Abstract
This study sought to evaluate the impact of virtual reality (VR) tools in procedural planning of transcatheter aortic valve replacement. A prospective study involving 11 patients referred for transcatheter aortic valve replacement was conducted. A multidetector computed tomography was used to acquire and segment the anatomy of the access route and landing zone. From the information obtained with the multidetector computed tomography in DICOM format, we built a virtual platform (VisuaMed, Techer Team, Valencia, Spain) that contains all the clinical information of the patients and a virtualized model of their anatomy. Wearing VR devices, the professional was able to ‘walk inside’ the anatomy in an interactive and immersive way. Decisions after the evaluation of routine clinical images were compared with those after experience with VR models and intraprocedural findings.
Keywords: Transcatheter aortic valve replacement, Virtual reality, Imaging, Procedural planning
Patients referred for transcatheter aortic valve replacement (TAVR) usually underwent a multidetector computed tomography imaging (MDCT) and also three-dimensional (3D) reconstruction of MDCT images of the access route and landing zone using different planning tools like semi-automated 3mensio (3mensio Medical Imaging BV, Bilthoven, The Netherlands) or full-automated Heart Navigator, Phillips [1].
INTRODUCTION
Patients referred for transcatheter aortic valve replacement (TAVR) usually underwent a multidetector computed tomography imaging (MDCT) and also three-dimensional (3D) reconstruction of MDCT images of the access route and landing zone using different planning tools like semi-automated 3mensio (3mensio Medical Imaging BV, Bilthoven, The Netherlands) or full-automated Heart Navigator, Phillips [1].
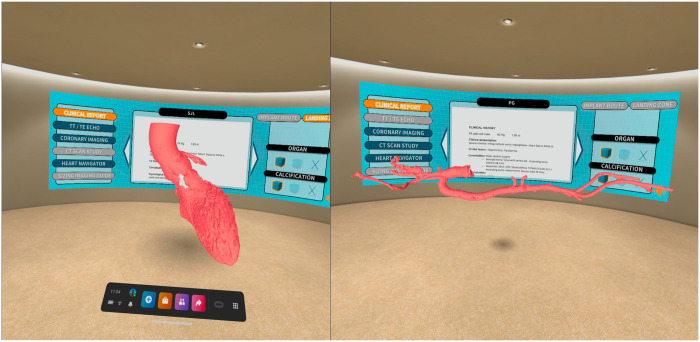
Virtual reality is a computer-generated environment perceived through a device known as a virtual reality (VR) headset [2]. From the information obtained with the MDCT in a DICOM format, we built a virtual platform (VisuaMed, Techer Team, Valencia, Spain) that contains all the clinical information of the patients and a virtualized model of their anatomy (Fig. 1). The VR has the ability to ‘transport the user inside the human body’ and provides an unlimited view of anatomy from every angle in an immersive experience with high fidelity and accuracy (Videos 1 and 2).
Figure 1:
Virtual room platform with all the clinical and image information of the patient on a panel and virtualized tridimensional model of the access route and landing zone. We can ‘walk around and inside’ the model analysing all anatomical details, the pattern of calcification and coronary ostia location. In addition, there is availability of tools to turn, expand, measure, draw, segment and visualize specific parts of the anatomy by enabling the transparency of tissues or take a pictures/videos and save them to the computer.
The goal of this study was to evaluate the utility of VR in individualized scenarios for TAVR planning.
MATERIALS AND METHODS
Study design
We hypothesized that VR improves the information about the patient target anatomy and could have an impact on the decisions about implant strategy.
A prospective study was conducted between May 2020 and May 2021 on 11 consecutive patients with severe aortic valvular disease and referred for TAVR procedure. The study complies with the Declaration of Helsinki and all patients signed a consent form for the use of personal medical images for research to protect the privacy of subjects and the confidentiality of their personal information. This study has been approved by the ethics committee of the Centre (Clinical Trials registration number NCT04944667).
Our study used a case-crossover design in which each patient serves as its own control. Each case was first evaluated with routine imaging techniques (echocardiography, angiography, MDCT) and specialized software packages (3D, Heart Navigator). Then, each case was re-evaluated by the same Heart Team with the addition of VR models (Fig. 1). The Heart Team members responded to a questionnaire about the usefulness of VR (Supplementary Material).
RESULTS
Eleven patients were included in the study from 1 single centre. Patient demographics, clinical characteristics and image data are described in Table 1.
Table 1.
Patient demographics, clinical and image data
| Case | Description | Areas of concern |
|---|---|---|
| 1 | 79-Year-old-female. Severe aortic regurgitation. NYHA III–IV. Previous cardiac surgery (septal myectomy). Breast cancer (surgery + RT). Severe COPD. Tricuspid aortic valve. Preserved LVEF. | Pure aortic regurgitation. Distance annulus-LM coronary ostia: 10 mm. Severe tortuosity and calcification in bilateral femoral-iliac route |
| 2 | 71-Year-old-man. Severe AS. NYHA III. Severe COPD. Obesity. Chronic CAD. Preserved LVEF. Tricuspid aortic valve. | None |
| 3 | 50-Year-old-male. Severe bicuspid aortic stenosis. Huge calcification. Anomalous origin of the RCA. | Bicuspid valve with huge calcification in valve, mitral annulus and LVOT. Anomalous origin of RCA from the left coronary sinus. |
| 4 | 49-Year-old-man. Previous cardiac surgery for bioAVR (Trifecta Abbott 23) + ascending aorta replacement. Severe-massive intra prosthetic regurgitation. Dilated LV. NYHA III. Annulus-LCA distance: 8 mm. Annulus-RCA distance: 12 mm. | Valve-in-valve procedure |
| Short LCA-prosthesis distance | ||
| 5 | 80-Year-old man. Severe aortic regurgitation. NYHA II–III. Previous CABG × 4 (LIMA + RIMA). Preserved LVEF. | None |
| 6 | 78-Year-old-man. Severe AS. NYHA III. Chronic CAD. Severe COPD. Preserved LVEF. Severe peripheral arteriopathy. | None |
| 7 | 74-Year-old female. Critical AS, low-gradient, low-flow. LVEF: 28%. Severe pulmonary hypertension. Previous cardiac surgery for mechanical mitral valve replacement. Short | Depth implant target |
| 8 | 77-Year-old male. Severe AS. NYHA III. Obesity. Poor mobility. Spine surgery/arthrosis. Preserved LVEF. | None |
| 9 | 70-Year-old-female. Previous SAVR (Crown 21) + CABG. Severe aortic bioprosthesis dysfunction. NYHA III. Distance annulus-LMCA: 8 mm. Distance annulus-RCA: 7 mm. Sinus of Valsalva: 29 × 29 × 29. | Valve-in-valve procedure |
| Short distance LMCA/RCA-prosthesis | ||
| 10 | 78-Year-old-female. Severe AS. NYHA III. Severe Sd Sjögren. Tricuspid aortic valve. Preserved LVEF. | None |
| 11 | 73-Year-old male. Severe AS low gradient. LVEF: 35%. Previous cardiac surgery for CABG × 4. | None |
All MDCT studies provided adequate image quality for image segmentation and all cases were successfully converted to the VR model. The average waiting time between sending the DICOM files and receiving the models in VR was >24 h.
All cases survived with good clinical outcome and no major complications. Two patients had a mild paravalvular leak. There were no neurological or vascular complications. There was also no need for a permanent pacemaker implantation, although 1 patient had a new LBBB. The average hospital stay was 4.2 ± 1.2 days including 18 ± 3 h in ICU.
In 6 cases, the information provided by the VR experience did not lead to any changes to the team planning based on the conventional imaging planning. On the other hand, VR models modified the implant strategy in 5 cases (45.4% of the cases).
There were 2 cases of TAVR valve in valve in which the MDCT and 3D measurements showed a short distance between the malfunctional previous bioprosthesis and the coronary ostia. After the virtual experience, in both cases, we were sure that the Valsalva sinuses were wide enough and that the coronary ostia were further away than previously stated (Fig. 2). We could do the procedure without coronary protection.
A 74-year-old female patient with severe aortic stenosis and prior mechanical mitral valve replacement was evaluated. After the virtual experience, we were able to appreciate more clearly that the mitral-aortic distance was very short, and a higher implant was recommended to minimize the possibility of TAVR migration.
One patient who was initially considered for TAVR was referred for surgical replacement after VR experience. The reasons for the change were the presence of a bicuspid aortic valve with huge calcification protruding towards the LVOT and an anomalous origin of the right coronary artery (Table 2).
Figure 2:
Visualization of specific parts of the anatomy (calcium pattern) by enabling transparency. High origin of the left main coronary artery. Anomalous origin of the RCA from the left main coronary artery (arrow).
Table 2.
Strategic procedural plans with/without use of virtual reality models
| Case | Stage 1 (plan) | Stage 2 (plan + VR) | Changes |
|---|---|---|---|
| 1. 78-Year-old female | Femoral approach | Left carotid approach | Access route |
| 2. 71-Year-old male | No | ||
| 3. 50-Year-old male | Femoral TAVR | Open cardiac surgery | Procedure approach |
| 4. 50-Year-old male V-in-V | PCI coronary protection | No | No need for coronary protection |
| 5. 80-Year-old male | No | ||
| 6. 78-Year-old male | No | ||
| 7. 74-Year-old female (previous mechanical MVR) | Deep implantation planned | Implanted higher than initially planned | Modified depth of implantation |
| 8. 77-Year-old female | No | ||
| 9. 70-Year-old female V-in-V | PCI coronary protection | No | No need for coronary protection |
| 10. 80-Year-old female | No | ||
| 11. 74-Year-old male | No |
TAVR: transcatheter aortic valve replacement; VR: virtual reality.
DISCUSSION
Before any TAVR procedure, preprocedural planning is key to prevent potentially catastrophic complications. The MDCT is now the gold standard tool for TAVR planning [3]. Advances in imaging in the last 15 years have led to 3D recreations of anatomy, but they could only be viewed on a two-dimensional display, limiting the ability to fully interact with the model and to assess the patient’s anatomy with full accuracy.
In recent years, there was a growing interest in 3D-printing models to enhance the outcomes of complex cardiovascular cases including TAVR procedures [4]. 3D-printing models could be helpful, but the interaction with the model is limited. The VR allows to take a step forward and cross the barrier into a different universe where we could interact with the anatomy. The VR has the ability to ‘transport the user inside the human body’. Furthermore, VR technology provides unlimited views of anatomy from every angle in an immersive experience with high fidelity and accuracy and a stronger understanding of medical scenarios [5].
We hypothesized that VR could have an impact on the decisions about the implant strategy.
To the best of our knowledge, this is the first preliminary study to investigate the use of VR in the planning of TAVR procedures.
Our study suggests that in TAVR patients with complex scenarios, the immersive experience that VR provides could contribute to achieve the best possible plan for each patient. In fact, the objective of VR models is not to replace the software packages used widely but to add more information in an immersive and interactive way.
Limitations
A limitation of this approach is that it is only sensitive to the impact of the VR use on procedural planning, not on morbidity and mortality nor on mid- and long-term outcomes.
Another limitation is that with current technology we do not have information about the dynamic changes in geometry that occur during the cardiac cycle. Furthermore, we must take into account the limited number of patients included in the present study.
CONCLUSION
We evaluated the impact of VR in the planning of TAVR procedures. To the date, this is the first study of this new technology clinically applied to this group of patients.
In almost half of the cases, the consideration of new information provided by the VR models led the team to redesign the procedure plan. In all procedures, this additional information increased the confidence of the Heart Team during implantation.
Nevertheless, this is an initial study and further research might be considered to better describe the benefits of VR as a complementary tool for TAVR preprocedural planning.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Conflict of interest: The authors have no conflicts of interest.
Supplementary Material
ACKNOWLEDGEMENTS
We want to thank Victor Herrera and Helena Ortiz (Techer Team SL. Valencia. Spain) for the development of virtual reality models and for their constant collaboration with our team.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks the anonymous reviewer(s) for their contribution to the peer review process of this article.
Contributor Information
Xavier Ruyra, Cardiac Surgery Department, Quironsalud Teknon Heart Institute, Barcelona, Spain.
Eduard Permanyer, Cardiac Surgery Department, Quironsalud Teknon Heart Institute, Barcelona, Spain; Human Anatomy Department, Pompeu Fabra University, Barcelona, Spain.
Marina Huguet, Department of Diagnostic Imaging, Quironsalud Teknon Heart Institute, Barcelona, Spain.
Giuliana Maldonado, Cardiology Department, Quironsalud Teknon Heart Institute, Barcelona, Spain; Cardiology Department, Quironsalud Hospital General de Catalunya, Sant Cugat, Spain.
REFERENCES
- 1.Szekely G, Satava RM.. Virtual reality in medicine. BMJ 1999;13319:1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer A, Kofler M, Montagner M, Unbehaun A, Sündermann S, Buz S. et al. Reliability and influence on decision-making of fully-automated vs. semi-automated software packages for procedural planning in TAVI. Sci Rep 2020;10:10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleakley C, Monaghan MJ.. The pivotal role of imaging in TAVR procedures. Curr Cardiol Rep 2018;20:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin D, Burkhard G, Reisman M, McCabe JM, Dvir D, Ripley B.. 3D printing applications for transcatheter aortic valve replacement. Curr Cardiol Rep 2020;22:23. [DOI] [PubMed] [Google Scholar]
- 5.Yeung AWK, Tosevska A, Klager E, Eibensteiner F, Laxar D, Stoyanov J. et al. Virtual and augmented reality applications in medicine: analysis of the scientific literature. J Med Internet Res 2021;23:e25499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.