Abstract
Our laboratory has previously shown that after immunization with a strain of Salmonella typhimurium, SL3235, made avirulent by a blockage in the pathway of aromatic synthesis, murine splenocytes were profoundly suppressed in their capacity to mount an in vitro antibody plaque-forming cell (PFC) response to sheep erythrocytes. Evidence indicated that suppression was mediated by nitric oxide (NO), since the in vitro addition of NG-monomethyl-l-arginine blocked suppression. The present studies examined the effect of blocking NO production on Salmonella-induced immunosuppression by in vivo administration of aminoguanidine hemisulfate (AG). AG was administered to C3HeB/FeJ mice in their drinking water (2.5% solution) for 7 days prior to intraperitoneal inoculation with SL3235. AG treatment inhibited the increase in nitrate and nitrite levels in plasma and nitrite levels in the spleen seen in immunized mice. Importantly, AG treatment completely blocked suppression of the splenic PFC response and markedly attenuated the suppression of the response to concanavalin A in immunized mice, providing further evidence that Salmonella-induced immunosuppression is mediated by NO. AG treatment also alleviated the majority of the splenomegaly associated with SL3235 inoculation, which correlated with a blockage of influx of neutrophils and macrophages into spleens, as assessed by flow cytometry. AG treatment unexpectedly resulted in 90% mortality in mice injected with the highly attenuated vaccine strain of Salmonella, SL3235. Increased mortality in AG-treated mice correlated with inability to clear organisms from the spleen by day 15 postinoculation and with persistent bacteremia, compared with control mice. Collectively, these in vivo results underscore the dual biological consequences of NO production following Salmonella infection, with NO being necessary for host defense, but also having the potentially adverse effect of immunosuppression. A unifying hypothesis to explain how these seemingly paradoxical effects could both result from NO production is presented.
A variety of attenuated strains of Salmonella typhimurium with precise genetic lesions are being considered as live, oral vaccines for typhoid fever (10, 32, 39). Attenuated Salmonella strains also have potential as vectors for presentation of heterologous passenger antigens of unrelated pathogens to the immune system (10). Mouse models of Salmonella infection have been used extensively to evaluate the feasibility of various attenuation strategies (22) and guest antigen immunization (10).
Our laboratory has previously shown that inoculation with SL3235, an avirulent strain of S. typhimurium, developed by Hoseith and Stocker (32) that has a defined, nonreverting blockage in the aroA gene, induced long-term protection against challenge with virulent Salmonella and transient cross-protection against Listeria monocytogenes (37). Paradoxically, SL3235 also induced profound suppression in splenocyte immune functions (19). Other strains of Salmonella with different attenuating mutations also produced immunosuppression (20). Splenocytes from SL3235-immunized mice were suppressed in their ability to mount an in vitro plaque-forming cell (PFC) response to sheep erythrocytes (SRBC) (2, 3, 20) and to proliferate in response to mitogens (19, 38). Evidence indicated that the suppression was mediated by macrophages, since removal of adherent cells markedly reduced suppression and purified adherent cells added to normal cultures were suppressive (2, 34, 38). Further, suppression did not require cell contact, since immune cells suppressed normal cells across a filter (2). Macrophage-derived nitric oxide was implicated as the suppressor factor, based on the observations that spleen cells isolated from mice immunized 7 days after SL3235 infection produced high levels of nitric oxide and that in vitro addition of the nitric oxide synthase (NOS) inhibitor NG-monomethyl-l-arginine (NMMA) blocked the suppression of the PFC response (4). Addition of NMMA to concanavalin A (ConA)-stimulated cultures of immune spleen cells also reversed suppression when immune splenocytes were cocultured with normal splenocytes to decrease the number of nitric oxide-producing cells under l-arginine-limited conditions (34).
To further examine the role of nitric oxide in vivo in immunosuppression, we have administered the inducible NOS (iNOS) inhibitor aminoguanidine sulfate (AG) to mice in their drinking water. AG treatment completely prevented suppression of the splenic PFC response and partially reduced the suppression of the response to ConA in immunized mice, providing further evidence that immunosuppression is mediated by nitric oxide. Unexpectedly, AG treatment also resulted in a high degree of mortality of mice inoculated with the attenuated vaccine strain of Salmonella, SL3235. This result is of interest because there is some controversy about the role of NO in host resistance to Salmonella. These results show that NO or its derivatives mediate immunosuppression following attenuated Salmonella inoculation and that NO is vital in the host defense against even attenuated Salmonella.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free female C3HeB/FeJ (ityr) mice were purchased at 6 weeks of age from Jackson Laboratory (Bar Harbor, Maine) and housed in our animal facility for at least 1 week prior to use. The mice were fed rodent chow (Purina, St. Louis, Mo.), and fresh water was available ad libitum. C3HeB/FeJ mice are inherently susceptible to virulent Salmonella infection even though they are ityr (17).
Bacteria.
S. typhimurium SL3235, provided by Bruce A. D. Stocker (Stanford University School of Medicine, Stanford, Calif.), a smooth avirulent aroA mutant strain (50% lethal dose, >107 CFU/mouse when given intraperitoneally [i.p.]) was used for all experiments (32). Lyophilized organisms were rehydrated with brain heart infusion broth and grown to log phase as described previously (37).
AG treatment and infection protocol.
To inhibit nitric oxide production by macrophages, mice were given a 2.5% solution of AG hemisulfate (Sigma, St. Louis, Mo.) in sterilized drinking water beginning 7 days prior to Salmonella immunization as previously described (8). On day 7 after the start of AG treatment, mice were immunized i.p. with live, log-phase SL3235 in 0.5 ml of pyrogen-free 0.9% sodium chloride injection, USP (saline) (Abbott Laboratories, North Chicago, Ill.), at doses in the range of 2 × 105 to 4 × 105 CFU/mouse (AG-SL3235). Control groups were as follows: SL3235-inoculated mice given sterile drinking water (H2O-SL3235), mice injected i.p. with 0.5 ml of saline and given sterile drinking water (H2O-saline), and mice treated with AG for 7 days and injected with 0.5 ml of saline (AG-saline). AG-treated animals continued to receive AG in their drinking water until the time of sacrifice.
Collection of plasma and spleen cells.
At 5 days after Salmonella injection, plasma was collected by cardiac puncture with heparin-coated needles (Accurate Chemical & Scientific Corp, Westbury, N.Y.) from mice anesthetized with Nembutal sodium solution (Abbott Laboratories) (2.5 mg/ml). The blood was centrifuged at 10,000 × g for 3 min. The supernatants were frozen at −70°C for use in nitrate-nitrite reduction assays. The mice were then sacrificed by cervical dislocation. Their spleens were aseptically removed, weighed, and teased into single-cell suspensions. Cells were prepared as previously described for use in in vitro Mishell-Dutton cultures and for mitogen studies (2). Cell numbers were determined with a Coulter Counter (Coulter Electronics, Hialeah, Fla.), and the cultures were adjusted to the desired cell concentration. Cells were used for determination of nitrite production, their ability to respond to ConA, or their capacity to make antibody in Mishell-Dutton cultures.
Nitrate and nitrite levels in plasma.
Nitric oxide was quantified in plasma by using the Griess reagent to measure nitrite ion concentration. The procedure of Schmidt et al. was used to reduce nitrate concentrations to nitrite (50). The plasma (25 μl) was diluted fourfold with sterile water. Fifty micromolar NADPH tetrasodium salt, 5 μM flavin adenine dinucleotide disodium salt, and 0.1 U of nitrite reductase from Aspergillus per ml (Boehringer Mannheim Biochemicals, Indianapolis, Ind.) were added, and the mixture was incubated for 20 min at 37°C. Then, 10 U of lactate dehydrogenase per ml (Boehringer Mannheim) and 10 mM sodium pyruvate (M.A. Bioproducts, Walkersville, Md.) were added, and the mixture was incubated for 5 min at 37°C. A 30% solution of zinc sulfate (Sigma) was made and centrifuged at 10,000 × g for 5 min. Then 100 μl of supernatant was transferred to each well of a 96-well plate (Nunc Inc., Naperville, Ill.), to which an equal volume of Griess reagent (1% sulfanilamide, 0.1% naphthylethylene diamine dihydrochloride, 2% H3PO4 [Sigma]) was added. The absorbance at 550 nm was determined, and NO2− was quantitated by using NaNO2 as a standard.
Nitrite levels in spleens.
Splenocytes were suspended in RPMI 1640 containing 10% heat-inactivated fetal bovine serum (HyClone, Logan, Utah) 50 U of penicillin-streptomycin per ml (GIBCO), and 2 mM l-glutamine (Sigma) and plated at 107 cells/ml in 96-well plates (Costar, Cambridge, Mass.). The cells were cultured for 48 h at 37°C in 5% CO2. Then 100 μl of cell-free supernatant was collected and mixed with 100 μl of Griess reagent. The absorbance at 550 nm was determined, and NO2− was quantitated with using NaNO2 as a standard (27).
Mishell-Dutton PFC assay.
Splenocytes (107 per ml) in RPMI 1640 with HEPES buffer (GIBCO), with 1 mM nonessential amino acids (GIBCO), 1 mM sodium pyruvate (M.A. Bioproducts), 0.05 mM 2-mercaptoethanol (Sigma), 2 mM l-glutamine (GIBCO), 10 mg each of guanosine, uridine, adenosine, and cytosine (Sigma), 10% endotoxin-free fetal bovine serum (HyClone), and 50 μg of gentamicin per ml (GIBCO) were plated into 24-well plates (Costar). SRBC (Rockland Inc., Gilbertsville, Pa.) were added at 3.5 × 106 per ml, and the cultures were incubated at 37°C in 7% O2− 10% CO2− 83% N2 for 5 days in a sealed chamber. The number of antibody-producing cells based on plaque production was determined by the method of Cunningham and Szenberg (11). In some cultures 1.25 mM NMMA (Sigma), a nitric oxide inhibitor, was added in 50 μl. Data are expressed as a suppression index, which is the ratio of PFC responses from H2O-SL3235- or AG-SL3235-treated mice to those from H2O-saline control animals from the same experiment.
Responses to ConA.
Spleen cells were placed in cocultures consisting of 2 × 105 cells from infected spleens mixed with 8 × 105 normal spleen cells. Control wells had 106 normal spleen cells alone in a final volume of 100 μl of RPMI in flat-bottom 96-well Costar plates. ConA (0.1 μg/well) was added in 50 μl of RPMI. In some wells, 1.25 mM NMMA was added in 50 μl of RPMI. The final volume in each well was brought to 200 μl with RPMI. Cells were cultured for 42 h at 37°C with 5% CO2. They were then pulsed with [3H]thymidine (0.5 μCi in 50 μl) (Amersham Life Science Inc., Arlington Heights, Ill.) for 6 h and harvested with a multichannel harvester (Inotech, Lansing, Mich.). Thymidine incorporation was determined by counting filters placed in CytoScint (ICN, Irvine, Calif.) liquid scintillation solution with a beta counter (Packard, Downers Grove, Ill.). To calculate [3H]thymidine uptake, background counts of wells without addition of ConA were subtracted from those of mitogen-stimulated cells. Mitogen responses are expressed as a suppression index, which is the ratio of cpm from H2O-SL3235- or AG-SL3235-treated mice to those from H2O-saline control animals from the same experiment.
Flow cytometry.
Spleen cells were placed into V-bottom microtiter plates (Costar) at 106 cells/well, and Fc receptors were blocked by addition of 10% heat-inactivated rabbit serum (Rockland Inc.) for 20 min. The cells were washed in 200 μl of phosphate-buffered saline (PBS) with 1% bovine serum albumin (BSA) (Sigma) and 0.1% NaN3 (PBS-BSA). A 200-μl volume of biotinylated primary antibody was added to each sample, and the mixtures were incubated on ice for 30 min. Macrophages and polymorphonuclear leukocytes (PMNs) were quantitated with biotinylated rat anti-mouse CD11b (Mac-1) (Pharmingen, San Diego, Calif.). B cells were detected with biotinylated goat anti-mouse immunoglobulin (Pharmingen), and T cells were quantitated with biotinylated hamster CD3-ɛ (Pharmingen). The cells were then washed twice with PBS-BSA and incubated with streptavidin R-phycoerythrin (Biosource International, Camarillo, Calif.) for 30 min. The cells were washed and fixed in 1% paraformaldehyde for 20 min and resuspended in 0.5 ml of PBS-BSA. A minimum of 10,000 cells were analyzed on an Epics Elite flow cytometer (Coulter Corp). Data are expressed as the percentage of positive cells (minus background fluorescence) ± standard error of the mean.
Survival.
Groups of mice were treated for 7 days with water containing 2.5% AG or sterile water with no additive. On day 7, they were inoculated i.p. with 7 × 105 CFU of SL3235. AG treatment continued through the course of the experiment. The mice were observed daily, and mortality was scored.
Determination of the bacterial burden.
Groups of mice were given sterile drinking water or water treated with AG. At 7 days after the start of AG treatment, the animals were infected with SL3235. AG treatment was continued until the mice were sacrificed on the designated day postinfection. The animals were anesthetized with Nembutal sodium (2.5 mg/ml), and blood was obtained by cardiac puncture. Duplicate plates of 0.1 ml of blood or appropriate dilution in sterile saline solution were made by using Levine eosin-methylene blue (EMB) agar plates (DIFCO), and the number of Salmonella colonies was counted. The mice were then sacrificed by cervical dislocation. The spleens and/or livers were aseptically removed and weighted. The organs were homogenized with an SDT Tissuemizer (Tekmar Co., Cincinnati, Ohio) in sterile water at a final volume of 5 ml for spleen samples and 10 ml for liver samples. A 0.1-ml sample of homogenate or appropriate dilution was plated on EMB plates and grown overnight, and the colonies were counted.
Statistics.
The significance of differences observed in nitrate and nitrite levels in plasma, flow cytometry, spleen weights, and immune responses of control and AG-treated mice was assessed by analysis of variance followed by Tukey’s honestly significant test. For nitrite levels, and bacterial burdens, Student’s t test for independent samples was used. Differences were considered significant for P ≤ 0.05.
RESULTS
AG treatment reduces nitrate and nitrite levels in plasma.
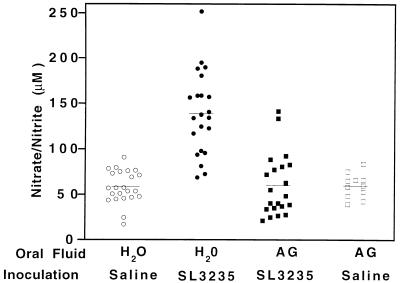
Mice inoculated with 2 × 105 to 4 × 105 SL3235 organisms and given water to drink (H2O-SL3235) showed significant elevation of nitrate and nitrite levels in plasma by day 5 postinoculation (139 μM) (Fig. 1). AG treatment of mice inoculated with SL3235 (AG-SL3235) blocked this mean elevation in the nitrate and nitrite levels (59.9 μM). The mean levels of nitrate and nitrite in AG-treated, uninfected mice (AG-saline) (59.7 μM) were not significantly different from those in control (H2O-saline) mice (58.3 μM). These results demonstrate that the AG treatment regime was effective in inhibiting the elevation of nitrate and nitrite levels induced in plasma by Salmonella immunization and did not affect the constitutive levels of nitrate and nitrite in plasma.
FIG. 1.
Effect of AG treatment on nitrate and nitrite levels in plasma. Mice were treated for 7 days with AG in their drinking water prior to i.p. inoculation with saline (AG-saline) or SL3235 (AG-SL3235). AG-treated animals continued to receive AG in their drinking water until the time of sacrifice. Control groups received water without AG and were given SL3235 (H2O-SL3235) or saline (H2O-saline) i.p. At 5 days postinfection, plasma was obtained by cardiac puncture and nitrate and nitrite levels were assessed. Data are pooled from four experiments, except for the AG-saline group, where data are from two experiments. The total number of animals included in each group was 23 for H2O-saline, 22 for H2O-SL3235, 21 for AG-SL3235, and 11 for AG-saline. Horizontal lines indicate mean values. P ≤ 0.0001, AG-SL3235 versus H2O-SL3235; not significant, AG-SL3235 versus H2O-saline or AG-saline.
AG inhibition of splenic nitric oxide and blockage of immunosuppression.
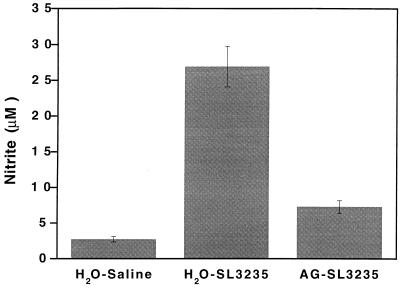
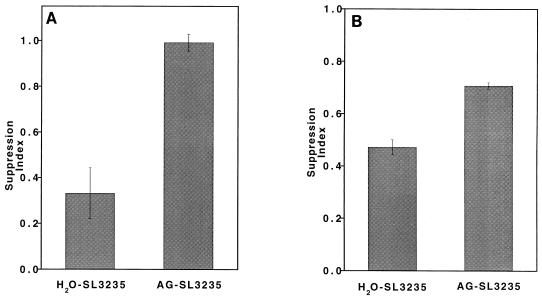
We have previously reported that SL3235 inoculation results in the induction of both high levels of NO in the spleen, as assessed by nitrite concentrations in the supernatant of cultured splenocytes, and immunosuppression of the primary PFC response to SRBC (2–4, 34). The effect of AG treatment in vivo on these two parameters was assessed. In animals treated with AG (AG-SL3235), splenocyte nitrite levels were greatly reduced (Fig. 2), although baseline levels were not obtained. Treatment with AG also completely blocked suppression of the PFC response induced by SL3235 inoculation (Fig. 3A). AG did not affect the PFC response of mice which were not given SL3235, since the mean response of AG-saline mice was 1,818 PFC/107 cells and that of H2O-saline mice was 1,932 PFC/107 cells, which is not statistically significantly different. AG treatment partially blocked suppression of the response to ConA when cells from immunized mice were cocultured with normal splenocytes (Fig. 3B).
FIG. 2.
Effect of in vivo AG treatment on nitrite production by spleen cells in vitro. The nitrite level in spleen cells was determined in mice treated for 7 days with AG in their drinking water prior to i.p. inoculation with SL3235 (AG-SL3235). AG-treated animals continued to receive AG in their drinking water until the time of sacrifice. Controls were given water and inoculated with SL3235 (H2O-SL3235) or saline (H2O-saline). Splenocytes were isolated 5 days after i.p. injection and cultured for 2 days at 2 × 106 cells/well. The nitrite concentrations in cell-free supernatants were assessed. Data are expressed as the mean ± standard error of the mean for a minimum of triplicate wells from four experiments. P ≤ 0.0001, AG-SL3235 versus H2O-SL3235 or H2O-saline.
FIG. 3.
Effect of in vivo AG treatment on SL3235-induced suppression of the response to heterologous antigens. Mice were treated for 7 days with AG in their drinking water prior to i.p. inoculation with SL3235 (AG-SL3235). AG-treated animals continued to receive AG in their drinking water until the time of sacrifice. Controls were given water and inoculated with SL3235 (H2O-SL3235) or saline (H2O-saline). Splenocytes were isolated 5 days postinjection. (A) Suppression of the in vitro PFC response. Splenocytes were cultured with SRBC. Five days later, the number of PFC/107 cells was determined. Data are expressed as a ratio of H2O-SL3235 or AG-SL3235 compared with H2O-saline. Data are the mean and standard error of the mean for a minimum of triplicate wells from four experiments. P ≤ 0.0001, AG-SL3235 versus H2O-SL3235; not significant, AG-SL3235 versus H2O-saline (the H2O-saline group has a suppression index of 1.0). (B) Suppression of responses to ConA. SL3235-infected splenocytes (2 × 105/well) were cultured with splenocytes from H2O-saline mice (6 × 105/well) for 48 h in the presence of ConA. Splenocytes were pulsed with [3H]thymidine to assess proliferative responses. Data are expressed as a ratio of H2O-SL3235 or AG-SL3235 compared with H2O-saline and are the mean and standard error of the mean for a minimum of triplicate wells from four experiments. P ≤ 0.05, AG-SL3235 versus H2O-SL3235 or H2O-saline (the H2O-saline group has a suppression index of 1.0).
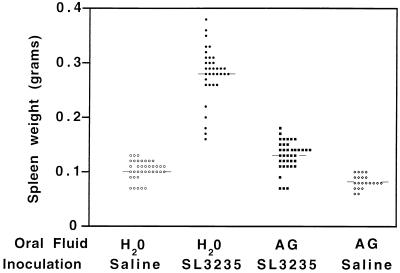
AG treatment prevents splenomegaly and inhibits cellular infiltration in the spleen.
We have previously shown that 7 days after inoculation with SL3235 there is marked splenomegaly (37). As shown in Fig. 4, splenomegaly is also observed 5 days after inoculation with SL3235. Treatment of SL3235-immunized mice with AG greatly reduced the splenomegaly to levels near those in saline-inoculated mice. The kinetics of splenomegaly after SL3235 inoculation with or without AG treatment were monitored by using five mice per time point. AG treatment effectively inhibited splenomegaly over the 15 days of the study (data not shown).
FIG. 4.
Effect of AG treatment on spleen weight on day 5 after i.p. immunization. Mice were treated for 7 days with AG in their drinking water prior to i.p. inoculation with saline (AG-saline) or SL3235 (AG-SL3235). AG-treated animals continued to receive AG in their drinking water until the time of sacrifice. Control groups received water without AG and were given SL3235 (H2O-SL3235) or saline (H2O-saline) i.p. The mice were sacrificed 5 days postinjection, and their spleens were weighed. Data were collected from individual mice, at least five per experiment, and are the pool of seven experiments, with the exception of the data for the AG-saline group, which is the pool of two experiments. The horizontal lines indicate mean values. P ≤ 0.05, AG-SL3235 versus H2O-SL3235, AG-saline, and H2O-saline.
Our previous studies showed that splenomegaly induced by SL3235 at 7 days postimmunization was accompanied by increased numbers of neutrophils, macrophages, and precursor macrophages in the spleen (2). In the present experiments, the cellular composition of the spleen was examined by flow cytometry 5 days after SL3235 inoculation in infected and in AG-treated immunized mice. Table 1 shows that AG treatment blocked the influx of neutrophils and macrophages (Mac-1+) into the spleen 5 days after SL3235 immunization. The decrease in the percentage of CD3+ cells observed in spleens of SL3235-inoculated mice was also not apparent in AG-treated animals. The percentage of Ig+ cells in the spleen was not altered by SL3235 inoculation or by AG treatment.
TABLE 1.
Effect of aminoguanidine on spleen cell composition in Salmonella-immunized mice
| Cell type | Antibody target | % of total cellsa
|
||
|---|---|---|---|---|
| H2O-saline | H2O-SL3235 | AG-SL3235 | ||
| Macrophages and PMNs | Mac-1 (CD11b) | 18 ± 1 | 45 ± 2b | 20 ± 2c |
| T cells | CD3 | 34 ± 1 | 21 ± 2b | 31 ± 2c |
| B cells | sIg | 49 ± 2 | 55 ± 4d | 53 ± 3 |
Determined by flow cytometry on day 5 postimmunization. Results are mean ± standard error for six experiments.
P ≤ 0.05, H2O-SL3235 versus AG-SL3235 or H2O-saline.
Not significant, AG-SL3235 versus H2O-saline.
Not significant, H2O-SL3235 versus AG-SL3235 or H2O-saline.
Nitric oxide and resistance to Salmonella infection.
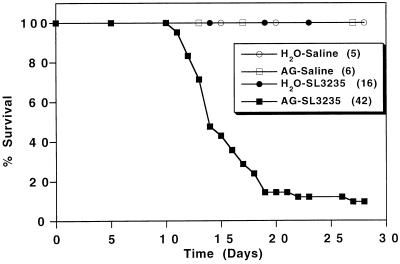
It was observed that mice treated with AG and inoculated with SL3235 exhibited decreased body weight, a reduction in water intake, ruffled fur, glassy swollen eyes, and listless behavior. These symptoms were not present in AG-treated uninoculated mice (AG-saline) or mice given SL3235 without AG treatment (H2O-SL3235), whose appearance was similar to that of the controls (H2O-saline). To systematically assess the affect of AG on infection with an attenuated strain of Salmonella, survival of SL3235-inoculated mice after AG treatment was tested. In two separate experiments, mice were treated with AG for 7 days prior to inoculation with approximately 7 × 105 SL3235 organisms and AG treatment was continued throughout the experiment. The combined results, presented in Fig. 5, show that treatment with AG followed by infection with SL3235 resulted in the death of 38 of 42 mice. Death occurred between days 10 and 27. Mice receiving SL3235 but no AG (H2O-SL3235) all survived. Treatment with AG followed by i.p. saline injection (AG-saline) was not toxic, since all the mice in this group survived, with no apparent symptoms, for over 90 days.
FIG. 5.
Effect of AG treatment on mouse survival. Mice were treated for 7 days with AG in their drinking water prior to i.p. inoculation with saline (AG-saline) or 7 × 105 SL3235 organisms (AG-SL3235). AG treatment was continued through the course of the experiment. Control groups received water without AG and were given 7 × 105 SL3235 organisms (H2O-SL3235) or saline (H2O-saline) i.p. The mice were observed daily, and deaths were recorded. Results are scored as percent survival. The data represent combined results from two experiments.
Effect of AG treatment on the bacterial burden in SL3235-immunized mice.
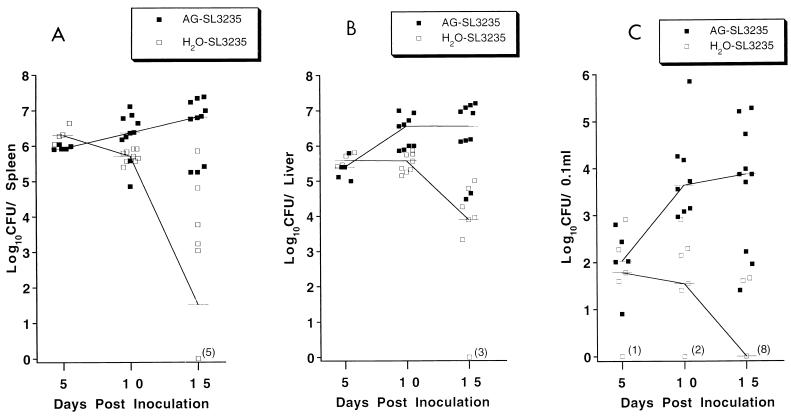
To examine whether mortality in AG-treated, Salmonella-infected mice (AG-SL3235) correlated with increased bacterial replication, parallel groups of mice were treated with AG for 7 days and also inoculated with approximately 7 × 105 SL3235 organisms. As above, AG treatment continued until the time of sacrifice. At various time points postinfection, animals were sacrificed and the bacterial burdens in blood, liver, and spleen were determined (Fig. 6). In AG-treated animals sacrificed on day 5 after SL3235 injection (AG-SL3235), there was little effect on the bacterial burdens in the spleen, liver, or blood compared with those in mice given drinking water without AG (H2O-SL3235). By day 10 there was a significantly greater number of bacteria in the spleens and livers of AG-treated animals (AG-SL3235) compared to infected controls (H2O-SL3235), with the effect on numbers of organisms in the blood being the most pronounced. Small numbers of bacteria were recovered from the blood of five of seven control mice inoculated with SL3235 (H2O-SL3235), and two mice were sterile. More than 9.2 × 102 bacteria per 0.1 ml were recovered from all eight AG-treated mice, with a median number of 4.5 × 103/0.1 ml. By day 15, the number of Salmonella in AG-treated mice was 10,000-fold greater in spleens and 1000-fold greater in livers compared with the numbers for control mice. The most dramatic difference between AG-treated and nontreated animals was observed in the number of Salmonella in the blood. By day 15, 8 of 10 control mice had sterile blood whereas all 10 AG-treated mice had large numbers of organisms in the blood, with a median of 7.4 × 103 organisms/0.1 ml. An increased incidence of peritonitis was observed in AG-treated immune mice (AG-SL3235) on day 15, with 5 of 10 mice being positive. The untreated, infected mice (H2O-SL3235) showed no signs of peritonitis.
FIG. 6.
Effect of AG treatment on bacterial burden over time after i.p. immunization. Mice were treated for 7 days with AG in their drinking water prior to i.p. inoculation with SL3235 (AG-SL3235). AG-treated animals continued to receive AG in their drinking water until the time of sacrifice. The control group received water without AG and was given SL3235 (H2O-SL3235) i.p. At various days postinoculation, at least five mice were anesthetized and blood was obtained by cardiac puncture. The mice were then sacrificed, and the spleens and livers were aseptically removed, weighed, and homogenized. Appropriate dilutions in sterile saline were plated on EMB agar plates and grown overnight, and the number of Salmonella colonies was counted. Data represent combined results from two experiments. In one of the experiments, no mice were sacrificed on day 5. Lines connect the median values for each group. (A) Splenic bacterial burden per organ. P ≤ 0.016, AG-SL3235 versus H2O-SL3235 on day 10; P ≤ 0.0001, AG-SL3235 versus H2O-SL3235 on day 15; not significant, AG-SL3235 versus H2O-SL3235 on day 5. (B) Liver bacterial burden per organ. P ≤ 0.0001, AG-SL3235 versus H2O-SL3235 on days 10 and 15; not significant, AG-SL3235 versus H2O-SL3235 on day 5. (C) Blood bacterial burden/0.1 ml of blood. P ≤ 0.0001, AG-SL3235 versus H2O-SL3235 on days 10 and 15; not significant, AG-SL3235 versus H2O-SL3235 on day 5.
DISCUSSION
Consistent with our previous findings (obtained 7 days after inoculation of mice with SL3235), 5 days after SL3235 injection there was a profound suppression of lymphocyte function as assessed by measuring the ability to mount an in vitro PFC response to SRBC (2, 3, 20) and the ability to proliferate in response to ConA (34, 38). To further support the role of nitric oxide in mediating immunosuppression following attenuated Salmonella inoculation, the in vivo role of nitric oxide on lymphocyte suppression was examined. The iNOS inhibitor AG, administered in the drinking water of C3HeB/FeJ mice prior to and during infection with attenuated Salmonella, completely inhibited the increase in nitrate and nitrite levels in plasma observed in SL3235-inoculated mice, indicating that the compound was effective in blocking iNOS activity. The AG treatment also greatly diminished in vitro splenic nitrite production observed in infected mice. A major finding of this study is that AG treatment completely abrogated suppression of the splenic PFC response of SL3235-inoculated splenocytes. In addition, suppression of the response to ConA observed in splenocytes of SL3235-immunized mice was partially reversed by AG treatment. Differences in the ease of reversing the suppression of PFC responses and responses to mitogens have been addressed previously (34). The inability of AG treatment to completely block the suppression of the response to ConA is most likely due to the capacity of ConA to upregulate gamma interferon (IFN-γ) production, resulting in increased nitric oxide production. Huang et al. showed that for complete reversal of the responses to ConA, it was necessary to use cocultures of normal and immune cells and to add NMMA to the medium under l-arginine-limited conditions (34). The observations that AG treatment in vivo completely blocks suppression of the PFC response or partially blocks suppression of the response to ConA supports the conclusion that nitric oxide is the suppressor factor induced by attenuated Salmonella.
We have previously shown that inoculation of SL3235 resulted in profound splenomegaly (37), which was due to a massive influx of neutrophils, macrophages, and precursor macrophages (2). The splenomegaly is a measure of the inflammatory response associated with the infection and correlates with NO levels (20). In the present study, AG treatment was found to inhibit splenomegaly and to block macrophage and PMN infiltration into the spleens of SL3235-inoculated mice. The approximately 75% reduction in NO production by immune spleen cells from AG-treated mice correlates with the decreased inflammatory response. These results are in contrast to our previous observations with in vivo anti-IL-12 treatment prior to SL3235 inoculation, which also reduced nitric oxide levels in the spleen by 75% and completely prevented suppression of the PFC response. However, anti-IL-12 only marginally reduced the splenomegaly and had no effect on SL3235-induced changes in splenocyte composition (51). The present study is consistent with the histological analysis of Umezawa et al., who showed that NMMA, given in vivo, reduced granuloma formation in the livers of mice infected with virulent Salmonella (55). However, since NMMA is not isotype specific, it is unclear whether cNOS or iNOS regulated this inflammatory response. In other infection models using nitric oxide inhibitors, the investigators did not find alterations in cellular inflammatory responses to Listeria monocytogenes (7, 43), Mycobacterium tuberculosis (8, 26), Klebsiella pneumonia (54), or Toxoplasma gondii (30, 36). One explanation for the effect of AG in blocking cellular influx into the spleen is that NO may be directly chemotactic. It has been reported that NO donors in vitro can induce chemoattractant locomotion of human neutrophils though agarose (6). However, there are also studies which do not support this premise (44, 47). Alternatively, nitric oxide might regulate chemokines responsible for cell trafficking into sites of inflammation. It has been reported that NO donors enhance MIP-1α release and that NMMA can block MIP-1α production in vitro (45). A recent report showed that organisms differ in their capacity to induce MIP-1α and that Salmonella is a potent inducer, while other organisms induce the chemokine to a lesser degree (29), providing a possible explanation for the differences observed in infection models regarding cellular inflammation after NO inhibition.
An unexpected finding of the present study was the AG-mediated sensitization of mice to the lethality of the attenuated vaccine strain, SL3235. SL3235 has a 50% lethal dose of >107 CFU in normal C3HeB/FeJ mice (37). AG treatment resulted in a 91% mortality rate at a dose of 7 × 105 CFU. Increased mortality correlated with higher bacterial burdens in the spleen, liver, and blood by day 10 postimmunization in AG-treated mice compared to controls and with an inability to clear the organisms from the liver and spleen by day 15. Persistent bacteremia was observed in AG-treated mice compared with untreated controls. Note that bacteria did not replicate to significantly higher levels in the spleens and livers of AG-treated mice; they failed to be cleared. These results show that NO is crucial in protection against even this attenuated Salmonella strain. Previous in vivo studies also supported a role for NO in controlling virulent Salmonella infection, since NO blockage by AG or NMMA treatment enhanced mortality (14, 55). Blocking NO in vivo also sensitizes mice to other facultative intracellular pathogens of macrophages, including M. tuberculosis (8), Leishmania major (23, 42), and Trypanosoma cruzi (33). Furthermore, AG treatment also increases the mortality of mice infected with an attenuated Salmonella strain that is deficient in a Cu,Zn-SOD (superoxide dismutase) which renders them hypersusceptible to superoxide and nitric oxide (13). The in vivo observations presented in this paper extend these studies by showing that AG treatment sensitized mice to infection with SL3235, a highly attenuated vaccine strain of Salmonella blocked in aromatic synthesis, a pathway which would not be expected to be related to sensitivity to NO.
The most straightforward interpretation of the in vivo observations showing such dramatic effects of blockage of NO by AG on the ability to control attenuated Salmonella infection is that macrophage-derived NO plays a major role in the salmonellacidal pathway. The observations that mice lacking CD4+ T-cell receptor (TCR) αβ cells (31), athymic mice (53), or mice unresponsive to IFN-γ (31) are highly susceptible to aroA inoculation could be interpreted as suggesting that each of these defects in the immune system interferes with the capacity to generate or respond to IFN-γ and thus to generate NO, in order to resolve an aroA infection. The failure to recruit inflammatory effector cells to the spleen and other sites of infection may also contribute to the lack of bactericidal activity. The lack of PMNs and macrophages in the spleens of AG-treated mice may allow bacteria to escape into the bloodstream, resulting in bacteremia and death.
Nitric oxide has been reported to be cytotoxic for many organisms, particularly those that are intracellular pathogens of macrophages. In vitro studies have shown that M. bovis (25), M. tuberculosis (9), M. leprae (1), M. avium (15), L. major (28, 40–42), Trypanosoma species (46, 56), Brucella abortus (35), and Francisella tularensis (5) are all killed through an NO-mediated pathway. However, the literature suggests that nitric oxide alone is insufficient to kill Salmonella (48). DeGroote et al. have reported that peroxynitrite (ONOO−), formed by the reaction of NO with superoxide, killed virulent Salmonella, as well as Salmonella mutants deficient in antioxidant defenses (12, 14). This group also reported that the reaction of NO with thiol-containing molecules to form S-nitrosothiols results in an oxygen-independent Salmonella cytostasis (12, 24). The complexity of the macrophage-killing mechanisms against Salmonella is demonstrated by a recent study showing that macrophages from iNOS knockout mice were still salmonellacidal, in contrast to macrophages lacking the 91-kDa subunit of the respiratory burst oxidase, which were unable to kill Salmonella (52). Since there may be several pathways to salmonellacidal activity and they may intersect, the in vivo studies of the role of NO in host defense against Salmonella addressed in the present paper are of added interest.
SL3235 inoculation induces both a profound immunosuppression and high levels of protection against challenge with virulent Salmonella, a finding we have called paradoxical (16). The results with AG point to the conclusion that NO mediates both immunosuppression and resistance to infection (16, 18, 21), since AG reverses suppression and sensitizes mice to Salmonella infection. We have previously suggested that the induction of macrophage NO represents a primitive alarm response of the host to a life-threatening microbial infection that targets macrophages (18). Whether NO or reactive nitrogen intermediates are directly microbicidal or act by inducing influx of inflammatory cells, inhibition of this pathway leads to failure to reduce bacterial burdens. With regard to immunosuppression, we have proposed that it results from inhibition of lymphocyte function due to a bystander cytostasis mechanism, in which lymphocytes in the vicinity of NO-secreting macrophages are inactivated (18). While the present paper does not address the mechanism of action of NO, the results show that the activity of NO on lymphocytes consists of suppression of the ability of the host to respond to new antigens or mitogens. This undesirable immunosuppression can be viewed as a side effect of macrophage activation, which is necessary to contain the invading pathogen, but may provide a window of immunologic immunosuppression to other pathogens or antigens. Our studies indicate that the suppression is transient, since it begins to wane about 3 weeks postinfection (38). It should be emphasized that the suppression we observed is to heterologous antigens presented to the host after vaccination. This issue is particularly important, since oral vaccines with passenger antigens are being considered for use in less developed parts of the world, where malaria, leishmaniasis, and other parasitic diseases are endemic. These target populations might be expected to be at greater risk for exposure to some other infectious agents in the period following immunization than are populations in the industrialized world. On the other hand, we have shown that maximal suppression occurs at the same time as the induction of tumoricidal and leishmaniacidal activity in peritoneal macrophages and cross-protection against challenge with Listeria (37, 49). If macrophage activation and NO production are responsible for both the protective and the suppressive aspects of vaccination with attenuated Salmonella, it might be expected that during the initial weeks after immunization vaccinated populations might actually have a period of nonspecific resistance to other, unrelated intracellular pathogens of macrophages. At present, there is no experimental evidence about whether immunosuppression to heterologous antigens occurs after oral inoculation of aroA strains in mice or humans. Immunomodulation as a consequence of vaccination is a crucial issue that should be further addressed in Salmonella vaccine development.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant AI15613. M. G. Schwacha was supported by a postdoctoral trainee fellowship (National Institutes of Health grant T32 AI07101).
The help of John P. Gaughan in carrying out the statistical analysis and the technical assistance of Joseph J. Meissler, Jr., are gratefully acknowledged.
REFERENCES
- 1.Adams L B, Franzblau S G, Vavrin Z, Hibbs J B, Jr, Krahenbuhl J L. l-Arginine-dependent macrophage effector functions inhibit metabolic activity of Mycobacterium leprae. J Immunol. 1991;147:1642–1646. [PubMed] [Google Scholar]
- 2.Al-Ramadi B K, Brodkin M A, Mosser D M, Eisenstein T K. Immunosuppression induced by attenuated Salmonella: evidence for mediation by macrophage precursors. J Immunol. 1991;146:2737–2746. [PubMed] [Google Scholar]
- 3.Al-Ramadi B K, Greene J M, Meissler J J, Jr, Eisenstein T K. Immunosuppression induced by attenuated Salmonella: effect of LPS responsiveness on development of suppression. Microb Pathog. 1992;12:267–278. doi: 10.1016/0882-4010(92)90045-p. [DOI] [PubMed] [Google Scholar]
- 4.Al-Ramadi B K, Meissler J J, Jr, Huang D, Eisenstein T K. Immunosuppression induced by nitric oxide and its inhibition by interleukin-4. Eur J Immunol. 1992;22:2249–2254. doi: 10.1002/eji.1830220911. [DOI] [PubMed] [Google Scholar]
- 5.Anthony L S D, Morrissey P J, Nano F E. Growth inhibition of Francisella tularensis live vaccine strain by IFN-γ-activated macrophages is mediated by reactive nitrogen intermediates derived from l-arginine metabolism. J Immunol. 1992;148:1829–1834. [PubMed] [Google Scholar]
- 6.Beauvais F, Michel L, Dubertret L. Exogenous nitric oxide elicits chemotaxis of neutrophils in vitro. J Cell Physiol. 1995;165:610–614. doi: 10.1002/jcp.1041650319. [DOI] [PubMed] [Google Scholar]
- 7.Boockvar K S, Granger D L, Poston R M, Maybodi M, Washington M K, Hibbs J B, Jr, Kurlander R L. Nitric oxide produced during murine listeriosis is protective. Infect Immun. 1994;62:1089–1100. doi: 10.1128/iai.62.3.1089-1100.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan J, Tanaka K, Carroll D, Flynn J, Bloom B R. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan J, Xing Y, Magliozzo R S, Bloom B R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatfield S, Roberts M, Londono P, Cropley I, Douce G, Dougan G. The development of oral vaccines based on live attenuated Salmonella strains. FEMS Immunol Med Microbiol. 1993;7:1–8. doi: 10.1111/j.1574-695X.1993.tb00374.x. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham A, Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968;14:599–600. [PMC free article] [PubMed] [Google Scholar]
- 12.De Groote M A, Granger D, Xu Y, Campbell G, Prince R, Fang F C. Genetic and redox determinants of nitric oxide cytotoxicity in a Salmonella typhimurium model. Proc Natl Acad Sci USA. 1995;92:6399–6403. doi: 10.1073/pnas.92.14.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Groote M A, Ochsner U A, Shiloh M U, Nathan C, McCord J M, Dinauer M C, Libby S J, Vazquez-Torres A, Xu Y, Fang F C. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc Natl Acad Sci USA. 1997;94:13997–14001. doi: 10.1073/pnas.94.25.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Groote M A, Testerman T, Xu Y, Stauffer G, Fang F C. Homocysteine antagonism of nitric oxide-related cytostasis in Salmonella typhimurium. Science. 1996;272:414–416. doi: 10.1126/science.272.5260.414. [DOI] [PubMed] [Google Scholar]
- 15.Denis M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J Leukoc Biol. 1991;49:380–387. doi: 10.1002/jlb.49.4.380. [DOI] [PubMed] [Google Scholar]
- 16.Eisenstein T K, Dalal N, Killar L, Lee J-C, Schafer R. Paradoxes of immunity and immunosuppression in Salmonella infection. Adv Exp Med Biol. 1988;239:353–366. doi: 10.1007/978-1-4757-5421-6_34. [DOI] [PubMed] [Google Scholar]
- 17.Eisenstein T K, Deakins L W, Killar L, Saluk P H, Sultzer B M. Dissociation of innate susceptibility to Salmonella infection and endotoxin responsiveness in C3HeB/FeJ mice and other strains in the C3H lineage. Infect Immun. 1982;36:696–703. doi: 10.1128/iai.36.2.696-703.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenstein T K, Huang D, Meissler J J, Jr, Al-Ramadi B K. Macrophage nitric oxide mediates immunosuppression in infectious inflammation. Immunobiology. 1994;191:493–502. doi: 10.1016/S0171-2985(11)80455-9. [DOI] [PubMed] [Google Scholar]
- 19.Eisenstein T K, Killar L M, Stocker B A D, Sultzer B M. Cellular immunity induced by avirulent Salmonella in LPS-defective C3H/HeJ mice. J Immunol. 1984;133:958–961. [PubMed] [Google Scholar]
- 20.Eisenstein T K, Meissler J J, Jr, Miller S I, Stocker B A D. Immunosuppression and nitric oxide production induced by parenteral live Salmonella vaccines do not correlate with protective capacity: a phoP::Tn10 mutant does not suppress but does protect. Vaccine. 1998;16:24–32. doi: 10.1016/s0264-410x(97)00160-6. [DOI] [PubMed] [Google Scholar]
- 21.Eisenstein T K, Schwacha M G, Huang D, Meissler J J., Jr . Attenuated Salmonella vaccines are immunosuppressive by macrophage-mediated production of nitric oxide. In: Keusch G T, Kawakami M, editors. Cytokines, cholera, and the gut. Ohmsha, Japan: IOS Press; 1997. pp. 353–359. [Google Scholar]
- 22.Eisenstein T K, Sultzer B M. Immunity to Salmonella infection. Adv Exp Med Biol. 1983;162:261–296. doi: 10.1007/978-1-4684-4481-0_26. [DOI] [PubMed] [Google Scholar]
- 23.Evans T G, Thai L, Granger D L, Hibbs J B., Jr Effect of in vivo inhibition of nitric oxide production in murine leishmaniasis. J Immunol. 1993;151:907–915. [PubMed] [Google Scholar]
- 24.Fang F C. Perspective series: host/pathogen interactions: mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flesch I E A, Kaufmann S H E. Mechanisms involved in Mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect Immun. 1991;59:3213–3218. doi: 10.1128/iai.59.9.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flynn J L, Scanga C A, Tanaka K E, Chan J. Effects of aminoguanidine on latent murine tuberculosis. J Immunol. 1998;160:1796–1803. [PubMed] [Google Scholar]
- 27.Green L C, Wagner D A, Glognowski J, Skipper P L, Wishnok J S. Analysis of nitrate, nitrite and (I5N) in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 28.Green S J, Meltzer M S, Hibbs J B, Jr, Nacy C A. Activated macrophages destroy intracellular Leishmania major amastigotes by an l-arginine-dependent killing mechanism. J Immunol. 1990;144:278–283. [PubMed] [Google Scholar]
- 29.Hachicha M, Rathanaswami P, Naccache P H, McColl S R. Regulation of chemokine gene expression in human peripheral blood neutrophils phagocytosing microbial pathogens. J Immunol. 1998;160:449–454. [PubMed] [Google Scholar]
- 30.Hayashi S, Chan C C, Gazzinelli R, Roberge F G. Contribution of nitric oxide to the host parasite equilibrium in toxoplasmosis. J Immunol. 1996;156:1476–1481. [PubMed] [Google Scholar]
- 31.Hess J, Ladel C, Miko D, Kaufmann S H E. Salmonella typhimurium aroA− infection in gene-targeted immunodeficient mice. J Immunol. 1996;156:3321–3326. [PubMed] [Google Scholar]
- 32.Hoiseth S K, Stocker B A D. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 33.Holscher C, Kohler G, Muller U, Mossmann H, Schaub G A, Brombacher F. Defective nitric oxide effector functions lead to extreme susceptibility of Trypanosoma cruzi-infected mice deficient in gamma interferon receptor or inducible nitric oxide synthase. Infect Immun. 1998;66:1208–1215. doi: 10.1128/iai.66.3.1208-1215.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang D, Schwacha M G, Eisenstein T K. Attenuated Salmonella vaccine-induced suppression of murine spleen cell responses to mitogen is mediated by macrophage nitric oxide: quantitative aspects. Infect Immun. 1996;64:3786–3792. doi: 10.1128/iai.64.9.3786-3792.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang X, Leonard B, Benson R, Baldwin C L. Macrophage control of Brucella abortus: role of reactive oxygen intermediates and nitric oxide. Cell Immunol. 1993;151:309–319. doi: 10.1006/cimm.1993.1241. [DOI] [PubMed] [Google Scholar]
- 36.Kersten-Scharton T M, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Killar L M, Eisenstein T K. Immunity to Salmonella typhimurium infection in C3H/HeJ and C3H/HeNCrlBR mice: studies with an aromatic-dependent live S. typhimurium strain as a vaccine. Infect Immun. 1985;47:605–612. doi: 10.1128/iai.47.3.605-612.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J-C, Gibson C W, Eisenstein T K. Macrophage-mediated mitogenic suppression induced in mice of the C3H lineage by a vaccine strain of Salmonella typhimurium. Cell Immunol. 1985;91:75–91. doi: 10.1016/0008-8749(85)90033-4. [DOI] [PubMed] [Google Scholar]
- 39.Levine M M, Hone D M. Typhoid fever. In: Cryz S J, editor. Vaccines and immunotherapy. New York, N.Y: Pergamon Press; 1991. pp. 59–72. [Google Scholar]
- 40.Liew F Y, Li Y, Millott S. Tumor necrosis factor-α synergizes with IFN-γ in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol. 1990;145:4306–4310. [PubMed] [Google Scholar]
- 41.Liew F Y, Li Y, Moss D, Parkinson C, Rogers M V, Moncada S. Resistance to Leishmania major infection correlates with the induction of nitric oxide synthase in murine macrophages. Eur J Immunol. 1991;21:3009–3014. doi: 10.1002/eji.1830211216. [DOI] [PubMed] [Google Scholar]
- 42.Liew F Y, Millot S, Parkinson C, Palmer R M J, Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from l-arginine. J Immunol. 1990;144:4794–4797. [PubMed] [Google Scholar]
- 43.MacMicking J D, Nathan C, Hom G, Chartrain N, Fletcher D S, Trumbauer M, Stevens K, Xie -W Q, Sokol K, Hutchinson N, Chen H, Mudgett J S. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 44.Malawista S E, de Boisfleury Chevance A. Chemotaxis by human neutrophils and their cytokineplasts treated with inhibitors of nitric oxide synthase: no suppression of orientation or tajectory. J Leukoc Biol. 1997;61:58–62. doi: 10.1002/jlb.61.1.58. [DOI] [PubMed] [Google Scholar]
- 45.Muhl H, Dinarello C A. Macrophage inflammatory protein-1α production in lipopolysaccharide-stimulated human adherent blood mononuclear cells is inhibited by the nitric oxide synthase inhibitor NG-monomethyl-l-arginine. J Immunol. 1997;159:5063–5069. [PubMed] [Google Scholar]
- 46.Munoz-Fernandez M A, Fernandez M A, Fresno M. Synergism between tumor necrosis factor-α and interferon-γ on macrophage activation for the killing of intracellular Trypanosoma cruzi through a nitric oxide-dependent mechanism. Eur J Immunol. 1992;22:301–307. doi: 10.1002/eji.1830220203. [DOI] [PubMed] [Google Scholar]
- 47.Perretti M, Szabo C, Thiemermann C. Effect of interleukin-4 and interleukin-10 on leukocyte migration and nitric oxide production in the mouse. Br J Pharmacol. 1995;116:2251–2257. doi: 10.1111/j.1476-5381.1995.tb15061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saito S, Onozuka K, Shinomiya H, Nakano M. Sensitivity of bacteria to NaNO2 and to l-arginine-dependent system in murine macrophages. Microbiol Immunol. 1991;354:325–329. doi: 10.1111/j.1348-0421.1991.tb01561.x. [DOI] [PubMed] [Google Scholar]
- 49.Schafer R, Nacy C A, Eisenstein T K. Induction of activated macrophages in C3H/HeJ mice by avirulent Salmonella. J Immunol. 1988;140:1638–1644. [PubMed] [Google Scholar]
- 50.Schmidt H H H W, Warner T D, Nakane M, Forstermann U, Murad F. Regulation and subcellular location of nitrogen oxide synthases in RAW264.7 macrophages. Mol Pharmacol. 1992;41:615–624. [PubMed] [Google Scholar]
- 51.Schwacha M G, Eisenstein T K. Interleukin-12 is critical for induction of nitric oxide-mediated immunosuppression following vaccination of mice with attenuated Salmonella typhimurium. Infect Immun. 1997;65:4897–4903. doi: 10.1128/iai.65.12.4897-4903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shiloh M, Ruan J, Nathan C. Evaluation of bacterial survival and phagocyte function with a fluorescence-based microplate assay. Infect Immun. 1997;65:3193–3197. doi: 10.1128/iai.65.8.3193-3198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinha K, Mastroeni P, Harrison J, Demarco de Hormaeche R, Hormaeche C E. Salmonella typhimurium aroA, htrA, and aroD hatrA mutants cause progressive infections in athymic (nu/nu) BALB/c mice. Infect Immun. 1997;65:1566–1569. doi: 10.1128/iai.65.4.1566-1569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsai W C, Strieter R M, Zisman D A, Wilkowski J M, Bucknell K A, Chen G, Standiford T J. Nitric oxide is required for effective innate immunity against Klebsiella pneumoniae. Infect Immun. 1997;65:1870–1875. doi: 10.1128/iai.65.5.1870-1875.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Umezawa K, Akaike T, Fujii S, Suga M, Setoguchi K, Ozawa A, Maeda H. Induction of nitric oxide synthesis and xanthine oxidase and their roles in the antimicrobial mechanism against Salmonella typhimurium in mice. Infect Immun. 1997;65:2932–2940. doi: 10.1128/iai.65.7.2932-2940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vincendeau P, Daulouede S, Veyret B, Darde M L, Bouteille B, Lemesre J L. Nitric oxide-mediated cytostatic activity on Trypanosoma brucei gambiense and Trypanosoma brucei brucei. Exp Parasitol. 1992;75:353–360. doi: 10.1016/0014-4894(92)90220-5. [DOI] [PubMed] [Google Scholar]