Background:
Chronic kidney disease is frequently associated with hypertension and poorly controlled blood pressure can lead to chronic kidney disease progression. Finerenone, a nonsteroidal mineralocorticoid receptor antagonist, significantly improves cardiorenal outcomes in patients with chronic kidney disease and type 2 diabetes. This analysis explored the relationship between office systolic blood pressure (SBP) and cardiorenal outcomes with finerenone in FIDELIO-DKD trial (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease).
Methods:
Patients with type 2 diabetes, urine albumin-to-creatinine ratio 30 to 5000 mg/g, and estimated glomerular filtration rate of 25 to <75 mL/min per 1.73 m2 receiving optimized renin-angiotensin system blockade, were randomized to finerenone or placebo. For this analysis, patients (N=5669) were grouped by baseline office SBP quartiles.
Results:
Finerenone reduced office SBP across the baseline office SBP quartiles, including patients with baseline office SBP of >148 mm Hg. Overall, patients with lower baseline office SBP quartile and greater declines from baseline in SBP were associated with better cardiorenal outcomes. The risk of primary kidney and key secondary cardiovascular composite outcomes was consistently reduced with finerenone versus placebo irrespective of baseline office SBP quartiles (P for interaction 0.87 and 0.78, respectively). A time-varying analysis revealed that 13.8% and 12.6% of the treatment effect with finerenone was attributed to the change in office SBP for the primary kidney composite outcome and the key secondary cardiovascular outcome, respectively.
Conclusions:
In FIDELIO-DKD, cardiorenal outcomes improved with finerenone irrespective of baseline office SBP. Reductions in office SBP accounted for a small proportion of the treatment effect on cardiorenal outcomes.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02540993.
Keywords: blood pressure, chronic kidney diseases, finerenone, mineralocorticoid receptor antagonist, systolic blood pressure, type 2 diabetes
Novelty and Relevance.
What Is New?
In FIDELIO-DKD trial (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease), finerenone reduced office systolic blood pressure (SBP) in patients on antihypertensive therapy across baseline office SBP quartiles, including patients with baseline office SBP of >148 mm Hg
What Is Relevant?
Finerenone’s cardiorenal benefits may be partially attributed to antihypertensive effects as measured in the office in patients with chronic kidney disease and type 2 diabetes; however, it remains to be determined whether the effects with finerenone are hemodynamic or nonhemodynamic in nature.
Clinical/Pathophysiological Implications?
Finerenone’s cardiorenal benefits were consistent in patients with chronic kidney disease and type 2 diabetes, irrespective of baseline office SBP and were unaffected by arterial hypertension.
Office SBP reduction was noted in patients with elevated blood pressure on optimized renin–angiotensin system blockade.
Arterial hypertension is highly prevalent in patients with chronic kidney disease (CKD) and type 2 diabetes (T2D).1,2 CKD commonly coexists with hypertension and poorly controlled blood pressure (BP) contributes to the progression of CKD.1,2 A urine albumin-to-creatinine ratio (UACR) over 30 mg/g and an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 are reported to be strongly associated with a high prevalence of hypertension, diabetes, or both.3
Existing evidence suggests that BP lowering to <130/80 mm Hg is associated with renoprotection in patients with albuminuric CKD.4 The Kidney Disease Improving Global Outcomes clinical practice guidelines propose a target systolic BP (SBP) up to 120 mm Hg, quantified with standardized office BP measurement, for the treatment of high BP in patients with nondialysis CKD with or without T2D.5 Renin-angiotensin system inhibitors, such as angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, are recommended as first-line agents for the treatment of hypertension in patients with albuminuric CKD because of their antihypertensive and renoprotective effects.1,3 Steroidal mineralocorticoid receptor antagonists (MRAs) such as spironolactone and eplerenone have been shown to be effective for the treatment of resistant or refractory hypertension,6–8 as well as improving albuminuria and proteinuria.9 Steroidal MRAs were also reported to improve endothelial dysfunction and reduce vascular stiffening in clinical trial settings.10
Finerenone is a novel, selective, nonsteroidal MRA antagonist that significantly delayed kidney disease progression and reduced the risk of cardiovascular outcomes in patients with CKD and T2D in the FIDELIO-DKD trial (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease).11,12 In this report, we analyze the relationship between office SBP and the effect of finerenone on cardiorenal outcomes in FIDELIO-DKD.
Methods
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Participants
The study design, inclusion and exclusion criteria, and the treatment protocols of FIDELIO-DKD were previously described in detail and further information are available in the Supplemental Material.11,13 In brief, FIDELIO-DKD was a global, phase 3, randomized, double-blind, placebo-controlled, parallel-group, event-driven trial comparing finerenone versus placebo in patients treated with the maximum tolerated labeled dose of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Eligible participants were aged 18 years or more, and had a clinical diagnosis of T2D, with either: a UACR ≥30 to <300 mg/g, an eGFR ≥25 to <60 mL/min per 1.73 m2 and a history of diabetic retinopathy; or a UACR ≥300 to ≤5000 mg/g and an eGFR ≥25 to <75 mL/min per 1.73 m2.
Procedures and Outcomes
Patients were randomized (1:1) to once-daily oral treatment with finerenone or matching placebo. Office BP was recorded at baseline and was measured at all visits throughout the trial to the end-of-study visit. During each visit, office BP was measured in accordance with published guidelines, with patients in sitting position after a 5-minute rest.14,15 The mean of 3 consecutive office BP readings, each reading with an interval of 1 minute or more in-between, and all 3 readings recorded within 20 minutes, were used in this analysis.14,15 If office BP was considered uncontrolled during the study period, it was permitted to add non-potassium-sparing diuretics as a first treatment choice. Thereafter, the addition and titration of antihypertensive medication was performed according to local guideline recommendations at the discretion of the investigator.
The primary composite outcome of FIDELIO-DKD was time to first occurrence of kidney failure, sustained eGFR decline ≥40% from baseline over 4 weeks or more, or renal death. The key secondary composite outcome was time to first occurrence of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure. Other secondary outcomes included a composite of time to first occurrence of kidney failure, a sustained decrease of at least 57% in eGFR from baseline over 4 weeks or more, or renal death (secondary kidney outcome) and change in UACR from baseline to month 4. Both primary and secondary efficacy and safety outcomes have been previously reported.11,12
Statistical Analysis
Baseline characteristics of patients, grouped by quartiles of baseline office SBP, were expressed as means (SD), medians and quartiles for continuous variables, or absolute numbers and percentages for categorical variables. Treatment differences of office SBP (and office diastolic BP) over the course of the trial were analyzed using a mixed model in each quartile of baseline office SBP and in the overall population.
To assess any potential heterogeneity of the treatment effect on efficacy outcome events regarding baseline office SBP, a Cox proportional hazards model (stratified by region, eGFR, and albuminuria categories at screening), which included quartiles of baseline office SBP and their interaction with treatment as covariates, was used. Treatment effects were expressed as hazard ratios (HRs) with corresponding 95% CIs.
To examine whether the finerenone treatment effect on efficacy outcomes was mediated by office SBP changes, a separate stratified Cox model was used, considering the complete time course of office SBP by means of a time-varying covariate for office SBP. Alternatively, to avoid increased numbers of values potentially missing not at random at later visits in the study, a landmark analysis, at the time point of maximal placebo-adjusted office SBP change (ie, the month 4 visit) and including treatment, baseline office SBP and change in office SBP from baseline to month 4 as covariates, was performed. For both the time-updated Cox model and the landmark analysis, the proportion of treatment effect explained by office SBP was calculated as 100% × ([HR0-HR1]/[HR0-1]), where HR1 and HR0 represent the HRs based on the models with and without the adjustment for office SBP values, respectively). In a second step, an interaction term between treatment and time-varying office SBP or office SBP change to month 4 was added into the adjusted Cox models to evaluate whether the size of the treatment effect further depended on office SBP values. Furthermore, the relationship of cardiorenal outcomes with office SBP was investigated by means of unstratified Cox models by treatment group, using cubic B-splines of time-varying office SBP with 3 equally spaced knots and adjusted for age, race, sex, smoking history, history of cardiovascular disease and baseline body mass index, heart rate, UACR, eGFR, and glycated hemoglobin.
Results
Patients
Among the 5734 patients, a total of 5669 patients (98.9%) without critical violations of Good Clinical Practice and with available office SBP data were analyzed over a median follow-up of 2.6 years. In the overall study population, the baseline mean (SD) values for office SBP and office diastolic BP were 138.0 (14.4) mm Hg and 75.8 (9.7) mm Hg, respectively. The study population was grouped into quartiles (Q) of office SBP at baseline (Q1, ≤128.7 mm Hg; Q2, >128.7 to ≤138.3 mm Hg; Q3, >138.3 to ≤148.0 mm Hg; Q4, >148.0 mm Hg).
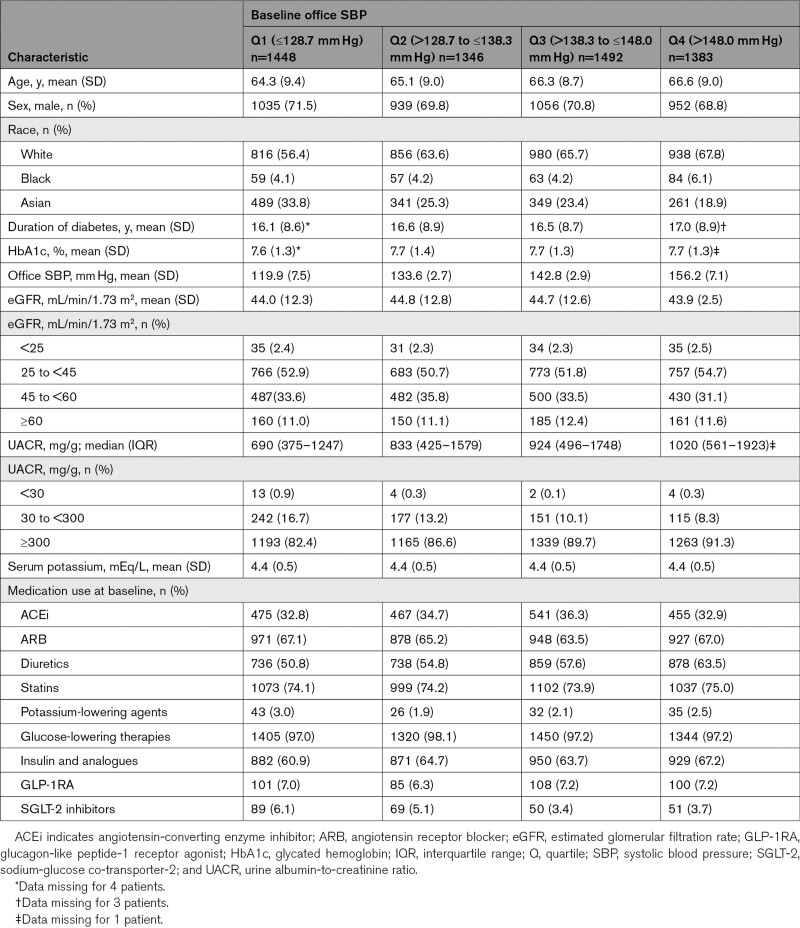
Baseline characteristics were generally similar between office SBP quartiles and treatment groups (Table 1; Table S1). As expected, the use of antihypertensive agents increased with higher office SBP quartiles (Table S2). Overall, patients received an average of 3.3 antihypertensive therapies at baseline.
Table 1.
Patient Baseline Characteristics by Baseline Office SBP Quartile
Effect of Finerenone on Office BP
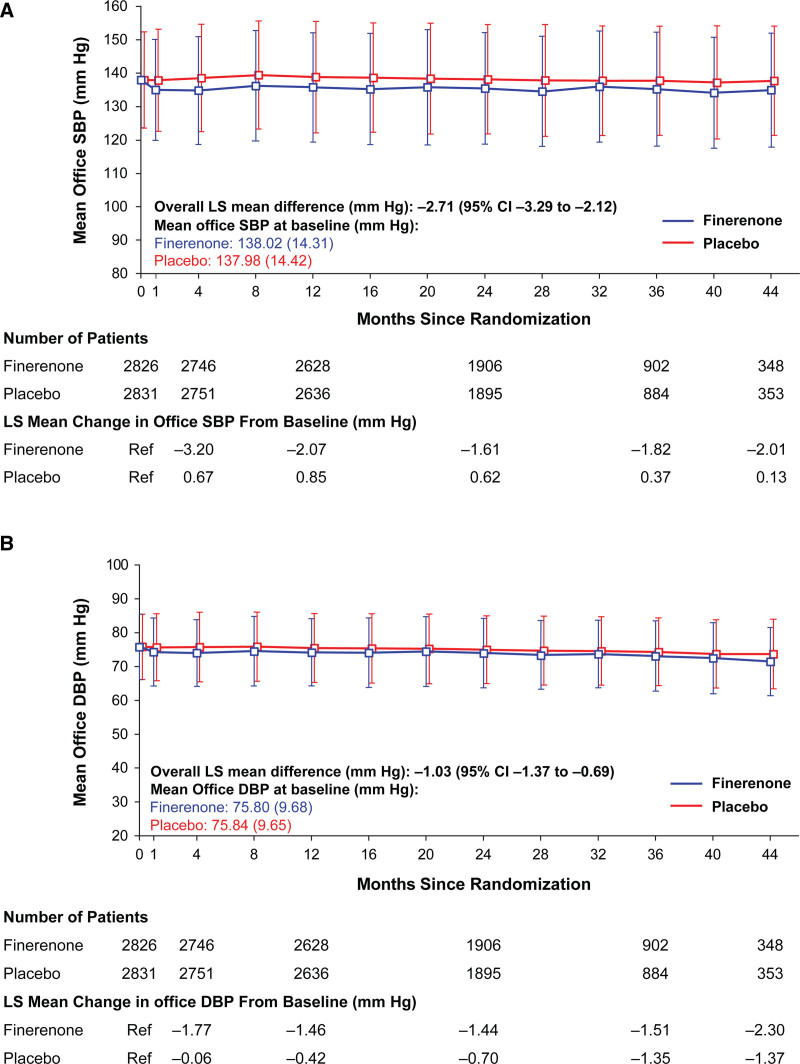
In the mixed model analysis, treatment with finerenone resulted in a modest and consistent reduction from baseline in office SBP compared with placebo, with an overall least squares (LS) mean difference between treatment groups of –2.71 mm Hg (95% CI, –3.29 to –2.12) over the course of the trial (Figure 1A). There was also a slight reduction in office diastolic BP from baseline with finerenone compared with placebo throughout the trial (LS mean difference, –1.03 mm Hg [95% CI, –1.37 to –0.69]; Figure 1B).
Figure 1.
Change in office systolic blood pressure (SBP) and office diastolic blood pressure (DBP) over the course of FIDELIO-DKD trial (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease). Effect of finerenone and placebo on (A) office SBP and (B) office DBP. A modest and consistent reduction in office SBP was observed with finerenone compared with placebo. There was also a slight reduction in office DBP with finerenone compared with placebo over the duration of the trial. Data expressed as mean (SD). Overall least squares (LS) mean difference is provided for the change from baseline.
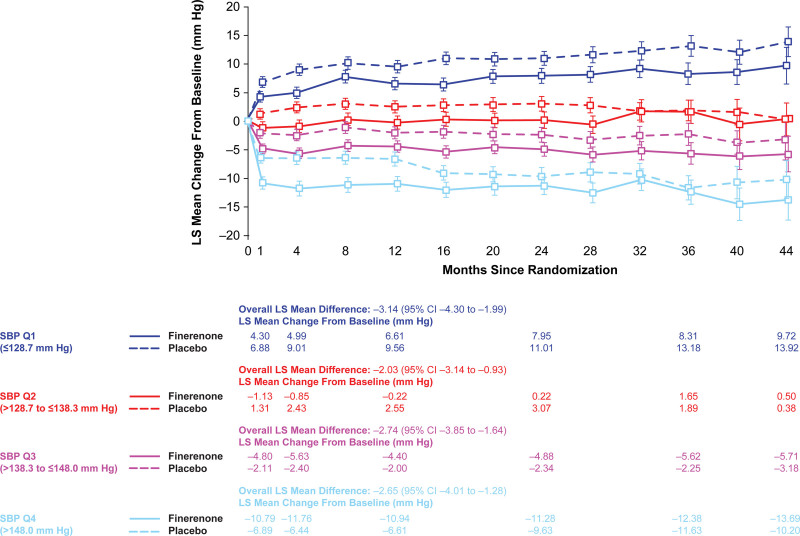
A maximum treatment difference of –3.84 mm Hg (95% CI, –4.59 to –3.10) in LS mean change in office SBP from baseline between finerenone and placebo was observed at month 4. In both groups, increasing quartiles of office SBP had greater declines in office SBP; however, declines were greater in the finerenone group. The LS mean changes in office SBP from baseline to month 4 across increasing quartiles of office SBP in the finerenone group was: +4.99, –0.85, –5.63, and –11.76 mm Hg, compared with the following LS mean changes in the placebo group: +9.01, +2.43, –2.40, –6.44 mm Hg (Figure 2). Over the duration of the trial, office SBP was lower with finerenone compared with placebo, and this effect was relatively stable after month 4. Differences between finerenone and placebo by office SBP quartiles were more pronounced from month 1 to 36 for Q1 and Q3, month 1 to 28 for Q2, and month 1 to 16 for Q4, as illustrated by the P for the treatment group comparison being ≤0.05.
Figure 2.
Change in office systolic blood pressure (SBP) by baseline office SBP quartiles during FIDELIO-DKD trial (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease). Effect of finerenone and placebo on office SBP by office SBP quartile at baseline. Data expressed as least squares (LS) mean (±95% CI). Overall LS mean difference is provided. Q indicates quartile.
The proportion of patients with treatment-resistant hypertension defined as office BP ≥140/90 mm Hg and taking 3 or more antihypertensives (including a diuretic) was consistently lower for finerenone versus placebo throughout the trial, with the largest difference observed at month 4 (Figure S1). Overall, the number of patients taking 4 or more antihypertensives increased as the trial progressed, and this was most evident in the placebo group. The largest difference between the finerenone and placebo group was observed at month 24, when 47.4% and 53.4% of patients, respectively, were taking 4 or more antihypertensives (Figure S2). At baseline, a total of 23.3% of patients in the highest office SBP quartile (Q4) received more than 3 antihypertensives excluding diuretics compared with 16.5% in Q1, 17.8% in Q2, and 19.3% in Q3; values were similar between the treatment groups. By month 36, a total of 40.5% of patients in the highest office SBP quartile (Q4) received more than 3 antihypertensives excluding diuretics (finerenone, 36.4% versus placebo, 43.9%) compared with 24.8% in office SBP Q1 (finerenone, 21.3% versus placebo, 28.4%), 29.2% in Q2 (finerenone, 27.7% versus placebo, 30.6%), and 30.8% in Q3 (finerenone, 29.0% versus placebo, 33.0%). Additionally, the proportions of patients achieving a target office BP of <140/90 mm Hg or <130/80 mm Hg was consistently higher for finerenone versus placebo over the course of the trial (Figure S3).
Kidney and Cardiovascular Outcomes by Baseline Office SBP Quartiles
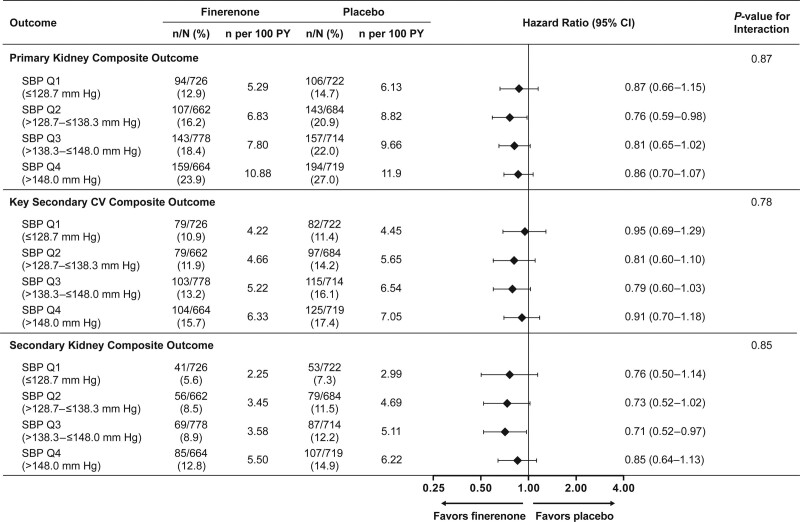
Increasing office SBP quartiles were associated with increasing incidence rates of kidney and cardiovascular outcomes. Finerenone lowered the risk of primary kidney and key secondary cardiovascular outcome events compared with placebo (HR, 0.82 [95% CI, 0.73–0.93], and HR, 0.86 [95% CI, 0.75–0.99], respectively). These effects were consistent across baseline office SBP quartiles (P for interaction, 0.87 and 0.78, respectively; Figure 3). The risk for the secondary kidney composite outcome was also lower with finerenone (HR, 0.76 [95% CI, 0.65–0.90]) in the primary analysis, and this effect was again similar across the different office SBP quartiles (P for interaction, 0.85; Figure 3). A greater reduction in UACR from baseline to month 4 with finerenone compared with placebo was also observed, and this treatment effect was consistent across the office SBP quartiles (Figure S4).
Figure 3.
Primary and key secondary outcomes by baseline office systolic blood pressure (SBP) quartile. Effect of finerenone on kidney and cardiovascular (CV) outcomes across baseline office SBP quartiles. A similar benefit was observed for the primary kidney composite (time to first occurrence of kidney failure, sustained estimated glomerular filtration rate (eGFR) decline ≥40% from baseline over 4 weeks or more, or renal death), the key secondary CV composite (time to first occurrence of CV death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure) and the secondary kidney composite (time to first occurrence of kidney failure, a sustained decrease of at least 57% in eGFR from baseline over 4 weeks or more, or renal death) outcomes. PY indicates patient-years; and Q, quartile.
Treatment Effect of Finerenone on Kidney and Cardiovascular Outcomes Adjusted for Change in Office SBP
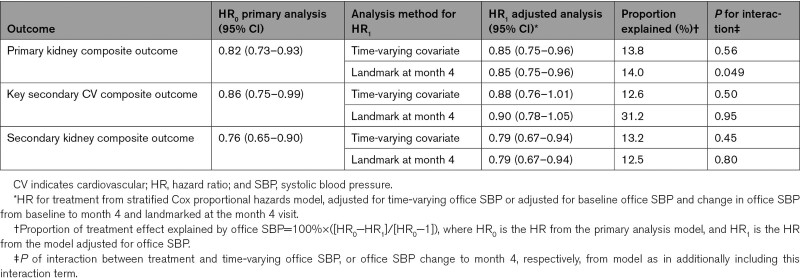
Assessment of the treatment effect of the primary kidney composite outcome adjusted for time-varying office SBP showed that 13.8% of the finerenone treatment effect was explained by office SBP, and no indication of treatment effect variation across the office SBP values (P for interaction, 0.56; Table 2). Spline-modeling showed an increased risk of the kidney outcome event with increasing time-varying office SBP in both treatment groups with a high degree of overlap between CIs (Figure S5A). Given that the maximum placebo-adjusted office SBP change from baseline was observed at month 4 and was generally maintained throughout the trial, a landmark analysis at month 4 was also used to assess the relationship between treatment with finerenone and the efficacy outcomes adjusted for baseline office SBP and change in office SBP from baseline to month 4. Results for the landmark analysis at month 4 were comparable with that observed in the time-varying analysis (14.0% of the treatment effect being explained by office SBP; P for interaction, 0.049). Similar findings were observed for the secondary kidney composite outcome (Table 2; Figure S5C). A correlation analysis also showed that log UACR change from baseline to month 4 correlated weakly with office SBP change from baseline to month 4 (Figure S6).
Table 2.
Cox Proportional Hazards Model Including Time-Varying Office SBP, or Baseline Office SBP and Office SBP Changes From Baseline to Month 4
For the key secondary cardiovascular composite outcome, the HR for treatment adjusted for time-varying office SBP showed that 12.6% of the treatment effect was explained by office SBP (P for interaction, 0.50). Adjustment for baseline office SBP and change in office SBP from baseline to month 4 led to a higher HR for the cardiovascular composite outcome, corresponding to 31.2% of the treatment effect being explained by office SBP (P for interaction, 0.95; Table 2); this can be attributed mainly to early occurrences of cardiovascular events and exclusion of time at risk in the first 4 months for this landmark analysis. While spline-modeling indicated a steeper increase in risk of the cardiovascular composite outcome with increasing time-varying office SBP for placebo compared with finerenone, the high degree of overlap between CIs did not imply an interaction between treatment with finerenone and office SBP for the cardiovascular composite outcome (Figure S5B).
Safety Outcomes in Patients by Baseline Office SBP Quartiles
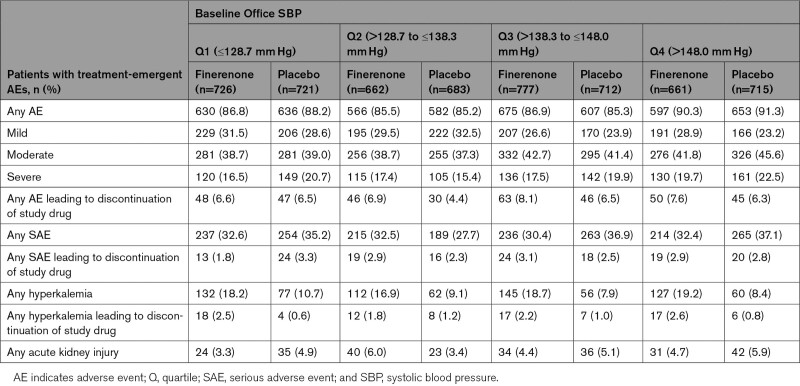
Incidence of any treatment-emergent adverse event was similar between treatment arms across the office SBP quartile groups and serious adverse events were generally less common in the finerenone treatment arm (Table 3). Incidence of investigator-reported treatment-emergent hyperkalemia was similarly increased in patients treated with finerenone across the office SBP quartiles, that is, around 2-fold compared with placebo. Incidence of treatment-emergent acute kidney injury was low across all office SBP quartiles and treatment groups (Table 3; Table S3).
Table 3.
Safety Outcomes by Baseline Office SBP Quartile
Discussion
In the overall FIDELIO-DKD cohort, finerenone has a modest effect on office BP in patients with CKD and T2D. A reduction in office SBP was observed across the baseline office SBP quartiles of the study population, with the greatest decline observed in patients treated with finerenone in the highest office SBP quartile of >148.0 mm Hg. The benefit of finerenone on kidney and cardiovascular outcomes was similar across quartiles of baseline office SBP and an analysis adjusted for change in office SBP demonstrated a persistent efficacy of finerenone. This suggests that in addition to reduction in BP as measured in the office, other mechanisms are likely to improve cardiorenal outcomes with finerenone. However, it remains to be determined to what extent cardiorenal protection with finerenone is due to effects on non-hemodynamic or hemodynamic factors such as 24-hour BP or other measures of BP load on the vasculature.
Exploratory analysis adjusting for change in office SBP suggested that a small proportion of the effect of finerenone on the kidney and cardiovascular outcomes (<14% in the time-varying analysis) may be attributed to changes in office SBP. Therefore, the benefit observed in delaying CKD progression and reducing cardiovascular events with finerenone would seem to also be associated with other mechanisms in addition to office BP. During FIDELIO-DKD, the cardiovascular benefits of finerenone were apparent earlier than the kidney benefits (with separation of the Kaplan-Meier curves occurring from ≈ 4 and 12 months, respectively).11,12 One potential hypothesis is that early cardiovascular benefits may be partly driven by short-term natriuretic or hemodynamic mechanisms (involving a decrease of hemodynamic stress biomarkers B-type natriuretic peptide and amino-terminal pro-B-type natriuretic peptide),11,16 whereas a reduction in inflammation and fibrosis may underlie the long-term kidney and cardiovascular protection. The aldosterone-mineralocorticoid receptor axis is associated with the pathogenesis and progression of CKD and cardiovascular disease, as well as resistant hypertension6,17,18 and overactivation of mineralocorticoid receptor is known to result in downstream transcription of profibrotic and hypertrophic genes.19,20 In preclinical studies, finerenone was found to reduce proinflammatory mediators and fibrosis in the kidney and the heart at doses that had just a mild effect on BP reduction.21,22 This is in contrast with the steroidal MRA eplerenone, with which little or no effect on these parameters were found.21,22 However, it must be noted that these preclinical animal studies used suboptimal methods for measuring BP and did not assess treatment effects on 24-hour BP load on the vasculature. Therefore, further clinical evidence is required to confirm that the anti-inflammatory and antifibrotic effects of finerenone played a contributing role toward the cardiorenal benefits observed in this trial.
In addition to the kidney and cardiovascular benefits found in FIDELIO-DKD, finerenone lowered the mean UACR in patients, and this was maintained over the duration of the trial.11 However, no meaningful correlation between change in UACR and office SBP change to month 4 was observed in the present study. Thus, it could be hypothesized that over the course of disease, the better preservation of kidney function in the finerenone group could have allowed for easier control of BP. Alternatively, it is possible that a stronger correlation might exist between the change in UACR with 24-hour BP to month 4 than with office SBP to month 4. Thus, we cannot exclude the possibility that a substantial reduction of 24-hour BP with finerenone might have largely accounted for the reduction in UACR throughout the trial.
Over the duration of FIDELIO-DKD, there was an overall increase in office SBP for patients in the lowest baseline office SBP quartile and an overall decrease in office SBP for patients in the highest office SBP quartile for both treatment arms; these were likely because of a regression-to-the-mean effect (Figure 2). A factor that may have played a role was the modification of background antihypertensive treatment throughout the trial, which was permitted at the discretion of the investigators in line with local guideline recommendations. Thus, interruption or down-titrations of antihypertensive medications could have occurred for patients with low office BP, and the opposite for patients with high office BP. The number of antihypertensive medications taken throughout the trial was broadly similar across treatment arms (Figure S2), with a slightly lower proportion of patients receiving finerenone receiving ≥4 medications as the trial progressed; this may have resulted in a more conservative estimate in the difference in office BP lowering observed with finerenone versus placebo.
There are limitations in this analysis that must be acknowledged. While patients with CKD commonly experience nocturnal hypertension,23–25 this trial only recorded office BP. Furthermore, in FIDELIO-DKD, baseline office BP was better controlled than the BP of patients typically seen in everyday nephrology practice.26 It should be noted that FIDELIO-DKD included patients with predominantly stage 3 or 4 CKD with severely elevated albuminuria and T2D. The FIGARO-DKD trial (Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease), which is complimentary in design to FIDELIO-DKD but included patients with T2D and stage 2 to 4 CKD with moderately elevated albuminuria or stage 1 or 2 CKD with severely elevated albuminuria also showed a similar modest reduction in office SBP with finerenone, suggesting a similar effect of finerenone on office BP across a broader CKD population.27
Conclusions
In FIDELIO-DKD, the overall benefit of finerenone on kidney and cardiovascular outcomes occurred independently of baseline office SBP in the presence of a modest reduction in office SBP. Analyses adjusted for office SBP changes in FIDELIO-DKD suggest that office SBP change with finerenone may have contributed to a proportion of its outcome. Thus, we postulate that the mechanisms underlying the treatment effect of finerenone and contributing to kidney and cardiovascular benefits in patients with CKD and T2D could be of hemodynamic and non-hemodynamic nature. However, it remains to be determined to what extent the cardiorenal protection with finerenone demonstrated in FIDELIO-DKD is due to effects on non-hemodynamic or hemodynamic factors such as 24-hour BP or other measures of BP load on the vasculature.
Perspectives
The novel, selective, nonsteroidal MRA, finerenone reduced the risk of kidney and cardiovascular events in patients with CKD and T2D while having a modest effect on office BP in the phase 3 FIDELIO-DKD trial. This analysis sought to further investigate the relationship between office SBP and the cardiorenal benefits of finerenone in FIDELIO-DKD. Results reported here show lower baseline office SBP and greater declines in office SBP were associated with better cardiorenal outcomes. However, the treatment effects with finerenone were similar across different office SBP levels, and an exploratory analysis adjusting for change in office SBP suggested that a small proportion of the cardiorenal benefits were attributed to the effect of finerenone on office BP reduction. Since the results described here are based on the assessment of office BP, it remains to be determined to what extent the cardiorenal protection with finerenone is due to effects on non-hemodynamic or hemodynamic factors such as 24-hour BP or other measures of BP load on the vasculature.
Article Information
Acknowledgments
We are indebted to the patients who have participated in this trial, the FIDELIO-DKD trial (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease) investigators, the study centers who supported the trial, and the study teams.
Sources of Funding
FIDELIO-DKD trial (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease) was conducted and funded by Bayer AG. The funder participated in study design, data collection, data analysis, data interpretation, and approval of the article. Analyses were conducted by the sponsor, and all authors had access to and participated in the interpretation of the data. Medical writing assistance was provided by Connie Lam, PhD, of Chameleon Communications International, and was funded by Bayer AG.
Disclosures
L.M. Ruilope has received consultancy fees from Bayer. R. Agarwal reports personal fees and nonfinancial support from Bayer Healthcare Pharmaceuticals, Inc during the conduct of the study; he also reports personal fees and nonfinancial support from Akebia Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Fresenius, Janssen, Relypsa, Inc, Sanofi, and Vifor Pharma; he has received personal fees from Ironwood Pharmaceuticals, Lexicon, Merck & Co., and Reata Pharmaceuticals, and nonfinancial support from E. R. Squibb & Sons, OPKO Health, Inc, and Otsuka America Pharmaceutical, Inc; he is a member of data safety monitoring committees for Amgen, AstraZeneca, and Celgene; a member of steering committees of randomized trials for Akebia Therapeutics, Bayer, Janssen, and Relypsa, Inc; a member of adjudication committees for AbbVie, Bayer, Boehringer Ingelheim, and Janssen; and he has received research grants from the US Veterans Administration and the National Institutes of Health. S.D. Anker has received research support from Abbott Vascular and Vifor Pharma, and personal fees from Abbott Vascular, Bayer, Boehringer Ingelheim, BRAHMS, Cardiac Dimensions, Impulse Dynamics, Novartis, Servier, and Vifor Pharma. G. Filippatos reports lectures fees and/or that he is a committee member of trials and registries sponsored by Amgen, Bayer, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor Pharma. He has received research support from the European Union. B. Pitt reports consultant fees for Ardelyx, AstraZeneca, Bayer, Boehringer Ingelheim, Brainstorm Medical, Cereno Scientific, G3 Pharmaceuticals, KBP Biosciences, PhaseBio, Sanofi/Lexicon, Sarfez Pharmaceutical, Inc, scPharmaceuticals, SQ Innovation, Tricida, and Vifor Pharma/Relypsa, Inc; he has stock options for Ardelyx, Brainstorm Medical, Cereno Scientific G3 Pharmaceuticals, KBP Biosciences, SQ Innovation, Sarfez Pharmaceutical, Inc., scPharmaceuticals, Tricida, and Vifor Pharma/Relypsa, Inc; he also holds a patent for site-specific delivery of eplerenone to the myocardium (US patent No. 9931412) and a provisional patent for histone-acetylation–modulating agents for the treatment and prevention of organ injury (provisional patent US 63/045,784). P. Rossing reports personal fees from Bayer during the conduct of the study; he has received research support and personal fees from AstraZeneca and Novo Nordisk, and personal fees from Astellas Pharma, Inc, Boehringer Ingelheim, Eli Lilly and Company, Gilead, Merck, Merck Sharp and Dohme, Mundipharma, Sanofi, and Vifor Pharma. All fees are given to Steno Diabetes Center Copenhagen. P. Sarafidis reports consultant and speaker fees from Amgen, AstraZeneca, Boehringer Ingelheim, Elpen Pharmaceuticals, Genesis Pharma, Innovis Pharma, Menarini, Sanofi, and Winmedica, and research support from AstraZeneca; he is a committee member of trials sponsored by Bayer. R.E. Schmieder has received grants, consultancy fees, and honoraria from Bayer. N. Mentenich and C. Nowack are full-time employees of Bayer AG, Division Pharmaceuticals, Germany. A. Joseph was a full-time employee of Bayer AG, Division Pharmaceuticals, Germany at the time of the studies and analysis; he is now a full-time employee of Chiesi Farmaceutici S.p.A, Parma, Italy. G.L. Bakris reports research funding, paid to the University of Chicago Medicine, from Bayer during the conduct of the study; he also reports research funding, paid to the University of Chicago Medicine, from Novo Nordisk and Vascular Dynamics; he acted as a consultant and received personal fees from Alnylam, Merck, and Relypsa, Inc; he is an Editor of American Journal of Nephrology, Nephrology, and Hypertension.
Supplemental Material
Tables S1–S3
Figures S1–S6
Supplementary Material
Nonstandard Abbreviations and Acronyms
- BP
- blood pressure
- CKD
- chronic kidney disease
- eGFR
- estimated glomerular filtration rate
- FIDELIO-DKD
- Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease
- FIGARO-DKD
- Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease
- HR
- hazard ratio
- LS
- least squares
- MRA
- mineralocorticoid receptor antagonist
- SBP
- systolic blood pressure
- T2D
- type 2 diabetes
- UACR
- urine-albumin-to-creatinine ratio
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.122.19744.
For Sources of Funding and Disclosures, see page 2694.
References
- 1.Ku E, Lee BJ, Wei J, Weir MR. Hypertension in CKD: core curriculum 2019. Am J Kidney Dis. 2019;74:120–131. doi: 10.1053/j.ajkd.2018.12.044 [DOI] [PubMed] [Google Scholar]
- 2.Van Buren PN, Toto R. Hypertension in diabetic nephropathy: epidemiology, mechanisms, and management. Adv Chronic Kidney Dis. 2011;18:28–41. doi: 10.1053/j.ackd.2010.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levey AS, Astor BC, Stevens LA, Coresh J. Chronic kidney disease, diabetes, and hypertension: what’s in a name?. Kidney Int. 2010;78:19–22. doi: 10.1038/ki.2010.115 [DOI] [PubMed] [Google Scholar]
- 4.Sarafidis PA, Ruilope LM. Aggressive blood pressure reduction and renin-angiotensin system blockade in chronic kidney disease: time for re-evaluation?. Kidney Int. 2014;85:536–546. doi: 10.1038/ki.2013.355 [DOI] [PubMed] [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99:S1–S87. doi: 10.1016/j.kint.2020.11.003 [DOI] [PubMed] [Google Scholar]
- 6.Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J., et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059–2068. doi: 10.1016/S0140-6736(15)00257-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calhoun DA, White WB. Effectiveness of the selective aldosterone blocker, eplerenone, in patients with resistant hypertension. J Am Soc Hypertens. 2008;2:462–468. doi: 10.1016/j.jash.2008.05.005 [DOI] [PubMed] [Google Scholar]
- 8.Ouzan J, Perault C, Lincoff AM, Carre E, Mertes M. The role of spironolactone in the treatment of patients with refractory hypertension. Am J Hypertens. 2002;15:333–339. doi: 10.1016/s0895-7061(01)02342-1 [DOI] [PubMed] [Google Scholar]
- 9.Alexandrou ME, Papagianni A, Tsapas A, Loutradis C, Boutou A, Piperidou A, Papadopoulou D, Ruilope L, Bakris G, Sarafidis P. Effects of mineralocorticoid receptor antagonists in proteinuric kidney disease: a systematic review and meta-analysis of randomized controlled trials. J Hypertens. 2019;37:2307–2324. doi: 10.1097/HJH.0000000000002187 [DOI] [PubMed] [Google Scholar]
- 10.Sakima A, Arima H, Matayoshi T, Ishida A, Ohya Y. Effect of mineralocorticoid receptor blockade on arterial stiffness and endothelial function: a meta-analysis of randomized trials. Hypertension. 2021;77:929–937. doi: 10.1161/HYPERTENSIONAHA.120.16397 [DOI] [PubMed] [Google Scholar]
- 11.Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A., et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219–2229. doi: 10.1056/NEJMoa2025845 [DOI] [PubMed] [Google Scholar]
- 12.Filippatos G, Anker SD, Agarwal R, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Schloemer P, Tornus I, Joseph A., et al. Finerenone and cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes. Circulation. 2021;143:540–552. doi: 10.1161/CIRCULATIONAHA.120.051898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Nowack C, Kolkhof P, Ferreira AC, Schloemer P, Filippatos G. Design and baseline characteristics of the Finerenone in reducing kidney failure and disease progression in diabetic kidney disease trial. Am J Nephrol. 2019;50:333–344. doi: 10.1159/000503713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6 [DOI] [PubMed] [Google Scholar]
- 15.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A., et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc [DOI] [PubMed] [Google Scholar]
- 16.Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, Nowack C, Kolkhof P, Kim SY, Zannad F. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34:2453–2463. doi: 10.1093/eurheartj/eht187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaisser F, Farman N. Emerging roles of the mineralocorticoid receptor in pathology: toward new paradigms in clinical pharmacology. Pharmacol Rev. 2016;68:49–75. doi: 10.1124/pr.115.011106 [DOI] [PubMed] [Google Scholar]
- 18.Agarwal R, Kolkhof P, Bakris G, Bauersachs J, Haller H, Wada T, Zannad F. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42:152–161. doi: 10.1093/eurheartj/ehaa736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolkhof P, Jaisser F, Kim SY, Filippatos G, Nowack C, Pitt B. Steroidal and novel non-steroidal mineralocorticoid receptor antagonists in heart failure and cardiorenal diseases: comparison at bench and bedside. Handb Exp Pharmacol. 2017;243:271–305. doi: 10.1007/164_2016_76 [DOI] [PubMed] [Google Scholar]
- 20.Barrera-Chimal J, Girerd S, Jaisser F. Mineralocorticoid receptor antagonists and kidney diseases: pathophysiological basis. Kidney Int. 2019;96:302–319. doi: 10.1016/j.kint.2019.02.030 [DOI] [PubMed] [Google Scholar]
- 21.Kolkhof P, Delbeck M, Kretschmer A, Steinke W, Hartmann E, Barfacker L, Eitner F, Albrecht-Kupper B, Schafer S. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64:69–78. doi: 10.1097/FJC.0000000000000091 [DOI] [PubMed] [Google Scholar]
- 22.Grune J, Beyhoff N, Smeir E, Chudek R, Blumrich A, Ban Z, Brix S, Betz IR, Schupp M, Foryst-Ludwig A., et al. Selective mineralocorticoid receptor cofactor modulation as molecular basis for finerenone’s antifibrotic activity. Hypertension. 2018;71:599–608. doi: 10.1161/HYPERTENSIONAHA.117.10360 [DOI] [PubMed] [Google Scholar]
- 23.Agarwal R, Andersen MJ. Correlates of systolic hypertension in patients with chronic kidney disease. Hypertension. 2005;46:514–520. doi: 10.1161/01.HYP.0000178102.85718.66 [DOI] [PubMed] [Google Scholar]
- 24.Fukuda M, Mizuno M, Kimura G. Nocturnal hypertension and chronic kidney disease. Curr Hypertens Rev. 2011;7:5–8. doi: 10.2174/157340211795909025 [Google Scholar]
- 25.Drawz PE, Alper AB, Anderson AH, Brecklin CS, Charleston J, Chen J, Deo R, Fischer MJ, He J, Hsu CY., et al. Masked hypertension and elevated nighttime blood pressure in CKD: prevalence and association with target organ damage. Clin J Am Soc Nephrol. 2016;11:642–652. doi: 10.2215/CJN.08530815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Triolo L, Cattaruzza MS, Sicoli R, Ansali F, Malaguti M, Osborn J, Biagini M. Blood pressure control and comorbidity in a nephrology clinic. J Nephrol. 2004;17:808–812. [PubMed] [Google Scholar]
- 27.Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, Joseph A, Kolkhof P, Nowack C, Schloemer P., et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385:2252–2263. doi: 10.1056/NEJMoa2110956 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.