; TAF- based regimen, n = 213; week 144: DTG/3TC, n = 243; TAF-based regimen, n = 230), excluding participants with lipid-modifying agent administered at baseline (lipid data collected after initiation of a lipid-modifying agent were censored and a last observation carried forward method was applied so that the last available fasted, on-treatment lipid value before initiation of a lipid-modifying agent was used). dNCEP categories at weeks 96 and 144 versus baseline. Abbreviations: CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NA, not applicable; TC, total cholesterol; TGL, triglycerides.
DISCUSSION
Week 144 results of the TANGO study demonstrate long-term, durable virologic efficacy after switching to the 2-drug regimen DTG/3TC, as evidenced by its sustained noninferiority (ITT-E) in maintaining virologic suppression, compared with continuing 3- or 4-drug TAF-based regimens. The supportive analysis in the per-protocol population favored DTG/3TC at weeks 96 and 144. Importantly, the rate of Snapshot virologic failure at week 144 remained <1% in the DTG/3TC group (ITT-E). Virologic suppression rates were high and sustained and were similar between treatment groups across subgroups. Differences in small subgroups should be interpreted with caution, especially where Snapshot failures were driven by nonvirologic causes. This demonstrates the efficacy of DTG/3TC regardless of baseline treatment, participant, or disease characteristics. No participants who switched to DTG/3TC met CVW criteria, and no resistance development was reported in either treatment group through week 144. The overall rates of AEs were similar between groups, and after week 48, the rates of AEs, SAEs, drug-related AEs, and AEs leading to study discontinuation were similar between groups.
Few participants had HIV-1 RNA levels ≥50 copies/mL in the present study, consistent with results from the SALSA study, in which DTG/3TC demonstrated efficacy noninferior to that of continuing any current antiretroviral regimen for 1 year in treatment-experienced participants [8]. Virologic response rates were also high in the GEMINI-1 and GEMINI-2 studies, in which DTG + 3TC demonstrated durable efficacy for 3 years in treatment-naive participants [3–5]. In addition, the low rate of virologic failure observed through week 144 in TANGO is consistent with findings from a meta-analysis of 6 real-world observational studies, which found that DTG/3TC as maintenance therapy resulted in virologic failure (defined as 2 consecutive viral load measurements ≥50 copies/mL and/or 1 measurement >1000 copies/mL) in 1% of treatment-experienced patients (n = 2033) at either week 48 or week 96 [10].
No participants met CVW criteria through week 144 in the DTG/3TC group. In addition, the post hoc analysis of baseline proviral genotyping found that the 4 DTG/3TC participants with preexisting archived M184M/V/I mutations at baseline maintained virologic suppression at their last on-study viral load measurement through the 144-week study period. When historical plasma RNA resistance reports are not available, proviral DNA testing results may be informative but should be interpreted with caution. Proviral DNA resistance testing can show the presence of resistance mutations but is not definitive in showing the absence of preexisting resistance, so its clinical utility has not been fully determined [7]. A systematic literature review of real-world evidence showed that across 7 studies reporting on virologically suppressed adults with historical M184I/V substitution, 3% of study participants (5 of 154) experienced virologic failure, and none had treatment-emergent resistance-associated integrase strand transfer inhibitor substitutions at virologic failure [11]. Overall, the TANGO results, along with real-world evidence for DTG/3TC, provide further confidence in the high barrier to resistance of DTG/3TC.
Through week 144, the rate of drug-related AEs was higher with DTG/3TC than with TAF-based regimens, which is expected in a switch study setting where participants were stable on their preexisting regimens. In TANGO, 90% of participants were switched to 2 new drugs in the DTG/3TC group at baseline. However, in the post–week 48 AE analysis, rates of drug-related AEs and AEs leading to discontinuation were low and similar in both groups, and rates of AEs and SAEs were comparable between groups, supporting the transient impact of adjusting to new treatment regimens. From baseline to week 144, weight gains were comparable between groups. Of note, weight loss in participants with BMI categorized as obese at baseline in the TAF-based regimen group was observed between weeks 48 and 96 with weight regained by week 144; in the DTG/3TC group, a smaller weight loss was observed from day 1, with weight regained by week 48. The dynamic of weight changes over time may be related to the impact of external lifestyle interventions, as evidenced by the post hoc analysis of dietetic counseling/weight management program referrals. The numbers of participants withdrawing because of an AE of increased weight (2 of 369 receiving DTG/3TC and 1 of 371 receiving TAF-based regimens) and the proportions with ≥10% weight increase from baseline (13% and 12% for DTG/3TC and TAF-based regimens, respectively) were similar between groups.
Although treatment differences in insulin resistance, as measured by HOMA-IR, were observed in the week 48 analysis, none were observed at week 144, with levels approximately 10%–11% higher than baseline in both groups. Differences in diet/exercise intervention referrals, which occurred more frequently in participants in the TAF-based regimen group with baseline BMI categorized as obese, and in weight fluctuations over time limit interpretation of results. Changes in lipid parameters favored the DTG/3TC group and were sustained through 3 years of treatment, while the proportions of participants who started treatment with lipid-lowering agents since baseline were similar between groups. After 144 weeks of study treatment, metabolic health (as assessed by weight, lipid values, and insulin resistance) was generally stable across both groups. Changes in renal biomarkers did not indicate any significant clinical impact on kidney function in either group. Small and similar changes between groups in bone biomarkers were observed in the present study. Similar and minimal changes in inflammatory biomarkers were also observed in the 2 groups. Inflammatory biomarker results are in line with the high and comparable rates of virologic suppression in both groups, including rates of virologic suppression below qualitative detectable levels (“target not detected”).
The TANGO study has some limitations. Most participants in the study were white (79%), male (92%), aged <50 years (77%), and used boosted elvitegravir-based regimens at baseline (73%); regardless, no apparent differences were observed in post hoc subgroup analyses of efficacy and safety [12]. Since the week 48 analysis, the COVID-19 pandemic posed a barrier to data collection, with week 96 data unavailable for 44 of 741 participants (6%), with numbers imbalanced between groups. At week 144, only 7 participants had data missing owing to the COVID-19 pandemic. The impact of COVID-19 was accounted for in the efficacy evaluable population Snapshot sensitivity analyses, which were consistent with the main Snapshot analysis.
In conclusion, week 96 and 144 secondary analyses of the TANGO study demonstrate durable, noninferior efficacy of DTG/3TC compared with continuing TAF-based regimens in virologically suppressed adults, with no confirmed virologic failure or observed resistance in the DTG/3TC group through 3 years. These results provide further support for the use of this 2-drug regimen as a treatment option for treatment-experienced, virologically suppressed individuals with HIV-1 infection and no history of virologic failure or documented resistance to DTG or 3TC.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Clinical Trials Registration: NCT03446573
Acknowledgments. The authors thank the study participants and their families and caregivers; the investigators and site staff who participated in the study; and the ViiV Healthcare, GlaxoSmithKline, Pharmaceutical Product Development, and Phastar study team members. They also thank Maria Claudia Nascimento for her contributions to the study design, medical monitoring, and data analysis. Editorial assistance was provided under the direction of the authors by Aarthi Gobinath, PhD, and Jennifer Rossi, MA, ELS, MedThink SciCom, and was funded by ViiV Healthcare.
Financial support. This work was supported by ViiV Healthcare.
Contributor Information
Olayemi Osiyemi, Triple O Research Institute PA, West Palm Beach, Florida, USA.
Stéphane De Wit, CHU Saint-Pierre, Université Libre de Bruxelles, Brussels, Belgium.
Faïza Ajana, Centre Hospitalier de Tourcoing, Tourcoing, France.
Fiona Bisshop, Holdsworth House Medical Brisbane, Queensland, Australia.
Joaquín Portilla, Hospital General Universitario de Alicante, Alicante, Spain.
Jean Pierre Routy, McGill University Health Centre, Montreal, Quebec, Canada.
Christoph Wyen, Praxis am Ebertplatz, Cologne, Germany.
Mounir Ait-Khaled, ViiV Healthcare, Brentford, United Kingdom.
Peter Leone, ViiV Healthcare, Research Triangle Park, North Carolina, USA.
Keith A Pappa, ViiV Healthcare, Research Triangle Park, North Carolina, USA.
Ruolan Wang, ViiV Healthcare, Research Triangle Park, North Carolina, USA.
Jonathan Wright, GlaxoSmithKline, Brentford, United Kingdom.
Nisha George, GlaxoSmithKline, Bangalore, India.
Brian Wynne, ViiV Healthcare, Research Triangle Park, North Carolina, USA.
Michael Aboud, ViiV Healthcare, Brentford, United Kingdom.
Jean van Wyk, ViiV Healthcare, Brentford, United Kingdom.
Kimberly Y Smith, ViiV Healthcare, Research Triangle Park, North Carolina, USA.
References
- 1.Back D. 2-Drug regimens in HIV treatment: pharmacological considerations. Germs 2017; 7:113–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saag MS, Gandhi RT, Hoy JF, et al. . Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society–USA Panel. JAMA 2020; 324:1651–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cahn P, Sierra Madero J, Arribas JR, et al. . Three-year durable efficacy of dolutegravir plus lamivudine in antiretroviral therapy-naive adults with HIV-1 infection. AIDS 2022; 36:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahn P, Sierra Madero J, Arribas JR, et al. . Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019; 393:143–55. [DOI] [PubMed] [Google Scholar]
- 5.Cahn P, Sierra Madero J, Arribas JR, et al. . Durable efficacy of dolutegravir plus lamivudine in antiretroviral treatment–naive adults with HIV-1 infection: 96-week results from the GEMINI-1 and GEMINI-2 randomized clinical trials. J Acquir Immune Defic Syndr 2020; 83:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European AIDS Clinical Society. EACS guidelines version 11.0. October 2021. Available at: https://www.eacsociety.org/media/final2021eacsguidelinesv11.0_oct2021.pdf. Accessed 1 December 2021.
- 7.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. 16 August 2021. Available at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf. Accessed 22 September 2021.
- 8.Llibre JM, Brites CA, Cheng CY, et al. . Switching to the 2-drug regimen of dolutegravir/lamivudine (DTG/3TC) fixed-dose combination is non-inferior to continuing a 3-drug regimen through 48 weeks in a randomized clinical trial (SALSA). In: Program and abstracts of the 11th IAS Conference on HIV Science (virtual). Geneva, Switzerland: International AIDS Society, 2021:72. Abstract OALB0303. [Google Scholar]
- 9.van Wyk J, Ajana F, Bisshop F, et al. . Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose 2-drug regimen vs continuing a tenofovir alafenamide–based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: phase 3, randomized, noninferiority TANGO study. Clin Infect Dis 2020; 71:1920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Punekar YS, Parks D, Joshi M, et al. . Effectiveness and safety of dolutegravir two-drug regimens in virologically suppressed people living with HIV: a systematic literature review and meta-analysis of real-world evidence. HIV Med 2021; 22:423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel R, Evitt L, Mariolis I, et al. . HIV treatment with the two-drug regimen dolutegravir plus lamivudine in real-world clinical practice: a systematic literature review. Infect Dis Ther 2021; 10:2051–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benson P, Kinder CA, Pérez-Elías MJ, et al. . Switching to DTG/3TC FDC is noninferior to TBR for 96 weeks: TANGO subgroup analyses. In: Program and abstracts of the 28th Conference on Retroviruses and Opportunistic Infections (virtual). San Francisco, CA: International Antiviral Society–USA, 2021:152. Abstract 417. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.