Abstract
Background
Secondary bacterial and fungal coinfections have been reported among critically ill coronavirus disease-19 (COVID-19) patients and are associated with increased disease severity and mortality incidence (MI) rates.
Aims
This study aimed to track bacterial and fungal coinfections among COVID-19 patients in the intensive care unit (ICU) and to assess the impact of these infections on disease prognosis and patient outcomes in Jordan.
Materials and Methods
This was a single-center study that enrolled 46 ICU patients diagnosed with COVID-19. Microbiological and antimicrobial susceptibility results and inflammatory biomarker data were retrospectively analyzed.
Results
The MI rate attributed to bacterial and fungal coinfections was 84.8%, and the highest rate was reported among patients older than 70 years (66.7%). The MI rate related to bacterial coinfections was 95.2%, whereas that of fungal coinfections was 4.8%. The most commonly isolated bacterium in the blood was a coagulase-negative staphylococcus (41%), followed by Klebsiella pneumoniae in nasopharyngeal swabs (34%) and Acinetobacter baumannii in sputum samples (31%). Candida species were the sole cause of fungal coinfections in the studied population. In particular, Candida albicans was isolated from 3% of patients with bacteremia, whereas Candida glabrata was isolated from 8% of nasopharyngeal swabs. Klebsiella pneumoniae was considered the major cause of upper respiratory tract infections (34%). Multifactorial infection was significantly associated with increased MI (p value <0.001).
Conclusion
COVID-19 MI is associated with respiratory bacterial/fungal coinfections. The ability to predict bacterial and fungal coinfections in ICU patients may be crucial to their survival and prognosis.
1. Introduction
A large proportion of patients with respiratory viral infections develop secondary bacterial and/or fungal coinfections, leading to increased severity and mortality due to the synergistic interaction of microbial pathogenesis and the host immune system [1]. The risk of death among COVID-19 patients increases by more than twofold in the presence of bacterial and fungal coinfections [2]. Additionally, several studies have reported low rates of confirmed bacterial coinfections in COVID-19 patients, which were attributed to the lack of prompt diagnosis and administration of broad-spectrum empirical antibiotics in COVID-19 patients at the time of hospital administration [3].
The exact mechanism by which COVID-19 predisposes patients toward coinfections with other microorganisms is not yet fully understood. However, there are different hypotheses to explain this relationship; one of them is that infected respiratory cells are induced to release anti-inflammatory cytokines [4]; these cytokines inhibit the link between the adaptive and innate immune systems, leading to delayed or inhibited bacterial clearance [4]. Another hypothesis is that the production of viral enzymes such as neuraminidase and sialidase promotes bacterial and fungal colonization [5].
The prevalence of COVID-19 coinfection varies in different situations. The highest prevalence of coinfections has been detected among hospitalized immune-suppressed patients (who were exposed to central lines and mechanical ventilators) and patients who had an underlying history of diabetes or other chronic diseases [6, 7]. Such infection may be caused by a single microbe or multiple microbes [8, 9], with various complications such as severe pneumonia [8], epidermal signs [10], alterations in the gastrointestinal microbiome [11], bacteremia [12], and hospital-acquired bacterial infections in the intensive care unit (ICU) [13]. Additionally, the presence of multidrug-resistant bacteria creates an additional crisis in the treatment of critically ill COVID-19 patients [14]. Complications of coinfections in COVID-19 patients depend on the etiology of coinfected microorganisms, viral load, “severity of viral infection,” and the host immune response to the infection [1].
A computerized tomography (CT) scan plays a vital role among critically ill COVID-19 patients admitted to the ICU because it is used in the monitor of patients, evaluating patient disease severity and prognosis, informing the treating physician about laboratory tests required, and classifying patients into different risk groups [15, 16].
Several studies have reported that clinicians tend to administer empirical broad-spectrum antibiotics to treat or avoid suspected bacterial coinfection [17, 18]. This, in turn, results in many adverse effects that change the normal microflora, leading to the emergence of new antibiotic-resistance mutations [19]. Therefore, it is important to begin empirical therapies to ensure patient survival and then to apply narrower-spectrum antibiotics after receiving microbiological lab results [20, 21]. Therefore, this study aimed to investigate isolated bacterial and fungal coinfections among COVID-19 patients in the ICU and to assess the impact of such coinfections on disease prognosis and patient outcomes in a private hospital in Amman, Jordan.
2. Materials and Methods
Eighty critically ill patients were admitted to the ICU between January 2020 and June 2021 in a private hospital in Amman, Jordan. All of them were assessed for COVID-19 infection by real-time reverse transcription-polymerase chain reaction (RT‒PCR). Only 56 were diagnosed with COVID-19, and they were included in the study. Based on the definition of coinfection, namely, infections that occur ≤48–72 h after hospital admission [22], only 46 patients fulfilled the criterion and were included in the study. In addition, 10 patients were excluded from the study owing to missing data regarding the date of administration and the date of bacterial and fungal growth (Figure 1).
Figure 1.
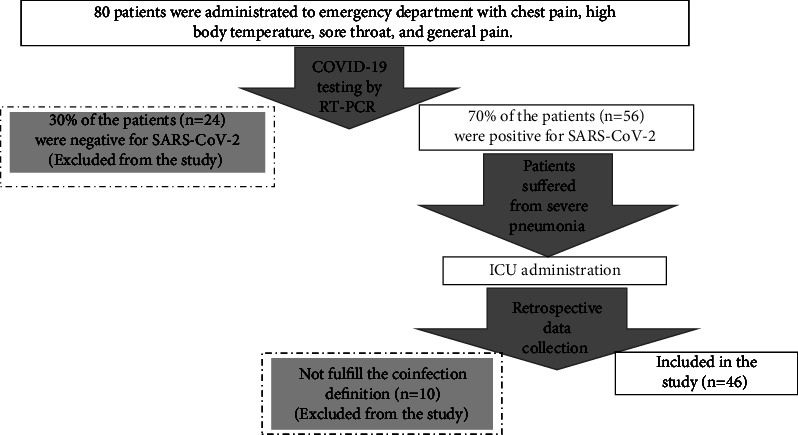
Flow chart for inclusion of the study participants.
At the time of ICU administration, required clinical, radiological, and microbiological assessments were performed. Bacterial isolates were identified based on colony characteristics and biochemical tests using a VITEK 2 system (bioMérieux)® (USA); fungal isolates were determined by colony morphology and colony characteristics. A galactomannan test to detect bacteremia caused by aspergillosis infections was not performed. Data collected included age, sex, patient outcome (died or survived), treatment provided, coinfection outcomes (upper respiratory infection, lower respiratory infection, bacteremia, and meningitis), and antimicrobial susceptibility results. Additionally, data on inflammatory biomarkers, including C-reactive protein (C-RP), ferritin, d-dimer, procalcitonin (PCT), globulin, white blood cell count (WBC), neutrophil count, and lymphocyte count, were selected to be included in the study to indicate the impact of bacterial and fungal coinfections due to missing data for other parameters of hematological, biochemical, and serological test profiles.
Ethical approval was obtained from the Ethics Committee for Scientific Research (ECSR) at Zarqa University based on the requirement for the protection of human subjects and the ethical principles related to research studies (no. 2/8/2021). Data were analyzed using IBM SPSS Statistics (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp). The chi-square (χ2) test was performed for categorical variables and the independent samples t-test and Mann‒Whitney U test for continuous variables; results are presented as the mean and standard deviation. Statistical significance was defined as a p value <0.05.
3. Results
A total of 46 ICU COVID-19 patients were included in this study. The types of isolated microorganisms for each and patient demographic data (age, sex, and patient outcomes) are presented in Table 1. The major isolated infectious bacteria were Klebsiella pneumoniae, Staphylococcus aureus, Enterococcus faecalis, Serratia marcescens, Pseudomonas aeruginosa, Acinetobacter baumannii, coagulase-negative staphylococci, Coryneform bacilli, and Staphylococcus epidermidis. Among the isolated fungi, only two species of Candida were detected: Candida glabrata and C. albicans.
Table 1.
General characteristics of bacterial and/or fungal coinfections among ICU COVID-19 patients.
| Patient # | Isolated microorganisms (type of specimen) | Gender | Age | Patient outcomes |
|---|---|---|---|---|
| 1 |
Klebsiella pneumoniae (sputum and nasopharyngeal swab) Serratia marcescens (blood) |
Male | 72 | Died |
| 2 | Klebsiella pneumoniae (blood) | Female | 66 | Died |
| 3 |
Staphylococcus aureus (nasopharyngeal swab) Pseudomonas aeruginosa (nasopharyngeal swab) |
Male | 56 | Died |
| 4 |
Acinetobacter baumannii (sputum) Coagulase-negative staphylococci (sputum) Serratia marcescens (sputum) |
Female | 63 | Died |
| 5 | Coagulase-negative staphylococci (blood) | Female | 85 | Died |
| 6 | Coagulase-negative staphylococci (blood) | Female | 85 | Died |
| 7 | Enterococcus faecalis (pus) | Male | 66 | Died |
| 8 | Klebsiella pneumoniae (blood) | Female | 28 | Died |
| 9 | Coagulase-negative staphylococci (blood) Staphylococcus aureus (blood) Enterococcus faecalis (blood) Klebsiella pneumoniae (blood) |
Male | 79 | Died |
| 10 | Coagulase-negative staphylococci (blood) Pseudomonas aeruginosa (blood) |
Male | 89 | Died |
| 11 | Candida glabrata (sputum) | Male | 61 | Died |
| 12 | Pseudomonas aeruginosa (sputum) | Male | 79 | Survived |
| 13 | Pseudomonas aeruginosa (sputum) | Male | 77 | Died |
| 14 | Staphylococcus aureus (blood) | Female | 29 | Died |
| 15 | Coagulase-negative staphylococci (blood, and nasopharyngeal swab) | Male | 83 | Died |
| 16 | Klebsiella pneumoniae (nasopharyngeal swab) | Female | 76 | Survived |
| 17 | Coagulase-negative staphylococci (blood) | Female | 82 | Died |
| 18 |
Staphylococcus aureus (blood) Klebsiella pneumoniae (blood) |
Female | 85 | Died |
| 19 | Coagulase-negative staphylococci (blood) | Male | 77 | Died |
| 20 | Coagulase-negative staphylococci (blood) | Male | 78 | Survived |
| 21 | Klebsiella pneumoniae (sputum) | Male | 55 | Died |
| 22 | Pseudomonas aeruginosa (sputum) | Male | 78 | Died |
| 23 |
Acinetobacter baumannii (blood) Klebsiella pneumoniae (blood) |
Female | 15 | Died |
| 24 | Coagulase-negative staphylococci (blood) | Female | 80 | Died |
| 25 | Klebsiella pneumoniae (blood) | Male | 77 | Died |
| 26 | Candida albicans (cerebrospinal fluid) | Female | 82 | Survived |
| 27 | Enterococcus faecium (blood) | Female | 64 | Died |
| 28 | Coryneform bacilli (blood) | Female | 67 | Died |
| 29 | Staphylococcus epidermidis (blood) | Male | 57 | Died |
| 30 |
Candida albicans (blood) Coagulase-negative staphylococci (blood) |
Female | 80 | Died |
| 31 | Acinetobacter baumannii (sputum) | Male | 74 | Died |
| 32 | Coagulase-negative staphylococci (blood) | Female | 84 | Died |
| 33 |
Acinetobacter baumannii (sputum) Klebsiella pneumoniae (sputum) |
Male | 83 | Died |
| 34 | Staphylococcus epidermidis (blood) | Female | 80 | Died |
| 35 | Coagulase-negative staphylococci (blood) | Male | 83 | Died |
| 36 | Enterococcus faecalis (nasopharyngeal swab) | Female | 29 | Died |
| 37 | Enterococcus faecalis (nasopharyngeal swab) | Male | 89 | Died |
| 38 | Staphylococcus aureus (nasopharyngeal swab) | Female | 86 | Died |
| 39 | Staphylococcus epidermidis (blood) | Male | 87 | Died |
| 40 | Coagulase-negative staphylococci (blood) | Female | 29 | Survived |
| 41 | Klebsiella pneumoniae (nasopharyngeal swab) | Male | 78 | Survived |
| 42 | Enterococcus faecium (blood) | Female | 66 | Survived |
| 43 | Coagulase-negative staphylococci (blood) | Male | 76 | Died |
| 44 |
Enterobacter aerogenes (blood) Coagulase-negative staphylococci (blood) |
Male | 89 | Died |
| 45 | Staphylococcus aureus (blood) | Female | 88 | Died |
| 46 | Acinetobacter baumannii (sputum) | Male | 82 | Died |
The MI attributed to coinfection with bacteria and fungi in the overall studied population was 84.8% (Table 2) and was higher among male patients (53.8% vs. 46.2% for females) and those older than 70 years (66.7%). The MI due to bacterial infection was 94.9%, while it was 2.6% for fungal infections and 2.6% due to dual bacterial and fungal coinfections. In addition, the MI as a result of bacterial coinfection was 75.7% and 24.3% for multi- and monobacterial coinfections, respectively (p < 0.001). Based on sites of coinfection, the MI was 13.0% for upper respiratory infection, 24.1% for lower respiratory infection, and 63.0% for bacteremia. Moreover, levels of serum PCT, C-RP, ferritin, D-dimer, WBC count, and lymphocyte counts were higher in patients who died.
Table 2.
Impact of bacterial and/or fungal coinfections among ICU COVID-19 patients.
| Variable | Patient's outcomes | p value∗ | ||||
|---|---|---|---|---|---|---|
| Survived | Died | |||||
| Sex | Male | 42.9% | 53.8% | 0.466 | ||
| Female | 57.1% | 46.2% | ||||
|
| ||||||
| Age | Less than 29 years | 0.0% | 5.1% | 0.642 | ||
| 29–49 years | 14.3% | 5.1% | ||||
| 50–65 years | 0.0% | 15.4% | ||||
| 66–70 years | 14.3% | 7.7% | ||||
| More than 70 years | 71.4% | 66.7% | ||||
|
| ||||||
| Cause of coinfections | Fungi (sole) | 14.3% | 2.6% | 0.366 | ||
| Dual (bacterial and fungal coinfections) | 0.0% | 2.6% | ||||
| Bacteria | 85.7% | 94.9% | ||||
| Monobacterial | 0.0% | 24.3% | <0.001 | |||
| Multibacterial | 100.0% | 75.7% | ||||
|
| ||||||
| Antimicrobial susceptibility test | Antibiotic resistance | Yes | 28.6% | 51.3% | 0.149 | |
| No | 71.4% | 48.7% | ||||
| Resistance to antibiotics | Oxacillin resistance | Yes | 28.6% | 38.5% | 0.618 | |
| No | 71.4% | 61.5% | ||||
| Methicillin resistance | Yes | 14.3% | 15.4 | 0.268 | ||
| No | 85.7% | 84.6% | ||||
|
| ||||||
| Inflammatory biomarkers (mean ± SD) | Procalcitonin (g/dl) | 0.93 ± 0.47 | 22.77 ± 0.87 | 0.585 | ||
| C-reactive protein (mg/L) | 48.15 ± 0.36 | 95.88 ± 0.93 | 0.404 | |||
| Ferritin (ng/ml) | 838.18 ± 0.98 | 4230.96 ± 0.78 | 0.692 | |||
| D-dimer (ng/ml) | 607.00 ± 2.03 | 9200.40 ± 7.50 | 0.446 | |||
| White blood cells count (×109 cells/L) | 12.63 ± 5.12 | 18.73 ± 3.48 | 0.586 | |||
| Neutrophils counts (%) | 89.75 ± 1.26 | 81.48 ± 7.89 | 0.326 | |||
| Lymphocyte counts (%) | 5.50 ± 1.53 | 13.07 ± 2.54 | 0.356 | |||
|
| ||||||
| Site of coinfection | Upper respiratory infection | 28.6% | 13.0% | 0.171 | ||
| Lower respiratory infection | 14.3% | 24.1% | ||||
| Bacteremia | 42.9% | 63.0% | ||||
| Meningitis | 14.3% | 0.0% | ||||
|
| ||||||
| Total | 15.2% | 84.8% | — | |||
Normal range: procalcitonin: less than 0.5 ng/ml: low risk of severe sepsis, 0.5–2.0 ng/ml: clinical suspicion of sepsis, more than 2.0–10 ng/ml: high risk of severe sepsis, more than 10 ng/ml: high likelihood of septic shock; C-reactive protein: less than 5.0 mg/L; ferritin: male: 22–322 ng/ml, female: 10–291 ng/ml; D-dimer: less than 500 ng/ml; globulin: 2–3 g/dl; white blood cell count: 4.5–11 × 109/L; neutrophil count: 30–75%; lymphocytes: 20–40%. ∗Statistically significant at p < 0.05.
The patterns of bacterial and fungal coinfections based on the type of specimen where the microorganism was isolated are illustrated in Figure 2. The most numerous bacterial types were isolated from blood; coagulase-negative staphylococci had the highest proportion (41%), followed by Klebsiella pneumoniae (14%), Staphylococcus aureus (11%), and Staphylococcus epidermidis (8%). In sputum, the highest proportion of isolated bacteria was Acinetobacter baumannii (31%), followed by Pseudomonas aeruginosa and Klebsiella pneumoniae (23% for each). Candida glabrata was the sole cause of fungal coinfection in lower respiratory tract infections, with an 8% prevalence in the studied population. Regarding nasopharyngeal swabs, Klebsiella pneumoniae was considered the major isolated microorganism, with a 34% prevalence, followed by Staphylococcus aureus and Enterococcus faecalis (22% for each).
Figure 2.
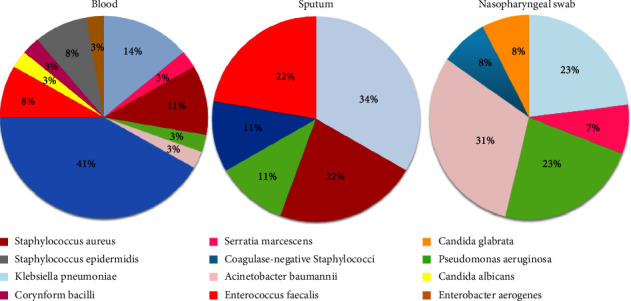
Bacterial and fungal coinfection distribution based on the type of specimen.
Table 3 shows the microorganism distribution among different specimen types based on sex. In females, the highest proportion of isolated microorganisms in sputum was Klebsiella pneumoniae, Serratia marcescens, Acinetobacter baumannii, and coagulase-negative staphylococci (25% for each), whereas coagulase-negative staphylococci had the highest prevalence of bacteria isolated from blood (36.8%). The highest proportions of isolated bacteria from nasopharyngeal swabs were Klebsiella pneumoniae, Staphylococcus aureus, and Enterococcus faecalis, with a 33.3% proportion for each. However, Candida albicans was the only fungus isolated from cerebrospinal fluids in both males and females. In males, the prevalence of Pseudomonas aeruginosa and Acinetobacter baumannii was higher in sputum (33.3% for each), followed by Klebsiella pneumoniae (22.2%). Similar to females, coagulase-negative staphylococci recorded in males had the highest prevalence of bacteria isolated from blood (47.1%). However, the higher proportion of isolated bacteria from nasopharyngeal swabs was Klebsiella pneumoniae (33.3%).
Table 3.
Microorganism distribution among different specimen types based on sex.
| Isolated microorganism | Type specimen | Total (%) | |||
|---|---|---|---|---|---|
| Sputum | Blood | Nasopharyngeal swab | Cerebrospinal fluid | ||
| $ Females | |||||
| Klebsiella pneumoniae | 25.0% | 15.8% | 33.3% | 0.0% | 18.5 |
| Serratia marcescens | 25.0% | 0.0% | 0.0% | 0.0% | 3.7 |
| Staphylococcus aureus | 0.0% | 15.8% | 33.3% | 0.0% | 14.8 |
| Acinetobacter baumannii | 25.0% | 5.3% | 0.0% | 0.0% | 7.4 |
| Coagulase-negative staphylococci | 25.0% | 36.8% | 0.0% | 0.0% | 29.6 |
| Enterococcus faecalis | 0.0% | 10.5% | 33.3% | 0.0% | 11.1 |
| Candida albicans | 0.0% | 5.3% | 0.0% | 100.0% | 7.4 |
| Coryneform bacilli | 0.0% | 5.3% | 0.0% | 0.0% | 3.7 |
| Staphylococcus epidermidis | 0.0% | 5.3% | 0.0% | 0.0% | 3.7 |
|
| |||||
| ∗ Males | |||||
| Klebsiella pneumoniae | 22.2% | 11.8% | 33.3% | 0.0% | 18.2 |
| Serratia marcescens | 0.0% | 5.9% | 0.0% | 0.0% | 2.9 |
| Staphylococcus aureus | 0.0% | 5.9% | 16.7% | 0.0% | 5.9 |
| Pseudomonas aeruginosa | 33.3% | 5.9% | 16.7% | 0.0% | 14.7 |
| Acinetobacter baumannii | 33.3% | 0.0% | 0.0% | 0.0% | 8.8 |
| Coagulase-negative staphylococci | 0.0% | 47.1% | 16.7% | 0.0% | 26.5 |
| Enterococcus faecalis | 0.0% | 5.9% | 16.7% | 100.0% | 8.8 |
| Candida glabrata | 11.1% | 0.0% | 0.0% | 0.0% | 2.9 |
| Staphylococcus epidermidis | 0.0% | 11.8% | 0.0% | 0.0% | 5.9 |
| Enterobacter aerogenes | 0.0% | 5.9% | 0.0% | 0.0% | 2.9 |
$ p value was 0.329; ∗p value was 0.246.
4. Discussion
Bacterial coinfection was common during previous respiratory viral pandemics. It was associated with a poor prognosis of the viral disease and considered a risk factor for death [23]. The MI of bacterial and fungal coinfection in COVID-19 patients was 50%, with more concern for antibiotic-resistant bacteria [3, 5]. The present data revealed that coinfections among COVID-19 in the ICU resulted from different types of bacteria and fungi, confirming what have been reported in other studies. Overall, coinfection among ICU COVID-19 patients results from bacteria [3], fungi [3], or other respiratory viruses [24]. In agreement with our findings, Silva et al. (2021) reported that the MI among COVID-19 patients in the ICU was 50.47% (83.14% being coinfected by fungi and/or bacteria) [25].
In addition, the present results found that the MI as a result of bacterial coinfection was 94.9%, 13.0% for upper respiratory infection, 24.1% for lower respiratory infection, and 63.0% for bacteremia. These results confirm what had been reported by Hughes et al., i.e., that bacteremia is considered a serious complication of bacterial superinfection and a risk factor for mortality in COVID-19 patients [10]. Bacteremia among COVID-19 patients has been reported worldwide with various percentages: 1.8% in Michigan City [26], 6% in New York City [27], 7.1% in the United Kingdom [10], 25% in Pennsylvania [28], and 37.0% in Milan [12], with mortality rates ranging between 9.8% and 47.5%. The worst-case scenario for critically ill COVID-19 patients is the development of polymicrobial coinfections [17], especially if one of the bacteria is multidrug-resistant, with a significant risk of complicating multiple organ failure and septic shock [8]. The main influence of bacterial coinfection in COVID-19 patients is mainly attributed to septicemia, multiorgan failure, septic shock, and respiratory, cardiac, kidney and liver dysfunction [27, 29, 30].
The present study shows an increase in inflammatory biomarkers, including PCT, C-RP, D-dimer, and ferritin, among patients who did not survive, in line with previous findings [27] and correlating significantly with respiratory failure distress, extended mechanical ventilation, and an increased MI rate [31]. Lymphocytopenia among our participants can be explained by interleukin upregulation and cytokine storms that cause lymphocyte apoptosis [7, 9], which have been considered risk factors for coinfection during the COVID‐19 pandemic [6].
Antibiotic resistance was detected among approximately half of the patients who died (51.3%), which creates additional challenges in controlling patient outcomes and prognosis [14]. The prophylactic use of broad-spectrum antibiotics among such patients must be taken into consideration to avoid the drawbacks of long-term broad-spectrum antibiotic misuse [28]. The protocols regarding broad-spectrum antibiotic use are different at the national level; for example, the Chinese Institutes of Health do not recommend the use of broad-spectrum antibiotics even in critically ill patients without bacterial coinfection [17], whereas in the Netherlands, empirical antibiotics are recommended for all ICU and mechanically ventilated patients [32].
Among the study strengths, this study is the first in Jordan and included accurately identified bacterial and fungal isolates that caused coinfection among COVID-19 patients in the ICU, and it is the first study to clinically characterize ICU COVID-19 patients in the Middle East and North Africa region (MENA). Additionally, the samples were obtained from different types of specimens. Among limitations, this was a single-center study, and the present findings should be considered with caution. The small sample size is attributed to the nature of the studied population, namely, ICU patients.
In conclusion, bacterial/fungal coinfections hurt critically ill COVID-19 patients and may lead to worse disease prognosis and increased MI. Timely prediction of bacterial and fungal coinfection among critically ill ICU patients through monitoring of inflammatory biomarkers before microbiological culture and antibiotic susceptibility results may play a critical role in improving patient prognosis and increasing the survival rate.
Acknowledgments
We would like to thank the Jordan Hospital health team and managers for facilitating the data collection.
Data Availability
The database analyzed for the current study is available from the corresponding author upon reasonable request.
Ethical Approval
The study protocol was carried out following the principles of the Declaration of Helsinki as revised in 2000. Ethical approval for this research was obtained from the Ethics Committee for Scientific Research (ECSR) at Zarqa University by the requirement of the protection of human subjects and the ethical principles related to research studies (no. 2/8/2021).
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
All the authors have taken responsibility for the entire content of this manuscript and authorized its submission.
References
- 1.Bakaletz L. O. Viral-bacterial co-infections in the respiratory tract. Current Opinion in Microbiology . 2017;35:30–35. doi: 10.1016/j.mib.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martins-Filho P. R., Tavares C. S. S., Santos V. S. Factors associated with mortality in patients with COVID-19. A quantitative evidence synthesis of clinical and laboratory data. European Journal of Internal Medicine . 2020;76:97–99. doi: 10.1016/j.ejim.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawson T. M., Moore L. S., Zhu N., et al. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support covid-19 antimicrobial prescribing. Clinical Infectious Diseases . 2020;71 doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet . 2020;395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet . 2020;395(10229):1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Zt, Chen Zm, Chen Ld, et al. Coinfection with SARS‐CoV‐2 and other respiratory pathogens in patients with COVID‐19 in Guangzhou, China. Journal of Medical Virology . 2020;92(11):2381–2383. doi: 10.1002/jmv.26073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arentz M., Yim E., Klaff L., et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA . 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lescure F.-X., Bouadma L., Nguyen D., et al. Clinical and virological data of the first cases of covid-19 in europe: a case series. The Lancet Infectious Diseases . 2020;20(6) doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards M. J., Edwards J. R., Culver D. H., Gaynes R. P. Nosocomial infections in medical intensive care units in the United States. Critical Care Medicine . 1999;27(5):887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Hughes S., Troise O., Donaldson H., Mughal N., Moore L. S. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clinical Microbiology and Infections . 2020;26(10):1395–1399. doi: 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y., Cao Y., Wang S., Cai K., Xu K. COVID‐19 and gastrointestinal symptoms. British Journal of Surgery . 2020;107(10):e382–e383. doi: 10.1002/bjs.11821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zangrillo A., Beretta L., Scandroglio A. M., et al. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Critical care and resuscitation: Journal of the Australasian Academy of Critical Care Medicine . 2020;22(3):200–211. doi: 10.1016/S1441-2772(23)00387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory Medicine . 2020;8(5):475–481. doi: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manohar P., Loh B., Nachimuthu R., Hua X., Welburn S. C., Leptihn S. Secondary bacterial infections in patients with viral pneumonia. Frontiers of Medicine . 2020;7:p. 420. doi: 10.3389/fmed.2020.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandi N., Ciccarese F., Balacchi C., et al. Co-infections and superinfections in COVID-19 critically ill patients are associated with CT imaging abnormalities and the worst outcomes. Diagnostics . 2022;12(7):p. 1617. doi: 10.3390/diagnostics12071617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandi N., Ciccarese F., Rimondi M. R., et al. An imaging overview of COVID-19 ards in ICU patients and its complications: a pictorial review. Diagnostics . 2022;12(4):p. 846. doi: 10.3390/diagnostics12040846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diez-Sampedro A., Gonzalez A., Delgado V., Flowers M., Maltseva T., Olenick M. COVID-19 and advanced practice registered nurses: frontline update. The Journal for Nurse Practitioners . 2020;16(8):551–555. doi: 10.1016/j.nurpra.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gautret P., Lagier J.-C., Parola P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. International Journal of Antimicrobial Agents . 2020;56(1) doi: 10.1016/j.ijantimicag.2020.105949.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Chakraborty S. San Diego County Nanopore SARS-Cov2 Sequencing Data Shows Metagenomic Prevotella, Haemophilus Parainfluenzae, a Lot of Unknown Species and Chimeric Reads . 2020. [Google Scholar]
- 20.World Health Organization. Strengthening Response to Pandemics and Other Public-Health Emergencies: Report of the Review Committee on the Functioning of the International Health Regulations (2005) and on Pandemic Influenza (H1N1) 2009 . Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 21.Rawson T. M., Moore L. S. P., Castro-Sanchez E., et al. COVID-19 and the potential long-term impact on antimicrobial resistance. Journal of Antimicrobial Chemotherapy . 2020;75(7):1681–1684. doi: 10.1093/jac/dkaa194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lansbury L., Lim B., Baskaran V., Lim W. S. Co-infections in people with COVID-19: a systematic review and meta-analysis. Journal of Infection . 2020;81(2):266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chertow D. S., Memoli M. J. Bacterial coinfection in influenza: a grand rounds review. JAMA . 2013;309(3):275–282. doi: 10.1001/jama.2012.194139. [DOI] [PubMed] [Google Scholar]
- 24.Aghbash P. S., Eslami N., Shirvaliloo M., Baghi H. B. Viral coinfections in COVID‐19. Journal of Medical Virology . 2021;93(9):5310–5322. doi: 10.1002/jmv.27102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva D. L., Lima C. M., Magalhães V. C., et al. Fungal and bacterial coinfections increase mortality of severely ill COVID-19 patients. Journal of Hospital Infection . 2021;113:145–154. doi: 10.1016/j.jhin.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaughn V. M., Gandhi T., Petty L. A., et al. Empiric antibacterial therapy and community-onset bacterial Co-infection in patients hospitalized with COVID-19: a multi-hospital cohort study. Clinical Infectious Diseases . 2020;72 doi: 10.1093/cid/ciaa1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goyal P., Choi J. J., Pinheiro L. C., et al. Clinical characteristics of Covid-19 in New York city. New England Journal of Medicine . 2020;382(24):2372–2374. doi: 10.1056/nejmc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goncalves Mendes Neto A., Lo K. B., Wattoo A., et al. Bacterial infections and patterns of antibiotic use in patients with COVID‐19. Journal of Medical Virology . 2021;93(3):1489–1495. doi: 10.1002/jmv.26441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henry B. M., De Oliveira M. H. S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clinical Chemistry and Laboratory Medicine . 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 30.Ruscitti P., Berardicurti O., Di Benedetto P., et al. Severe COVID-19, another piece in the puzzle of the hyperferritinemic syndrome. An immunomodulatory perspective to alleviate the storm. Frontiers in immunology . 2020;1130 doi: 10.3389/fimmu.2020.01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heer R. S., Mandal A. K., Kho J., et al. Elevated procalcitonin levels in severe Covid-19 may not reflect bacterial co-infection. Annals of Clinical Biochemistry . 2021;58(5):520–527. doi: 10.1177/00045632211022380. [DOI] [PubMed] [Google Scholar]
- 32.Alhazzani W., Møller M. H., Arabi Y. M., et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Medicine . 2020;46(5):854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The database analyzed for the current study is available from the corresponding author upon reasonable request.


