Abstract
Effective host defense against Pneumocystis carinii depends upon the integrated actions of inflammatory cells and mediators in the lungs. Using immunocompetent and immunosuppressed mice, our laboratory has defined inflammatory changes in the lungs in response to P. carinii. However, the essential molecules and mechanisms required for cellular recruitment and for host defense against P. carinii are undefined. We hypothesized that urokinase-type plasminogen activator (uPA), a protease intimately involved in inflammatory cell migration and activation, is required for clearance of P. carinii. To test this hypothesis in vivo, we compared the intensity of P. carinii infection and inflammation in the lungs of mice lacking the uPA gene (uPA knockout mice) and in the lungs of wild-type mice. After intratracheal inoculation with P. carinii organisms, uPA knockout mice developed uniformly heavy P. carinii pneumonia while wild-type mice cleared the P. carinii inoculum. Bronchoalveolar lavage fluid from uPA knockout mice contained significantly smaller numbers of cells than did lavage fluid from wild-type mice. We conclude that deletion of the uPA gene prevents the clearance of P. carinii and reduces inflammatory cell recruitment. Therefore, uPA is an important participant in the network of inflammatory events required for the clearance of P. carinii, confirming an important role for this molecule in pulmonary host defense against opportunistic pathogens.
Pneumocystis carinii remains a frequent cause of serious pneumonia in individuals with impaired cell-mediated immunity, both in human immunodeficiency virus (HIV)-infected individuals (38) and in patients receiving immunosuppressive therapy (40). Because failures of prophylaxis and therapy against P. carinii continue to occur with conventional antimicrobial agents, recent investigations have focused on the immune and inflammatory events that occur during P. carinii pneumonia (39). An improved understanding of these immune and inflammatory events could lead to novel immunotherapies directed against this important opportunistic pathogen.
Effective host defense against P. carinii depends upon the integrated actions of inflammatory cells and mediators. Given the inherent difficulties in examining host responses in humans, immunologically relevant animal models provide powerful tools to examine defense against P. carinii in vivo. Our laboratory has examined the defense against P. carinii in mice specifically depleted of CD4+ T lymphocytes, which mimics an immunologic hallmark of HIV infection (35). Previously, we defined inflammatory responses in mouse lungs in response to P. carinii, comparing responses in mice depleted of CD4+ T lymphocytes to responses in intact mice (4). We demonstrated that accumulation of CD4+ T lymphocytes and macrophages accompanies the clearance of P. carinii from the lungs of intact mice and that accumulation of CD8+ T lymphocytes occurs in the lungs of CD4-depleted mice. More recently, we demonstrated that these CD8+ T lymphocytes can provide partial host defense against P. carinii during prolonged depletion of CD4+ T lymphocytes (3). Additionally, alveolar macrophages act as effector cells against P. carinii, probably by phagocytosis and killing of the organisms (11, 22, 24). However, the signals required for effective recruitment of immune and inflammatory cells in response to P. carinii are unknown.
Urokinase-type plasminogen activator (uPA), a protease intimately involved in inflammatory-cell migration and activation, participates in the degradation of matrix proteins by leukocytes during recruitment to inflammatory sites (10, 15, 31). Based on previous work examining host defense against P. carinii, several lines of evidence suggest an important role for uPA in defense. First, uPA is expressed by macrophages and by activated, but not resting, lymphocytes (6, 12, 27). Second, the uPA receptor (uPAR, CD87) is present on monocytes, neutrophils, and activated lymphocytes (6, 28, 31). Third, uPAR clusters to the leading edge of migrating monocytes during chemotactic responses (15). Fourth, uPA is required for pulmonary clearance of Cryptococcus neoformans, an opportunistic fungus, from the lungs of mice (14). Finally, plasminogen activator activity is markedly decreased in alveolar macrophages and in bronchoalveolar lavage (BAL) fluid obtained from HIV-infected individuals and plasminogen activator inhibitor activity is increased in BAL fluid obtained from HIV-infected individuals during P. carinii pneumonia (1).
We hypothesized that the important host defense molecule uPA could mediate inflammation and clearance in response to P. carinii infection. To test this hypothesis in vivo, we examined P. carinii infection in the lungs of mice deficient of the uPA gene and in the lungs of background-matched, wild-type mice. We demonstrated that uPA knockout mice become heavily infected with P. carinii after intratracheal inoculation with the organism while wild-type mice clear the infection completely. Additionally, uPA knockout mice demonstrate reduced accumulation of inflammatory cells in response to P. carinii, compared with wild-type mice, providing a mechanistic explanation for the failure of host defense against P. carinii in the absence of uPA.
MATERIALS AND METHODS
Animals.
The uPA knockout mice were a generous gift of Peter Carmeliet, Leuven, Belgium (8). By using standard techniques, the uPA gene was deleted by homologous recombination to yield heterozygotes. Breeding of these heterozygotes was used to establish homologous uPA knockout and wild-type mice (C57BL/6 × 129). The mouse genotypes were confirmed by analysis of tail DNA by PCR with gene-specific primers (14). Background-matched, wild-type mice were used as controls for each group of knockout mice. We have previously demonstrated that these wild-type mice have inflammatory responses indistinguishable from those of either background strain (14).
P. carinii-infected athymic mice (nu/nu on a BALB/c background [Taconic Laboratories, Germantown, N.Y.]) were used as a source of P. carinii in all experiments (4). P. carinii organisms were passaged into the lungs of uninfected athymic mice at monthly intervals. To exclude alloantigen as a confounding cause of inflammation, uninfected athymic mice were used as a source of sham inoculum for the uPA knockout and wild-type mice. Lungs from these athymic mice were handled and prepared identically to those from P. carinii-infected athymic mice.
All the mice were housed in microisolator caging under laminar-flow conditions and were maintained in barrier-protected rooms. Sentinel animals were regularly monitored for common rodent diseases. Variously aged wild-type, uPA knockout, and uninfected athymic mice were always histologically negative for spontaneous P. carinii infection. All the procedures were approved by the Animal Care Subcommittee, Department of Veterans Affairs Medical Center, Ann Arbor, Mich.
Intratracheal inoculations.
Both wild-type and uPA knockout mice were inoculated intratracheally with P. carinii as previously described (4). We performed intratracheal inoculations under direct vision to ensure that each mouse received an identical inoculum at an identical time point. P. carinii-infected athymic mouse lungs were removed under sterile conditions, frozen at −20°C for 2 h, homogenized mechanically, filtered, and centrifuged. Smears were stained with modified Giemsa stain for quantitation of P. carinii organisms, and touch preparations of the lungs were examined with Gram stain to exclude tissue contaminated with bacteria. During pentobarbital anesthesia, the tracheas of recipient mice were exposed by a midline neck incision and a blunted needle was passed through the mouth into the mid-trachea under direct vision. A polyethylene catheter was passed through this needle, and 0.1 ml of inoculum (2 × 105 P. carinii organisms) was injected, followed by 0.4 ml of air. The incision was sutured, and the mice were allowed to recover in a prone position.
Sham inoculations of wild-type and uPA knockout mice were performed in parallel. The lungs from uninfected athymic mice were removed and prepared in an identical manner but did not demonstrate P. carinii organisms on slides stained with modified Giemsa stain. Preparations were brought to identical volumes, and intratracheal inoculations were performed.
BAL fluid analysis.
At 4 weeks after intratracheal inoculation, wild-type and uPA knockout mice were exsanguinated during deep pentobarbital anesthesia. This time point was chosen based on previous work from our laboratory examining the development of infection in CD4-depleted mice and clearance of infection in immunologically intact mice (4). A polyethylene catheter was inserted into the trachea, and the lungs were lavaged with 11 ml of warmed calcium- and magnesium-free phosphate-buffered saline–0.6 mM EDTA in 0.5-ml aliquots (4). The BAL fluid was centrifuged at 500 × g for 10 min at 4°C, the pellets were washed twice in phosphate-buffered saline and the cells were enumerated with a hemocytometer. For flow cytometry, the cells were resuspended and stained for 30 min with fluorescein- or phycoerythrin-conjugated antibodies directed against CD3, CD4, or CD8 or with irrelevant, isotype-matched control antibodies (PharMingen, San Diego, Calif.) (4). For differential counting, 105 cells per BAL sample were washed onto nitrocellulose filters with 5-μm pores (Millipore, South San Francisco, Calif.) and the filters were mounted onto glass slides, fixed overnight in 10% buffered formalin, and stained with hematoxylin and eosin (H&E). Blinded differential counts were performed on at least 200 cells per slide (4).
Measurement of the intensity of P. carinii infection.
For scoring of infection, the mouse lungs were inflated with 10% buffered formalin through the same catheter used for BAL, fixed overnight, and embedded in paraffin. Histologic sections (5 μm thick), stained with H&E and with Gomori methenamine silver, were graded blindly for the extent of P. carinii infection (33) by using modifications of semiquantitative scales previously described and validated (3, 4). These scales are based upon grading of the entire lung surface area present on the slide rather than numbers of microscopic fields, and they range from 0 (no cysts identified and no foamy alveolar exudate in any field) to 4 (cysts and foamy alveolar exudate throughout the alveoli in most regions). Our laboratory and others have demonstrated that histologic grading of infection correlates closely with organism counts from homogenized lungs (3, 20).
Statistical methods.
Infection scores between experimental groups were compared with the Mann-Whitney test, and cell counts and cell numbers in BAL fluid were compared with t tests (42). Testing was performed with StatView software (Abacus Concepts, Berkeley, Calif.), and significance was accepted at P < 0.05.
RESULTS
Intensity of P. carinii infection.
At 4 weeks after intratracheal inoculation with P. carinii organisms, the intensity of infection in the lungs of wild-type and uPA knockout mice was compared (Fig. 1). Wild-type mice cleared the P. carinii inoculum by 4 weeks after inoculation, with only a single mouse demonstrating several individual, widely scattered P. carinii cysts. This finding is comparable to previous observations made with other immunocompetent mice (4). In contrast, the lungs of uPA knockout mice were uniformly heavily infected with P. carinii, with all mice demonstrating grade 3 (cysts throughout the alveoli in many regions) or grade 4 (cysts and foamy alveolar exudate throughout the alveoli in most regions) infections. To ensure that P. carinii infections were induced by inoculation and did not occur spontaneously, wild-type and uPA knockout mice were inoculated with sham homogenates prepared from the lungs of uninfected athymic mice. None of the lungs from sham-inoculated wild-type mice or uPA knockout mice (n = 3 for each group) contained P. carinii organisms.
FIG. 1.
Intensity of P. carinii infection. Wild-type (n = 9) and uPA knockout (n = 10) mice were inoculated intratracheally with P. carinii organisms, and the intensity of infection was scored 4 weeks after inoculation, using a histologic grading scale. Wild-type mice cleared the P. carinii inoculum, with only a single mouse demonstrating several individual, widely scattered P. carinii cysts. Conversely, uPA knockout mice were uniformly heavily infected with P. carinii, with all mice demonstrating grade 3 or grade 4 infections. Data represent medians obtained from two individual experiments. ∗, P < 0.007 by the Mann-Whitney test compared with wild-type mice.
Histology of P. carinii infection.
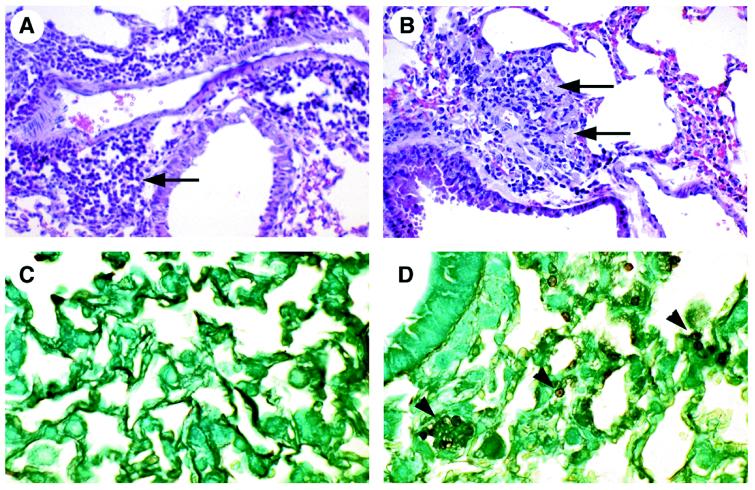
At 4 weeks after P. carinii inoculation, the lungs of wild-type mice demonstrated mild perivascular and peribronchiolar infiltrates composed of mononuclear cells but demonstrated no alveolar exudates (Fig. 2A). Lung sections stained with the Gomori methenamine silver stain did not show P. carinii organisms (Fig. 2C). In comparison, the lungs of uPA knockout mice demonstrated markedly fewer inflammatory infiltrates but demonstrated widespread granular, eosinophilic exudates indicative of P. carinii infection (Fig. 2B). Lung sections stained with the Gomori methenamine silver stain revealed widespread clusters of P. carinii organisms with typical morphology (Fig. 2D). These data demonstrate that clearance of P. carinii organisms from the lungs requires the presence of uPA. To ensure that histologic changes and accumulation of inflammatory cells were caused by P. carinii infection rather than by host material inoculated with the organisms, wild-type and uPA knockout mice were inoculated with sham homogenates prepared from the lungs of uninfected athymic mice. The lungs of sham-inoculated wild-type mice and uPA knockout mice were histologically normal and contained no P. carinii organisms (data not shown).
FIG. 2.
Histology of P. carinii infection. Lungs of wild-type mice inoculated with P. carinii demonstrated perivascular and peribronchiolar mononuclear cell infiltrates (arrow) (A) but no P. carinii organisms (C). Lungs of uPA knockout mice inoculated with P. carinii demonstrated markedly reduced mononuclear cell inflammation but contained alveolar exudate indicative of P. carinii infection (arrows) (B), confirmed by the presence of P. carinii organisms (arrowheads) (D). (A and B) H&E stain; magnification, ×200. (C and D) Gomori methenamine silver stain; magnification, ×400.
Accumulation of inflammatory cells.
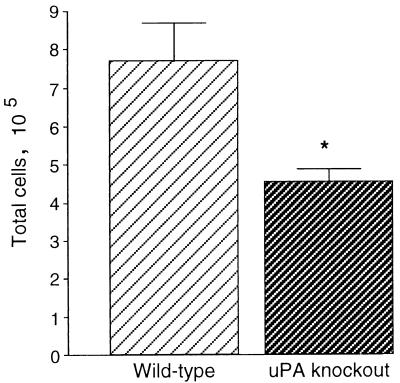
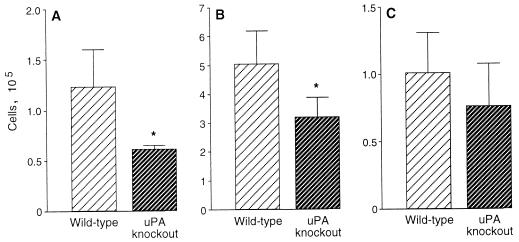
At 4 weeks after P. carinii inoculation, inflammatory cells in BAL fluid were counted and identified to quantitate cellular accumulation. BAL fluid from wild-type mice contained significantly greater numbers of cells than did BAL fluid from uPA knockout mice (Fig. 3). When the cell types were enumerated, the numbers of lymphocytes and macrophages were significantly decreased in BAL fluid from uPA knockout mice compared with wild-type mice (Fig. 4). Although the numbers of neutrophils present in the lungs of uPA knockout mice were also reduced compared with the numbers of neutrophils in the lungs of wild-type mice, this difference was not statistically significant. When lymphocytes were subjected to phenotype analysis by flow cytometry, no differences occurred in the percentages of CD4+ and CD8+ lymphocytes in the lungs of wild-type and uPA knockout mice. BAL fluid from wild-type mice contained 32.1 ± 3.0% CD4+ cells and 29.3 ± 5.6% CD8+ cells, while BAL fluid from uPA knockout mice contained 33.6 ± 4.4% CD4+ cells and 29.1 ± 3.2% CD8+ cells (mean ± standard error of the mean; n = 5 for each determination). Thus, the total numbers of T lymphocytes accumulating in the lungs during P. carinii infection were reduced in the absence of uPA, and this reduction was reflected equally in the CD4+ and CD8+ populations.
FIG. 3.
Inflammatory cell accumulation. Wild-type (n = 5) and uPA knockout (n = 5) mice were inoculated intratracheally with P. carinii organisms, and cells in BAL fluid were enumerated 4 weeks after inoculation. Data represent means ± SEM; ∗, P < 0.02 by t test compared with wild-type mice.
FIG. 4.
Cellular constituents of BAL fluid. Wild-type (n = 5) and uPA knockout (n = 5) mice were inoculated intratracheally with P. carinii organisms, and cells in BAL fluid were characterized 4 weeks after inoculation. Total numbers of lymphocytes (A), macrophages (B), and neutrophils (C) are indicated. Data represent means ± SEM; ∗, P < 0.05 by t test compared with wild-type mice.
DISCUSSION
These data demonstrate that in wild-type mice, clearance of P. carinii occurs during successful recruitment of inflammatory cells to the lungs. In contrast, uPA knockout mice are unable to provide effective defense against P. carinii, develop severe pneumonia characterized by alveolar exudates and large numbers of P. carinii organisms, and exhibit decreased accumulation of inflammatory cells in the lungs. The numbers of both lymphocytes and macrophages are significantly decreased in the lungs of the uPA knockout mice compared with wild-type mice. While total numbers of lymphocytes are reduced in the lungs of uPA knockout mice, the relative proportions of CD4+ and CD8+ populations are comparable to the proportions measured in the lungs of wild-type mice. Thus, deletion of the uPA gene prevents the clearance of P. carinii from the lungs of mice and significantly reduces the accumulation of immune and inflammatory cells required for defense.
These changes in accumulation of immune and inflammatory cells after challenge with P. carinii must be placed into context with our previous work examining cellular recruitment to the lungs of immunologically intact BALB/c mice during this opportunistic infection. We demonstrated accumulation of inflammatory cells in the lungs during clearance of P. carinii (4), resulting in increases in the numbers of lymphocytes and macrophages in BAL fluid. This finding confirms the central roles of CD4+ lymphocytes (4, 17, 19, 35), CD8+ lymphocytes (3), and macrophages (11, 22, 24) in host defense against P. carinii. In the present experiments, the lungs of wild-type mice demonstrated increased numbers of lymphocytes and macrophages in BAL fluid compared with the lungs of uPA knockout mice. These data are entirely consistent with the proposed requirement for uPA in inflammatory-cell recruitment, since uPA and uPAR (which permits cells to focus pericellular proteolysis) are both expressed by macrophages and activated lymphocytes (6, 12, 27, 28, 31). Thus, accumulation of inflammatory cells is required for effective clearance of P. carinii, and this accumulation is significantly decreased in the absence of uPA.
The uPA knockout mice demonstrated impaired recruitment of both CD4+ and CD8+ lymphocytes in response to P. carinii challenge in comparison to wild-type mice, consistent with previous data demonstrating that the expression of uPA and of uPAR are activation events in T lymphocytes. Resting T lymphocytes from healthy donors do not express uPAR, and only low levels of uPA are detected, but stimulation via T-cell receptors (TCRs) results in upregulation of both uPAR and uPA in T lymphocytes (6, 12, 27). Simultaneous clustering of TCR with the β2-integrin LFA-1 is synergistic in inducing uPAR expression (6), suggesting that T-lymphocyte binding to matrix counterligands further induces uPAR expression and T-lymphocyte activation. In vitro examinations of T lymphocytes demonstrate that activation and invasion can be inhibited by blocking uPA activity or by blocking uPAR with a specific antibody (6). While spleens from uPA knockout mice show effacement of lymphoid centers (34), lymphocyte development and repertoire have not yet been characterized. Thus, there is substantial but fragmentary evidence that expression of uPA and of uPAR are intrinsically involved in T-lymphocyte activation and migration.
The role that uPA plays in lymphocyte recruitment may not be limited to enhancing matrix proteolysis during cellular migration, since uPA may modulate interactive cytokine networks during inflammatory responses. For example, uPA has been shown to enhance the release of active interleukin-1 (IL-1) from macrophages (25), and uPAR is coexpressed with IL-2 receptors, establishing its expression in TCR-mediated T-lymphocyte activation (27). IL-2 expression is inhibited in the presence of serine protease inhibitors, suggesting that proteases such as uPA or plasmin are involved in the modulation of IL-2 (2). Inadequate production of IL-2 results in diminished lymphocyte proliferation and therefore may account for the blunted T-lymphocyte response seen in the uPA knockout mice in response to P. carinii and C. neoformans. IL-2 stimulation also upregulates uPAR expression by T lymphocytes (27). Therefore, IL-2 not only activates T lymphocytes but also endows them with the ability to focus uPA proteolytic activity through cell surface uPAR expression during entry into inflammatory foci. Thus, at least two uPA-mediated processes, proteolytic capacity and cell activation, may be impaired in the T-lymphocyte population of the uPA knockout mice. The present data indicate, in an in vivo model of infection, that uPA is indeed crucial for T-lymphocyte accumulation in the lungs.
The uPA knockout mice also demonstrated diminished recruitment of macrophages in response to P. carinii infection. Macrophages express both uPA and uPAR constitutively, and the expression of both is increased on exposure to inflammatory mediators (13, 15, 16, 36). In response to a chemotactic gradient, monocytes/macrophages tightly cluster uPAR at the leading edge of migration, focusing uPA proteolytic activity during directed migration (15). In addition to permitting matrix degradation during recruitment to inflammatory sites, uPA may be involved in macrophage activation, including signal transduction. Roles for uPA catalytic activity, uPA growth factor and binding domain, and uPA glycosylation have all been proposed (21, 29, 32). Therefore, it appears that uPA binding may play a contributing role in monocyte/macrophage chemotaxis, even if its catalytic activity is not required. Supportive of these findings are the results of a study demonstrating that the binding of uPA to monocytes/macrophages results in the phosphorylation of several protein tyrosine kinases (7). Our previous work, demonstrating that macrophage transcription of tumor necrosis factor requires uPA, supports the hypothesis that uPA-mediated signaling modulates expression of inflammatory cytokines (37).
The present data significantly broaden the scope and implications of our previous work demonstrating that the absence of uPA prevents the accumulation of inflammatory cells in response to the opportunistic fungus C. neoformans and also prevents the clearance of this fungus (14). Thus, uPA knockout mice have diminished inflammatory and immune responses to both P. carinii and C. neoformans. These opportunistic pathogens are similar in that successful host defense depends upon cell-mediated immunity (5, 18). Additionally, defective defense against both organisms occurs with quantitative impairment of inflammatory cell recruitment, rather than during the absolute depletion of these cells induced in previous investigations (3). The susceptibility of these knockout mice to opportunistic infection cannot be explained by baseline depletion of lymphocytes, since we previously demonstrated that numbers of intrapulmonary CD4+ and CD8+ T lymphocytes are increased in naive uPA knockout mice compared with wild-type mice (14). Conversely, the organisms differ significantly in important aspects of host defense and pathogenesis. P. carinii is predominantly an extracellular organism, with trophozoite forms causing alveolar injury during tight adherence to epithelial cells (41). Alveolar macrophages can ingest and kill P. carinii organisms (11, 22, 24), and impairment of macrophage uptake or phagocytosis in the uPA knockout mice could result in susceptibility. However, the lack of accumulation of viable organisms within alveolar macrophages of infected animals suggests that susceptibility is not wholly dependent upon impaired intracellular killing but, rather, is dependent on an impairment in extracellular killing (30). In contrast, the killing of C. neoformans is predominantly an intracellular event (9, 23, 26) and the alveolar macrophages of susceptible animals contain large numbers of viable C. neoformans organisms (14). Therefore, uPA is a critical modulator of cell-mediated immunity for both intracellular and extracellular killing in two disparate models of opportunistic infection.
Several methodologic aspects of the current study deserve comment. First, both wild-type and uPA knockout mice were inoculated intratracheally with identical preparations of P. carinii organisms on the same day. Therefore, each mouse received a challenge of equal organism numbers and virulence. As in our previous work, sham-inoculated mice did not develop infection, indicating that spontaneous P. carinii infection does not occur in this model. Second, we evaluated the possible confounding influence of alloantigen in these studies. Given the unavoidable presence of small amounts of BALB/c host materials present in the P. carinii preparations for inoculation, the exclusion of alloantigen as a cause of inflammation was essential. However, neither wild-type nor uPA knockout mice demonstrated histologic inflammation or P. carinii infection after receiving the sham inoculum. This observation agrees with our recently published work examining host defense in other gene-deleted mice of similar genetic background (33).
We conclude that deletion of the uPA gene prevents the clearance of P. carinii and reduces inflammatory-cell recruitment in vivo. These results confirm a central role for uPA in inflammation and host defense directed against P. carinii. Because plasminogen activator activity appears to be decreased in the lungs of HIV-infected individuals (1), our findings suggest that modulation of uPA in vivo could provide a novel immunotherapeutic approach to improving host defense against this important opportunistic pathogen.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants HL-57011 and HL-59823 (to J.M.B.) and HL-51082 (to M.R.G.) from the National Heart, Lung, and Blood Institute and by Merit Review Funds from the Medical Research Service, Department of Veterans Affairs (to M.R.G.).
We thank Jennifer A. Fuller for expert technical assistance and Jeffrey L. Curtis and Galen B. Toews for helpful discussion and review of the manuscript.
REFERENCES
- 1.Angelici E, Contini C, Romani R, Epifano O, Serra P, Canipari R. Production of plasminogen activator and plasminogen activator inhibitors by alveolar macrophages in control subjects and AIDS patients. AIDS. 1996;10:283–290. doi: 10.1097/00002030-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Auberger P, Sonthonnax S, Peyron J-F, Mari B, Fehlmann M. A chymotryptic-type serine protease is required for IL-2 production by Jurkat T cells. Immunology. 1990;70:547–550. [PMC free article] [PubMed] [Google Scholar]
- 3.Beck J M, Newbury R L, Palmer B E, Warnock M L, Byrd P K, Kaltreider H B. Role of CD8+ lymphocytes in host defense against Pneumocystis carinii in mice. J Lab Clin Med. 1996;128:477–487. doi: 10.1016/s0022-2143(96)90044-x. [DOI] [PubMed] [Google Scholar]
- 4.Beck J M, Warnock M L, Curtis J L, Sniezek M J, Arraj-Peffer S M, Kaltreider H B, Shellito J E. Inflammatory responses to Pneumocystis carinii in mice selectively depleted of helper T lymphocytes. Am J Respir Cell Mol Biol. 1991;5:186–197. doi: 10.1165/ajrcmb/5.2.186. [DOI] [PubMed] [Google Scholar]
- 5.Beck J M, Warnock M L, Kaltreider H B, Shellito J E. Host defense against Pneumocystis carinii in mice selectively depleted of CD4+ lymphocytes. Chest. 1993;103:116S–118S. doi: 10.1378/chest.103.2_supplement.116s-a. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi E, Ferrero E, Fazioli F, Mangeili F, Wang J, Bender J, Blasi F, Pardi R. Integrin-dependent induction of functional urokinase receptors in primary T lymphocytes. J Clin Investig. 1996;98:1133–1141. doi: 10.1172/JCI118896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohuslav J, Horejesi V, Hansmann C, Stockl J, Weidle U H, Majdic O, Bartke I, Knapp W, Stockinger H. Urokinase plasminogen activator receptor, β2-integrins, and Src-kinases within a single receptor complex of human monocytes. J Exp Med. 1995;181:1381–1390. doi: 10.1084/jem.181.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmeliet P, Schoonjans L, Kieckens L, Ream B, Degen J, Bronson R, De Vos R, van den Oord J J, Collen D, Mulligan R C. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994;368:419–424. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- 9.Domer J E, Carrow E W. Immunity to fungal infections. Adv Exp Med Biol. 1983;162:383–403. doi: 10.1007/978-1-4684-4481-0_36. [DOI] [PubMed] [Google Scholar]
- 10.Estreicher A, Mühlhause J, Carpentier J, Orci L, Vassalli J. The receptor for urokinase type plasminogen activator polarizes expression of the protease to the leading edge of migrating monocytes and promotes degradation of enzyme inhibitor complexes. J Cell Biol. 1990;111:783–792. doi: 10.1083/jcb.111.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ezekowitz R A, Williams D J, Koziel H, Armstrong M Y, Warner A, Richards F F, Rose R M. Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature. 1991;351:155–158. doi: 10.1038/351155a0. [DOI] [PubMed] [Google Scholar]
- 12.Gunderson D, Tran-Thang C, Sordat B, Mourali F, Ruegg C. Plasmin-induced proteolysis of Tenascin-C. Modulation by T lymphocyte-derived urokinase-type plasminogen activator and effect on T lymphocyte adhesion, activation, and cell clustering. J Immunol. 1997;158:1051–1060. [PubMed] [Google Scholar]
- 13.Gyetko M, Shollenberger S, Sitrin R. Urokinase expression in mononuclear phagocytes: cytokine-specific modulation by interferon gamma and tumor necrosis factor-alpha. J Leukoc Biol. 1992;51:256–263. doi: 10.1002/jlb.51.3.256. [DOI] [PubMed] [Google Scholar]
- 14.Gyetko M R, Chen G-H, McDonald R A, Goodman R, Huffnagle G B, Wilkinson C C, Fuller J A, Toews G B. Urokinase is required for the pulmonary inflammatory response to Cryptococcus neoformans: a murine transgenic model. J Clin Investig. 1996;97:1818–1826. doi: 10.1172/JCI118611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gyetko M R, Todd III R F, Wilkinson C C, Sitrin R G. The urokinase receptor is required for human monocyte chemotaxis in vitro. J Clin Investig. 1994;93:1380–1387. doi: 10.1172/JCI117114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gyetko M R, Wilkinson C C, Sitrin R G. Monocyte urokinase expression: Modulation by interleukins. J Leukoc Biol. 1993;53:598–601. doi: 10.1002/jlb.53.5.598. [DOI] [PubMed] [Google Scholar]
- 17.Harmsen A G, Stankiewicz M. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J Exp Med. 1990;172:937–945. doi: 10.1084/jem.172.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huffnagle G B, Yates J L, Lipscomb M F. T cell-mediated immunity in the lung: a Cryptococcus neoformans pulmonary infection model using SCID and athymic nude mice. Infect Immun. 1991;59:1423–1433. doi: 10.1128/iai.59.4.1423-1433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishimine T, Kawakami K, Nakamoto A, Saito A. Analysis of cellular response and gamma interferon synthesis in bronchoalveolar lavage fluid and lung homogenate of mice infected with Pneumocystis carinii. Microbiol Immunol. 1995;39:49–58. doi: 10.1111/j.1348-0421.1995.tb02167.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim C K, Foy J M, Cushion M T, Stanforth D, Linke M J, Hendrix H L, Walzer P D. Comparison of histologic and quantitative techniques in evaluation of therapy for experimental Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1987;31:197–201. doi: 10.1128/aac.31.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirchheimer J C, Wojta J, Christ G, Binder B R. Proliferation of a human epidermal tumor cell line stimulated by urokinase. FASEB J. 1987;1:125–128. doi: 10.1096/fasebj.1.2.3038646. [DOI] [PubMed] [Google Scholar]
- 22.Limper A H, Hoyte J S, Standing J E. The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J Clin Investig. 1997;99:2110–2117. doi: 10.1172/JCI119384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipscomb M F, Huffnagle G B, Lovchik J A, Lyons C R, Pollard A M, Yates J L. The role of T lymphocytes in pulmonary microbial defense mechanisms. Arch Pathol Lab Med. 1993;117:1225–1232. [PubMed] [Google Scholar]
- 24.Masur H, Jones T C. The interaction in vitro of Pneumocystis carinii with macrophages and L-cells. J Exp Med. 1978;147:157–170. doi: 10.1084/jem.147.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsushima K, Taguchi M, Kovacs E J, Young H A, Oppenheim J J. Intracellular localization of human monocyte associated interleukin-1 (IL-1) activity and release of biologically active IL-1 from monocytes by trypsin and plasmin. J Immunol. 1986;136:2883–2891. [PubMed] [Google Scholar]
- 26.Mody C H, Tyler C L, Sitrin R G, Jackson C, Toews G B. Interferon-gamma activates rat alveolar macrophages for anticryptococcal activity. Am J Respir Cell Mol Biol. 1991;5:19–26. doi: 10.1165/ajrcmb/5.1.19. [DOI] [PubMed] [Google Scholar]
- 27.Nykjaer A, Møller B, Todd III R F, Christensen T, Andreasen P A, Gliemann J, Petersen C M. Urokinase receptor: an activation antigen in human T lymphocytes. J Immunol. 1994;152:505–516. [PubMed] [Google Scholar]
- 28.Nykjaer A, Petersen C, Christensen E, Davidsen O, Gliemann J. Urokinase receptors in human monocytes. Biochim Biophys Acta. 1990;1052:399–407. doi: 10.1016/0167-4889(90)90149-8. [DOI] [PubMed] [Google Scholar]
- 29.Odekon L E, Blasi F, Rifkin D B. Requirement for receptor-bound urokinase in plasmin-dependent cellular conversion of latent TGF-beta to TGF-beta. J Cell Physiol. 1994;158:398–407. doi: 10.1002/jcp.1041580303. [DOI] [PubMed] [Google Scholar]
- 30.Pesanti E L. Interaction of cytokines and alveolar cells with Pneumocystis carinii in vitro. J Infect Dis. 1991;163:611–616. doi: 10.1093/infdis/163.3.611. [DOI] [PubMed] [Google Scholar]
- 31.Plesner T, Ploug M, Ellis V, Ronne E, Høyer-Hansen G, Wittrup M, Pedersen T L, Tscherning T, Danø K, Hansen N E. The receptor for urokinase-type plasminogen activator and urokinase is translocated from two distinct intracellular compartments to the plasma membrane on stimulation of human neutrophils. Blood. 1994;83:808–815. [PubMed] [Google Scholar]
- 32.Rabban S A, Mazar A P, Berneir S M, Haq M, Bolivar I, Henkin J, Goltzman D. Structural requirements for the growth factor activity of the amino-terminal domain of urokinase. J Biol Chem. 1992;267:14151–14156. [PubMed] [Google Scholar]
- 33.Rudmann D G, Preston A M, Moore M W, Beck J M. Susceptibility to Pneumocystis carinii in mice depends on simultaneous depletion of interferon-gamma and type 1 and 2 tumor necrosis factor receptor genes. J Immunol. 1998;161:360–366. [PubMed] [Google Scholar]
- 34.Shapiro R L, Duquette J G, Nunez I, Roses D F, Harris M N, Wilson E L, Rifkin D B. Animal model: Urokinase-type plasminogen activator-deficient mice are predisposed to staphylococcal botryomycosis, pleuritis, and effacement of lymphoid follicles. Am J Pathol. 1997;150:359–369. [PMC free article] [PubMed] [Google Scholar]
- 35.Shellito J, Suzara V V, Blumenfeld W, Beck J M, Steger H J, Ermak T H. A new model of Pneumocystis carinii infection in mice selectively depleted of helper T lymphocytes. J Clin Investig. 1990;85:1686–1693. doi: 10.1172/JCI114621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sitrin R, Todd III R, Mizukami I, Gross T, Shollenberger S, Gyetko M. Cytokine-specific regulation of urokinase receptor (CD87) expression by U937 mononuclear phagocytes. Blood. 1994;84:1268. [PubMed] [Google Scholar]
- 37.Sitrin R G, Shollenberger S B, Strieter R M, Gyetko M R. Endogenously produced urokinase activity amplifies tumor necrosis factor-α secretion by THP-1 mononuclear phagocytes. J Leukoc Biol. 1996;59:302–311. doi: 10.1002/jlb.59.2.302. [DOI] [PubMed] [Google Scholar]
- 38.Stansell J D. Pneumocystis carinii pneumonia. In: Rosen M J, Beck J M, editors. Human immunodeficiency virus and the lung. New York, N.Y: Marcel Dekker, Inc.; 1998. pp. 271–312. [Google Scholar]
- 39.Walzer P D. Pathogenic mechanisms. In: Walzer P D, editor. Pneumocystis carinii pneumonia. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1994. pp. 251–265. [Google Scholar]
- 40.Yale S H, Limper A H. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illnesses and prior corticosteroid therapy. Mayo Clin Proc. 1996;71:5–13. doi: 10.4065/71.1.5. [DOI] [PubMed] [Google Scholar]
- 41.Yoneda K, Walzer P D. Mechanism of pulmonary alveolar injury in experimental Pneumocystis carinii pneumonia in the rat. Br J Exp Pathol. 1981;62:339–346. [PMC free article] [PubMed] [Google Scholar]
- 42.Zar J H. Biostatistical analysis. 2nd ed. Englewood Cliffs, N.J: Prentice-Hall; 1984. [Google Scholar]