Abstract
The COVID-19 pandemic has disproportionately affected certain groups, such as older people (ie, >65 years), minority ethnic populations, and people with specific chronic conditions including diabetes, cardiovascular disease, kidney disease, and some respiratory diseases. There is now evidence of not only direct but also indirect adverse effects of COVID-19 in people with diabetes. Recurrent lockdowns and public health measures throughout the pandemic have restricted access to routine diabetes care, limiting new diagnoses, and affecting self-management, routine follow-ups, and access to medications, as well as affecting lifestyle behaviours and emotional wellbeing globally. Pre-pandemic studies have shown that short-term delays in delivery of routine care, even by 12 months, are associated with adverse effects on risk factor control and worse microvascular, macrovascular, and mortality outcomes in people with diabetes. Disruptions within the short-to-medium term due to natural disasters also result in worse diabetes outcomes. However, the true magnitude of the indirect effects of the COVID-19 pandemic on long-term outcomes and mortality in people with diabetes is still unclear. Disasters tend to exacerbate existing health disparities; as we recover ambulatory diabetes services in the aftermath of the pandemic, there is an opportunity to prioritise those with the greatest need, and to target resources and interventions aimed at improving outcomes and reducing inequality.
Introduction
As of Oct 21, 2022, there had been 6 553 936 deaths attributable to COVID-19 globally, with more than 623 million PCR-confirmed and lateral-flow-confirmed infections.1 The disease has disproportionately affected certain groups, such as older people (ie, >65 years) and those from some minority ethnic populations2 some of whom might live in a suboptimal home environment (eg, an overcrowded space) or have little access to medical coverage.3 These populations are also known to have a high prevalence of chronic conditions such as diabetes, cardiovascular disease, kidney disease, and some respiratory diseases. People with these conditions have faced some of the worst COVID-19 outcomes.4 The complex interaction of medical, environmental, and socioeconomic disadvantage, has been termed syndemic rather than just epidemic, and is known to worsen outcomes and impose the double burden of poor medical outcomes and poor socioeconomic status on these populations.5, 6 In this Personal View, we discuss the direct and indirect effects of COVID-19 on people with type 1 and type 2 diabetes and ways in which these effects can be alleviated now and in the immediate future. We also provide some recommendations to prepare for the challenges that people with diabetes might face in a post-pandemic world and in any future pandemic or natural crisis.
Direct impact of COVID-19 on people with diabetes
Evidence suggests that hyperglycaemia is a substantial contributor that links diabetes to the severity of COVID-19.7 A population-level study of over 61 million people in England during the first wave of the pandemic reported that after adjusting for age, sex, deprivation, ethnicity, and geographical region, found increased risk for in-hospital COVID-19-related death in people with diabetes compared with people without diabetes. Specifically, the odds ratios for in-hospital COVID-19-related death, compared with the general population, were 3·51 (95% CI 3·16–3·90) in people with type 1 diabetes and 2·03 (1·97–2·09) in people with type 2 diabetes. A linked study of over 2·8 million people with type 2 diabetes and over 250 000 people with type 1 diabetes during the same period reported that weekly death registrations increased by 59·1% in people with type 1 diabetes and 64·3% in people with type 2 diabetes compared with what would have been expected given death rates in the same weeks in the previous 3 years.8, 9 A narrative review from 2020 of data from 112 articles reported consistent evidence that diabetes was a risk factor for severe COVID-19 outcomes, including intensive care unit admission and death. In people with diabetes, high blood glucose levels reported both before or during COVID-19 illness were associated with poor outcomes.10
Initial portrayal of COVID-19 as another form of viral pneumonia has now been superseded by evidence that the disease affects multiple systems and not just the respiratory system. Manifestations of severe COVID-19 include widespread endothelial dysfunction, haematological disorders, hyperimmune responses,11 as well as the development, in some people, of what has been termed long COVID.12 Data on the epidemiology of long COVID in people with diabetes are scarce; however, some evidence has shown possible implications for this population.13 Since soon after the emergence of COVID-19 as a global pandemic, there has also been a research interest in newly diagnosed, and potentially new onset, diabetes in people who have had COVID-19. Although the precise mechanisms for new-onset diabetes in people with COVID-19 are not known, several inter-related processes have been hypothesised. These processes include identification of previously undiagnosed diabetes, stress hyperglycaemia,12, 14 steroid-induced hyperglycaemia, and direct or indirect effects of SARS-CoV-2 on the β cell. The function and survival of β cells are positively influenced by the ACE-2-Ang-(1-7)-Mas axis.15 SARS-CoV2, which internalises and depletes ACE-2 and activates the renin–angiotensin system (RAS) pathway, docks at ACE-2 receptors.16 This produces cell dysfunction and promotes inflammation insulin resistance, which has a favourable impact on the RAS pathway. Additionally, a hyperglycaemic state might upregulate ACE-2 receptors for viral infection, making the diabetic situation more severe.14
Symptoms of COVID-19 can last longer than one month in a subset of people with acute COVID-19 disease, with some patients reporting symptoms up to 6 months later.17 Originally known as post-acute COVID-19 after-effects or post-COVID-19 syndrome,18 this occurrence is now more widely termed as long COVID.19 The WHO defined long COVID as occurring 3 months after the onset of COVID-19 and lasting at least 2 months.20 The symptoms of long COVID are highly varied, ranging from physical symptoms such as generalised fatigue to mental health problems.21 Over the past year, small studies in people with diabetes have reported some of these long COVID symptoms including increased risk of suicide for 12 months,22 and persistence of symptoms of fatigue, chest pain, and breathlessness at 9 months.23
Finally, the effects of the financial cost of the COVID-19 pandemic need to be considered, as the pandemic and related public health restrictions imposed major challenges on societies and health-care systems. An analysis from May, 2022, including 41 studies, showed that the economic burden of COVID-19 has been substantial for individuals, health-care systems, and tax payers due to both direct (eg, hospitalisation and intensive care unit admission, and COVID-19 testing, screening, and vaccination) and indirect (eg, quarantine, isolation, physical distancing, and restriction policies) costs.24 Exacerbation of health and economic costs related to COVID-19 can compound health equity issues particularly among underprivileged groups in society and among older people with frailty, minority ethnic populations, and people with certain chronic conditions such as diabetes, cardiovascular disease, kidney disease, and some respiratory diseases.4, 25 This huge economic burden might also hamper prompt introduction of new and more effective forms of treatments for people with diabetes. Over the past few years, randomised clinical trials have shown that new glucose-lowering drugs, namely SGLT2-inhibitors and GLP-1 receptor agonists reduce the risk of major adverse cardiovascular events, hospitalisation for heart failure, and progression of diabetic kidney disease in people with type 2 diabetes.26, 27 However, these drugs come at a higher cost than traditional, often off-patent glucose-lowering agents, and require large initial investments that might not be affordable because of the ongoing financial resource consumption imposed by the COVID-19 pandemic.
Indirect effects of COVID-19 in people with diabetes
For people with diabetes, recurrent lockdowns and public health measures throughout the pandemic have restricted access to routine diabetes care, limiting new diagnoses, and affecting self-management, care-seeking behaviour, and access to medications.28, 29 These effects have compromised care for optimising glycaemic control. Across Europe, and in the UK, approximately 47% of health-care professionals with an interest in diabetes, including those working in primary care, reported substantial service disruption.30 Another global survey of health-care professionals from 47 countries including low-income and middle-income countries (LMICs) reported that diabetes was the chronic condition most affected by COVID-19 due to disruptions in care.31
Two surveillance studies, one on a cohort of adult patients in the USA,32 and another on health-care professionals in the UK,33 reported adverse effects on routine care. In the study from the USA,32 researchers collected self-reported data each month for 12 months and identified substantial deficiencies in routine diabetes care. Participants in the study reported rationing of diabetes therapies (95 [17%] of 547) and reduced monitoring (106 [16%] of 667). Furthermore, 243 (36%) of 667 respondents reported challenges consulting with their diabetes-care providers. Due to unemployment or lost income during the pandemic, people living with diabetes faced financial hardship that could reduce their ability to afford medications, especially in countries and areas where private insurance, pay-for-service, or co-payments are predominant medical schemes. Even if eligible to receive free medications, they might be required to claim them in-person, which could create a barrier in some low-income countries where there are few, if any, home-delivery services. In low socioeconomic areas, food security and homelessness due to inability to pay rent are among the social factors that can exacerbate the effects of a pandemic in people with pre-existing health conditions such as diabetes.34
In some high-income countries, since the pandemic, health-care professionals have reduced face-to-face contact with patients, adapted new ways of delivering care, and adopted technological support for self-management of chronic conditions such as diabetes. Given the importance of continuity of care, disruptions in assessment and management of care processes and risk factors, are grounds for concern.
In a UK national audit, completion of eight regular diabetes care assessments (ie, weight, blood pressure, cholesterol, smoking status, HbA1c, urinary albumin, serum creatinine, and foot examinations) were associated with reduced mortality, whereas increased hazards of mortality (hazard ratio 1·37, 95% CI 1·28–1·46) was observed in people who completed five or fewer.35 Pre-COVID-19 pandemic also showed that completion of more care processes was associated with reduced rates of lower extremity amputations,36 sight-threatening diabetic retinopathy,37 emergency hospital admission, cardiovascular disease-related admissions, and all-cause mortality.38 In Hong Kong, the implementation of a territory-wide systematic risk assessment and management programme in 1995 changed how diabetes care is provided there. The health authority had established a territory-wide diabetes database for quality assurance by instructing nurses and health-care assistants to complete protocol-guided assessments every 2 years, including eye, foot, blood, and urine examination. This programme led to a 70% decline in death rate among people with diabetes in 2000–16, the highest rate of decline among 19 high-income countries.39, 40, 41 Nevertheless, overcrowded living conditions, insufficient risk communication to patients, and resistance to vaccination among people more at risk of severe complications (eg, minority ethnic populations, young patients [ie, <18 years], older patients, and patients with other chronic disease) have adversely affected people with diabetes.
Many people with chronic diseases, including diabetes, avoided or delayed seeking medical attention for routine non-COVID-19-related problems due to fear of infection or to help reduce strain on health-care services already overburdened by COVID-19.42 Analysis of routine primary-care data in the UK of 618 161 patients with type 2 diabetes, showed statistically significant reductions in evidence-based care processes and prescribing in primary care during the first lockdown.43 Further analysis of this database showed an increased rate of non-COVID-19-related deaths in people with diabetes in England linked with the decreased completion of routine diabetes care processes.44 Another UK study reported a reduction in diagnosis of type 2 diabetes (rate reduction [RR] 0·21, 95% CI 0·16–0·26), reduction in assessment of glycaemic control (RR 0·77, 0·76–0·78), and reduction in new prescriptions of metformin (RR 0·20, 0·16–0·23).43
There is also evidence of adverse effects of COVID-19 on mental health45 and health-promoting lifestyles during the pandemic. In a follow-up of the Look AHEAD study of 2829 older adults (age 75·6 years [SD 6]) with type 2 diabetes in the USA, the authors reported a 1·6-fold increased prevalence for depressive symptoms and 1·8-fold increased prevalence for loneliness during the pandemic compared with pre-pandemic rates.46 Greater psychological distress and increased rates of anxiety have been reported among both the general population and those with chronic diseases such as diabetes.30 Apart from restrictions on human movements during the pandemic, the feeling of negative emotions have also been associated with reduced motivation, and increased physical inactivity and sedentary behaviour.47 Several reports indicated weight gain among people with or at risk of having diabetes, especially in young people, women, and those from low socioeconomic backgrounds during the pandemic.48 In one global survey of 1829 diabetes nurses from 27 countries, 873 (48%) of the respondents reported seeing some adverse effects of COVID-19 on the physical and psychological risks of people with diabetes.30 These included an increase in anxiety (1486 [82%]), diabetes distress (1189 [65%]), and depression (893 [49%]).30
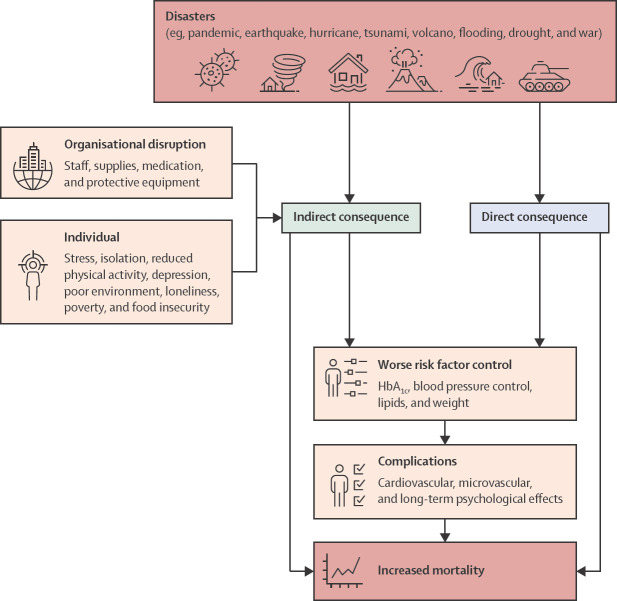
The direct and indirect consequences of natural disasters and wars on people with diabetes can be seen in figure 1 .
Figure 1.
Indirect and direct consequences of natural disasters and wars
Lessons from previous natural disasters
Disasters (eg, natural disasters and wars) tend to exacerbate existing health disparities.49 Although it is too early to determine the indirect effects (ie, long-term outcomes and mortality) of the COVID-19 pandemic on people with diabetes, previous natural disasters have shown that disruptions within the short-to-medium term, results in increases in worse outcomes, including cardiovascular and diabetes complications (table ).10, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65
Table.
Effects of previous natural disasters on people with diabetes
| Name of event | Date of event | Follow-up after disaster | Effects | Population studied | |
|---|---|---|---|---|---|
| Japan | |||||
| Kirizuka et al (1997)50 | Kobe earthquake | Jan 17, 1995 | 8 months | Increase in the mean value of HbA1c (8·34% [SD 2·07] in March, 1995 vs 7·74% [1·82] in December, 1994; p<0·01) | 177 people living with diabetes |
| Inui et al (1998)51 | Kobe earthquake | Jan 17, 1995 | From 2 months after event to 1 year after the event | Increase in HbA1c after the disaster (7·44% [SD 0·15] vs 7·64% [0·11], n=110; p<0·01); after the event HbA1c levels peaked at 3–4 months after the earthquake; there was a significant increase in stress-related somatic symptoms, sleep disturbances and anxiety, and social dysfunction | 157 adults (mean age 59·3 years [SD 1·2]) living with diabetes |
| Fujihara et al (2012)52 | Tōhoku earthquake and tsunami | March 11, 2011 | 3 months after event | Worsening glycaemic control Total General Health Questionnaire scores (OR 1·03) and interruption of drug intake (OR 4·48) associated with worsening of glycaemic control | 320 adults (mean age 65 years [SD 13]) with type 2 diabetes |
| Satoh et al (2015)53 | Tōhoku earthquake and tsunami | March 11, 2011 | Up to 1·6 years after event | Increased incidence of diabetes among evacuees (9·3–11·0%; p<0·0001) | 27 486 Japanese adults (mean age 66·3 years [SD 9·4]) |
| Leppold et al (2016)54 | Tōhoku earthquake and tsunami | March 11, 2011 | 4 weeks after event | HbA1c was 6·76 (SD 0·69) in 2010, 6·87 (SD 0·83) in 2011, and 6·93 (SD 0·87) in 2012; the proportion of participants with poor glycaemic control (HbA1c ≥7·0%) increased significantly from 31·9% in 2010 to 41·4% in 2012 (p=0·028) | 404 adults (mean age 71·0 years [SD 10·6]) living with diabetes |
| Kondo et al (2019)55 | Kumamoto earthquake | April 16, 2016 | 13 months after event | No change in glycaemic control in people with type 1 diabetes; increase in HbA1c after the disaster in people with type 2 diabetes; initially HbA1c decreased by 0·11% at 1–2 months after disaster, but at a later stage it increased (p=0·013) | 557 people living with diabetes |
| USA | |||||
| Quast and Feng (2019)56 | Hurricane Katrina | Aug 23–31, 2005 | 3 years after event | Reduced health-care use, particularly reduced screening for cholesterol disorders | Seniors living with diabetes |
| Fonseca et al (2009)57 | Hurricane Katrina | Aug 23–31, 2005 | 6−16 months after event (March 1–Dec 31, 2006) | Increased HbA1c, systolic blood pressure and LDL; systolic blood pressure increased then declined, HbA1c increased throughout study period; modelling studies found Hurricane Katrina increased direct, indirect, and total health-care costs, and reduced life expectancy and quality-adjusted life expectancy; existing disparities in health, related to socioeconomic status, were exacerbated after the disaster, with potential long-term consequences | 1795 adults (mean age 61·9 years [SD 11·6]) living with diabetes |
| Cefalu et al (2006)58 | Hurricane Katrina | Aug 23–31, 2005 | Days to few weeks after event | Inadequate diabetes supplies; increase in cases of depression; changes in meal composition and pattern | People living with diabetes |
| Quast and Mortensen (2015)59 | Hurricane Katrina | Aug 23–31, 2005 | 2006; 1 year after event | The proportion of children (aged ≤17 years) who received recommended tests (eg, HbA1c, eye exams, and microalbuminuria) fell or grew at a much slower rate compared with control group; the rate of diabetes keto acidosis increased | Children (aged ≤17 years) with diabetes on Medicaid |
| Velez-Valle et al (2016)60 | Hurricane Sandy | Oct 22–Nov 2, 2012 | The week after the event | Increase in emergency department visits | Adults living with diabetes and older adults (>65 years) living with type 2 diabetes |
| Lee et al (2016)61 | Hurricane Sandy | Oct 22–Nov 2, 2012 | First week after event (Oct 29, 2012) | Increase in emergency department visits for all reasons | People living with diabetes |
| Heptulla et al (2016)62 | Hurricane Sandy | Oct 22–Nov 2, 2012 | 3–6 months after event; February–July, 2013 | More symptoms of post-traumatic stress p<0·006 | 142 families caring for children (mean age 13·3 years [SD 2]) with type 1 diabetes on 100% insulin therapy |
| Türkiye | |||||
| Sengül et al (2004)63 | Marmara earthquake | Aug 17, 1999 | ·· | Increased insulin requirements, HbA1c, and decreased quality of life | People with type 1 diabetes |
| Sierra Leone | |||||
| Koroma et al (2019)64 | Ebola outbreak | Dec 26, 2013–June 9, 2016 | June–December, 2015 | Increase in number of people with diabetes | People with non-communicable diseases |
| Puerto Rico | |||||
| Cruz-Cano et al (2019)65 | Hurricane Maria | Sept 20, 2017 | 1 year after event (up to Oct 1, 2017) | Excess deaths due to diabetes | Older people (≥60 years) living with diabetes |
OR=odds ratio.
One small study of 88 people with type 1 diabetes living in the Marmara earthquake zone in northwest Türkiye reported that the natural disaster had a negative effect on both glycaemic control and quality of life for people with type 1 diabetes.63 3 months after the earthquake, the average HbA1c increased from 7·4% (57 mmol/mol) to 8·5% (69 mmol/mol; p<0·05) and insulin requirements increased significantly from 0·58 IU/kg per day to 0·77 IU/kg per day (p<0·05). A study of 1795 adults who lived through Hurricane Katrina in the USA showed the adverse health and economic effects on diabetes management from a short-term natural disaster with possible lasting negative health and economic effects. 57 On the basis of increased HbA1c, blood pressure, and blood lipids in people with diabetes during previous natural events, modelling done at the time estimated that Hurricane Katrina had increased direct, indirect, and total health-care costs, and reduced life expectancy.57
In Japan, within 2 months of the Kobe earthquake in January, 1995, there was an increase in psychological problems and worsening of glycaemic control in people with diabetes.51 In a study of 44 people with type 2 diabetes, the Croatian War of Independence (1991–95) was associated with long-lasting stress and depression.66, 67 The Russia–Ukraine conflict has also been reported to have affected diabetes care through the disruption of diabetes-care facilities, including pharmaceutical supplies and food supplies.68 These barriers can worsen the emotional effect that is inherent to war and ultimately negatively affect diabetes control. Approximately 130 000 people with type 1 diabetes and more than 2·3 million people with type 2 diabetes are currently struggling to get their condition under control in Ukraine.69 Keeping diabetes under control is becoming increasingly difficult because of a shortage of medications and obstacles in contacting medical professionals. People with diabetes—and even the manufacturers—are finding it extremely difficult to get insulin and other medications, as well as glucose testing strips and meters.69 Active combat zones, precarious humanitarian corridors, and destruction of transportation systems represent major hurdles. Access to medications and diagnostics is only one aspect of diabetes daily management.70 The military conflict makes it impractical, if not unfeasible, to control diet and lifestyle, which are the cornerstones of diabetes treatment. Furthermore, the difficult situations that people with chronic diseases are living in and the perceived deterioration of health resources are likely to generate anxiety and depression, conditions that have been reported to increase the risk of diabetes complications and to reduce life expectancy.71
Although some lessons regarding health-care provision have been learned from previous disasters, the impact of the COVID-19 pandemic will probably be much more deleterious for people living with diabetes than the impact of previous disasters on this population for a number of reasons. First, although previous natural disasters have been limited to specific geographical regions, COVID-19 is having a global impact. Second, although previous disasters have only been brief, the COVID-19 pandemic has already lasted for more than 2 years, and is still ongoing. Thus, the negative effect on diabetes care is likely to be more severe and also global. To help to alleviate this negative effect, it is crucial that the lessons from previous natural disasters and learnings from COVID-19 research, are shared globally for implementation strategies to be adapted to local needs and to help to prepare for any future pandemics.
Recovery: the evolution of the traditional health-care model
As we learn more about SARS-CoV-2 and its consequences, the multidimensional effects on people with diabetes, especially as they relate to healthy lifestyle behaviours and emotional wellbeing, will exacerbate the effects of delayed intervention with an expected increased incidence of diabetes-related complications in years to come.72 Diabetes self-management is essential for diabetes care and has the potential to counter these effects.73, 74 In this regard, delays in delivery of self-management education programmes is of major concern as these programmes have been shown to have positive benefits on both physical and pychological health, and thus might play an important role in minimising detrimental health effects of the pandemic.
The rapid transition to remote consultations via telehealth, telephone, video, and electronic consultation, initially came from necessity, with an aim to limit COVID-19 transmission while still maintaining contact with patients. As services are being restored, health-care professionals continue to face many challenges. Tasked with recovering all aspects of clinical care and addressing backlogs accrued across all domains, many services face workforce pressures due to clinician fatigue, illness, and self-isolation during the ongoing pandemic. Furthermore, people with diabetes remain fearful of attending health-care facilities, because of the ongoing threat due to emergence of mutant strains of COVID-19.75 Consequently, although the number of face-to-face appointments is increasing, a hybrid model with both virtual and face-to-face consultations is likely to remain.76
Provided there is availability of technology, emerging evidence suggests that telehealth interventions in diabetes care could also offer opportunities to improve patient care and clinical outcomes compared with regular care.77 One longitudinal study conducted over 15 weeks of an inpatient virtual diabetes management service portayed the feasibility of such a transition, while providing similar outcomes to traditional face-to-face care.78 However, disadvantages include difficulty in establishing rapport because of poor expression of emotional wellbeing, a tendency of the patient to safeguard concerns, or insufficient non-verbal cues to enhance communication in a virtual environment.79 When clinical tests or physical examinations are required, and cannot be conducted remotely, direct contact will still be essential.
For routine diabetes care, increased efficiency can be achieved through advanced preparation with a two-stepped approach, ensuring that all clinical care processes and examinations are done and recorded in a single one-stop visit, with either face-to-face or remote review when test results are available.80 Questionnaires in preparation for these reviews can provide information on emotional, as well as physical wellbeing,63 and data on physical measurements can also be provided by self-report such as weight, blood pressure, and blood glucose. Given the size of the population of people with diabetes, their multiple needs and long-term nature of the condition, global experts in the Lancet Commission report on diabetes81 advocate using non-physician personnel to collect data systematically to stratify risk. These data can then be used to empower self-management and inform decision making while establishing a register for quality assurance to identify unmet needs.81
An individual's present state of health, health-related beliefs and habits, poor environmental factors, previous diseases, psychosocial stressors, and low socioeconomic status could lead to digital exclusion.82, 83 The higher proportion of COVID-19 mortality in people with diabetes compared with people without diabetes highlights their clinical vulnerability, including older age (>65 years), frailty, comorbidity, ethnicity, and socioeconomic disadvantage.9, 84 Similarly, disparity in literacy or access to digital care can also widen care gaps,82 especially for older people and those with low education. Even in high-income countries, socioeconomic inequality is associated with digital poverty, poor access to technologies, and poor digital literacy, making remote consultations and telehealth inaccessible.83, 85 For some minority ethnic groups, who might already face socioeconomic disadvantage, systemic discrimination, or disparities in diabetes care,86 cultural or language barriers could add additional complexity in accessing remote consultations.82 People living with disabilities, such as sensory impairment, cognitive impairment, learning disability, or serious mental illness, are also at risk of digital exclusion and might benefit from face-to-face review.79, 80 Additionally, restricted direct or walk-in access to health-care facilities will disproportionately affect people living with these and other disabilities that hinder them from accessing clinic appointments.
Health-care professionals, therefore, need to be aware of the varying social and personal circumstances encountered in everyday life and be sensitive towards nuances in communication that might suggest a concern that cannot be voiced within a virtual consultation. Ensuring sufficient time, space, and privacy to talk is essential in the care of vulnerable people. When virtual consultation is offered, video consultation confers advantage over telephone consultation, with sight of the environment and non-verbal cues;76, 79 however, this is also dependent on the willingness of the patients and sometimes practitioners in sharing their videos during teleconference. Despite the convenience that remote consultation offers, many people with diabetes report a preference for face-to-face attendance. People with diabetes cite the importance of establishing relationships with clinicians, being able to see who they are talking to, fluency of conversation, privacy, and the better opportunity to ask questions.76, 79
Although electronic consultation has been widely advocated, there are other non-medical barriers such as administrative support, which need to be overcome before this method can become a part of routine care. To maximise engagement, evaluation of a hybrid implementation model of yearly clinical and laboratory assessment combined with online consultations and medication refill based on self-monitored indexes versus usual care could provide definitive evidence regarding its feasibility, acceptability, and utility in different patient segments and in different health-care settings. These natural experiments with evaluation are examples in which best practice can be shared, and lessons can be learned to transform the experience of virtual care due to the pandemic into an innovative solution for optimising diabetes care and education. Technologies are also not likely to be widely available or practical in many LMICs, which have large populations of people with diabetes.31 Governments need to invest in infrastructures to support telemedicine and in alternative models of care including the use of pharmacists to deliver the backlog of care.87
Risk stratification for the routine review of patients post-pandemic
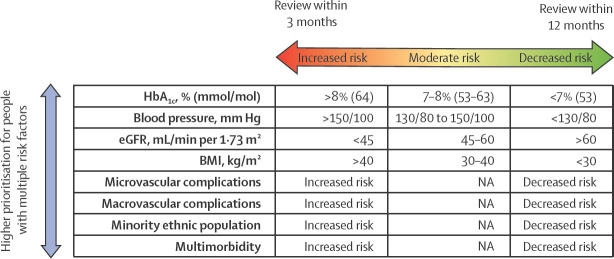
The COVID-19 pandemic has amplified inequalities in health care and health outcomes. People with diabetes have been disproportionately affected, with higher rates of hospital and intensive care unit admissions and higher mortality associated with SARS-CoV-2 infection.72 Identifiable vulnerabilities should direct clinical care, but despite what is known, there is evidence of inequity in diabetes care.86, 88 As we recover ambulatory diabetes services during and in the aftermath of the pandemic, there is an opportunity to prioritise those with the greatest need and to target resources and interventions aimed at improving outcomes and reducing inequality. Diabetes professional bodies have offered guidance on how to stratify risk and who to prioritise for diabetes review.80, 89 Computerised clinical record systems commonly offer search facilities that can be used to identify subgroups of patients on the basis of coded clinical or biometric data. Prioritisation should be based on socioeconomic status, education levels, established complications, comorbidities, and modifiable risk factors that are closely associated with progression of diabetes-related complications or risk of adverse outcomes from SARS-CoV-2 infection (figure 2 ). People with multiple risk factors that predispose them to severe COVID-19 outcomes should be offered more frequent 3-monthly reviews. In those with less risk, the review frequency can be relaxed to up to once a year. People with the highest (red) risk should be prioritised, followed by those with intermediate (amber) risk, and finally, those with the lowest identifiable (green) risk (figure 2).
Figure 2.
A strategy for prioritising recall for diabetes review, based on identifiable clinical need
3-monthly reviews of high-risk populations is advocated to prevent adverse outcomes. eGFR=estimated glomerular filtration rate. NA=not applicable.
Other integrated risk assessment and management strategies have been shown to improve self-management. The web-based Joint Asia Diabetes Evaluation platform for example, provides a template for collecting data during a structured assessment with the issue of a personalised report. It contains risk categories, trends, and targets of modifiable risk factors and individualised decision support. Additionally, these integrated risk assessment and management strategies have also been shown to improve multiple risk factors, such as reduce therapeutic inertia, and clinical events in a broad range of settings including LMICs.90, 91
Although these programmes and guidelines suggest a time duration for review,80, 89 in practice, what can be offered within a given timeframe will depend on capacity, competing pressures, and local circumstances, particularly regarding COVID-19 resurgence. Internationally there are further ongoing waves due to new variants of SARS-CoV-2 causing further disruptions in care and recovery. Clinical judgement remains essential in ensuring appropriate prioritisation and safety of people with diabetes. Priority consideration should also be given to vulnerable people, particularly where a specific need might not be identifiable by a coded clinical or biometric parameter.
Vaccination and long COVID
Global efforts have been made to develop vaccines against SARS-CoV-2, and since December, 2020, vaccines have been authorised on a national basis to help reduce transmission, hospitalisation, and mortality. There have been few data on effectiveness of vaccines in people with diabetes and hyperglycaemia.92, 93, 94 However, it must be emphasised that the frequent coexistence of diabetes and obesity has created a highly inflammatory milieu with atypical energy metabolism. Insulin is the only hormone that reduces blood glucose, whereas there are many stress hormones that can elevate blood glucose. People with diabetes are most likely to decompensate during acute emergencies with reduced ability to use energy optimally resulting in multisystem organ failure, especially in the presence of hypoxia.
In this context, the increased risk of cardiovascular disease after post-influenza pneumonia, caused by a proinflammatory and prothrombotic state, which can be prevented by vaccination, has been well proven. A report from Italy published in January, 2022, suggested that COVID-19 vaccinations only produced a mild immunity in people with type 2 diabetes with poor glycaemic control. This mild immune response is in contrast to normoglycaemic people and people with type 2 diabetes with good glycaemic control. Neutralising antibody titres and CD4 cytokine responses involving type 1 helper T cells were found to be lower in people with type 2 diabetes with HbA1c levels greater than 7% at 21 days following the first vaccine dose than in people with HbA1c levels of 7% at baseline.92 A UK national prospective cohort study has shown that while the relative risks of COVID-19-related to hospitalisation and death remain elevated in those with type 2 diabetes following vaccination, the absolute risks are greatly reduced and similar to that of a population without diabetes.95 Thus, this pandemic has offered an opportunity for health-care professionals to educate people with diabetes regarding their vulnerability especially in the presence of suboptimal risk factor control.
International actions and recommendations
A major crisis, such as a pandemic, is an opportunity to reflect on things that are taken for granted. This concept is encapsulated by the building back better strategy espoused by the UN to reduce the risk of future disasters on nations and communities.96 There have been several major initiatives to consider what a post-pandemic world might look like. In the Pan European Commission on Health and Sustainable Development, which reported to the WHO Director for Europe, experts call for actions that go beyond the immediate priorities, advocate strengthening preparedness for pandemics, and ask how societies can be better prepared for the wide range of future health threats.97 Several of its recommendations have relevance to diabetes. These recommendations place increased focus on health inequalities, specifically citing the need for improved data on ethnicity, and a comprehensive approach to health defined by WHO as physical, mental, and social wellbeing.98
Due to the fact that people with type 2 diabetes often present without symptoms in the initial stages when glycaemic levels are not very high, it is often seen as important but not urgent (ie, no immediate risk of death). Nevertheless, together with other chronic diseases (eg, cardiovascular disease, chronic kidney disease, and cancer), they account for 70% of mortality and morbidities globally.81 The COVID-19 pandemic reminds the global community of the importance of prevention, often seen as a less urgent issue to health-care providers than treatment. We, therefore, recommend that all stakeholders and governments should implement strategies aimed at preventing and optimising diabetes care so that the world is more prepared to minimise the adverse outcomes associated with diabetes before the next global health threat. In the UK, the National Health Service Diabetes Prevention Programme has provided remote and digital delivery of lifestyle interventions by non-health-care professionals, fostering programme resilience throughout the 2 years of the pandemic.99
Everywhere, socioenocomically underprivileged communities have suffered most from poor care for diabetes during the pandemic. As we move forward—hopefully—to a post-COVID era, we call on all concerned to ensure that achieving universal health coverage, as already committed to by governments in the Sustainable Development Goals100 and in international declarations,101 assumes even greater priority. Universal health coverage is essential if we are to ensure access to essential medications and consumables and the continuing care and support that people living with diabetes need but so often do not receive.102 We also call for renewed efforts to implement the UN High Level Declaration on Prevention and Control of Non-Communicable Diseases,103 taking measures that will improve our ecosystem by reducing poverty, empowering those in need of care, and creating a health-enabling environment that promotes healthy diet and physical activity.
To effectively reduce and manage diabetes epidemic, we must go further than these declarations, which inevitably represent compromises reflecting the commercial influences brought to bear on some governments.104 We call on the international community to recognise that manufacturers of harmful products—notably energy dense foods and sugar-sweetened beverages—are a large part of the problem,104 and recommend that they introduce comprehensive legislative and regulatory packages that tackle the drivers of consumption, price, availability, and marketing. As the Non-communicable Disease Alliance and others have argued cogently, this is a necessary step in reducing the double burden of obesity and diabetes.105, 106
Conclusion
The COVID-19 pandemic exemplifies the collective effects of politics, economics, social factors, and technology on a pandemic, whereby acute infectious disease and chronic disease meet. Whether at the individual or the community level, acute infections such as COVID-19 and chronic diseases (eg, diabetes) are inexorably related. The response to them needs to be coordinated similarly.
Declaration of interests
KK has acted as a consultant, speaker or received grants for investigator-initiated studies for AstraZeneca, Novartis, Novo Nordisk, Sanofi, Eli Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie/Menarini Group, Janssen, and Napp Pharmaceuticals. VRA has served as a consultant for Applied Therapeutics, Fractyl, Novo Nordisk, Pfizer, Sanofi; has a spouse employed at Janssen; and has received research support through their institution for clinical trial investigator, clinical trial leadership roles, or both from Applied Therapeutics, Eli Lilly, Fractyl, Novo Nordisk, and Sanofi. SS reports personal fees from Amgen, AstraZeneca, Napp Pharmaceuticals, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Roche, Sanofi, and Boehringer Ingelheim. Additionally, SS reports grants from AstraZeneca, Sanofi, Servier, and Janssen. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
KK and SS are supported by the National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands and the NIHR Leicester Biomedical Research Centre.
Contributors
KK had the initial idea for the Personal View, which was developed with SS. KK and SS drafted the section contents and all authors contributed to it.
References
- 1.WHO Corona Virus (Covid-19) Dashboard. Geneva, World Health Organization. https://covid19.who.int
- 2.Martin CA, Jenkins DR, Minhas JS, et al. Socio-demographic heterogeneity in the prevalence of COVID-19 during lockdown is associated with ethnicity and household size: results from an observational cohort study. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barboza M, Marttila A, Burström B, Kulane A. Covid-19 and pathways to health inequities for families in a socioeconomically disadvantaged area of Sweden—qualitative analysis of home visitors' observations. Int J Equity Health. 2021;20:215. doi: 10.1186/s12939-021-01556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh AK, Gillies CL, Singh R, et al. Prevalence of co-morbidities and their association with mortality in patients with COVID-19: a systematic review and meta-analysis. Diabetes Obes Metab. 2020;22:1915–1924. doi: 10.1111/dom.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yancy CW. COVID-19 and African Americans. JAMA. 2020;323:1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 6.Douglas M, Katikireddi SV, Taulbut M, McKee M, McCartney G. Mitigating the wider health effects of COVID-19 pandemic response. BMJ. 2020;369 doi: 10.1136/bmj.m1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–1077. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8:813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8:823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann-Boyce J, Morris E, Goyder C, et al. Diabetes and COVID-19: Risks, management, and learnings from other national disasters. Diabetes Care. 2020;43:1695–1703. doi: 10.2337/dc20-1192. [DOI] [PubMed] [Google Scholar]
- 11.Roberts CM, Levi M, McKee M, Schilling R, Lim WS, Grocott MPW. COVID-19: a complex multisystem disorder. Br J Anaesth. 2020;125:238–242. doi: 10.1016/j.bja.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajan S, Khunto K, Alwan N, et al. European Observatory on Health Systems and Policies; Copenhagen: 2021. In the wake of the pandemic: preparing for long COVID. [PubMed] [Google Scholar]
- 13.Rizvi AA, Kathuria A, Al Mahmeed W, et al. Post-COVID syndrome, inflammation, and diabetes. J Diabetes Complications. 2022;36 doi: 10.1016/j.jdiacomp.2022.108336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khunti K, Del Prato S, Mathieu C, Kahn SE, Gabbay RA, Buse JB. COVID-19, Hyperglycemia, and new-onset diabetes. Diabetes Care. 2021;44:2645–2655. doi: 10.2337/dc21-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ni W, Yang X, Yang D, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24:422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou P, Yang X, Wang X, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patient-Led Research Collaborative Report: what does COVID-19 recovery actually look like? https://patientresearchcovid19.com/research/report-1
- 18.National Institute for Health and Care Exellence COVID-19 rapid guideline: managing the long-term effects of COVID-19. Dec 18, 2020. https://www.nice.org.uk/guidance/ng188 [PubMed]
- 19.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO A clinical case definition of post COVID-19 condition by a Delphi consensus. 6 October 2021. https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 [DOI] [PMC free article] [PubMed]
- 21.Perlis RH, Santillana M, Ognyanova K, et al. Prevalence and correlates of long COVID symptoms among US adults. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.38804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alessi J, Scherer GDLG, Erthal IN, et al. One in ten patients with diabetes have suicidal thoughts after 1 year of the COVID-19 pandemic: we need to talk about diabetes and mental health not only during suicide prevention awareness month. Acta Diabetol. 2022;59:143–145. doi: 10.1007/s00592-021-01807-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mechi A, Al-Khalidi A, Al-Darraji R, et al. Long-term persistent symptoms of COVID-19 infection in patients with diabetes mellitus. Int J Diabetes Dev Ctries. 2022;42:49–52. doi: 10.1007/s13410-021-00994-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vardavas C, Zisis K, Nikitara K, et al. The cost of the COVID-19 pandemic vs the cost-effectiveness of mitigation strategies in the EU/UK/EEA and OECD countries: a systematic review. medRxiv. 2022 doi: 10.1101/2022.05.31.22275813. published online May 31. (preprint). [DOI] [Google Scholar]
- 25.Chudasama YV, Zaccardi F, Gillies CL, et al. Patterns of multimorbidity and risk of severe SARS-CoV-2 infection: an observational study in the U.K. BMC Infect Dis. 2021;21:908. doi: 10.1186/s12879-021-06600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9:653–662. doi: 10.1016/S2213-8587(21)00203-5. [DOI] [PubMed] [Google Scholar]
- 27.McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6:148–158. doi: 10.1001/jamacardio.2020.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohseni M, Ahmadi S, Azami-Aghdash S, et al. Challenges of routine diabetes care during COVID-19 era: a systematic search and narrative review. Prim Care Diabetes. 2021;15:918–922. doi: 10.1016/j.pcd.2021.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valabhji J, Barron E, Gorton T, et al. Associations between reductions in routine care delivery and non-COVID-19-related mortality in people with diabetes in England during the COVID-19 pandemic: a population-based parallel cohort study. Lancet Diabetes Endocrinol. 2022;10:561–570. doi: 10.1016/S2213-8587(22)00131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forde R, Arente L, Ausili D, et al. The impact of the COVID-19 pandemic on people with diabetes and diabetes services: a pan-European survey of diabetes specialist nurses undertaken by the Foundation of European Nurses in Diabetes survey consortium. Diabet Med. 2021;38 doi: 10.1111/dme.14498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chudasama YV, Gillies CL, Zaccardi F, et al. Impact of COVID-19 on routine care for chronic diseases: a global survey of views from healthcare professionals. Diabetes Metab Syndr. 2020;14:965–967. doi: 10.1016/j.dsx.2020.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratzki-Leewing AA, Ryan BL, Buchenberger JD, Dickens JW, Black JE, Harris SB. COVID-19 hinterland: surveilling the self-reported impacts of the pandemic on diabetes management in the USA (cross-sectional results of the iNPHORM study) BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-049782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seidu S, Hambling C, Holmes P, et al. The impact of the COVID pandemic on primary care diabetes services in the UK: a cross-sectional national survey of views of health professionals delivering diabetes care. Prim Care Diabetes. 2022;16:257–263. doi: 10.1016/j.pcd.2021.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim D. Financial hardship and social assistance as determinants of mental health and food and housing insecurity during the COVID-19 pandemic in the United States. SSM Popul Health. 2021;16 doi: 10.1016/j.ssmph.2021.100862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holman N, Knighton P, O'Keefe J, et al. Completion of annual diabetes care processes and mortality: A cohort study using the National Diabetes Audit for England and Wales. Diabetes Obes Metab. 2021;23:2728–2740. doi: 10.1111/dom.14528. [DOI] [PubMed] [Google Scholar]
- 36.Gunn LH, Vamos EP, Majeed A, et al. Associations between attainment of incentivized primary care indicators and incident lower limb amputation among those with type 2 diabetes: a population-based historical cohort study. BMJ Open Diabetes Res Care. 2021;9 doi: 10.1136/bmjdrc-2020-002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKay AJ, Gunn LH, Sathish T, et al. Associations between attainment of incentivised primary care indicators and incident diabetic retinopathy in England: a population-based historical cohort study. BMC Med. 2021;19:93. doi: 10.1186/s12916-021-01966-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gunn LH, McKay AJ, Molokhia M, et al. Associations between attainment of incentivised primary care indicators and emergency hospital admissions among type 2 diabetes patients: a population-based historical cohort study. J R Soc Med. 2021;114:299–312. doi: 10.1177/01410768211005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan JCN, Lim LL, Luk AOY, et al. From Hong Kong diabetes register to JADE program to RAMP-DM for data-driven actions. Diabetes Care. 2019;42:2022–2031. doi: 10.2337/dci19-0003. [DOI] [PubMed] [Google Scholar]
- 40.Wu H, Lau ESH, Ma RCW, et al. Secular trends in all-cause and cause-specific mortality rates in people with diabetes in Hong Kong, 2001-2016: a retrospective cohort study. Diabetologia. 2020;63:757–766. doi: 10.1007/s00125-019-05074-7. [DOI] [PubMed] [Google Scholar]
- 41.Magliano DJ, Chen L, Carstensen B, et al. Trends in all-cause mortality among people with diagnosed diabetes in high-income settings: a multicountry analysis of aggregate data. Lancet Diabetes Endocrinol. 2022;10:112–119. doi: 10.1016/S2213-8587(21)00327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vamos EP, Khunti K. Indirect effects of the COVID-19 pandemic on people with type 2 diabetes: time to urgently move into a recovery phase. BMJ Qual Saf. 2021 doi: 10.1136/bmjqs-2021-014079. [DOI] [PubMed] [Google Scholar]
- 43.Carr MJ, Wright AK, Leelarathna L, et al. Impact of COVID-19 restrictions on diabetes health checks and prescribing for people with type 2 diabetes: a UK-wide cohort study involving 618 161 people in primary care. BMJ Qual Saf. 2021;31:503–514. doi: 10.1136/bmjqs-2021-013613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valabhji J, Barron E, Gorton T, et al. Associations between reductions in routine care delivery and non-COVID-19-related mortality in people with diabetes in England during the COVID-19 pandemic: a population-based parallel cohort study. Lancet Diabetes Endocrinol. 2022;10:561–570. doi: 10.1016/S2213-8587(22)00131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Violant-Holz V, Gallego-Jiménez MG, González-González CS, et al. Psychological health and physical activity levels during the COVID-19 pandemic: a systematic review. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17249419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chao AM, Wadden TA, Clark JM, et al. Changes in the Prevalence of Symptoms of Depression, Loneliness, and Insomnia in U.S. Older Adults With Type 2 Diabetes During the COVID-19 Pandemic: The Look AHEAD Study. Diabetes Care. 2022;45:74–82. doi: 10.2337/dc21-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stockwell S, Trott M, Tully M, et al. Changes in physical activity and sedentary behaviours from before to during the COVID-19 pandemic lockdown: a systematic review. BMJ Open Sport Exerc Med. 2021;7 doi: 10.1136/bmjsem-2020-000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valabhji J, Barron E, Bradley D, Bakhai C, Khunti K, Jebb S. Effect of the COVID-19 pandemic on body weight in people at high risk of type 2 diabetes referred to the English NHS Diabetes Prevention Programme. Lancet Diabetes Endocrinol. 2021;9:649–651. doi: 10.1016/S2213-8587(21)00218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khunti S, Khunti K, Seidu S. Therapeutic inertia in type 2 diabetes: prevalence, causes, consequences and methods to overcome inertia. Ther Adv Endocrinol Metab. 2019;10 doi: 10.1177/2042018819844694. 2042018819844694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kirizuka K, Nishizaki H, Kohriyama K, et al. Influences of The Great Hanshin-Awaji Earthquake on glycemic control in diabetic patients. Diabetes Res Clin Pract. 1997;36:193–196. doi: 10.1016/s0168-8227(97)00030-2. [DOI] [PubMed] [Google Scholar]
- 51.Inui A, Kitaoka H, Majima M, et al. Effect of the Kobe earthquake on stress and glycemic control in patients with diabetes mellitus. Arch Intern Med. 1998;158:274–278. doi: 10.1001/archinte.158.3.274. [DOI] [PubMed] [Google Scholar]
- 52.Fujihara K, Saito A, Heianza Y, et al. Impact of psychological stress caused by the Great East Japan Earthquake on glycemic control in patients with diabetes. Exp Clin Endocrinol Diabetes. 2012;120:560–563. doi: 10.1055/s-0032-1314873. [DOI] [PubMed] [Google Scholar]
- 53.Satoh H, Ohira T, Hosoya M, et al. Evacuation after the Fukushima Daiichi nuclear power plant accident is a cause of diabetes: results from the Fukushima health management survey. J Diabetes Res. 2015;2015 doi: 10.1155/2015/627390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leppold C, Tsubokura M, Ozaki A, et al. Sociodemographic patterning of long-term diabetes mellitus control following Japan's 3.11 triple disaster: a retrospective cohort study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-011455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kondo T, Miyakawa N, Motoshima H, et al. Impacts of the 2016 Kumamoto Earthquake on glycemic control in patients with diabetes. J Diabetes Investig. 2019;10:521–530. doi: 10.1111/jdi.12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quast T, Feng L. Long-term effects of disasters on health care utilization: Hurricane Katrina and older individuals with diabetes. Disaster Med Public Health Prep. 2019;13:724–731. doi: 10.1017/dmp.2018.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fonseca VA, Smith H, Kuhadiya N, et al. Impact of a natural disaster on diabetes: exacerbation of disparities and long-term consequences. Diabetes Care. 2009;32:1632–1638. doi: 10.2337/dc09-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cefalu WT, Smith SR, Blonde L, Fonseca V. The Hurricane Katrina aftermath and its impact on diabetes care: observations from “ground zero”: lessons in disaster preparedness of people with diabetes. Diabetes Care. 2006;29:158–160. doi: 10.2337/diacare.29.1.158. [DOI] [PubMed] [Google Scholar]
- 59.Quast T, Mortensen K. Diabetes care provided to children displaced by Hurricane Katrina. Disaster Med Public Health Prep. 2015;9:480–483. doi: 10.1017/dmp.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Velez-Valle EM, Shendell D, Echeverria S, Santorelli M. Type II diabetes emergency room visits associated with Hurricane Sandy in New Jersey: implications for preparedness. J Environ Health. 2016;79:30–37. [PubMed] [Google Scholar]
- 61.Lee DC, Gupta VK, Carr BG, et al. Acute post-disaster medical needs of patients with diabetes: emergency department use in New York City by diabetic adults after Hurricane Sandy. BMJ Open Diabetes Res Care. 2016;4 doi: 10.1136/bmjdrc-2016-000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heptulla R, Hashim R, Johnson DN, et al. Evaluating emergency preparedness and impact of a Hurricane Sandy in pediatric patients with diabetes. Disaster Mil Med. 2016;2:2. doi: 10.1186/s40696-016-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sengül A, Ozer E, Salman S, et al. Lessons learnt from influences of the Marmara earthquake on glycemic control and quality of life in people with type 1 diabetes. Endocr J. 2004;51:407–414. doi: 10.1507/endocrj.51.407. [DOI] [PubMed] [Google Scholar]
- 64.Koroma IB, Javadi D, Hann K, Harries AD, Smart F, Samba T. Non-communicable diseases in the Western Area District, Sierra Leone, following the Ebola outbreak. F1000 Res. 2019;8:795. doi: 10.12688/f1000research.18563.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cruz-Cano R, Mead EL. Causes of excess deaths in Puerto Rico after Hurricane Maria: a time-series estimation. Am J Public Health. 2019;109:1050–1052. doi: 10.2105/AJPH.2019.305015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pibernik-Okanović M, Roglić G, Prasek M, Metelko Z. War-induced prolonged stress and metabolic control in type 2 diabetic patients. Psychol Med. 1993;23:645–651. doi: 10.1017/s0033291700025423. [DOI] [PubMed] [Google Scholar]
- 67.Mortality GBD. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alessi J, Yankiv M. War in Ukraine and barriers to diabetes care. Lancet. 2022;399:1465–1466. doi: 10.1016/S0140-6736(22)00480-9. [DOI] [PubMed] [Google Scholar]
- 69.The PharmaLetter Russia experiences drug shortages despite ongoing supplies from abroad. May 4, 2022. https://www.thepharmaletter.com/article/russia-experiences-drug-shortages-despite-ongoing-supplies-from-abroad
- 70.The Lancet Diabetes & Endocrinology Ukraine: diabetes on the front line. Lancet Diabetes Endocrinol. 2022;10:231. doi: 10.1016/S2213-8587(22)00084-5. [DOI] [PubMed] [Google Scholar]
- 71.Wu CS, Hsu LY, Wang SH. Association of depression and diabetes complications and mortality: a population-based cohort study. Epidemiol Psychiatr Sci. 2020;29:e96. doi: 10.1017/S2045796020000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barone MTU, Harnik SB, de Luca PV, et al. The impact of COVID-19 on people with diabetes in Brazil. Diabetes Res Clin Pract. 2020;166 doi: 10.1016/j.diabres.2020.108304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chatterjee S, Davies MJ, Heller S, Speight J, Snoek FJ, Khunti K. Diabetes structured self-management education programmes: a narrative review and current innovations. Lancet Diabetes Endocrinol. 2018;6:130–142. doi: 10.1016/S2213-8587(17)30239-5. [DOI] [PubMed] [Google Scholar]
- 74.Fisher EB, Chan JC, Nan H, Sartorius N, Oldenburg B. Co-occurrence of diabetes and depression: conceptual considerations for an emerging global health challenge. J Affect Disord. 2012;142(suppl):S56–S66. doi: 10.1016/S0165-0327(12)70009-5. [DOI] [PubMed] [Google Scholar]
- 75.Caballero AE, Ceriello A, Misra A, et al. COVID-19 in people living with diabetes: an international consensus. J Diabetes Complications. 2020;34 doi: 10.1016/j.jdiacomp.2020.107671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kilvert A, Wilmot EG, Davies M, Fox C. Virtual consultations: are we missing anything? Pract Diabetes. 2020;37:143–146. [Google Scholar]
- 77.Phillip M, Bergenstal RM, Close KL, et al. The digital/virtual diabetes clinic: the future is now-recommendations from an international panel on diabetes digital technologies introduction. Diabetes Technol Ther. 2021;23:146–154. doi: 10.1089/dia.2020.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jones MS, Goley AL, Alexander BE, Keller SB, Caldwell MM, Buse JB. Inpatient transition to virtual care during COVID-19 pandemic. Diabetes Technol Ther. 2020;22:444–448. doi: 10.1089/dia.2020.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kilvert A, Fox C, Calladine L. What do people with diabetes really think about remote consultations. Pract Diabetes. 2020;38:51–56. [Google Scholar]
- 80.Diggle J, Brown P. How to undertake a remote diabetes review. DiabetesOnTheNet. 2020;22:43. [Google Scholar]
- 81.Chan JCN, Lim LL, Wareham NJ, et al. The Lancet Commission on diabetes: using data to transform diabetes care and patient lives. Lancet. 2021;396:2019–2082. doi: 10.1016/S0140-6736(20)32374-6. [DOI] [PubMed] [Google Scholar]
- 82.Crawford A, Serhal E. Digital health equity and COVID-19: the innovation curve cannot reinforce the social gradient of health. J Med Internet Res. 2020;22 doi: 10.2196/19361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Azeem M. EJ M, Sophie C-C. COVID-19 is magnifying the digital divide. BMJ Blogs. 2020. https://blogs.bmj.com/bmj/2020/09/01/covid-19-is-magnifying-the-digital-divide/
- 84.Hewitt J, Carter B, Vilches-Moraga A, et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5:e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O'Connor S, Hanlon P, O'Donnell CA, Garcia S, Glanville J, Mair FS. Understanding factors affecting patient and public engagement and recruitment to digital health interventions: a systematic review of qualitative studies. BMC Med Inform Decis Mak. 2016;16:120. doi: 10.1186/s12911-016-0359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mathur R, Farmer RE, Eastwood SV, Chaturvedi N, Douglas I, Smeeth L. Ethnic disparities in initiation and intensification of diabetes treatment in adults with type 2 diabetes in the UK, 1990–2017: a cohort study. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bullen C, McCormack J, Calder A, et al. The impact of COVID-19 on the care of people living with noncommunicable diseases in low- and middle-income countries: an online survey of physicians and pharmacists in nine countries. Prim Health Care Res Dev. 2021;22:e30. doi: 10.1017/S146342362100030X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ogunwole SM, Golden SH. Social determinants of health and structural inequities-root causes of diabetes disparities. Diabetes Care. 2021;44:11–13. doi: 10.2337/dci20-0060. [DOI] [PubMed] [Google Scholar]
- 89.Nagi D, Wilmott E, Owen K, et al. ABCD position statement on risk stratification of adult patients with diabetes during COVID-19 pandemic. Br J Diabetes. 2021;21:123–131. [Google Scholar]
- 90.Tutino GE, Yang WY, Li X, et al. A multicentre demonstration project to evaluate the effectiveness and acceptability of the web-based Joint Asia Diabetes Evaluation (JADE) programme with or without nurse support in Chinese patients with Type 2 diabetes. Diabet Med. 2017;34:440–450. doi: 10.1111/dme.13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lim LL, Lau ESH, Ozaki R, et al. Association of technologically assisted integrated care with clinical outcomes in type 2 diabetes in Hong Kong using the prospective JADE Program: a retrospective cohort analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marfella R, D'Onofrio N, Sardu C, et al. Does poor glycaemic control affect the immunogenicity of the COVID-19 vaccination in patients with type 2 diabetes: the CAVEAT study. Diabetes Obes Metab. 2022;24:160–165. doi: 10.1111/dom.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pal R, Bhadada SK, Misra A. COVID-19 vaccination in patients with diabetes mellitus: current concepts, uncertainties and challenges. Diabetes Metab Syndr. 2021;15:505–508. doi: 10.1016/j.dsx.2021.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dispinseri S, Lampasona V, Secchi M, et al. Robust neutralizing antibodies to SARS-CoV-2 develop and persist in subjects with diabetes and COVID-19 pneumonia. J Clin Endocrinol Metab. 2021;106:1472–1481. doi: 10.1210/clinem/dgab055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hippisley-Cox J, Coupland CA, Mehta N, et al. Risk prediction of COVID-19 related death and hospital admission in adults after covid-19 vaccination: national prospective cohort study. BMJ. 2021;374 doi: 10.1136/bmj.n2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dodson K. UN Foundation; Sept 30, 2021. Global health at UNGA: accountability is key to building back better.https://unfoundation.org/blog/post/global-health-at-unga-accountability-is-key-to-building-back-better/ [Google Scholar]
- 97.Forman R, Azzopardi-Muscat N, Kirkby V, et al. Drawing light from the pandemic: rethinking strategies for health policy and beyond. Health Policy. 2022;126:1–6. doi: 10.1016/j.healthpol.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.WHO Basic documents 47th edn. 2009. http://whqlibdoc.who.int/hist/official_records/constitution.pdf
- 99.Valabhji J. Excess inpatient mortality for those with diabetes in England. Diabet Med. 2013;30:1391–1392. doi: 10.1111/dme.12311. [DOI] [PubMed] [Google Scholar]
- 100.UN Make the SDGs a reality. UN Department of Economic and Social Affairs. 2022. https://sdgs.un.org/
- 101.UN Political declaration of the high-level meeting on universal health coverage “universal health coverage: moving together to build a healthier world”. UN Department of Economic and Social Affairs. 2019. https://www.un.org/pga/73/wp-content/uploads/sites/53/2019/07/FINAL-draft-UHC-Political-Declaration.pdf
- 102.Klatman EL, McKee M, Ogle GD. Documenting and visualising progress towards universal health coverage of insulin and blood glucose test strips for people with diabetes. Diabetes Res Clin Pract. 2019;157 doi: 10.1016/j.diabres.2019.107859. [DOI] [PubMed] [Google Scholar]
- 103.UN . UN General Asseembly; 2018. Political declaration of the third high-level meeting of the General Assembly on the prevention and control of non-communicable diseases.https://documents-dds-ny.un.org/doc/UNDOC/GEN/N18/315/40/PDF/N1831540.pdf?OpenElement [Google Scholar]
- 104.Mialon M, Vandevijvere S, Carriedo-Lutzenkirchen A, et al. Mechanisms for addressing and managing the influence of corporations on public health policy, research and practice: a scoping review. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-034082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alliance NCD. Signalling virtue, promoting harm - unhealthy commodity industries and COVID-19. 2020. https://ncdalliance.org/resources/signalling-virtue-promoting-harm
- 106.van Schalkwyk MC, Maani N, McKee M. Public health emergency or opportunity to profit? The two faces of the COVID-19 pandemic. Lancet Diabetes Endocrinol. 2021;9:61–63. doi: 10.1016/S2213-8587(21)00001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]