Abstract
Background
Despite continuing efforts to improve the inclusion of underserved groups in clinical research, gaps in diversity remain. Participation of special populations is especially important when facing problems of unprecedented complexity such as the COVID-19 pandemic. A better understanding of factors associated with the immune response in diverse populations would advance future preventive and curative approaches.
Objective
The objective of this study was to investigate the factors potentially responsible for adverse events following COVID-19 immunization. The study population included adults from rural areas, transitional countries, and those with medically understudied conditions, across a broad age range.
Methods
The study evolved from peer support networks developed during the COVID-19 pandemic. Participants were recruited digitally through online neighborhood and health communities. Some of the participants volunteered as study investigators assisting with offline recruitment and safety monitoring. Individuals who consented to participate were asked to share their vaccination experiences either using constantly evolving web-based surveys or via one-on-one communication. Inferential statistical analysis to estimate differences between study groups was performed using parametric and nonparametric tests.
Results
Of 1430 participants who shared their vaccination experiences, 648 had outcome measures at their 1.5-year follow-up. Significant differences were found between age groups, types of vaccine adverse events (VAEs), incidences of breakthrough infections, and health conditions linked to the microbiome. Pairwise comparisons showed that VAEs interfering with daily activities were significantly higher in both younger (18-59 years) and older age groups (80-100 years, P<.001) than in the 60-79–year age group. Short-term VAEs were associated with lower incidence of breakthrough COVID-19 infections relative to those who reported either minimal or long-term adverse events (P<.001). A genetic origin was suggested for some adverse reactions.
Conclusions
The findings of this study demonstrate that vaccine adverse reactions in older individuals are being overlooked, and the incidence of VAEs impairing immunity may be higher than previously perceived. Better preventive measures are needed for all those at risk for life-threatening and long-term adverse events due to vaccination. Supportive community-based studies focusing on these populations could add important data to the current body of knowledge. Further and more comprehensive studies should follow.
Trial Registration
ClinicalTrials.gov NCT04832932; https://clinicaltrials.gov/ct2/show/NCT04832932
International Registered Report Identifier (IRRID)
RR2-10.1101/2021.06.28.21256779
Keywords: COVID-19, COVID-19 vaccines, vaccine adverse events, breakthrough infections, decentralized participatory study, elderly, older individuals, medically underserved populations, aging, elderly population, vaccination, genetic disparity, microbiome disparity, impaired immunity
Introduction
Background
While COVID-19 vaccines are highly effective, they can lead to a range of vaccine adverse events (VAEs). The mechanisms underlying VAEs and their association with vaccination efficacy are not completely clear.
Traditional clinical trials are essential for understanding the safety profiles of new interventions, but many people would not enroll themselves. The COVID-19 Citizen Science study [1] and social media analytics [2,3] address some knowledge gaps, but not social- and age-related barriers to participation. Passive surveillance data [4] are subject to the same limitations and insufficient follow-up. Alternative research strategies are needed to complement the existing evidence base.
Research Objectives
The idea for this study evolved as minority populations were struggling to make vaccination appointments owing to digital inequality, or seeking answers to questions about their pre-existing conditions, which were not addressed by funded research. A previously reported protocol for an ambispective study [5] hypothesized that the safety profile and immune response to COVID-19 vaccines depend on pre-existing health conditions, metabolism, and microbiomes. The objective of this study was to investigate the factors influencing adverse events following COVID-19 immunization in communities that include underrepresented groups.
Methods
Study Population
Participants were recruited from private e-neighborhood networks and health support groups via direct emails and social media posts. Particular efforts were made to recruit populations underrepresented in existing research data sets. Individuals with socially debilitating metabolic body odor (MEBO) [6], including extraoral halitosis, “People Allergic to me,” and trimethylaminuria were invited to contribute to the goals of this study along with their relatives. Support groups for autoimmune disorders, neuropathy, tinnitus, and irritable bowel syndrome were also contacted. To ensure the inclusion of digitally disadvantaged individuals, several volunteers served as study investigators providing face-to-face support. To prevent double-counting, investigators had access to lists of subjects maintained by other investigators in their community but not to personally identifiable information. Cookies were not used to guarantee the anonymity of those sharing information without enrollment. To boost engagement, research insights were periodically communicated via the internet. Data collection was automated using rule-based parsing of emails, alerts from social networks, survey spreadsheets, and group application programming interfaces. An inquiry into medical history was made at initial and follow-up data collections.
Inclusion Criteria
Inclusion criteria were age≥18 years, intention to get vaccinated, and intended availability throughout the study period. No one was excluded for reasons other than age.
Outcomes
The study’s primary outcome was the incidence of adverse reactions within 14 days of immunization. The secondary outcome was long-term health conditions and the incidence of breakthrough COVID-19 infections that occurred post vaccination with either a single dose of a COVID-19 vaccine or with 2 or more doses.
Ethical Considerations
Details of the study procedures (the optional nature of all questions, how the information will be used, the ability to withdraw from research, risks, and benefits) were explained to the participants, and informed e-consent was obtained. Ethics approval for primary and secondary analysis was granted by the institutional review board of MEBO Research (IRB00010169, protocol 20210103MEBO). To ensure participant confidentiality, all their data were coded and stored in a decentralized manner with no individual having complete access to sensitive information. All identifiable data were removed from survey and interview responses. Neither participants nor investigators were compensated.
Data Analysis
Demographic and clinical characteristics of study groups were compared using the Fisher exact test and relative risk calculations for categorical variables and the Mann-Whitney U test for continuous variables. All statistical tests were 2-sided, for which a P value of ≤.05 and a 95% CI were used to indicate statistical significance. All analyses were conducted using Python (version 3.10).
Results
Participant Characteristics
Participant characteristics are described in Table 1. Of the 1430 vaccinated adults and the 648 participants who were followed up, 51% (n=732 and n=329, respectively) were female. Prevalence of chronic disease was age- and sex-matched between the study cohort and the general population. The age at vaccination ranged from 18 to 119 years. The age group of ≥100 years includes 20 vaccinated semisuper- and supercentenarians (10 men and 10 women) with official social media and Gerontology Wiki accounts [7]. These individuals were added to the study database and followed up from early 2021. Since no information is available about their postimmunization symptoms, this group is not included in the VAE analysis.
Table 1.
Basic descriptive and inferential statistics of the study population.
| Characteristics | Vaccinated (n=1430) | 1-year follow up (n=648) | All-cause mortality (n=30) | ||||||||||||||||||||
|
|
Total (n=1430) | No or minimum VAEsa (n=1113) | VAEs (n=317) | P value | Total (n=648) | No COVID-19 infection post vaccination (n=389) | Breakthrough COVID-19 infection (n=259) | P value | Mortality (n=30) | P value | |||||||||||||
| Age at receipt of the first dose (years), median (IQR) | |||||||||||||||||||||||
|
|
All | 62 (40-70) | 65 (49-72) | 42 (30-61) | <.001 | 58 (38-70) | 61 (47-62) | 42 (30-66) | <.001 | 95 (75-110) | <.001 | ||||||||||||
|
|
Female | 63 (41-70) | 65 (49-72) | 45 (31-64) | Ref.b | 59 (40-70) | 63 (50-72) | 43 (32-65) | Ref. | 104 (84-114) | Ref. | ||||||||||||
|
|
Male | 62 (38-71) | 65 (46-72) | 40 (27-55) | <.001 | 56 (35-71) | 60 (43-72) | 42 (29-67) | .90 | 91 (75-108) | .07 | ||||||||||||
| Sex, n (%) | |||||||||||||||||||||||
|
|
Female | 732 (51) | 558 (50) | 174 (55) | Ref. | 329 (51) | 201 (52) | 128 (49) | Ref. | 12 (40) | Ref. | ||||||||||||
|
|
Male | 698 (49) | 555 (50) | 143 (45) | .20 | 319 (49) | 188 (48) | 131 (51) | .60 | 18 (60) | .30 | ||||||||||||
| Adverse events, n (%) | |||||||||||||||||||||||
|
|
Short-term | 174 (12) | 0 (0) | 174 (56) | N/Ac | 93 (15) | 77 (20) | 16 (6) | <.001 | 1 (3.5) | .20 | ||||||||||||
|
|
Long-term | 143 (10) | 5 (0) | 138 (44) | N/A | 103 (16) | 52 (13) | 51 (20) | .20 | 1 (3.5) | .40 | ||||||||||||
|
|
No or minimal | 1113 (78) | 1113 (100) | 0 (0) | N/A | 450 (69) | 260 (67) | 187 (74) | Ref. | 26 (93) | Ref. | ||||||||||||
| Age groups (years), n (%) | |||||||||||||||||||||||
|
|
18-29 | 169 (7) | 97 (9) | 72 (23) | <.001 | 85 (13) | 29 (7) | 56 (22) | <.001 | 0 (0) | N/A | ||||||||||||
|
|
30-39 | 173 (9) | 102 (9) | 71 (22) | <.001 | 85 (13) | 34 (9) | 51 (21) | <.001 | 0 (0) | N/A | ||||||||||||
|
|
40-49 | 139 (12) | 93 (9) | 46 (15) | <.001 | 81 (13) | 47 (12) | 34 (13) | .10 | 0 (0) | N/A | ||||||||||||
|
|
50-59 | 164 (17) | 127 (11) | 37 (12) | <.001 | 90 (14) | 63 (17) | 27 (11) | >.99 | 0 (0) | N/A | ||||||||||||
|
|
60-69 | 401 (24) | 356 (32) | 45 (14) | Ref. | 135 (21) | 93 (24) | 42 (16) | Ref. | 2 (7) | Ref. | ||||||||||||
|
|
70-79 | 301 (20) | 272 (24) | 29 (9) | .50 | 116 (17) | 78 (20) | 39 (14) | .70 | 8 (29) | .02 | ||||||||||||
|
|
80-100 | 62 (7) | 45 (4) | 17 (5) | <.001 | 35 (6) | 27 (7) | 8 (3) | .90 | 7 (23) | <.001 | ||||||||||||
|
|
>100 | 21 (4) | N/A | N/A | N/A | 21 (3) | 20 (4) | 1 (0) | .06 | 13 (41) | <.001 | ||||||||||||
aVAE: vaccine adverse event.
bRef.: Reference.
cN/A: not applicable.
Evaluation Outcomes
The CONSORT (Consolidated Standards of Reporting Trials) flow diagram in Figure 1 shows the progression of the study, including that of the 1430 subjects who received their first vaccine dose between December 2020 and August 2022 and the 648 individuals whose most recent update was between May and October 2022.
Figure 1.
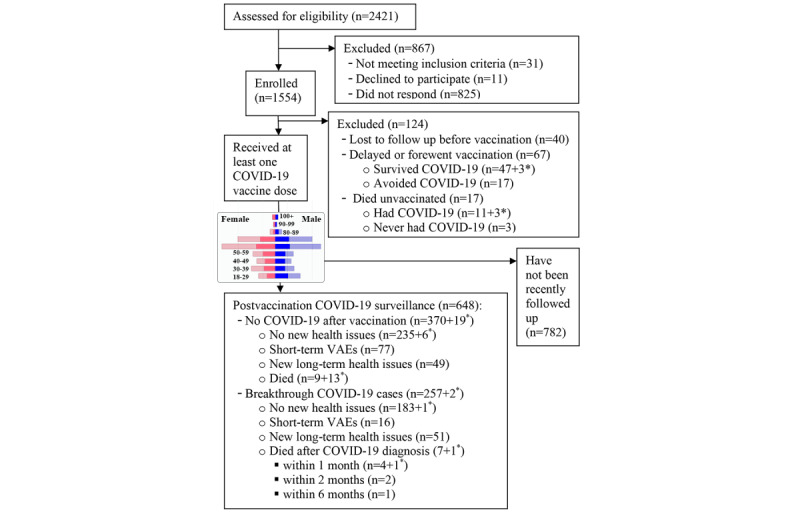
CONSORT (Consolidated Standards of Reporting Trials) flow diagram. Participant flow through the study. Asterisks denote data for the centenarians with publicly available profiles, followed up from early 2021. The population pyramid chart (blue: males; pink: females; transparent shades: no follow-up) shows age and sex distribution of vaccinated individuals (n=1430) and those who were recently followed up (n=648). VAE: vaccine adverse event.
The bar charts in Figure 1 illustrate a balanced representation of both sexes in all age groups, which is also evident in Table 1. Out of 1430 study participants who self-reported outcomes after vaccination, 317 (22%) experienced side effects that prevented them from performing daily activities after receiving at least one of the doses (Table 1). The overall Kruskal-Wallis comparison of VAEs in all age groups was significant (P<.001). Pairwise comparisons showed that while the rate of adverse events was similar in the 60-69–year and 70s-79–year age group, the incidence of VAEs in both younger (18-59 years) and older age groups (80-100 years) was significantly higher (P<.001).
Figure 2 displays the distribution of 648 participants by adverse events, or the absence thereof, for each age group.
Figure 2.
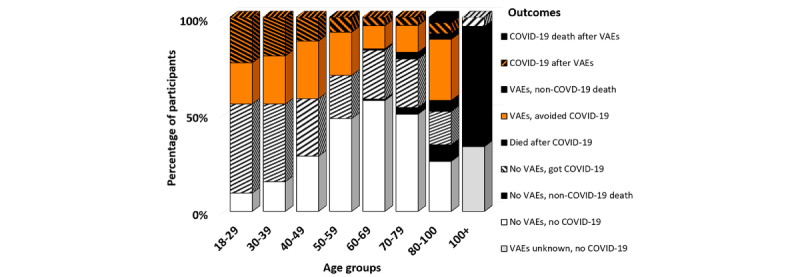
Distribution of participants by adverse event. A white background indicates no or minimal VAEs, while an orange background depicts participants who reported VAEs that prevented them from performing daily activities, diagonal stripes denote breakthrough COVID-19 infection, and solid black represents fatal outcomes. VAE: vaccine adverse event.
Incidence of breakthrough infections was significantly higher in participants younger than 40 years (Figure 3), as observed previously in many different healthy cohorts [8-10].
Figure 3.
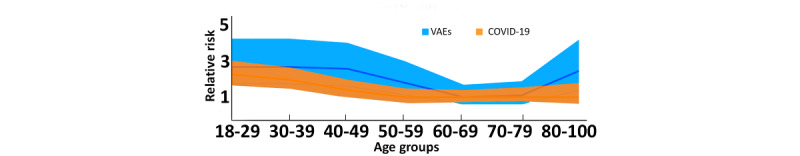
Area charts of CIs for relative risk of vaccine adverse events (VAEs; light blue) vs breakthrough infections (orange) shown for the 648 individuals with recently updated information. Relative risk is shown as a solid line (dark blue: VAEs; orange: COVID-19).
Another significant association was found between adverse reactions and the ability to avoid infection, but only if symptoms did not last longer than a week (Table 1). We observed higher incidence of pulmonary VAEs in the MEBO [6] subgroup of individuals with halitosis when compared to the group with the second highest prevalence (P=.03). We also identified 5 pairs of first-degree relatives with nearly identical sets of VAEs including cardiological events or severe nausea.
Overall, 5%-10% of individuals in chronic disease groups experienced exacerbation of their respective conditions following COVID‐19 immunization. The difference in the incidence of VAEs between healthy individuals and those with chronic conditions was not significant for all age groups (P>.05).
There were no significant gender differences in the incidence of VAEs, but age distributions revealed significant differences: a greater risk of VAEs among younger males and higher all-cause mortality in older individuals (Table 1).
Discussion
Principal Findings
In summary, we found that adverse events following the COVID-19 immunization are likely to be influenced by a combination of demographic, genetic, and environmental factors. The principal findings of this study were (1) higher incidence of VAEs in both younger and the “oldest old” groups than in “younger old” populations, (2) association of VAEs with immunogenicity observed for short-term but not long-term adverse reactions, and (3) indications that disparities in host genetics and microbiomes in VAEs may exist.
Comparison With Prior Work
Age is a known factor contributing to reactogenicity. The heterogeneity is commonly addressed by splitting the sample in 2 groups with the cutoff age between 50 and 65 years [11]. Our study suggests that this simple assumption is not sufficient.
Reporting of VAEs is higher in younger and more educated individuals [12], but it does not appear to translate to higher rates of hospitalization or life-threatening events [13]. We observed a higher incidence of VAEs in both younger and older age groups than in the “young old” populations. We speculate that more adverse events are identified when younger and more educated individuals monitor the oldest participants. The oldest-old participants were more likely to either ignore the side effects or attribute them to aging; for example, duodenal bleeding, markedly decreased appetite [14], and transient amnesia [15] following immunization.
Systemic adverse reactions have been found to be associated with a higher antibody response in mostly healthy younger [16,17] and diseased populations [18,19]. We observed this association across all age groups, health conditions, and genders, but only with respect to short-term effects of vaccination. An impaired humoral immune response was observed after longer-lasting neurological side effects [20,21]. Lower antibody titers were also associated with depressive symptoms after the first and before the second dose of mRNA vaccines [22]. We suggest that more studies are needed on all types of serious and longer-lasting side effects of vaccination.
Host genetic factors are known to contribute to the severity of COVID-19 [23] and stronger short-term reactions to COVID-19 vaccines [24]. Genetic contributions are also being considered for several serious adverse reactions [25,26]. Our preliminary data support the contributions of genetic and microbiome to cardiological and respiratory VAEs. More comprehensive multiomic analyses are needed to draw definite conclusions.
Limitations
The primary limitation of this study was that the data were obtained from self-reports.
Conclusions
Our results demonstrate that vaccine adverse reactions in older populations can be easily overlooked. Long-term effects of vaccination in all age groups could outweigh the benefits of this preventive measure in some populations. More research is needed for genetic, epigenetic, metabolome-, and microbiome-associated risk factors of serious VAEs. The prohibitive cost of comprehensive studies [16-24] disproportionally affects underserved populations. Observational trials such as this study therefore represent an effective alternative prescreening strategy for multiomics research.
Acknowledgments
I thank all participants for helping others in their communities and contributing data for the study.
Abbreviations
- CONSORT
Consolidated Standards of Reporting Trials
- MEBO
metabolic body odor
- VAE
vaccine adverse event
CONSORT-eHEALTH checklist (V 1.6.1).
Data Availability
Raw deidentified data and case reports are available upon request to the author, subject to ethics approval.
Footnotes
Conflicts of Interest: None declared.
References
- 1.Beatty AL, Peyser ND, Butcher XE, Carton TW, Olgin JE, Pletcher MJ, Marcus GM. The COVID-19 Citizen Science study: protocol for a longitudinal digital health cohort study. JMIR Res Protoc. 2021 Aug 30;10(8):e28169. doi: 10.2196/28169. https://www.researchprotocols.org/2021/8/e28169/ v10i8e28169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarynowski A, Semenov A, Kamiński M, Belik V. Mild adverse events of Sputnik V vaccine in Russia: social media content analysis of Telegram via deep learning. J Med Internet Res. 2021 Nov 29;23(11):e30529. doi: 10.2196/30529. https://www.jmir.org/2021/11/e30529/ v23i11e30529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khademi Habibabadi S, Delir Haghighi P, Burstein F, Buttery J. Vaccine adverse event mining of Twitter conversations: 2-phase classification study. JMIR Med Inform. 2022 Jul 16;10(6):e34305. doi: 10.2196/34305. https://medinform.jmir.org/2022/6/e34305/ v10i6e34305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hause AM, Baggs J, Marquez P, Abara WE, Baumblatt J, Blanc PG, Su JR, Hugueley B, Parker C, Myers TR, Gee J, Shimabukuro TT, Shay DK. Safety monitoring of COVID-19 mRNA vaccine second booster doses among adults aged ≥50 years - United States, March 29, 2022-July 10, 2022. MMWR Morb Mortal Wkly Rep. 2022 Jul 29;71(30):971–976. doi: 10.15585/mmwr.mm7130a4. doi: 10.15585/mmwr.mm7130a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabashvili I. Community-based phenotypic study of safety, tolerability, reactogenicity and Immunogenicity of emergency-use-authorized vaccines against COVID-19 and viral shedding potential of post-vaccination infections: protocol for an ambispective study. medRxiv. doi: 10.1101/2021.06.28.21256779. Preprint posted online July 21, 2021. https://www.medrxiv.org/content/10.1101/2021.06.28.21256779v2.full.pdf . [DOI] [Google Scholar]
- 6.Gabashvili IS. Cutaneous bacteria in the gut microbiome as biomarkers of systemic malodor and People Are Allergic to Me (PATM) conditions: insights from a virtually conducted clinical trial. JMIR Dermatol. 2020 Nov 4;3(1):e10508. doi: 10.2196/10508. https://derma.jmir.org/2020/1/e10508/ [DOI] [Google Scholar]
- 7.List of oldest people vaccinated against COVID-19. Gerontology Wiki. [2021-10-01]. https://gerontology.fandom.com/wiki/List_of_oldest_people_vaccinated_against_COVID-19 .
- 8.Stouten V, Hubin P, Haarhuis F, van Loenhout J, Billuart M, Brondeel R, Braeye T, Van Oyen H, Wyndham-Thomas C, Catteau L. Incidence and risk factors of COVID-19 vaccine breakthrough infections: a prospective cohort study in Belgium. Viruses. 2022 Apr 13;14(4):802. doi: 10.3390/v14040802. https://www.mdpi.com/resolver?pii=v14040802 .v14040802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonelli M, Penfold RS, Merino J, Sudre CH, Molteni E, Berry S, Canas LS, Graham MS, Klaser K, Modat M, Murray B, Kerfoot E, Chen L, Deng J, Österdahl Marc F, Cheetham NJ, Drew DA, Nguyen LH, Pujol JC, Hu C, Selvachandran S, Polidori L, May A, Wolf J, Chan AT, Hammers A, Duncan EL, Spector TD, Ourselin S, Steves CJ. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022 Jan;22(1):43–55. doi: 10.1016/S1473-3099(21)00460-6. https://linkinghub.elsevier.com/retrieve/pii/S1473-3099(21)00460-6 .S1473-3099(21)00460-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimi K, Neylan TC, Bertenthal D, Seal KH, O'Donovan A. Association of psychiatric disorders with incidence of SARS-CoV-2 breakthrough infection among vaccinated adults. JAMA Netw Open. 2022 Apr 01;5(4):e227287. doi: 10.1001/jamanetworkopen.2022.7287. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2022.7287 .2791033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutton N, San Francisco Ramos A, Beales E, Smith D, Ikram S, Galiza E, Hsia Y, Heath PT. Comparing reactogenicity of COVID-19 vaccines: a systematic review and meta-analysis. Expert Rev Vaccines. 2022 Sep 15;21(9):1301–1318. doi: 10.1080/14760584.2022.2098719. https://www.tandfonline.com/doi/full/10.1080/14760584.2022.2098719 . [DOI] [PubMed] [Google Scholar]
- 12.Mangat HS, Musah A, Luedtke S, Syed AA, Maramattom BV, Maruthanal J, Bosman A, Kostkova P. Analyses of reported severe adverse events after immunization with SARS-CoV-2 vaccines in the United States: One year on. Front. Public Health. 2022 Oct 13;10:972464. doi: 10.3389/fpubh.2022.972464. https://discovery.ucl.ac.uk/id/eprint/10157354/1/2022%20Analyses%20of%20Reported%20Severe%20Adverse%20Events%20after%20Immunization%20with%20SARS-nCoV2%20Vaccines%20in%20the%20United%20States%20-%20One%20Year%20On.pdf . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton E, Oversby S, Kitchener S, Ratsch A. Post COVID-19 vaccination: AusVaxSafety survey participation and adverse events - a community-based regional Queensland study. Aust N Z J Public Health. 2022 Oct 03; doi: 10.1111/1753-6405.13300. https://onlinelibrary.wiley.com/doi/pdf/10.1111/1753-6405.13300 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osozawa S. Case report: anorexia as a new type of adverse reaction caused by the COVID-19 vaccination: a case report applying detailed personal care records. F1000Res. 2022 Jan 4;11:4. doi: 10.12688/f1000research.75277.1. https://f1000researchdata.s3.amazonaws.com/manuscripts/79125/e96d50ed-d585-4dfd-8f20-33c237f045fc_75277_-_soichi_osozawa.pdf . [DOI] [Google Scholar]
- 15.Katsuki M, Higo Y, Komagata S, Kashiwagi K, Koh A. Transient global amnesia related to the third coronavirus disease-19 (COVID-19) vaccination. Cureus. 2022 Jul;14(7):e27121. doi: 10.7759/cureus.27121. https://europepmc.org/abstract/MED/36004026 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uwamino Y, Kurafuji T, Sato Y, Tomita Y, Shibata A, Tanabe A, Yatabe Y, Noguchi M, Arai T, Ohno A, Yokota H, Yamasawa W, Uno S, Nishimura T, Hasegawa N, Saya H, Wakui M, Murata M, Keio Donner Project Team Young age, female sex, and presence of systemic adverse reactions are associated with high post-vaccination antibody titer after two doses of BNT162b2 mRNA SARS-CoV-2 vaccination: An observational study of 646 Japanese healthcare workers and university staff. Vaccine. 2022 Feb 11;40(7):1019–1025. doi: 10.1016/j.vaccine.2022.01.002. https://europepmc.org/abstract/MED/35033389 .S0264-410X(22)00002-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanizsai A, Molnar T, Varnai R, Zavori L, Tőkés-Füzesi M, Szalai Z, Berecz J, Csecsei P. Fever after vaccination against SARS-CoV-2 with mRNA-based vaccine associated with higher antibody levels during 6 months follow-up. Vaccines (Basel) 2022 Mar 14;10(3):447. doi: 10.3390/vaccines10030447. https://www.mdpi.com/resolver?pii=vaccines10030447 .vaccines10030447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braun E, Horowitz NA, Leiba R, Weissman A, Mekel M, Shachor-Meyouhas Y, Hussein K, Halberthal M, Azzam ZS, Berger G. Association between IgG antibody levels and adverse events after first and second Bnt162b2 mRNA vaccine doses. Clin Microbiol Infect. 2022 Jul 15;:S1198-743X(22)00366-4. doi: 10.1016/j.cmi.2022.07.002. https://europepmc.org/abstract/MED/35843565 .S1198-743X(22)00366-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin T, Hung N, Hung S. Association of reactogenicity with immunogenicity of the ChAdOx1 nCoV-19 vaccine in patients undergoing hemodialysis. Vaccines (Basel) 2022 Aug 21;10(8):1366. doi: 10.3390/vaccines10081366. https://www.mdpi.com/resolver?pii=vaccines10081366 .vaccines10081366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaulen LD, Doubrovinskaia S, Mooshage C, Jordan B, Purrucker J, Haubner C, Seliger C, Lorenz H, Nagel S, Wildemann B, Bendszus M, Wick W, Schönenberger S. Neurological autoimmune diseases following vaccinations against SARS-CoV-2: a case series. Eur J Neurol. 2022 Feb 31;29(2):555–563. doi: 10.1111/ene.15147. https://europepmc.org/abstract/MED/34668274 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cicalese MP, Ferrua F, Barzaghi F, Cerri F, Moro M, Aiuti A, Silvani P. Third cranial nerve palsy in an 88-year-old man after SARS-CoV-2 mRNA vaccination: change of injection site and type of vaccine resulted in an uneventful second dose with humoral immune response. BMJ Case Rep. 2022 Mar 08;15(2):e246485. doi: 10.1136/bcr-2021-246485. https://europepmc.org/abstract/MED/35135792 .15/2/e246485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneko H, Tsuboi H. Depressive symptoms predict antibody titers after a second dose of the SARS-CoV-2 BNT162b2 vaccine among hospital workers in Japan. Brain Behav Immun. 2022 Oct 15;:S0889-1591(22)00379-8. doi: 10.1016/j.bbi.2022.09.004. https://europepmc.org/abstract/MED/36116693 .S0889-1591(22)00379-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niemi MEK, Daly MJ, Ganna A. The human genetic epidemiology of COVID-19. Nat Rev Genet. 2022 Sep 02;23(9):533–546. doi: 10.1038/s41576-022-00478-5. https://europepmc.org/abstract/MED/35501396 .10.1038/s41576-022-00478-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolze A, Neveux I, Schiabor Barrett KM, White S, Isaksson M, Dabe S, Lee W, Grzymski JJ, Washington NL, Cirulli ET. HLA-A∗03:01 is associated with increased risk of fever, chills, and stronger side effects from Pfizer-BioNTech COVID-19 vaccination. HGG Adv. 2022 May 14;3(2):100084. doi: 10.1016/j.xhgg.2021.100084. https://linkinghub.elsevier.com/retrieve/pii/S2666-2477(21)00065-8 .S2666-2477(21)00065-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salter B, Jessome M, Tarnopolsky M, Yousuf H. Possible association between rhabdomyolysis and mRNA SARS-CoV-2 vaccination in a patient with gene mutation. CMAJ. 2022 Feb 22;194(7):E252–E256. doi: 10.1503/cmaj.211856. http://www.cmaj.ca/cgi/pmidlookup?view=long&pmid=35193861 .194/7/E252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishibayashi H, Kishimoto S, Sekiguchi K, Okada S, Ihara K. Myocarditis in 13-year-old monochorionic diamniotic twins after COVID-19 vaccination. J Clin Immunol. 2022 Aug 26;:1–3. doi: 10.1007/s10875-022-01360-z. https://europepmc.org/abstract/MED/36008643 .10.1007/s10875-022-01360-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT-eHEALTH checklist (V 1.6.1).
Data Availability Statement
Raw deidentified data and case reports are available upon request to the author, subject to ethics approval.


