Abstract
Haemophilus ducreyi lipooligosaccharide (LOS) is capable of inducing an inflammatory response in skin (A. A. Campagnari, L. M. Wild, G. Griffiths, R. J. Karalus, M. A. Wirth, and S. M. Spinola, Infect. Immun. 59:2601–2608, 1991) and likely contributes to the virulence of this sexually transmitted pathogen (B. A. Bauer, M. K. Stevens, and E. J. Hansen, Infect. Immun. 68:4290–4298, 1998). An open reading frame in H. ducreyi 35000 was found to encode a predicted protein that was 59% identical to the protein product of the rfaF (waaF) gene of Salmonella typhimurium. The H. ducreyi waaF gene was able to complement an S. typhimurium rfaF (waaF) mutant, a result which confirmed the identity of this gene. In contrast to the rfaF (waaF) gene of enteric bacteria, the H. ducreyi waaF gene was not located adjacent to other genes involved in lipopolysaccharide expression. Inactivation of the H. ducreyi waaF gene by insertion mutagenesis resulted in expression of a LOS that migrated much faster than wild-type LOS in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The LOS of this mutant also did not bind a monoclonal antibody directed against a cell surface-exposed epitope of wild-type H. ducreyi LOS. Testing of the wild-type H. ducreyi strain and its isogenic waaF mutant in the temperature-dependent rabbit model for dermal lesion production by H. ducreyi revealed that this waaF mutant was less virulent than the wild-type parent strain. Complementation of the H. ducreyi waaF mutant with the wild-type H. ducreyi waaF gene resulted in expression of both wild-type LOS and wild-type virulence by this mutant.
Haemophilus ducreyi, a gram-negative coccobacillus, is the organism which causes the sexually transmitted disease known as chancroid. This ulcerogenital disease is endemic in some developing countries in Africa and Asia, and outbreaks of chancroid in the United States have apparently increased since 1980 (76). Because genital ulcer diseases, including chancroid, have been well established to be significant risk factors for transmission of the human immunodeficiency virus (76), identification of the virulence factors of H. ducreyi has become the subject of increased research activity over the past 10 years.
Despite this research effort, little is known about the H. ducreyi gene products that are directly involved in the development of genital ulcers. Only recently have putative virulence factors of this organism been identified; they include a hemolysin with cytotoxic activity (2, 47–50, 74), a second cytotoxin (13, 56), a hemoglobin-binding outer membrane protein (16, 69), a novel pilus (7), and both a copper-zinc superoxide dismutase (39, 60) and a SodA homolog (61). In addition, gene products which have the potential to directly or indirectly regulate expression of H. ducreyi virulence factors have been described (12, 51), and there have been new model systems introduced for the study of the interaction of H. ducreyi with host cells (8, 28, 29, 38, 72, 73, 75).
As might be expected, the lipooligosaccharide (LOS) present in the H. ducreyi outer membrane has potent inflammatory activity (11). Structurally, H. ducreyi LOS has been shown to be very similar to the LOSs of other gram-negative mucosal surface pathogens, including Neisseria gonorrhoeae and Haemophilus influenzae (10, 22, 41–43, 64). However, only recently have attempts been made to address the possible involvement of the oligosaccharide portion of H. ducreyi LOS in the pathogenesis of chancroid. These efforts stemmed from the identification, cloning, and sequencing of several H. ducreyi genes whose encoded proteins are directly or indirectly involved in LOS expression (6, 21, 68, 79). These proteins include cytidine-5′-monophosphate N-acetylneuraminic acid synthetase, an enzyme involved in the ability of this organism to sialylate its LOS (42, 79), as well as homologs of RfaK (LbgB) (heptosyltransferase) (21, 68) and GmhA (phosphoheptose isomerase) (6). Only a few H. ducreyi mutants defective in LOS expression have been characterized (6, 9, 21, 68), and the lack of an intact LOS molecule has been shown to adversely affect both the ability of H. ducreyi to attach to and invade human keratinocytes in vitro (21) and the ability of this organism to cause skin lesions in an animal model (6).
In the present study, an H. ducreyi gene encoding a protein with significant identity to the Salmonella typhimurium RfaF (WaaF) (57, 65) protein was shown to be essential for expression of wild-type LOS by H. ducreyi 35000 and was shown to complement an S. typhimurium rfaF (waaF) mutant. Lack of expression of this WaaF homolog caused H. ducreyi to synthesize a truncated LOS molecule and to become attenuated in an animal model for experimental chancroid. (In accordance with a recent recommendation [57], the gene designation waaF will be used in place of rfaF in this report from this point forward.)
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli and S. typhimurium strains were grown in Luria-Bertani medium (59) supplemented, when appropriate, with tetracycline, chloramphenicol, or kanamycin at a final concentration of 15, 30, or 30 μg/ml, respectively. H. ducreyi strains were grown on chocolate agar (CA) plates containing 1% (vol/vol) IsoVitaleX (BBL Microbiological Systems, Cockeysville, Md.) as previously described (55). CA plates were supplemented either with chloramphenicol (2 μg/ml) for mutant selection or with both chloramphenicol (2 μg/ml) and kanamycin (30 μg/ml) for complementation experiments. A modified Haemophilus somnus liquid medium (31) was used in growth studies; it consisted of filter-sterilized Columbia broth (Difco Laboratories, Detroit, Mich.) supplemented with 2.5% (vol/vol) fetal bovine serum, 0.1% (wt/vol) Trizma base (Sigma Chemical Co. St. Louis, Mo.), 1% (vol/vol) IsoVitaleX, and heme (final concentration, 25 μg/ml). In these growth studies, H. ducreyi strains were grown at 33°C with slow shaking (90 rpm) in a water bath; growth was monitored by measurement of culture turbidity.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Bacterial strains | ||
| E. coli | ||
| DH5α | Host strain for cloning experiments | 59 |
| HB101 | Host strain essential for propagating plasmids carrying mutated H. ducreyi DNA inserts used in electroporation | 6, 59 |
| S. typhimurium | ||
| SL3770 | Expresses wild-type LPS | 58 |
| SL3789 | rfaF (waaF) mutant; expresses a truncated LPS molecule which migrates rapidly in SDS-PAGE relative to SL3770 LPS | 58 |
| H. ducreyi | ||
| 35000 | Wild-type strain isolated in Winnipeg, Manitoba, Canada; LOS binds MAb 3E6 | 23 |
| 35000.7 | Isogenic LOS mutant with a cat cartridge inserted into the rfaK (lbgB) gene; expresses a truncated LOS molecule that does not bind MAb 3E6 | 68 |
| 35000.252 | Isogenic LOS mutant with a cat cartridge inserted into the Ppu10I site within the gmhA gene; expresses a truncated LOS molecule that does not bind MAb 3E6 | 6 |
| 35000.10 | Isogenic LOS mutant with an Ω-Cm cartridge inserted into the MscI site within the waaF gene; expresses a truncated LOS molecule that does not bind MAb 3E6 | This study |
| Plasmids | ||
| pUC19 | Cloning vector; encodes Ampr | 59 |
| pHD1.3 | pUC19 containing a 1.5-kb insert of H. ducreyi 35000 chromosomal DNA with an incomplete waaF ORF | This study |
| pBR322 | Cloning vector; encodes Ampr, Tetr | 59 |
| pSLR1 | pBR322 with a 5.8-kb H. ducreyi 35000 DNA insert containing the waaF gene | This study |
| pBB10 | pSLR1 with an Ω-Cm cartridge inserted into the MscI site within the waaF gene | This study |
| pCR2.1 | Cloning vector; encodes Ampr | Invitrogen |
| pBB12 | pCR2.1 with a 1.5-kb PCR-derived insert containing the H. ducreyi waaF gene | This study |
| pLS88 | Cloning vector capable of replication in H. ducreyi; encodes Kanr, Smr, Sulr | 15 |
| pBB13 | pLS88 with a 1.5-kb PCR-derived insert containing the H. ducreyi waaF gene | This study |
PCR and recombinant DNA techniques.
PCR systems, employed to confirm the identities of certain mutant constructs and to produce double-stranded DNA probes, utilized Taq DNA polymerase (Promega, Madison, Wis.); these reactions were performed in accordance with the manufacturer’s instructions (71). A Gene Amp XL PCR kit (Perkin-Elmer Applied Biosystems, Foster City, Calif.) was used to identify mutated plasmids containing the Ω-chloramphenicol (Ω-Cm) cartridge. The Pfu DNA polymerase system (Stratagene) was employed to amplify DNA that was used to construct plasmids containing the wild-type H. ducreyi waaF gene. Boiled bacterial cell preparations (27) or chromosomal DNA purified from H. ducreyi was used as the template for PCR. Standard techniques such as restriction enzyme digestions, ligation reactions, transformations, electroporation of E. coli strains, and plasmid purifications have been previously described (5, 59).
H. ducreyi genomic libraries.
Two different H. ducreyi 35000 genomic libraries were used in this study. The first was constructed in pUC19 (69) and yielded the recombinant plasmid pHD1.3. The other consisted of a partial PstI digest of H. ducreyi 35000 chromosomal DNA that was ligated into the PstI site of pBR322 (59) and used to transform E. coli RR1. The recombinant plasmid pSLR1 was derived from the latter library.
Nucleotide sequence analysis.
A model 373A automated DNA sequencer (Applied Biosystems, Foster City, Calif.) was used to perform nucleotide sequence analysis. DNA sequence information was analyzed through the National Center for Biotechnology Information, using the BLAST network service to search the GenBank database (4), and with MacVector sequence analysis software (version 6; Oxford Molecular Group, Campbell, Calif.).
Construction of the isogenic H. ducreyi waaF mutant.
The Ω-Cm cartridge was excised from pHP45Ω-Cm (17) by digestion with BamHI, isolated by agarose gel electrophoresis, made blunt ended by treatment with the Klenow fragment of DNA polymerase I (New England Biolabs, Beverly, Mass.) and deoxynucleoside triphosphates, and blunt-end ligated into the MscI site within the waaF open reading frame (ORF) of pSLR1 (Fig. 1) to obtain pBB10. After propagation in E. coli HB101, plasmid pBB10 was purified by using a Wizard Plus Minipreps DNA purification system (Promega), linearized by digestion with PvuI, and used to electroporate H. ducreyi 35000 (24). The desired transformants were selected on CA plates supplemented with chloramphenicol.
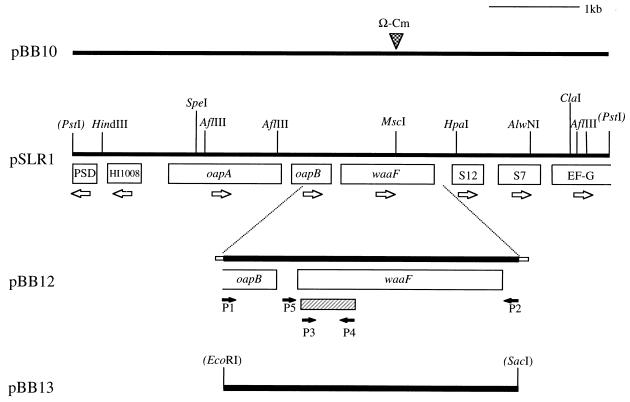
FIG. 1.
Partial restriction map of the H. ducreyi 35000 chromosomal DNA insert in pSLR1 and related plasmids. The eight ORFs present in this insert are indicated together in boxes, with open arrows designating the proposed directions of transcription. Restriction sites in parentheses indicate vector cloning sites. The closed arrows indicate various oligonucleotide primers used in PCR. The cross-hatched bar beneath the waaF gene indicates the 328-bp probe used for Southern blot analysis (see Fig. 4). The Ω-Cm cartridge was ligated into the MscI site of pSLR1 to construct pBB10. Plasmid pBB12 is pCR2.1 with a 1.5-kb PCR product containing the waaF gene and flanking DNA; the small boxes on the ends of this insert represent nucleotides involved in cloning it into the pCR2.1 vector. Plasmid pBB13 is pLS88 with the aforementioned 1.5-kb PCR product cloned into the EcoRI and SacI sites of this vector.
Complementation of an S. typhimurium waaF mutant with the H. ducreyi waaF gene.
A 1.5-kb PCR product containing the wild-type H. ducreyi waaF gene was prepared by using 600 ng of H. ducreyi 35000 chromosomal DNA template together with the oligonucleotide primers P1 (5′-CGGAATTCATCTCGCATTCAGCACTCCC-3′) and P2 (5′-CCGAGCTCCAAGTATCAAAGATTTCCCTGC-3′) (Fig. 1) (the underlined sequences indicate EcoRI and SacI sites, respectively). This 1.5-kb fragment was ligated into the pCR2.1 vector (Invitrogen, Carlsbad, Calif.) and used to transform E. coli DH5α. A plasmid (pBB12) from one of these transformants was used to electroporate the waaF mutant strain S. typhimurium 3789 (58); the desired recombinant strain was selected with ampicillin.
Complementation of the H. ducreyi waaF mutant.
After digestion with both EcoRI and SacI, the 1.5-kb PCR product described above was ligated into pLS88 (15) and used to transform E. coli DH5α; transformants were selected with kanamycin. A plasmid (pBB13) from one of these transformants was used to electroporate the waaF mutant H. ducreyi 35000.10. Selection of the complemented H. ducreyi mutants was accomplished by using CA containing both kanamycin and chloramphenicol.
MAb and colony blot radioimmunoassay.
Monoclonal antibody (MAb) 3E6 is specific for a surface-exposed epitope of H. ducreyi 35000 LOS (25). Hybridoma culture supernatant containing this MAb was used as the source of the primary antibody in the colony blot radioimmunoassay (34, 52).
Southern blot analysis.
AflIII or both ClaI and SpeI were used to digest purified H. ducreyi chromosomal DNA. Southern blot analysis was performed as previously described (59), using two different DNA probes. A 328-bp probe internal to the H. ducreyi waaF gene was constructed by PCR with the oligonucleotide primers P3 (5′-AGATTTTGATTATAGGACCC-3′) and P4 (5′-CTTTCACCTTTCCAACCG-3′) (Fig. 1). The 3.8 kb Ω-Cm cartridge was also used as a probe in Southern blot analyses. These probes were radiolabeled with [32P]dCTP by using a Random Primed DNA Labeling Kit (Boehringer-Mannheim, Indianapolis, Ind.).
Analysis of S. typhimurium LPS and H. ducreyi LOS.
Proteinase K was used to treat whole-cell lysates of S. typhimurium and H. ducreyi strains (52); then Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (40) was performed to resolve the lipopolysaccharide (LPS) or LOS in these preparations. Silver staining (77) and Western blot analysis with MAb 3E6 (6) were accomplished as described elsewhere.
Analysis of outer membrane proteins.
Proteins present in the Sarkosyl-insoluble fraction from H. ducreyi cell envelopes (19, 36) were resolved by SDS-PAGE and stained with Coomassie blue.
Virulence testing.
The relative virulence of the H. ducreyi strains used in this study was assessed in the temperature-dependent rabbit model for experimental chancroid (55). These animal studies were approved by the Institutional Animal Care and Research Advisory Committee of the University of Texas Southwestern Medical Center. Eight New Zealand White adult male rabbits were used in each experiment, and they were housed at a room temperature between 15 and 17°C. All other housing procedures and animal care procedures were performed in accordance with the standards of the U.S. Department of Agriculture and the Association for the Assessment and Accreditation of Laboratory Animal Care International. H. ducreyi cells were serially diluted and injected intradermally into each animal. Each rabbit was injected with at most three strains and with one injection of each dilution of the inoculum. To prevent bias in scoring of the lesions, the inocula were encoded by a second party prior to injection. Strain codes were broken after the conclusion of the experiment. Scoring of lesions on days 2, 4, and 7 postinjection involved the following numeric values: 0 = no change, 1 = erythema, 2 = induration, 3 = nodule, and 4 = pustule or necrosis (55). In addition, lesions were photographed on days 4 and 7 postinjection, and material excised from lesions caused by injection of 105 CFU of H. ducreyi was cultured on CA plates on day 7 postinjection. Organisms recovered on CA medium were then subcultured onto CA supplemented with appropriate antimicrobial compounds to confirm the presence of the relevant antimicrobial resistance markers and were also tested by colony blot radioimmunoassay with MAb 3E6 to confirm the LOS phenotype. Statistical analyses were performed as described previously (68, 69).
Nucleotide sequence accession number.
The nucleotide sequence of the 5.8-kb DNA fragment containing the H. ducreyi waaF gene was deposited in GenBank and assigned accession no. AF087414.
RESULTS
Identification of the waaF gene of H. ducreyi 35000.
Partial nucleotide sequence analysis of a recombinant clone [E. coli DH5α(pHD1.3)], derived from an H. ducreyi 35000 genomic library constructed in the plasmid vector pUC19, revealed the presence of an incomplete ORF encoding a predicted, truncated protein with homology to the WaaF protein of S. typhimurium (65). The 1.5-kb H. ducreyi DNA insert in pHD1.3 contained a putative translational start codon for this ORF but lacked a termination codon. To obtain the complete ORF, a double-stranded probe was constructed by PCR, using oligonucleotide primers P4 (see Materials and Methods) and P5 (5′-TTGCCATAATATAGCCCG-3′), with H. ducreyi 35000 chromosomal DNA as the template. The use of this probe to screen an H. ducreyi genomic library constructed in pBR322 resulted in the identification of the recombinant plasmid pSLR1, which contained a 5.8-kb H. ducreyi 35000 DNA insert (Fig. 1).
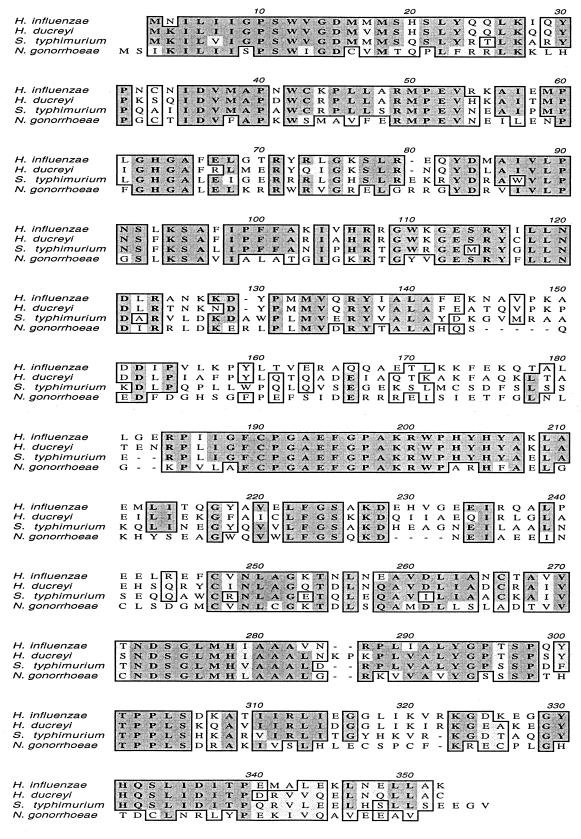
Nucleotide sequence analysis of the middle of this 5.8-kb DNA fragment revealed a complete ORF, 1,048 nucleotides in length, which encoded a predicted protein with a calculated molecular mass of 39,153 Da. This protein had 59% identity with the ADP-heptose:LPS heptosyltransferase II (WaaF) of S. typhimurium (65) and 70% identity with the WaaF protein of H. influenzae (Fig. 2) (20, 44). The WaaF protein from N. gonorrhoeae (63) was only 43% identical to the H. ducreyi WaaF molecule (Fig. 2). Two complete ORFs encoding predicted proteins with homology to the OapA and OapB proteins of H. influenzae (80) were located immediately upstream from this waaF homolog (Fig. 1). Two small ORFs encoding predicted proteins with similarity to a hypothetical protein (HI1008) and a phosphatidylserine decarboxylase from H. influenzae (20) were located on the opposite strand (Fig. 1). Two ORFs encoding predicted proteins with homology to the S12 (70) and S7 ribosomal proteins and an incomplete ORF encoding a predicted protein with homology to elongation factor G (20) were located downstream. These other ORFs were not analyzed further in this study.
FIG. 2.
Comparison of the deduced amino acid sequences of the WaaF proteins from H. influenzae (20, 44), H. ducreyi 35000, S. typhimurium (65), and N. gonorrhoeae (53, 63) by using the Clustal-W alignment program in MacVector version 6. Dark shading indicates residues that are identical; light shading indicates residues that are similar.
Complementation of an S. typhimurium waaF mutant with the H. ducreyi waaF gene homolog.
Complementation analysis was used to determine whether the similarity between the WaaF protein of S. typhimurium and the H. ducreyi WaaF homolog would allow the latter to function in this enteric organism. The H. ducreyi waaF gene was amplified by PCR and cloned into pCR2.1 to obtain pBB12 (Fig. 1). waaF mutant strain S. typhimurium 3789 (58) was electroporated with this recombinant plasmid, and one of the resultant transformants [strain 3789(pBB12)] was selected for further study.
Tricine-SDS-PAGE was used to analyze LPS molecules present in proteinase K-treated whole-cell lysates of the relevant S. typhimurium strains (Fig. 3). The LPS of the wild-type strain S. typhimurium 3770 (Fig. 3, lane 1) formed the ladder-like pattern indicative of the presence of the O antigen repeat unit, whereas the LPS of the S. typhimurium waaF mutant appeared as a single, very-fast-migrating band (Fig. 3, lane 2). The migration rate of the LPS of this mutant was not affected by the presence of the plasmid vector (Fig. 3, lane 3). However, when the wild-type H. ducreyi waaF was present in trans in strain 3789 (Fig. 3, lane 4), this S. typhimurium mutant synthesized an LPS which migrated in a pattern similar, if not identical, to that obtained with the LPS of the wild-type strain.
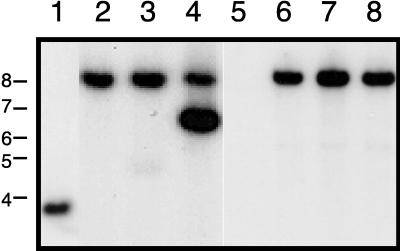
FIG. 3.
SDS-PAGE analysis of LPS expressed by the S. typhimurium waaF mutant strain 3789 and related S. typhimurium strains. LPS present in proteinase K-treated whole-cell lysates was resolved by Tricine-SDS-PAGE and stained with silver. Lanes: 1, S. typhimurium 3770, with a wild-type waaF gene; 2, S. typhimurium 3789; 3, S. typhimurium 3789 containing the pCR2.1 vector; 4, S. typhimurium 3789 containing pBB12 with the wild-type H. ducreyi waaF gene.
Construction of an isogenic H. ducreyi waaF mutant.
Plasmid pBB10 (Fig. 1), containing the Ω-Cm cartridge inserted within the MscI site in the middle of the H. ducreyi waaF ORF, was linearized with PvuI and used to electroporate H. ducreyi 35000. Thirty-four chloramphenicol-resistant H. ducreyi transformants were tested initially in the colony blot radioimmunoassay for reactivity with the H. ducreyi LOS-specific MAb 3E6. Nine of these proved to lack reactivity with this MAb (data not shown); one of these nine (isolate 35000.10) was selected at random for further study.
Southern blot analysis was performed to confirm that strain 35000.10 was an isogenic waaF mutant. When chromosomal DNA from the wild-type parent strain 35000 was probed with the 328-bp fragment from the H. ducreyi waaF gene (Fig. 1), a 3.9-kb SpeI-ClaI fragment was found to hybridize with this probe (Fig. 4, lane 1). This same probe hybridized with a 7.8-kb SpeI-ClaI fragment of chromosomal DNA from strain 35000.10 (Fig. 4, lane 2). This result was consistent with the replacement of the wild-type waaF gene with the mutated allele containing the 3.8-kb Ω-Cm cartridge. When the Ω-Cm cartridge was used as the probe, a 7.8-kb SpeI-ClaI fragment from strain 35000.10 also was found to hybridize (Fig. 4, lane 6). Chromosomal DNA from the wild-type parent strain 35000 failed to hybridize with the Ω-Cm probe (Fig. 4, lane 5).
FIG. 4.
Southern blot analysis of chromosomal DNA preparations from H. ducreyi wild-type, mutant, and complemented mutant strains. Chromosomal DNAs were digested with both SpeI and ClaI, resolved by agarose gel electrophoresis, and probed with either a 328-bp PCR product derived from the H. ducreyi waaF gene (lanes 1 to 4) or the Ω-Cm cartridge (lanes 5 to 8). Lanes: 1 and 5, strain 35000; 2 and 6, waaF mutant 35000.10; 3 and 7, 35000.10(pLS88); 4 and 8, 35000.10(pBB13). The positions of molecular size markers (in kilobases) are indicated on the left side of the figure.
Characterization of the isogenic waaF mutant.
The wild-type strain 35000 and the waaF mutant strain 35000.10 grew at the same rate and to the same final density in broth (data not shown). Analysis of the outer membrane protein profiles of these two strains revealed that the waaF mutant (Fig. 5, lane 3) did not appear to lack any proteins expressed by the wild-type parent strain (Fig. 5, lane 1), although these two strains did differ in the relative abundances of some of their outer membrane proteins (Fig. 5).
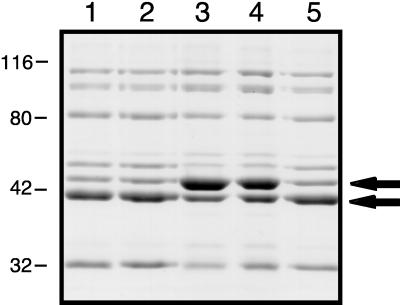
FIG. 5.
Comparison of outer membrane proteins from H. ducreyi wild-type, mutant, and complemented mutant strains. Proteins present in the Sarkosyl-insoluble cell envelope fraction were resolved by SDS-PAGE and stained with Coomassie blue. Lanes: 1, wild-type strain 35000; 2, strain 35000(pLS88); 3, waaF mutant strain 35000.10; 4, strain 35000.10(pLS88); 5, strain 35000.10(pBB13). The two arrows indicate outer membrane proteins whose relative abundances were different for the wild-type strain 35000 and the mutant strain 35000.10. The positions of molecular mass markers (in kilodaltons) are shown on the left side of this figure.
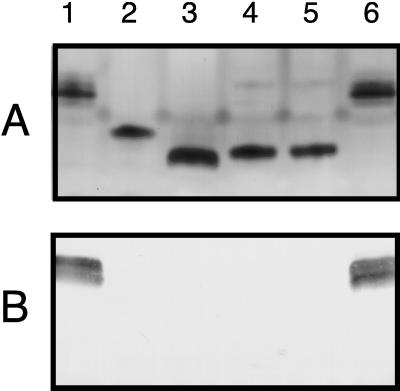
In SDS-PAGE, the LOS of the waaF mutant 35000.10 migrated at a rate different from those of the LOSs of the wild type and other previously described H. ducreyi LOS mutants. The LOS of the wild-type strain 35000 (Fig. 6A, lane 1) and that of the isogenic gmhA mutant 35000.252 (6) (lane 3) migrated at the slowest and fastest rates, respectively. The LOS of the waaF mutant 35000.10 (Fig. 6A, lane 4) migrated more rapidly than the LOS of the rfaK (lbgB) mutant 35000.7 (68) (Fig. 6A, lane 2) but more slowly than that of the gmhA mutant. In Western blot analysis with MAb 3E6, as previously shown, the wild-type strain 35000 (Fig. 6B, lane 1) bound this antibody (68). In contrast, all three of the LOS mutants failed to bind this MAb (Fig. 6B, lanes 2 to 4).
FIG. 6.
Characterization of the LOSs expressed by wild-type, mutant, and complemented mutant strains of H. ducreyi. LOSs present in proteinase K-treated whole-cell lysates were resolved by SDS-PAGE and either stained with silver (A) or transferred to nitrocellulose for Western blot analysis with the H. ducreyi LOS-specific MAb 3E6 (B). Lanes: 1, wild-type strain 35000; 2, rfaK (lbgB) mutant 35000.7; 3, gmhA mutant 35000.252; 4, waaF mutant 35000.10; 5, strain 35000.10(pLS88); 6, strain 35000.10(pBB13).
Complementation of the H. ducreyi waaF mutant.
The pLS88 vector and the recombinant plasmid pBB13, constructed by inserting the wild-type H. ducreyi waaF gene into pLS88 (Fig. 1), were used to electroporate the waaF mutant strain H. ducreyi 35000.10. Southern blot analysis of the waaF mutant containing only the pLS88 vector (Fig. 4, lanes 3 and 7) yielded a hybridization pattern identical to that of the mutant alone. In contrast, when the waaF mutant containing pBB13 was probed with the waaF-specific DNA fragment, two SpeI-ClaI fragments hybridized with this probe (Fig. 4, lane 4). The 7.8-kb fragment represented the mutated waaF gene in the chromosome, whereas the 6.5-kb fragment corresponded to the expected size of the linearized form of pBB13 (which contained a single ClaI site and no SpeI sites). As expected, only the 7.8-kb SpeI-ClaI fragment (containing the mutated waaF gene) from strain 35000.10(pBB13) bound to the Ω-Cm probe (Fig. 4, lane 8).
Successful complementation of the waaF mutant was confirmed by analysis of its LOS phenotype. The wild-type H. ducreyi waaF gene in trans in the waaF mutant [i.e., strain 35000.10(pBB13)] (Fig. 6, lanes 6) resulted in expression of a LOS molecule which had a migration rate and MAb 3E6 reactivity indistinguishable from those of wild-type LOS (Fig. 6, lanes 1). The migration rate and MAb 3E6 reactivity of the LOS of the H. ducreyi waaF mutant containing only the pLS88 vector [i.e., strain 35000.10(pLS88)] (Fig. 6, lanes 5) were not distinguishable from those of the waaF mutant 35000.10 (Fig. 6, lanes 4). Complementation of this mutant with the H. ducreyi waaF gene (Fig. 5, lane 5) also resulted in expression of an outer membrane protein profile similar, if not identical, to those of the wild-type parent strain (Fig. 5, lane 1) and the wild-type strain containing only the vector (Fig. 5, lane 2). The presence of the vector alone in the waaF mutant (Fig. 5, lane 4) did not affect the outer membrane profile of this strain relative to that of the mutant (Fig. 5, lane 3).
Effect of the waaF mutation on virulence expression.
The temperature-dependent rabbit model (55) was used to evaluate the virulence of the isogenic waaF mutant 35000.10. In the first experiment, which involved eight rabbits (Table 2, experiment A), by day 2 postinfection, five of eight rabbit lesions resulting from inoculation of 105 CFU of the wild-type strain had formed pustules and one lesion demonstrated necrosis; all six of these lesions were assigned a lesion score of 4. The two remaining lesions were nodular and received a lesion score of 3. By day 7 postinfection, seven of eight rabbit lesions that resulted from the inoculation of 105 CFU of the wild-type strain had progressed and necrosis with eschar formation was evident (lesion score = 4), whereas the eighth lesion was still at the nodular stage (lesion score = 3). In contrast, on day 2 postinfection, all eight lesions that resulted from inoculation of 105 CFU of the waaF mutant had only formed nodules, and seven of these lesions remained at this nodular stage on day 7 postinfection. Similar individual rabbit lesion scores were obtained in a second, independent experiment that also utilized eight animals (Table 2, experiment B). In these two experiments, mean lesion scores obtained with the wild-type strain 35000 were significantly higher than those obtained with the waaF mutant (Table 2). Finally, when lesions were sampled on day 7 postinfection, viable H. ducreyi 35000 organisms were recovered from 14 of 16 lesions produced by this wild-type strain; in contrast, viable H. ducreyi 35000.10 organisms were not recovered from any of 16 lesions produced by this waaF mutant.
TABLE 2.
Lesion formation by wild-type, waaF mutant, and complemented waaF mutant strains of H. ducreyi in the temperature-dependent rabbit modela
| Expt | Strain | Inoculum size | Mean lesion score ± SD on day:
|
P valueb | ||
|---|---|---|---|---|---|---|
| 2 | 4 | 7 | ||||
| A | 35000 (wild type) | 105 | 3.75 ± 0.46 | 3.88 ± 0.35 | 3.88 ± 0.35 | |
| 35000.10 (waaF mutant) | 105 | 3.00 ± 0 | 3.13 ± 0.35 | 3.13 ± 0.35 | 0.0006 | |
| 35000 | 104 | 3.00 ± 0 | 3.63 ± 0.52 | 3.75 ± 0.46 | ||
| 35000.10 | 104 | 3.00 ± 0 | 2.25 ± 0.46 | 2.63 ± 0.52 | ||
| B | 35000 | 105 | 3.75 ± 0.46 | 4.00 ± 0 | 3.88 ± 0.35 | |
| 35000.10 | 105 | 3.00 ± 0 | 2.63 ± 0.52 | 2.63 ± 0.74 | 0.0001 | |
| 35000.10(pBB13) | 105 | 3.63 ± 0.52 | 3.75 ± 0.46 | 3.88 ± 0.35 | 0.0518c | |
| 35000 | 104 | 3.25 ± 0.46 | 3.50 ± 0.76 | 3.50 ± 0.76 | ||
| 35000.10 | 104 | 1.88 ± 0.64 | 1.13 ± 0.83 | 1.13 ± 1.13 | ||
| 35000.10(pBB13) | 104 | 3.13 ± 0.35 | 3.13 ± 0.64 | 3.25 ± 0.71 | ||
Eight rabbits were used in each experiment.
P values, for differences between wild-type and test strain lesion scores, were calculated with the lesion scores for both inoculum sizes from all 3 days.
The complemented waaF mutant 35000.10(pBB13) was significantly more virulent than the uncomplemented waaF mutant (P ≤ 0.0001).
As part of the second experiment (Table 2, experiment B), complementation analysis was performed to determine whether the presence of the wild-type H. ducreyi waaF gene in trans would restore the virulence of the waaF mutant. The complemented waaF mutant, 35000.10(pBB13), produced lesions that were not significantly different from those obtained with the wild-type parent strain (Table 2, experiment B). In addition, viable H. ducreyi organisms were recovered from seven of eight lesions produced by the complemented waaF mutant 35000.10 (pBB13). Colony blot analysis with MAb 3E6 and antimicrobial resistance testing were used to confirm the identity of the H. ducreyi organisms recovered from lesions produced by strain 35000.10(pBB13).
Detection of the waaF gene among H. ducreyi strains.
The 328-bp fragment from the waaF gene of H. ducreyi 35000 (Fig. 1) was used to probe chromosomal DNA from 11 strains of H. ducreyi (35000, Hd9, Hd12, Hd13, 512, 1145, 1151, 1352, Cha-1, R018, and STD101) (68). All of these strains possessed a 3.3-kb AflIII fragment of chromosomal DNA which hybridized to this probe, suggesting the presence of the waaF gene in all 11 strains (data not shown).
DISCUSSION
The potential involvement of H. ducreyi LOS in the expression of virulence by this pathogen was first amply illustrated by demonstration of the potent inflammatory ability of this amphipathic molecule in animal model systems (11, 78). Elucidation of the basic structure of H. ducreyi LOS has revealed that this outer membrane antigen is remarkably similar to the LOS of H. influenzae (1, 21, 42, 43, 64). However, in contrast to the recent accumulation of important information concerning the genetics of H. influenzae LOS expression (30, 32, 44, 45, 54, 81), there is still relatively little known about the gene products involved in the synthesis of H. ducreyi LOS.
Only recently has mutant analysis been used to begin investigation of the involvement of H. ducreyi LOS in virulence expression. Initial efforts in this area resulted in the construction of rfaK (lbgB) mutants of H. ducreyi, which expressed an LOS with a modestly truncated oligosaccharide (9, 21, 68). Interestingly, these H. ducreyi LOS mutants were less able to both attach to and invade human keratinocytes in vitro (9, 21). Testing of the virulence potential of an rfaK (lbgB) mutant in the temperature-dependent rabbit model yielded equivocal results in that this mutation did not exert a statistically significant effect on the ability of H. ducreyi to produce skin lesions in this animal model (68). However, a gmhA mutant of H. ducreyi, which expressed a drastically truncated LOS that likely consisted of lipid A together with a single molecule of 2-keto-3-deoxyoctulosonic acid (KDO) (26), was shown to be markedly less virulent than its wild-type parent strain in this same animal model (6). This result suggests that certain truncations of the H. ducreyi LOS oligosaccharide chain can affect the virulence potential of this pathogen in an animal model system.
First characterized in enteric organisms (62, 65), the gene (waaF) encoding heptosyltransferase II has now been identified in a number of bacterial pathogens and subsequently characterized (3, 14, 33, 44, 53, 63). At least in H. influenzae (44), and likely also in H. ducreyi, the protein product of the waaF gene is involved in catalyzing the addition of a (second) heptose molecule onto the heptose linked directly to KDO. The absence of WaaF activity in H. ducreyi would be predicted to result in the synthesis of a truncated lipid A-KDO-heptose moiety instead of a complete LOS molecule. Evidence of the involvement of the waaF gene product in a process likely to be relevant to virulence expression has been obtained previously for N. gonorrhoeae, for which a waaF mutation resulted in a markedly reduced ability to adhere to and invade Chang conjunctival epithelial cells in vitro (63), although this same mutation in Neisseria meningitidis did not alter the toxicity of whole cells of this encapsulated pathogen for human umbilical vein endothelial cells in culture (33). In addition, a waaF mutant of H. influenzae type b was shown to have a greatly reduced ability to cause bacteremia in infant rats following intraperitoneal injection (30).
The H. ducreyi waaF mutant expressed a LOS that migrated much more rapidly in SDS-PAGE than the LOS of the wild-type parent strain and just slightly more slowly than the drastically truncated LOS expressed by the H. ducreyi gmhA mutant (Fig. 6) (6). When present in trans, the wild-type H. ducreyi waaF gene allowed an S. typhimurium waaF mutant to express an LPS molecule which appeared to be identical to that expressed by the wild-type S. typhimurium strain (Fig. 3). The latter result confirmed the identity of this H. ducreyi protein as a functional WaaF homolog.
Similar to the previously described H. ducreyi gmhA mutant, the H. ducreyi waaF mutant exhibited some changes in the relative abundance of a few outer membrane proteins (Fig. 5). These changes appeared to involve the same proteins as were affected in the H. ducreyi gmhA mutant (6), suggesting that the observed alteration in outer membrane protein abundance is likely a response to the presence of a truncated LOS molecule in the outer membrane rather than a specific response to the waaF mutation. Interestingly, a waaF mutant of N. meningitidis was reported to be invariant from its wild-type parent strain with regard to expression of major surface antigens (33). Complementation of the H. ducreyi waaF mutant with the wild-type H. ducreyi waaF gene did result in restoration of expression of an apparent wild-type outer membrane protein profile (Fig. 5). Changes in outer membrane protein expression have also been described for deep, rough LPS mutants of enteric bacteria (18, 37, 46). Whether the described changes in the relative abundance of a few outer membrane proteins of the H. ducreyi waaF mutant could have affected virulence expression cannot be determined directly from the available data.
It is also interesting that the H. ducreyi waaF gene, similar to the rfaK (lbgB) (21, 68) and gmhA genes of this pathogen, is not located amid a cluster of other genes whose products are likely involved in LOS biosynthesis. Instead, the H. ducreyi waaF gene is located immediately upstream from a gene involved in the synthesis of a ribosomal protein and immediately downstream from two genes encoding proteins with homology to the H. influenzae OapA and OapB proteins (Fig. 1) (80). The H. influenzae OapA protein recently has been shown to be involved in the ability of H. influenzae to colonize the nasopharynx of the infant rat (80). This placement of the H. ducreyi waaF gene is in contrast to the multiple, closely linked genes involved in enteric core LPS biosynthesis (i.e., the E. coli rfa [waa] genes) (35, 62). Even in most strains of H. influenzae (44), the waaF gene is located immediately downstream from rfaD (waaD), as in enteric bacteria (62, 65). Why all of the H. ducreyi LOS biosynthetic genes described to date are located in discrete sites in the H. ducreyi genome remains to be determined.
Whereas lack of expression of WaaF did not have an apparent effect on the ability of H. ducreyi to grow in vitro in broth medium, the waaF mutation had a pronounced effect on H. ducreyi in vivo. More specifically, the H. ducreyi waaF mutant was less virulent than its wild-type parent strain in the temperature-dependent rabbit model (Table 2) and could not be recovered from lesions at 7 days postinfection. Restoration of both wild-type LOS expression and virulence was accomplished by provision of the wild-type H. ducreyi waaF gene in trans, a result which also indicated that the H. ducreyi waaF mutant did not possess a secondary mutation(s) which could have affected LOS expression or virulence. Whether the waaF mutation will alter the virulence of H. ducreyi for humans will eventually have to be determined in the human challenge model for experimental chancroid (66, 67).
ACKNOWLEDGMENTS
This study was supported by U.S. Public Health Service grant AI-32011 to E.J.H. B.A.B. was supported by U.S. Public Health Service training grant RR-07031.
S. typhimurium 3770 and 3789 were provided by Kenneth Sanderson, and the Ω-Cm cartridge was obtained from Joachim Frey. We thank Christine Ward and David Lewis for careful assistance with the animal model and both Robert Munson and Anthony Campagnari for helpful discussions.
REFERENCES
- 1.Ahmed H J, Frisk A, Månsson J-E, Schweda E K H, Lagergård T. Structurally defined epitopes of Haemophilus ducreyi lipooligosaccharides recognized by monoclonal antibodies. Infect Immun. 1997;65:3151–3158. doi: 10.1128/iai.65.8.3151-3158.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfa M J, DeGagne P, Totten P A. Haemophilus ducreyi hemolysin acts as a contact cytotoxin and damages human foreskin fibroblasts in cell culture. Infect Immun. 1996;64:2349–2352. doi: 10.1128/iai.64.6.2349-2352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen A G, Isobe T, Maskell D J. Identification and cloning of waaF (rfaF) from Bordetella pertussis and use to generate mutants of Bordetella spp. with deep rough lipopolysaccharide. J Bacteriol. 1998;180:35–40. doi: 10.1128/jb.180.1.35-40.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 5.Ausubel F M, Brent R, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1990. [Google Scholar]
- 6.Bauer B A, Stevens M K, Hansen E J. Involvement of the Haemophilus ducreyi gmhA gene product in lipooligosaccharide expression and virulence. Infect Immun. 1998;66:4290–4298. doi: 10.1128/iai.66.9.4290-4298.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brentjens R J, Ketterer M, Apicella M A, Spinola S M. Fine tangled pili expressed by Haemophilus ducreyi are a novel class of pili. J Bacteriol. 1996;178:808–816. doi: 10.1128/jb.178.3.808-816.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brentjens R J, Spinola S M, Campagnari A A. Haemophilus ducreyi adheres to human keratinocytes. Microb Pathog. 1994;16:243–247. doi: 10.1006/mpat.1994.1025. [DOI] [PubMed] [Google Scholar]
- 9.Campagnari A A, Karalus R, Apicella M, Melaugh W, Lesse A J, Gibson B W. Use of pyocin to select a Haemophilus ducreyi variant defective in lipooligosaccharide biosynthesis. Infect Immun. 1994;62:2379–2386. doi: 10.1128/iai.62.6.2379-2386.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campagnari A A, Spinola S M, Lesse A J, Kwaik Y A, Mandrell R E, Apicella M A. Lipooligosaccharide epitopes shared among gram-negative non-enteric mucosal pathogens. Microb Pathog. 1990;8:353–362. doi: 10.1016/0882-4010(90)90094-7. [DOI] [PubMed] [Google Scholar]
- 11.Campagnari A A, Wild L M, Griffiths G E, Karalus R J, Wirth M A, Spinola S M. Role of lipooligosaccharides in experimental dermal lesions caused by Haemophilus ducreyi. Infect Immun. 1991;59:2601–2608. doi: 10.1128/iai.59.8.2601-2608.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carson S D B, Thomas C E, Elkins C. Cloning and sequencing of a Haemophilus ducreyi fur homolog. Gene. 1996;176:125–129. doi: 10.1016/0378-1119(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 13.Cope L D, Lumbley S R, Latimer J L, Klesney-Tait J, Stevens M K, Johnson L S, Purven M, Munson R S, Jr, Lagergård T, Radolf J D, Hansen E J. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Kievit T R, Lam J S. Isolation and characterization of two genes, waaC (rfaC) and waaF (rfaF), involved in Pseudomonas aeruginosa serotype O5 inner-core biosynthesis. J Bacteriol. 1997;179:3451–3457. doi: 10.1128/jb.179.11.3451-3457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixon L G, Albritton W L, Willson P J. An analysis of the complete nucleotide sequence of the Haemophilus ducreyi broad-host-range plasmid pLS88. Plasmid. 1994;32:228–232. doi: 10.1006/plas.1994.1060. [DOI] [PubMed] [Google Scholar]
- 16.Elkins C, Chen C-J, Thomas C E. Characterization of the hgbA locus encoding a hemoglobin receptor from Haemophilus ducreyi. Infect Immun. 1995;63:2194–2200. doi: 10.1128/iai.63.6.2194-2200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fellay R, Frey J, Krisch H M. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram negative bacteria. Gene. 1987;52:147–152. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 18.Ferro-Luzzi Ames G, Spudich E N, Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974;117:406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filip C, Fletcher G, Wulff J L, Earhart C F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973;115:717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J-F, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C, Gocayne J D, Scott J, Shirley R, Liu L-I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback R C, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Frichman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 21.Gibson B W, Campagnari A A, Melaugh W, Phillips N J, Apicella M A, Grass S, Wang J, Palmer K L, Munson R S., Jr Characterization of a transposon Tn916-generated mutant of Haemophilus ducreyi 35000 defective in lipooligosaccharide biosynthesis. J Bacteriol. 1997;179:5062–5071. doi: 10.1128/jb.179.16.5062-5071.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibson B W, Melaugh W, Phillips N J, Apicella M A, Campagnari A A, Griffiss J M. Investigation of the structural heterogeneity of lipooligosaccharides from pathogenic Haemophilus and Neisseria species and of R-type lipopolysaccharides from Salmonella typhimurium by electrospray mass spectrometry. J Bacteriol. 1993;175:2702–2712. doi: 10.1128/jb.175.9.2702-2712.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammond G W, Lian C J, Wilt J C, Ronald A R. Antimicrobial susceptibility of Haemophilus ducreyi. Antimicrob Agents Chemother. 1978;13:608–612. doi: 10.1128/aac.13.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen E J, Latimer J L, Thomas S E, Helminen M, Albritton W L, Radolf J D. Use of electroporation to construct isogenic mutants of Haemophilus ducreyi. J Bacteriol. 1992;174:5442–5449. doi: 10.1128/jb.174.16.5442-5449.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen E J, Lumbley S R, Saxen H, Kern K, Cope L D, Radolf J D. Detection of Haemophilus ducreyi lipooligosaccharide by means of an immunolimulus assay. J Immunol Methods. 1995;185:225–235. doi: 10.1016/0022-1759(95)00118-t. [DOI] [PubMed] [Google Scholar]
- 26.Helander I M, Lindner B, Brade H, Altmann K, Lindberg A A, Rietschel E T, Zahringer U. Chemical structure of the lipopolysaccharide of Haemophilus ducreyi strain I-69 Rd−/b+: description of a novel deep-rough chemotype. Eur J Biochem. 1988;177:483–492. doi: 10.1111/j.1432-1033.1988.tb14398.x. [DOI] [PubMed] [Google Scholar]
- 27.Hennessy K J, Iandolo J J, Fenwick B W. Serotype identification of Actinobacillus pleuropneumoniae by arbitrarily primed polymerase chain reaction. J Clin Microbiol. 1993;31:1155–1159. doi: 10.1128/jcm.31.5.1155-1159.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hobbs M M, Paul T R, Wyrick P B, Kawula T H. Haemophilus ducreyi infection causes basal keratinocyte cytotoxicity and elicits a unique cytokine induction pattern in an in vitro human skin model. Infect Immun. 1998;66:2914–2921. doi: 10.1128/iai.66.6.2914-2921.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hobbs M M, San Mateo L R, Orndorff P E, Almond G, Kawula T H. Swine model of Haemophilus ducreyi infection. Infect Immun. 1995;63:3094–3100. doi: 10.1128/iai.63.8.3094-3100.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hood D W, Deadman M E, Allen T, Masoud H, Martin A, Brisson J R, Fleischmann R, Venter J C, Richards J C, Moxon E R. Use of the complete genome sequence information of Haemophilus influenzae strain Rd to investigate lipopolysaccharide biosynthesis. Mol Microbiol. 1996;22:951–965. doi: 10.1046/j.1365-2958.1996.01545.x. [DOI] [PubMed] [Google Scholar]
- 31.Inzana T J, Corbeil L B. Development of a defined medium for Haemophilus somnus isolated from cattle. Am J Vet Res. 1987;48:366–369. [PubMed] [Google Scholar]
- 32.Jackson A D, Maskell D, Moxon E R, Wilson R. The effect of mutations in genes required for lipopolysaccharide synthesis on Haemophilus influenzae type b colonization of human nasopharyngeal tissue. Microb Pathog. 1996;21:463–470. doi: 10.1006/mpat.1996.0076. [DOI] [PubMed] [Google Scholar]
- 33.Jennings M P, Bisercic M, Dunn K L R, Virji M, Martin A, Wilks K E, Richards J C, Moxon E R. Cloning and molecular analysis of the lsi1 (rfaF) gene of Neisseria meningitidis which encodes a heptosyl-2-transferase involved in LPS mediated toxicity for human endothelial cells. Microb Pathog. 1995;19:391–407. doi: 10.1006/mpat.1995.0074. [DOI] [PubMed] [Google Scholar]
- 34.Kimura A, Hansen E J. Antigenic and phenotypic variations of Haemophilus influenzae type b lipopolysaccharide and their relationship to virulence. Infect Immun. 1986;51:69–79. doi: 10.1128/iai.51.1.69-79.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klena J D, Pradel E, Schnaitman C A. Comparison of lipopolysaccharide biosynthesis genes rfaK, rfaL, rfaY, and rfaZ of Escherichia coli K-12 and Salmonella typhimurium. J Bacteriol. 1992;174:4746–4752. doi: 10.1128/jb.174.14.4746-4752.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klesney-Tait J, Hiltke T J, Maciver I, Spinola S M, Radolf J D, Hansen E J. The major outer membrane protein of Haemophilus ducreyi consists of two OmpA homologs. J Bacteriol. 1997;179:1764–1773. doi: 10.1128/jb.179.5.1764-1773.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koplow J, Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974;117:527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lammel C J, Dekker N P, Palefsky J, Brooks G F. In vitro model of Haemophilus ducreyi adherence to and entry into eukaryotic cells of genital origin. J Infect Dis. 1993;167:642–650. doi: 10.1093/infdis/167.3.642. [DOI] [PubMed] [Google Scholar]
- 39.Langford P R, Kroll J S. Distribution, cloning, characterization and mutagenesis of sodC, the gene encoding copper/zinc superoxide dismutase, a potential determinant of virulence in Haemophilus ducreyi. FEMS Immunol Med Microbiol. 1997;17:235–242. doi: 10.1111/j.1574-695X.1997.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 40.Lesse A J, Campagnari A A, Bittner W E, Apicella M A. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol Methods. 1990;126:109–117. doi: 10.1016/0022-1759(90)90018-q. [DOI] [PubMed] [Google Scholar]
- 41.Mandrell R E, McLaughlin R, Kwaik Y A, Lesse A, Yamasaki R, Gibson B, Spinola S M, Apicella M A. Lipooligosaccharides (LOS) of some Haemophilus species mimic human glycosphingolipids, and some LOS are sialylated. Infect Immun. 1992;60:1322–1328. doi: 10.1128/iai.60.4.1322-1328.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melaugh W, Campagnari A A, Gibson B W. The lipooligosaccharides of Haemophilus ducreyi are highly sialylated. J Bacteriol. 1996;178:564–570. doi: 10.1128/jb.178.2.564-570.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Melaugh W, Phillips N J, Campagnari A A, Tullius M V, Gibson B W. Structure of the major oligosaccharide from the lipooligosaccharide of Haemophilus ducreyi strain 35000 and evidence of additional glycoforms. Biochemistry. 1994;33:13070–13078. doi: 10.1021/bi00248a016. [DOI] [PubMed] [Google Scholar]
- 44.Nichols W A, Gibson B W, Melaugh W, Lee N-G, Sunshine M, Apicella M A. Identification of the ADP-l-glycero-d-manno-heptose-6-epimerase (rfaD) and heptosyltransferase II (rfaF) biosynthesis genes from nontypeable Haemophilus influenzae 2019. Infect Immun. 1997;65:1377–1386. doi: 10.1128/iai.65.4.1377-1386.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nichols W A, Raetz C R H, Clementz T, Smith A L, Hanson J A, Ketterer M R, Sunshine M, Apicella M A. htrB of Haemophilus influenzae: determination of biochemical activity and effects on virulence and lipooligosaccharide toxicity. J Endotoxin Res. 1997;8:585–588. [Google Scholar]
- 46.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palmer K L, Goldman W E, Munson R S., Jr An isogenic haemolysin-deficient mutant of Haemophilus ducreyi lacks the ability to produce cytopathic effects on human foreskin fibroblasts. Mol Microbiol. 1996;21:13–19. doi: 10.1046/j.1365-2958.1996.00615.x. [DOI] [PubMed] [Google Scholar]
- 48.Palmer K L, Grass S, Munson R S., Jr Identification of a hemolytic activity elaborated by Haemophilus ducreyi. Infect Immun. 1994;62:3041–3043. doi: 10.1128/iai.62.7.3041-3043.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmer K L, Munson R S., Jr Cloning and characterization of the genes encoding the haemolysin of Haemophilus ducreyi. Mol Microbiol. 1995;18:821–830. doi: 10.1111/j.1365-2958.1995.18050821.x. [DOI] [PubMed] [Google Scholar]
- 50.Palmer K L, Thornton A C, Fortney K R, Munson R S, Jr, Spinola S M. Evaluation of an isogenic hemolysin-deficient mutant in the human model of Haemophilus ducreyi infection. J Infect Dis. 1998;178:191–199. doi: 10.1086/515617. [DOI] [PubMed] [Google Scholar]
- 51.Parsons L M, Limberger R J, Shayegani M. Alterations in levels of DnaK and GroEL result in diminished survival and adherence of stressed Haemophilus ducreyi. Infect Immun. 1997;65:2413–2419. doi: 10.1128/iai.65.6.2413-2419.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patrick C C, Kimura A, Jackson M A, Hermanstorfer L, Hood A, McCracken G H, Jr, Hansen E J. Antigenic characterization of the oligosaccharide portion of the lipooligosaccharide of nontypable Haemophilus influenzae. Infect Immun. 1987;55:2902–2911. doi: 10.1128/iai.55.12.2902-2911.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petricoin E F, III, Danaher R J, Stein D C. Analysis of the lsi region involved in lipooligosaccharide biosynthesis in Neisseria gonorrhoeae. J Bacteriol. 1991;173:7896–7902. doi: 10.1128/jb.173.24.7896-7902.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phillips N J, McLaughlin R, Miller T J, Apicella M A, Gibson B W. Characterization of two transposon mutants from Haemophilus influenzae type b with altered lipooligosaccharide biosynthesis. Biochemistry. 1996;35:5937–5947. doi: 10.1021/bi960059b. [DOI] [PubMed] [Google Scholar]
- 55.Purcell B K, Richardson J A, Radolf J D, Hansen E J. A temperature-dependent rabbit model for production of dermal lesions by Haemophilus ducreyi. J Infect Dis. 1991;164:359–367. doi: 10.1093/infdis/164.2.359. [DOI] [PubMed] [Google Scholar]
- 56.Purvén M, Frisk A, Lönnroth I, Lagergård T. Purification and identification of Haemophilus ducreyi cytotoxin by use of a neutralizing monoclonal antibody. Infect Immun. 1997;65:3496–3499. doi: 10.1128/iai.65.8.3496-3499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reeves P R, Hobbs M, Valvano M A, Skurnik M, Whitfield C, Coplin D, Kido N, Klena J, Maskell D, Raetz C R, Rick P D. Bacterial polysaccharide synthesis and gene nomenclature. Trends Microbiol. 1996;4:495–503. doi: 10.1016/s0966-842x(97)82912-5. [DOI] [PubMed] [Google Scholar]
- 58.Roantree R J, Kuo T T, MacPhee D G. The effect of defined lipopolysaccharide core defects upon antibiotic resistances of Salmonella typhimurium. J Gen Microbiol. 1977;103:223–234. doi: 10.1099/00221287-103-2-223. [DOI] [PubMed] [Google Scholar]
- 59.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 60.San Mateo L R, Hobbs M M, Kawula T H. Periplasmic copper-zinc superoxide dismutase protects Haemophilus ducreyi from exogenous superoxide. Mol Microbiol. 1998;27:391–404. doi: 10.1046/j.1365-2958.1998.00687.x. [DOI] [PubMed] [Google Scholar]
- 61.San Mateo L R, Toffer K L, Kawula T H. The sodA gene of Haemophilus ducreyi expresses a hydrogen peroxide-inhibitable superoxide dismutase. Gene. 1998;207:251–257. doi: 10.1016/s0378-1119(97)00642-2. [DOI] [PubMed] [Google Scholar]
- 62.Schnaitman C A, Klena J D. Genetics of lipopolysaccharide biosynthesis in enteric bacteria. Microbiol Rev. 1993;57:655–682. doi: 10.1128/mr.57.3.655-682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwan E T, Robertson B D, Brade H B, van Putten J P M. Gonococcal rfaF mutants express Rd2 chemotype LPS and do not enter epithelial host cells. Mol Microbiol. 1995;15:267–275. doi: 10.1111/j.1365-2958.1995.tb02241.x. [DOI] [PubMed] [Google Scholar]
- 64.Schweda E K H, Jonasson J A, Jansson P-E. Structural studies of lipooligosaccharides from Haemophilus ducreyi ITM 5535, ITM 3147, and a fresh clinical isolate, ACY1: evidence for intrastrain heterogeneity with the production of mutually exclusive sialylated or elongated glycoforms. J Bacteriol. 1995;177:5316–5321. doi: 10.1128/jb.177.18.5316-5321.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sirisena D M, MacLachlan P R, Liu S-L, Hessel A, Sanderson K E. Molecular analysis of the rfaD gene, for heptose synthesis, and the rfaF gene, for heptose transfer, in lipopolysaccharide synthesis in Salmonella typhimurium. J Bacteriol. 1994;176:2379–2385. doi: 10.1128/jb.176.8.2379-2385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spinola S M, Orazi A, Arno J N, Fortney K R, Kotylo P, Chen C-Y, Campagnari A A, Hood A F. Haemophilus ducreyi elicits a cutaneous infiltrate of CD4 cells during experimental human infection. J Infect Dis. 1996;173:394–402. doi: 10.1093/infdis/173.2.394. [DOI] [PubMed] [Google Scholar]
- 67.Spinola S M, Wild L M, Apicella M A, Gaspari A A, Campagnari A A. Experimental human infection with Haemophilus ducreyi. J Infect Dis. 1994;169:1146–1150. doi: 10.1093/infdis/169.5.1146. [DOI] [PubMed] [Google Scholar]
- 68.Stevens M K, Klesney-Tait J, Lumbley S, Walters K A, Joffe A M, Radolf J D, Hansen E J. Identification of tandem genes involved in lipooligosaccharide expression by Haemophilus ducreyi. Infect Immun. 1997;65:651–660. doi: 10.1128/iai.65.2.651-660.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stevens M K, Porcella S, Klesney-Tait J, Lumbley S, Thomas S E, Norgard M V, Radolf J D, Hansen E J. A hemoglobin-binding outer membrane protein is involved in virulence expression by Haemophilus ducreyi in an animal model. Infect Immun. 1996;64:1724–1735. doi: 10.1128/iai.64.5.1724-1735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stuy J H, Walter R B. Cloning, characterization, and DNA base sequence of the high-level streptomycin resistance gene strA1 of Haemophilus influenzae Rd. J Bacteriol. 1992;174:5604–5608. doi: 10.1128/jb.174.17.5604-5608.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Templeton N S. The polymerase chain reaction: history, methods, and applications. Diagn Mol Pathol. 1992;1:58–72. doi: 10.1097/00019606-199203000-00008. [DOI] [PubMed] [Google Scholar]
- 72.Totten P A, Lara J C, Norn D V, Stamm W E. Haemophilus ducreyi attaches to and invades human epithelial cells in vitro. Infect Immun. 1994;62:5632–5640. doi: 10.1128/iai.62.12.5632-5640.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Totten P A, Morton W R, Knitter G H, Clark A M, Kiviat N B, Stamm W E. A primate model for chancroid. J Infect Dis. 1994;169:1284–1290. doi: 10.1093/infdis/169.6.1284. [DOI] [PubMed] [Google Scholar]
- 74.Totten P A, Norn D V, Stamm W E. Characterization of the hemolytic activity of Haemophilus ducreyi. Infect Immun. 1995;63:4409–4416. doi: 10.1128/iai.63.11.4409-4416.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trees D L, Arko R J, Morse S A. Mouse subcutaneous chamber model for in vivo growth of Haemophilus ducreyi. Microb Pathog. 1991;11:387–390. doi: 10.1016/0882-4010(91)90025-6. [DOI] [PubMed] [Google Scholar]
- 76.Trees D L, Morse S A. Chancroid and Haemophilus ducreyi: an update. Clin Microbiol Rev. 1995;8:357–375. doi: 10.1128/cmr.8.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsai C-M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharide in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 78.Tuffrey M, Alexander F, Ballard R C, Taylor-Robinson D. Characterization of skin lesions in mice following intradermal inoculation of Haemophilus ducreyi. J Exp Pathol. 1990;71:233–244. [PMC free article] [PubMed] [Google Scholar]
- 79.Tullius M V, Munson R S, Jr, Wang J, Gibson B W. Purification, cloning, and expression of a cytidine 5′-monophosphate N-acetylneuraminic acid synthetase from Haemophilus ducreyi. J Biol Chem. 1996;271:15373–15380. doi: 10.1074/jbc.271.26.15373. [DOI] [PubMed] [Google Scholar]
- 80.Weiser J N, Chong S T H, Greenberg D, Fong W. Identification and characterization of a cell envelope protein of Haemophilus influenzae contributing to phase variation in colony opacity and nasopharyngeal colonization. Mol Microbiol. 1995;17:555–564. doi: 10.1111/j.1365-2958.1995.mmi_17030555.x. [DOI] [PubMed] [Google Scholar]
- 81.Weiser J N, Shchepetov M, Chong S T H. Decoration of lipopolysaccharide with phosphorylcholine: a phase-variable characteristic of Haemophilus influenzae. Infect Immun. 1997;65:943–950. doi: 10.1128/iai.65.3.943-950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]