Abstract
Recently, we demonstrated that human monocyte-derived macrophages (MDM) treated with chloroquine or ammonium chloride had markedly increased antifungal activity against the AIDS-related pathogen Cryptococcus neoformans. Both of these agents raise the lysosomal pH, which suggested that the increased antifungal activity was a function of alkalinizing the phagolysosome. Moreover, there was an inverse correlation between growth of C. neoformans in cell-free media and pH. These data suggested that C. neoformans was well adapted to survive within acidic compartments. To test this hypothesis, we performed studies to determine the pH of human MDM and neutrophil phagosomes containing C. neoformans. Fungi were labeled with the isothiocyanate derivatives of two pH-sensitive probes: fluorescein and 2′,7′-difluorofluorescein (Oregon Green). These probes have pKas of 6.4 and 4.7, respectively, allowing sensitive pH detection over a broad range. The phagosomal pH averaged approximately 5 after ingestion of either live or heat-killed fungi and remained relatively constant over time, which suggested that C. neoformans does not actively regulate the pH of its phagosome. The addition of 10 and 100 μM chloroquine resulted in increases in the phagosomal pH from a baseline of 5.1 up to 6.5 and 7.3, respectively. Finally, by immunofluorescence, colocalization of C. neoformans and the MDM lysosomal membrane protein LAMP-1 was demonstrated, establishing that fusion of C. neoformans-laden phagosomes with lysosomal compartments takes place. Thus, unlike many other intracellular pathogens, C. neoformans does not avoid fusion with macrophage lysosomal compartments but rather resides and survives in an acidic phagolysosome.
A key to the success of intracellular pathogens that parasitize macrophages is the ability to survive within the hostile environment of the phagolysosome or to evade the phagolysosome altogether. Following phagocytosis of a variety of particulate stimuli, the phagosomal pH rapidly decreases to below 5.5 (10, 42). This relatively low pH is thought to enhance host defenses by inhibiting microbial growth and enhancing the activity of a variety of degradative enzymes active only under acidic conditions. Acidity is also thought to be necessary for intracellular trafficking and antigen presentation (10). To avoid the hostile low pH of the phagolysosome, some organisms have evolved mechanisms to modulate acidification. For example, phagosomes containing Mycobacterium tuberculosis and M. avium do not acidify below 6.3 to 6.5, apparently as a result of a selective block in accumulation of vacuolar proton-ATPase (37, 42). Macrophage phagolysosomes containing live Histoplasma capsulatum also have a near neutral pH (10). Other microorganisms, including Legionella pneumophila and Toxoplasma gondii, avoid acidification by residing in vesicles that fail to fuse with lysosomal compartments (1, 17, 40, 45).
Inhibition or avoidance of acidification can be a two-edged sword for microorganisms, as mobilization of iron from transferrin and ferritin, the two major sources of iron in the mononuclear phagocyte, is dependent on an acidic environment (3, 6). At neutral pH, iron remains bound to transferrin and thus unavailable to the microbe (3, 6). Iron availability from ferritin is thought to occur following pH-dependent proteolysis in lysosomes (38, 39, 41). Chloroquine, a diprotic weak base which raises the endocytic and lysosomal pH of eukaryotic cells (3, 25), inhibits the growth of L. pneumophila, H. capsulatum, and Francisella tularensis in human mononuclear phagocytes by limiting iron availability in the phagolysosome (3, 12, 30).
The encapsulated fungus Cryptococcus neoformans is a significant cause of morbidity and mortality in patients with impaired cell-mediated immunity, especially those with AIDS (5, 7). While not an obligate intracellular pathogen, C. neoformans is able to gain entry into macrophages via complement receptors (26). In vitro, killing of C. neoformans by human macrophages, even following cytokine activation, has not been convincingly demonstrated (8, 22, 23). Recently, we demonstrated that human monocyte-derived macrophages (MDM) treated with chloroquine had markedly increased fungistatic and fungicidal activity against C. neoformans (25). As opposed to the above-mentioned pathogens, chloroquine exerted its effect on C. neoformans independent of iron deprivation. Nevertheless, the mechanism appeared to be dependent on raising the pH of the phagolysosome, as another alkalinizing agent, ammonium chloride, had a similar effect. Moreover, the growth of C. neoformans in cell-free tissue culture medium was inversely proportional to pH, suggesting that alkalinizing the pH could significantly retard cryptococcal growth.
In view of the above studies suggesting a critical role for phagosomal pH in the pathogenesis of cryptococcosis, we sought to determine the pH of C. neoformans-laden phagosomes. Most techniques to determine intracellular pH utilize fluorescein derivatives. The emission spectrum of fluorescein is pH sensitive when excited at 498 nm and pH insensitive when excited at 450 nm, allowing ratio imaging that is independent of probe concentration (44). However, fluorescein has a pKa of around 6.4 and thus cannot be used to accurately quantitate pH much below 5.5. Recently a new pH-sensitive probe, Oregon Green (2′,7′-difluorofluorescein), was synthesized. Oregon Green has the same excitation and emission wavelengths as fluorescein but has a pKa of 4.7, allowing accurate measurement of phagosomal pH below 5.5. In the present study, C. neoformans was labeled with the isothiocyanate derivatives of fluorescein and Oregon Green, and then the pH of MDM phagosomes containing C. neoformans in the presence and absence of chloroquine was determined by dual-fluorescence spectrofluorimetry. Moreover, fusion of C. neoformans phagosomes with lysosomal compartments was examined by immunofluorescence microscopy.
MATERIALS AND METHODS
Materials.
Unless otherwise noted, reagents were obtained from Sigma Chemical Co. (St. Louis, Mo.). All experiments were performed under conditions carefully designed to minimize endotoxin contamination as described previously (27). RPMI 1640 and phosphate-buffered saline (PBS) were obtained from BioWhittaker, Inc. (Walkersville, Md.). RPMI 1640 was supplemented with l-glutamine, penicillin, and streptomycin but was without phenol red. Pooled human serum (PHS) was obtained by combining serum from more than 10 healthy donors under ice-cold conditions and storing it at −70°C to preserve complement activity. Culture medium, unless otherwise stated, was RPMI 1640 containing 10% PHS. Chloroquine was prepared as a 10× stock solution in RPMI 1640 and filter sterilized prior to use. The monoclonal anticapsular antibody 2H1 (a gift from Arturo Casadevall, Albert Einstein College of Medicine, Bronx, N.Y.) was purified from ascites fluid by passage over a protein G column as described previously (15) and used at a final concentration of 12.5 μg/ml. Preliminary experiments determined that this concentration was subagglutinating and opsonic. Unless otherwise noted, cells were incubated at 37°C in humidified air supplemented with 5% CO2.
C. neoformans.
Serotype A strain 145 of C. neoformans was grown on Sabouraud dextrose agar at 30°C. Fungi were harvested after 4 days of growth, washed three times in PBS, counted in a hemocytometer, and resuspended at the desired concentration. Where indicated, fungi were heat killed by immersion in a 60°C water bath for 30 min.
Live C. neoformans fungi were surface labeled with fluorescein isothiocyanate (FITC) by incubation in a 5-mg/ml solution of FITC dissolved in 50 mM borate buffer (pH 8.0) for 2 h at 37°C. Live fungi were labeled with the isothiocyanate derivative of Oregon Green (OGITC; a gift from Molecular Probes, Eugene, Oreg.) by incubation in a 1-mg/ml solution OGITC dissolved in PBS for 2 h at 37°C. Live fungi were freshly labeled the day of the experiment. Labeling with FITC or OGITC did not affect the viability of the C. neoformans as measured by counting CFU following dilutions and spreading on Sabouraud dextrose agar. Heat-killed fungi were labeled in a similar manner, except that incubation was for 18 h. Fungi were washed five times in PBS prior to addition to phagocytes.
Isolation of phagocytes.
Peripheral blood was obtained by venipuncture from healthy volunteers not taking any medications. The same donor was not used more than once. Blood was anticoagulated with pyrogen-free heparin (Elkins-Sinn Inc., Cherry Hill, N.J.) and centrifuged at 500 × g for 15 min. Neutrophils and peripheral blood mononuclear cells (PBMC) were isolated by centrifugation of the leukocyte-rich buffy-coat cells on a Ficoll-Hypaque density gradient as in our previous studies (23, 24). The neutrophil-rich layer was rid of contaminating erythrocytes by hypotonic lysis. PBMC (108) were suspended in 20 ml of RPMI 1640 containing 10% PHS and added to 100- by 15-mm petri dishes containing 20 poly-l-lysine-coated 9- by 22-mm rectangular glass coverslips (23). This quantity of cells resulted in a monolayer on the coverslips. MDM were obtained by culturing the PBMC for 6 to 8 days. MDM cultured on surfaces coated with poly-l-lysine are able to inhibit the growth of, but not kill, C. neoformans (23).
pH of MDM phagosomes.
Nonadherent cells were washed free, and glass coverslips containing MDM were transferred to 35- by 10-mm petri dishes (two per dish) with fresh culture medium. C. neoformans fungi (2 × 106) either labeled with FITC or OGITC or left unlabeled were added to each dish and incubated for 3 to 18 h. Coverslips were then dipped in 0.1% trypan blue for 3 min to quench the fluorescence of bound but not internalized C. neoformans. Preliminary experiments demonstrated that under these conditions, trypan blue quenched over 99% of the fluorescence of the extracellular organisms without significantly affecting fluorescence of the internalized C. neoformans (data not shown).
Glass coverslips were placed in a holding device that aligns the coverslip at a 30° angle to the excitation beam to minimize the effect of light reflection (32) and then placed in cuvettes containing 1 ml of RPMI 1640 prewarmed to 37°C. With the emission wavelength set at 520 nm, the ratio of fluorescence excitation at 498 and 450 nm was obtained in a thermostated Hitachi F-4500 spectrofluorimeter. Then, for each condition, a standard curve was generated by obtaining ratios of excitation at 498 and 450 nm in C. neoformans-laden MDM equilibrated in solutions of defined pH composed of 2.8 × 10−5 M nigericin, 80 mM potassium chloride, 30 mM sodium chloride, 30 mM potassium phosphate, 5.5 mM glucose, 0.8 mM magnesium sulfate, and 1.3 mM calcium chloride. Nigericin is a K+- and H+-specific ionophore that rapidly equilibrates phagosomal and extracellular pH under these conditions (20). Background values for excitation at 498 and 450 nm, emission at 520 nm, were obtained for MDM challenged with unlabeled C. neoformans and subtracted from readings obtained for labeled cells.
To calculate the average intracellular pH of the samples, the ratio of excitation at 498 and 450 nm (following background subtraction) from unknowns was compared with the standard pH titration curve generated with nigericin-permeabilized MDM.
pH of neutrophil phagosomes.
Essentially the same procedure was used to determine the pH of neutrophil phagosomes containing C. neoformans, with the following exceptions. Neutrophils (4 × 106) and OGITC-labeled live C. neoformans (1.5 × 106) were tumbled at 37°C for 1, 2, or 3 h in polypropylene microcentrifuge tubes containing a final volume of 1 ml RPMI 1640 with 10% PHS. Extracellular fungi were quenched with trypan blue; the neutrophils were washed free of trypan blue and resuspended in 1.2 ml of RPMI 1640. The cells were placed in a cuvette containing a stir bar, and fluorescence emitted at 520 nm from excitation at 498 and 450 nm was obtained.
Immunofluorescence staining for lysosomal membrane protein LAMP-1.
MDM were cultured for 6 to 8 days as described above except in eight-chamber glass slides (Lab-Tek; Nalge Nunc International, Naperville, Ill.). MDM were washed to remove nonadherent cells and incubated for 3 h with 104 live C. neoformans in the presence of RPMI 1640 containing 10% PHS. Cells were fixed and stained for LAMP-1 (CD107a) and C. neoformans capsular polysaccharide by a modification of established protocols (1, 45). Briefly, MDM were fixed in buffered paraformaldehyde-periodate and permeabilized with −20°C methanol. Cells were then sequentially incubated in (i) blocking buffer (PBS containing 2% goat serum); (ii) 1:100 dilution of anti-LAMP-1 (mouse immunoglobulin G1 monoclonal antibody H4A3; Developmental Studies Hybridoma Bank, Iowa City, Iowa [4]), and a 1:2,000 dilution of rabbit polyclonal anticapsular antibody (a gift from Thomas Kozel, Reno, Nev.); (iii) blocking buffer; (iv) 1:100 dilution of Alexa 488-conjugated goat anti-mouse antibody (Molecular Probes) and 1:100 dilution of Alexa 594-conjugated goat anti-rabbit antibody; and (v) blocking buffer. Control wells were incubated with irrelevant isotype-matched antibody 36.65 (directed against the hapten p-azophenylarsonate; a gift from Arturo Casadevall) (35) in lieu of H4A3. Cells were then visualized by epifluorescence microscopy (IMT-2 microscope; Olympus, Lake Success, N.Y.), using the B and G dichroic mirror units for Alexa 488 and Alexa 594, respectively. Phagosome-lysosome fusion was considered to take place if colocalization of LAMP-1 and cryptococcal capsular polysaccharide was observed (1, 45).
Statistics.
Means and standard errors (SE) were compared by the two-tailed paired t test. Adjustments for significance based on multiple comparisons were made by using Bonferroni’s method. Statistical significance was considered achieved if the P value multiplied by the number of comparisons was less than 0.05. All statistical calculations were performed with SigmaStat statistical software (Jandel Corporation, San Rafael, Calif.).
RESULTS
Calibration curves with OGITC- and FITC-labeled C. neoformans.
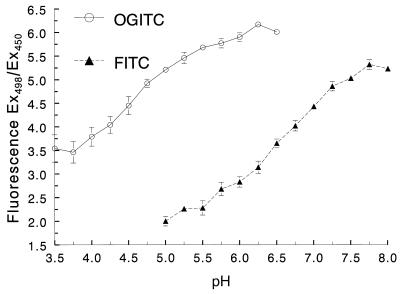
Initial experiments demonstrated that maximal pH-dependent fluorescence for both OGITC- and FITC-labeled C. neoformans occurred at 498 nm and that pH-independent fluorescence occurred at 450 nm (data not shown). These wavelengths were used for the remainder of the studies. Next, we generated standard curves to verify that OGITC and FITC could be used as probes to measure phagosomal pH. Coverslips containing adherent MDM were challenged with labeled C. neoformans for 3 h. Following quenching of the fluorescence of extracellular fungi with trypan blue, the coverslips were placed in cuvettes containing nigericin in a potassium-rich buffer of defined pH. This treatment equilibrates the pH in the phagosome with the pH in the buffer (10, 20). Following each pH change, we allowed 4 min for full equilibration to occur and then obtained the ratio of fluorescence emissions at 520 nm from excitation at 498 and 450 nm. As expected based on the known pKa of the two free probes, C. neoformans labeled with OGITC and FITC exhibited usable pH-dependent fluorescence over the ranges of approximately 3.75 to 5.50 and 5.50 to 7.50, respectively (Fig. 1). These calibration curves demonstrate that by using C. neoformans labeled with either OGITC or FITC, we could accurately determine the pH of phagosomes in MDM over a broad pH range.
FIG. 1.
Relationship between the ratio of excitation at 498 and 450 (Ex498/Ex450) and pH in MDM laden with C. neoformans labeled with OGITC and FITC. MDM were challenged for 3 h with C. neoformans labeled with OGITC or FITC and then permeabilized with nigericin-KCl in buffers at the pH indicated on the abscissa. Under such conditions, the pHs of the phagosome and the buffer are the same. Data are means ± SE of three independent experiments, each performed in duplicate.
pH of C. neoformans-containing phagosomes.
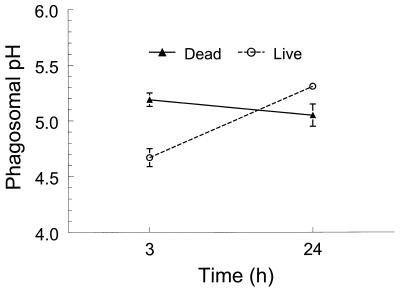
Having validated our methodology, we next determined the pH of MDM phagosomes containing C. neoformans. All experiments compared OGITC- and FITC-labeled C. neoformans. However, the data shown are for OGITC-labeled fungi, as the calculated phagosomal pH was consistently in the sensitive range for OGITC and insensitive range for FITC. Comparisons between live and dead C. neoformans were made 3 and 24 h following phagocytosis. For the dead fungi, the calculated phagosomal pH did not change significantly over time (5.2 ± 0.1 and 5.1 ± 0.2 at 3 and 24 h, respectively [Fig. 2]). However, over the same time course, the pH of phagosomes containing live fungi increased from 4.7 ± 0.2 to 5.3 ± 0.1 (P = 0.0056 by Student’s t test). By epifluorescence microscopy, FITC and OGITC remained strongly associated with C. neoformans throughout the 24-h incubation period with MDM. Thus, fluorescent label was observed only in association with the fungus and not in other locations in the MDM (data not shown). Nevertheless, the possibility that a small amount of label was shed outside the phagolysosome cannot be excluded.
FIG. 2.
Phagosomal pH of live and dead C. neoformans at various time points. MDM were challenged with OGITC-labeled live or dead fungi for 3 or 24 h, and then the pH of the intracellular fungi was determined as described in Materials and Methods. Data are means ± SE of four independent experiments, each performed in duplicate. P = 0.0004, comparing live fungi with dead fungi at 3 h; P = 0.0056, comparing live fungi at 3 and 24 h.
We next sought to determine whether changing the receptor-mediated route of entry of C. neoformans by the addition of the monoclonal anticapsular antibody 2H1 would affect the resulting phagosomal pH. However, in three independent experiments, the pH values were similar in the presence and absence of antibody (5.2 ± 0.1 and 5.1 ± 0.2, respectively).
Effect of chloroquine on the phagosomal pH.
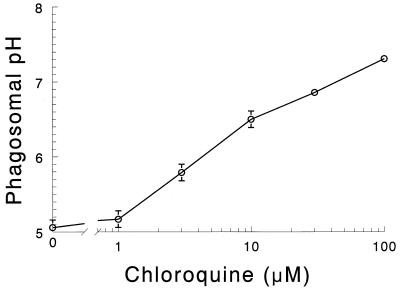
Our previous studies demonstrated that human MDM treated with chloroquine had markedly increased anticryptococcal activity (25). Therefore, we were interested in determining the pH in C. neoformans-laden phagosomes following chloroquine treatment. MDM were incubated with various concentrations of chloroquine for 30 min and then challenged with C. neoformans labeled with either OGITC or FITC. For calculated pH of <5.5, data obtained with OGITC were utilized; for values ≥5.5, data obtained with FITC were adopted. Phagosomal pH increased in a dose-dependent fashion over the concentration range, 1 to 100 μM, tested (Fig. 3). At 10 μM chloroquine, which was the concentration used in our previous studies to demonstrate enhancement of MDM-mediated anticryptococcal activity (25), the calculated pH was 6.5 ± 0.1.
FIG. 3.
Effect of chloroquine on the phagosomal pH. MDM were challenged with OGITC- or FITC-labeled heat-killed C. neoformans for 3 h in the presence of the indicated concentration of chloroquine. The pH of the intracellular fungi was determined as described in Materials and Methods. Data are means ± SE of three independent experiments, each performed in duplicate.
Phagosomal pH in neutrophils.
Neutrophils phagocytose and kill C. neoformans and are thought to participate in host defenses against C. neoformans, especially as part of first-line defenses before a cell-mediated immune response develops (7, 9). Therefore, we examined the pH of neutrophil phagosomes containing OGITC-labeled live C. neoformans. After 1, 2, and 3 h of incubation of neutrophils and C. neoformans, the phagosomal pH values were 5.2 ± 0.1, 5.2 ± 0.1, and 5.0 ± 0.04, respectively (means ± SE of four independent experiments, each of which had two to four replicate samples; P = nonsignificant, comparing any two groups).
Fusion of C. neoformans phagosomes with late endosomal compartments.
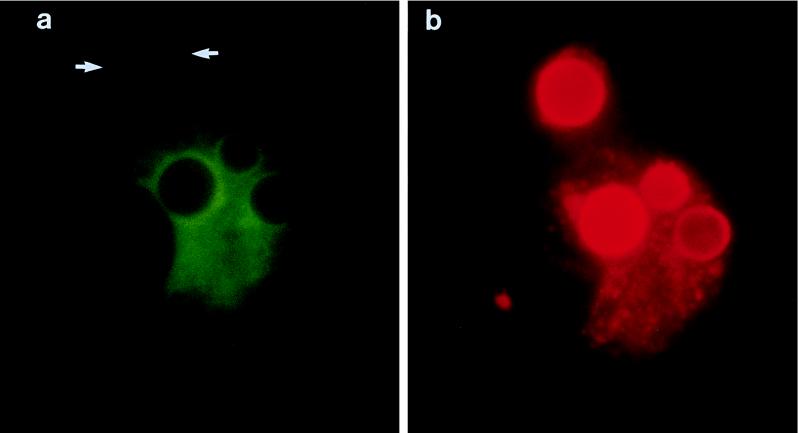
The above experiments demonstrating that acidification of C. neoformans phagosomes occurred strongly suggested that the vacuolar proton-ATPase fuses with the phagosome. In the final set of experiments, we sought to determine whether fusion of C. neoformans phagosomes with endosomal and lysosomal compartments occurred. To accomplish this, C. neoformans-laden MDM were simultaneously stained for LAMP-1 and cryptococcal capsule. LAMP-1 is a type I transmembrane glycoprotein that is localized in lysosomes and endosomes (34). In three independent experiments, intense LAMP-1 fluorescence surrounding the C. neoformans capsule was observed (Fig. 4). This pattern was seen in over 90% of the phagosomes. Other than very faint background autofluorescence, green fluorescence was not seen in cells incubated with the control antibody 36.65 in lieu of anti-LAMP-1 (not shown). Capsular polysaccharide staining was seen not only in association with the surface of C. neoformans but also scattered throughout the MDM (Fig. 4b).
FIG. 4.
Phagosome-lysosome fusion following MDM uptake of C. neoformans. MDM were challenged with live fungi for 3 h and then immunofluorescently stained for LAMP-1 with Alexa 488 and C. neoformans capsule with Alexa 494 as described in Materials and Methods. A single MDM that has phagocytosed four C. neoformans is shown. (a) LAMP-1 fluorescence (green) intensely distributed around the surface of the fungi (which faintly stains red). One fungus (arrows) appears to have been recently phagocytosed based on its appearance under phase-contrast microscopy (not shown) and has only a small rim of green fluorescence. LAMP-1 staining can also be appreciated in other parts of the cell except the nucleus. The same pattern of green fluorescence was seen in MDM stained for LAMP-1 with Alexa 488 but for which staining with the anticapsular antibody was not performed (not shown). (b) Capsule fluorescence (red) of the same cell. The C. neoformans capsule surrounding the four C. neoformans cells is readily apparent. Red fluorescence is also seen elsewhere in the MDM, mostly as a result of capsular polysaccharide secreted from the fungus. A similar pattern of fluorescence was seen in cells stained only for anticapsular antibody and not LAMP-1 (data not shown).
DISCUSSION
The studies presented herein demonstrate that C. neoformans resides in an acidic phagosome in human MDM and neutrophils. Although others have reported data for studies using FITC and related fluorescein dyes to determine phagosomal pH below 5.5 (10, 31, 32, 37, 42), we believe that our data presented in Fig. 1, as well as the calibration curves of others (10), demonstrate that accuracy is lost when the pH goes much below this value. The use of Oregon Green as a pH probe represents a significant advance in this regard in that accurate determination of phagosomal pH is still possible even when the phagosome is fully acidified. Moreover, by the use of both Oregon Green and traditional fluorescein as probes, the entire pH range encountered in mammalian phagosomes can be accurately quantitated.
The pH in MDM phagosomes containing live C. neoformans was similar to that seen with dead fungi over 24 h. These data suggest that unlike other pathogens such as M. tuberculosis and H. capsulatum, C. neoformans does not regulate the pH of its phagosome to an appreciable extent. C. neoformans grows more rapidly in acidic media than in neutral or alkaline media (18, 25) and appears to be able to resist the action of the macrophage lysosomal enzymes, which function optimally at acid pH (8, 25, 26). Thus, the pathogenicity of C. neoformans is not dependent on avoidance of an acidic pH. Other pathogens, including Coxiella burnetii, Leishmania amazonensis, and Salmonella typhimurium, have also adapted to survive within the acidic phagosome (2, 14, 33).
Although we and others have demonstrated that chloroquine enhances the antimicrobial activity of macrophages by presumed pH-dependent mechanisms (3, 12, 25, 30), to our knowledge, our data are the first to actually quantitate the pH in phagosomes following chloroquine treatment. MDM treated with 10 μM chloroquine, which was the concentration used to demonstrate anticryptococcal killing activity (25), raised the pH to 6.5. At that pH, some free iron should be present in the phagolysosome as a result of release from transferrin (6). This is consistent with our data demonstrating 10 μM chloroquine enhances the anticryptococcal activity of MDM independent of iron deprivation (25). Using mouse peritoneal macrophages that had endocytosed FITC-labeled dextran, Ohkuma and Poole determined that treatment with 100 μM chloroquine raised the intralysosomal pH to 6.4 (32). In our studies, treatment of human MDM with 100 μM chloroquine raised the pH of C. neoformans-laden phagosomes to 7.3. The discrepancy in pH obtained in our studies compared with those of Ohkuma and Poole may reflect disparate capacities of endosomes and phagosomes to concentrate chloroquine. Alternatively, there could be inherent differences between the macrophage populations studied.
The ability of MDM to acidify the C. neoformans phagosome strongly suggests that the vacuolar proton-ATPase responsible for phagosomal acidification is not excluded from the phagosome. Moreover, by immunofluorescence, colocalization of LAMP-1 with the C. neoformans phagosome was demonstrated. LAMP-1 is a highly glycosylated protein found in endosomal and lysosomal compartments that is commonly used as a marker for phagosome-lysosome fusion (4, 19, 36). In support of our findings, studies by Nessa et al. suggested that acidification and phagolysosomal fusion occur following phagocytosis of killed C. neoformans by rabbit alveolar macrophages in vivo (29). In contrast to these data with C. neoformans, other investigators have demonstrated that Mycobacterium phagosomes acquire LAMP-1 but not the vacuolar proton-ATPase (42). Finding LAMP-1 colocalized with cryptococcal phagosomes may have implications regarding antigen presentation by this fungus. Processing of protein antigens into peptides that bind to class II major histocompatibility complex molecules occurs in compartments containing LAMP-1 (36, 43).
By immunofluorescence staining, capsular polysaccharide was detected scattered throughout the MDM following phagocytosis of C. neoformans. Capsule is continuously shed from live C. neoformans and accumulates within macrophages. Thus, in autopsy studies of patients who died with cryptococcal meningoencephalitis, tissue capsular polysaccharide reactivity was localized in macrophages (21). In experimental rodent models, capsular polysaccharide also can be found inside macrophages (11, 13). Myriad immunological effects have been attributed to cryptococcal polysaccharide, including dysregulation of cytokine secretion, inhibition of leukocyte accumulation, induction of suppressor T cells, inhibition of antigen presentation, inhibition of lymphoproliferation, and induction of human immunodeficiency virus (16). The contribution of intramacrophage accumulation of cryptococcal polysaccharide to these processes is unknown.
The data presented herein demonstrate that unlike many other intracellular pathogens, C. neoformans does not avoid fusion with macrophage lysosomal compartments but rather resides and survives in the acidic phagolysosome. Taken together with results of previous studies demonstrating that chloroquine treatment enhances host defenses against cryptococcosis (25, 28), these data lend support for clinical trials of phagosome-alkalinizing agents (in particular, chloroquine) for the prophylaxis and treatment of cryptococcosis.
ACKNOWLEDGMENTS
This work was supported by grants AI37532, AI25780, DK31056, and DK51478 from the National Institutes of Health. S.M.L. is the recipient of a Burroughs Wellcome Fund Scholar Award in Pathogenic Mycology. T.S.H. is the recipient of an Advanced Training Fellowship from the Wellcome Trust.
We thank Arturo Casadevall and Thomas Kozel for the generous gifts of antibodies and Ralph R. Isberg for helpful suggestions regarding the LAMP-1 staining protocol.
REFERENCES
- 1.Andrews H L, Vogel J P, Isberg R R. Identification of linked Legionella pneumophila genes essential for intracellular growth and evasion of the endocytic pathway. Infect Immun. 1998;66:950–958. doi: 10.1128/iai.66.3.950-958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoine J C, Prina E, Jouanne C, Bongrand P. Parasitophorous vacuoles of Leishmania amazonensis-infected macrophages maintain an acidic pH. Infect Immun. 1990;58:779–787. doi: 10.1128/iai.58.3.779-787.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrd T F, Horwitz M A. Chloroquine inhibits the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. A potential new mechanism for the therapeutic effect of chloroquine against intracellular pathogens. J Clin Investig. 1991;88:351–357. doi: 10.1172/JCI115301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J W, Murphy T L, Willingham M C, Pastan I, August J T. Identification of two lysosomal membrane glycoproteins. J Cell Biol. 1985;101:85–95. doi: 10.1083/jcb.101.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuck S L, Sande M A. Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. N Engl J Med. 1989;321:794–799. doi: 10.1056/NEJM198909213211205. [DOI] [PubMed] [Google Scholar]
- 6.Dautry-Varsat A, Ciechanover A, Lodish H F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci USA. 1983;80:2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond R D. Cryptococcus neoformans. In: Mandell G L, Bennett J E, Dolin R, editors. Mandell, Douglas and Bennett’s principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone Inc.; 1995. pp. 2331–2340. [Google Scholar]
- 8.Diamond R D, Bennett J E. Growth of Cryptococcus neoformans within human macrophages in vitro. Infect Immun. 1973;7:231–236. doi: 10.1128/iai.7.2.231-236.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamond R D, Root R, Bennett J E. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J Infect Dis. 1972;125:367–375. doi: 10.1093/infdis/125.4.367. [DOI] [PubMed] [Google Scholar]
- 10.Eissenberg L G, Goldman W E, Schlesinger P H. Histoplasma capsulatum modulates the acidification of phagolysosomes. J Exp Med. 1993;177:1605–1611. doi: 10.1084/jem.177.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldmesser M, Casadevall A. Effect of serum IgG1 to Cryptococcus neoformans glucuronoxylomannan on murine pulmonary infection. J Immunol. 1997;158:790–799. [PubMed] [Google Scholar]
- 12.Fortier A H, Leiby D A, Narayanan R B, Asafoadjei E, Crawford R M, Nacy C A, Meltzer M S. Growth of Francisella tularensis LVS in macrophages: the acidic intracellular compartment provides essential iron required for growth. Infect Immun. 1995;63:1478–1483. doi: 10.1128/iai.63.4.1478-1483.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldman D L, Lee S C, Casadevall A. Tissue localization of Cryptococcus neoformans glucuronoxylomannan in the presence and absence of specific antibody. Infect Immun. 1995;63:3448–3453. doi: 10.1128/iai.63.9.3448-3453.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hackstadt T, Williams J C. Biochemical strategem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc Natl Acad Sci USA. 1981;78:3240–3244. doi: 10.1073/pnas.78.5.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison T S, Nong S, Levitz S M. Induction of human immunodeficiency virus type 1 expression in monocytic cells by Cryptococcus neoformans and Candida albicans. J Infect Dis. 1997;176:485–491. doi: 10.1086/514068. [DOI] [PubMed] [Google Scholar]
- 16.Hogan L H, Klein B S, Levitz S M. Virulence factors of medically important fungi. Clin Microbiol Rev. 1996;9:469–488. doi: 10.1128/cmr.9.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horwitz M A, Maxfield F R. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J Cell Biol. 1984;99:1936–1943. doi: 10.1083/jcb.99.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard D H. Some factors which affect the initiation of growth of Cryptococcus neoformans. J Bacteriol. 1961;82:430–435. doi: 10.1128/jb.82.3.430-435.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunziker W, Geuze H J. Intracellular trafficking of lysosomal membrane proteins. Bioessays. 1996;18:379–389. doi: 10.1002/bies.950180508. [DOI] [PubMed] [Google Scholar]
- 20.Krogstad D J, Schlesinger P H, Gluzman I Y. Antimalarials increase vesicle pH in Plasmodium falciparum. J Cell Biol. 1985;101:2302–2309. doi: 10.1083/jcb.101.6.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S C, Casadevall A, Dickson D W. Immunohistochemical localization of capsular polysaccharide antigen in the central nervous system cells in cryptococcal meningoencephalitis. Am J Pathol. 1996;148:1267–1274. [PMC free article] [PubMed] [Google Scholar]
- 22.Levitz S M. Macrophage Cryptococcus interactions. In: Zwilling B S, Eisenstein T K, editors. Macrophage pathogen interactions. New York, N.Y: Marcel Dekker; 1994. pp. 533–543. [Google Scholar]
- 23.Levitz S M, Farrell T P. Growth inhibition of Cryptococcus neoformans by cultured human monocytes: role of the capsule, opsonins, the culture surface, and cytokines. Infect Immun. 1990;58:1201–1209. doi: 10.1128/iai.58.5.1201-1209.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levitz S M, Farrell T P. Human neutrophil degranulation stimulated by Aspergillus fumigatus. J Leukoc Biol. 1990;47:170–175. doi: 10.1002/jlb.47.2.170. [DOI] [PubMed] [Google Scholar]
- 25.Levitz S M, Harrison T S, Tabuni A, Liu X. Chloroquine induces human mononuclear phagocytes to inhibit and kill Cryptococcus neoformans by a mechanism independent of iron deprivation. J Clin Investig. 1997;100:1640–1646. doi: 10.1172/JCI119688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levitz S M, Tabuni A. Binding of Cryptococcus neoformans by human cultured macrophages. Requirements for multiple complement receptors and actin. J Clin Investig. 1991;87:528–535. doi: 10.1172/JCI115027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levitz S M, Tabuni A, Kornfeld H, Reardon C C, Golenbock D T. Production of tumor necrosis factor alpha in human leukocytes stimulated by Cryptococcus neoformans. Infect Immun. 1994;62:1975–1981. doi: 10.1128/iai.62.5.1975-1981.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazzola R, Barluzzi R, Brozzetti A, Boelaert J R, Luna T, Saleppico S, Bistoni F, Blasi E. Enhanced resistance to Cryptococcus neoformans infection induced by chloroquine in a murine model of meningoencephalitis. Antimicrob Agents Chemother. 1997;41:802–807. doi: 10.1128/aac.41.4.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nessa K, Gross N T, Jarstrand C, Johansson A, Camner P. In vivo interaction between alveolar macrophages and Cryptococcus neoformans. Mycopathologia. 1997;139:1–7. doi: 10.1023/a:1006843202124. [DOI] [PubMed] [Google Scholar]
- 30.Newman S L, Gootee L, Brunner G, Deepe G S., Jr Chloroquine induces human macrophage killing of Histoplasma capsulatum by limiting the availability of intracellular iron and is therapeutic in a murine model of histoplasmosis. J Clin Investig. 1994;93:1422–1429. doi: 10.1172/JCI117119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh Y K, Straubinger R M. Intracellular fate of Mycobacterium avium: use of dual-label spectrofluorometry to investigate the influence of bacterial viability and opsonization on phagosomal pH and phagosome-lysosome interaction. Infect Immun. 1996;64:319–325. doi: 10.1128/iai.64.1.319-325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci USA. 1978;75:3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rathman M, Sjaastad M D, Falkow S. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect Immun. 1996;64:2765–2773. doi: 10.1128/iai.64.7.2765-2773.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rohrer J, Schweizer A, Russell D, Kornfeld S. The targeting of Lamp1 to lysosomes is dependent on the spacing of its cytoplasmic tail tyrosine sorting motif relative to the membrane. J Cell Biol. 1996;132:565–576. doi: 10.1083/jcb.132.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothstein T L, Gefter M L. Affinity analysis of idiotype-positive and idiotype-negative Ars-binding hybridoma proteins and Ars-immune sera. Mol Immunol. 1983;20:161–168. doi: 10.1016/0161-5890(83)90127-x. [DOI] [PubMed] [Google Scholar]
- 36.Rowell J F, Ruff A L, Guarnieri F G, Staveley-O’Carroll K, Lin X, Tang J, August J T, Siliciano R F. Lysosome-associated membrane protein-1-mediated targeting of the HIV-1 envelope protein to an endosomal/lysosomal compartment enhances its presentation to MHC class II-restricted T cells. J Immunol. 1995;155:1818–1828. [PubMed] [Google Scholar]
- 37.Schaible U E, Sturgill-Koszycki S, Schlesinger P H, Russell D G. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J Immunol. 1998;160:1290–1296. [PubMed] [Google Scholar]
- 38.Sibille J C, Ciriolo M, Kondo H, Crichton R R, Aisen P. Subcellular localization of ferritin and iron taken up by rat hepatocytes. Biochem J. 1989;262:685–688. doi: 10.1042/bj2620685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sibille J C, Kondo H, Aisen P. Uptake of ferritin and iron bound to ferritin by rat hepatocytes: modulation by apotransferrin, iron chelators and chloroquine. Biochim Biophys Acta. 1989;1010:204–209. doi: 10.1016/0167-4889(89)90162-6. [DOI] [PubMed] [Google Scholar]
- 40.Sibley L D, Weidner E, Krahenbuhl J L. Phagosome acidification blocked by intracellular Toxoplasma gondii. Nature. 1985;315:416–419. doi: 10.1038/315416a0. [DOI] [PubMed] [Google Scholar]
- 41.Starke P E, Gilbertson J D, Farber J L. Lysosomal origin of the ferric iron required for cell killing by hydrogen peroxide. Biochem Biophys Res Commun. 1985;133:371–379. doi: 10.1016/0006-291x(85)90916-7. [DOI] [PubMed] [Google Scholar]
- 42.Sturgill-Koszycki S, Schlesinger P H, Chakraborty P, Haddix P L, Collins H L, Fok A K, Allen R D, Gluck S L, Heuser J, Russell D G. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 43.Tulp A, Verwoerd D, Dobberstein B, Ploegh H L, Pieters J. Isolation and characterization of the intracellular MHC class II compartment. Nature. 1994;369:120–126. doi: 10.1038/369120a0. [DOI] [PubMed] [Google Scholar]
- 44.Vergne I, Constant P, Laneelle G. Phagosomal pH determination by dual fluorescence flow cytometry. Anal Biochem. 1998;255:127–132. doi: 10.1006/abio.1997.2466. [DOI] [PubMed] [Google Scholar]
- 45.Vogel J P, Andrews H L, Wong S K, Isberg R R. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]