Abstract
Background:
Acute SarsCov2 infection is associated with endothelial dysfunction and ‘endothelitis’, which might explain systemic microvascular impairment. The presence of endothelial damage may promote vasoconstriction with organ ischemia, inflammation, tissue oedema and a procoagulant state resulting in an increase in the incidence of cardiovascular and cerebrovascular events. Microvascular thrombosis has been demonstrated in postmortem autopsy of COVID-19 patients; however, few data are available about skin capillary alterations in these patients.
Materials and methods:
We evaluated skin microvascular alteration in 22 patients admitted to our hospital with SarsCov2 infection. Capillary density was evaluated by capillaroscopy in the nailfold and the dorsum of the finger in the acute phase of the disease. Capillaroscopy was repeated after 3 months (recovery phase). In addition, blood chemistry parameters and inflammatory markers were obtained during acute infection and at the recovery after 3 months.
Results:
Patients with COVID-19 showed skin microvascular complications, such as thrombosis, microhaemorrhages and neoangiogenesis, which were not detected after 3 months from the discharge. A significant reduction of capillary density in the dorsum was observed after 3 months from the acute infection (97.2 ± 5.3 vs. 75.81 ± 3.9 n/mm2P < 0.05). A significant inverse correlation between C-reactive protein and capillary density was observed in patients with acute SarsCov2 infection (r = 0.44, P < 0.05). Conversely a direct correlation between capillary density during the acute phase and lymphocyte number was detected (r = 0.49, P < 0.05).
Conclusion:
This is the first in-vivo evidence of skin capillary thrombosis, microhaemorrhages and angiogenesis in patients with acute SarsCov2 infection, which disappeared after 3 months, supporting the presence of endothelial dysfunction and inflammation. Capillary alterations might reflect systemic vascular effects of viral infection.
Keywords: capillary density, endothelial dysfunction, microcirculation, severe acute respiratory syndrome coronavirus 2 infection
INTRODUCTION
The ongoing pandemic emergency because of the rapid worldwide spread of severe acute respiratory syndrome coronavirus 2 (SarsCov2) infection has highlighted the need of an in-depth analysis of the pathogenic mechanisms determining the lung and extrapulmonary damage induced by the infection. Three distinct pathogenetic phases emerge from the clinical, blood chemistry and pathological data available to date: in the first phase a significant viral replication prevails, in the second phase lung damage related to the direct invasion of the bronchial mucosa and alveolar epithelium by the virus is present, while in the third phase an excessive inflammatory response of the host is prevalent and, in a high percentage of cases, it could determine the development of acute lung damage (acute lung injury, ALI) up to the most severe and often fatal picture of acute respiratory distress syndrome (ARDS).
Various studies have shown that patients with severe forms of coronavirus disease 2019 (COVID-19) have a high concentration of plasma cytokines, similar to the cytokine storm previously encountered in severe coronavirus infections (SARS) and Middle East respiratory syndrome (MERS) [1,2]. SarsCov2 infection can induce an ‘endothelitis’ in various organs both as a direct consequence of the viral invasion of the endothelium itself and following the inflammatory response of the host [3]. COVID-19 endothelitis could explain the microcirculatory functional impairment at the systemic level of different vascular districts and the related clinical consequences. The vascular endothelium is in fact a crucial organ for the regulation of vascular tone and the maintenance of vascular homeostasis [4]. The presence of endothelial damage can promote a greater vasoconstriction with consequent organ ischemia, inflammation, tissue oedema and a procoagulative state [5], resulting in an increase in the incidence of cardiovascular and cerebrovascular events. Thromboembolic complications have been reported during SarsCov2 infection by different groups [6–9], and these complications are associated to multiorgan failure and high mortality rate [10]. This could be particularly relevant in the presence of cardiovascular risk factors (cigarette smoking, high blood pressure, diabetes mellitus, obesity) and/or previous cardiovascular disease with preexisting endothelial dysfunction: all these risk factors are associated with unfavourable outcome in the patient suffering from COVID-19. Both pulmonary embolism and deep vein thrombosis are more frequent thromboembolic complications during COVID-19 disease [9]. Other macrovascular complications such as stroke, acute limb ischemia and acute coronary syndromes have been reported associated to SarsCov2 infection [11–13]. However, to current knowledge, very few data are available about microvascular alterations in that context, and most of them found microvascular thrombosis during autopsies as a complication of severe ARDS and platelet–fibrin thrombi as a common microscopic finding in the small pulmonary vasculature of lungs at necroscopy [14–17].
Microvascular alterations are very common in patients with cardiovascular diseases such as high blood pressure and diabetes mellitus. In particular, in these patients, an increase in the tunica media/internal lumen ratio (media–lumen ratio, MLR) is frequently observed in small resistance arteries, isolated from biopsies of the subcutaneous adipose tissue and evaluated with micromyography together with an increase in the vessel wall/internal lumen ratio (wall-to-lumen ratio, WLR) of the retinal arterioles evaluated with noninvasive methods (scanning laser–Doppler flowmetry or adaptive optics) [18–21]. Furthermore, it is well demonstrated that evaluation of imaging of retinal vessels by adaptive optics allows quantitative and qualitative microvascular phenotyping and their changes based on blood pressure levels, antihypertensive treatment, ageing and glycaemia [20,22–24] Another mechanism involved in the increase of vascular resistance is the reduction of microvascular density (rarefaction), which mainly affects the smaller vessels (less than 100 mm) such as arterioles and capillaries [25–27]. Rarefaction may be functional, when the microvessels are temporarily nonperfused or recruited, or anatomical, when the vessels are permanently absent. There is little information available about the mechanisms involved in the control of capillary density in the different vascular districts. Numerous studies have shown the presence of microvascular rarefaction in animal models [28]. In humans, several studies demonstrated a reduction of the number of capillaries in the skin of the dorsum of the finger of patients with essential hypertension [26,29]. It is not currently known whether capillary rarefaction may possess a prognostic significance per se, but there is evidence suggesting a correlation between capillary rarefaction and the MLR of subcutaneous small arteries [30]. Capillaroscopy is a reliable tool for the evaluation of capillary density in the cutaneous/subcutaneous vascular bed [26,27,31,32]. This noninvasive method permits to study morphological and functional characteristics of the distal microcirculation. In this study, we aim to evaluate the possible presence of skin capillary alterations or signs of microthrombosis with capillaroscopy, in patients with acute SarsCov2 infection and in the same patients after 3 months postdischarge.
PATIENTS AND METHODS
Study population
Patients admitted to the hospital in the months of March and April 2020 were eligible for the study if they had laboratory-confirmed SarsCov2 infection (see reference [31] for criteria adopted).
The protocol of the study was approved by the Ethics committee of our institution (Spedali Civili di Brescia, Medical School, University of Brescia), and a written informed consent was obtained from all patients. The procedures followed were in accordance with the declaration of Helsinki and the institutional guidelines.
Evaluation of vital and anthropometric parameters
In all patients the following parameters have been evaluated: clinical blood pressure, heart rate, oxyhaemoglobin saturation, weight, height and BMI.
The clinical blood pressure was measured during hospitalization and after 3 months as a follow-up visit with an automatic oscillometric device after 5 min of rest in a quiet environment; three measurements will be taken on the same arm and the average of the three values obtained will be considered.
Data were collected during all recoveries, including major comorbidities, previous therapy and current treatment. Data collected during acute infection after 2 days to admission included amount of respiratory support, markers of inflammation or progression of COVID-19, including C-reactive protein (CRP), lymphocytes count, serum glycemia, troponin T, ferritin, creatinine and D-dimer.
Assessment of capillary density
Skin capillary density was assessed by capillaroscopy, as described elsewhere [26,29]. Briefly, capillaroscopy was performed in a quiet and temperature-controlled room (21–22 °C) bedside after 2 days to admission and at recovery after 3 months plus 5–7 days. Basal capillary density (BCD) from the nailfold at the level of the first phalanx (basal periungueal capillaries:) and at the dorsum of the fourth finger (basal dorsal capillaries) of the nondominant hand were visualized using an epi-illuminated microscope containing a 100 W mercury vapor lamp light source, and pictures (final magnification of 200×) were obtained by video-microscopy (Videocap 3.0 D1 200; DS Medica, Milan, Italy) in baseline conditions (BCD). Capillary density was defined as the number of capillaries per square millimetres of the microscopic field and was counted by hand. The first row of the nailfold capillaries was considered. Capillary density was calculated by two independent operators, and the two findings were averaged.
The microvascular abnormalities such as thrombosis, microhaemorrhages and neoangiogenesis were detected in the nailfold network.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). Statistical significance within the group of patients and between times was assessed by Student's paired t-test. In order to be most conservative, capillary density between acute phase and recovery was also compared by Wilcoxon nonparametric test for paired data.
Univariate correlations were evaluated considering the Pearson coefficient. A P value less than 0.05 was considered as statistically significant. All analyses were performed using IBM SPSS software (version 23; IBM, Armonk, New York, USA) and MedCalc for Windows, version 15.0 (Medcalc Software, Ostend, Belgium).
RESULTS
Patients
The characteristics and laboratory parameters during acute infection are summarized in Tables 1 and 2. Twenty-two patients (14 men and 8 women) were evaluated: average age was 65 ± 2 years, BMI 25 ± 3 kg/m2. Twelve patients were hypertensive (54.5%), 6 were diabetic patients (27%), 4 were dyslipidaemic (18%) and 3 with cerebrovascular or cardiovascular disease history (13,6%). Seven patients were treated with intravenous steroids (31.8%), three with tocilizumab (13.6%) and 7 were treated with no invasive ventilation (31.8%). During the hospitalization, all patients presented SarsCov2-associated interstitial pneumonia, whereas vascular complications (ischemic stroke or pulmonary embolism) were observed in three patients (13.6%). One patient died before follow-up visit. Median CRP was 62.55 mg/dl, median lymphocyte count was 0,72 × 103/mm3, median ferritin was 1026.50 ug/l and median D-dimer was 447.5 mg/dl.
TABLE 1.
Clinical characteristics and laboratory parameters during acute infection
| Mean | |
| Age (years) | 64.99 ± 12 |
| BMI (kg/m2) | 25.81 ± 3.22 |
| SBP (mmHg) | 133.36 ± 20 |
| DBP (mmHg) | 77.45 ± 11.11 |
| Heart rate | 89.91 ± 11.73 |
| WBC (×103/mm3) | 10.32 ± 12.15 |
| Hb (g/dl) | 12.45 ± 1.55 |
| Platelets (×103/mm3) | 271.95 ± 118.91 |
| Neutrophils (×103/mm3) | 6.23 ± 4.97 |
| Lymphocytes (×103/mm3) | 3.44 ± 1.64 |
| Monocyte (×103/mm3) | 0.61 ± 0.61 |
| CRP (mg/dl) | 91.12 ± 84.39 |
| Glucose (mg/dl) | 125.00 ± 46.43 |
| Creatinine (mg/dl) | 0.95 ± 0.32 |
| Natrium (mmol/l) | 140.27 ± 3.6 |
| Kalium (mmol/l) | 3.74 ± 0.4 |
| Total calcium (mg/dl) | 8.70 ± 0.46 |
| Triglycerides (mg/dl) | 131.14 ± 43.99 |
| Total bilirubin (mg/dl) | 0.53 ± 0.28 |
| AST (U/l) | 53.59 ± 38.78 |
| ALT (U/l) | 57.55 ± 74.72 |
| GGT (U/l) | 79.48 ± 8.98 |
| LDH (U/l) | 355.14 ± 133.85 |
| CK (U/l) | 132.23 ± 126.93 |
| Troponin T (ng/ml) | 15.52 ± 14.7 |
| PT (INR) | 1.05 ± 0.11 |
| aPTT (s) | 30.32 ± 3.33 |
| Fibrinogen (mg/dl) | 532.05 ± 179.52 |
| D-Dimer (g/dl) | 1146.14 ± 1540.01 |
| Albumin (g/l) | 30.18 ± 6.69 |
| Circulating proteins (g/l) | 60.56 ± 4.81 |
| Ferritin (ug/l) | 1306.05 ± 1219.59 |
| Maximum ferritin (μg/l) | 1762.00 ± 1901.67 |
| Maximum CRP (mg/l) | 116.85 ± 113.62 |
| Minimum lymphocytes (x103/mm3) | 1.73 ± 4.41 |
| Maximun D-dimer (g/dl) | 1795.77 ± 2027.11 |
| Brixia score | 7.7 ± 2.2 |
ALT, alanine aminotransferase; aPTT, activated partial thromboplastin time; AST, aspartate transaminase; Brixia score, chest X-ray-scoring system; CK, total, creatine kinase; CRP, C-reactive protein; GGT, gamma-glutamyl transferase; Hb, hemoglobin; INR, international normalized ratio; LDH, lactate dehydrogenase; PT, prothrombin time; WBC, white blood cells.
TABLE 2.
Demographic characteristics and treatments during acute infection
| N (/22) | Percentage | |
| Female sex | 8 | 36.40 |
| Hypertension | 12 | 54.50 |
| Diabetes mellitus | 6 | 27.30 |
| Dyslipidemia | 4 | 18.20 |
| Ischemic heart disease | 1 | 4.50 |
| Obesity | 3 | 13.60 |
| Drug treatment | ||
| ACEi | 4 | 18.20 |
| ARB | 4 | 18.20 |
| Beta-blockers | 3 | 13.60 |
| CCB | 4 | 18.20 |
| Diuretics | 3 | 13.60 |
| Alfa-blockers | 1 | 4.50 |
| Thyroid hormone | 2 | 9.10 |
| PPI | 3 | 13.60 |
| ASA | 4 | 18.20 |
| Statin | 3 | 13.60 |
| Smoking (active/former) | 9 | 40.90 |
| noninvasive mechanical ventilation (NIMV) | 8 | 36.40 |
| Thrombotic events (i.e. stroke, PE) | 6 | 27.30 |
| Antiviral drug | 11 | 50.00 |
| Hydroxychloroquine | 22 | 100.00 |
| Steroids | 14 | 63.60 |
ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; ASA, acetylsalicylic acid; CCB, calcium channel blocker; PE, pulmonary embolism; PPI, proton pump inhibitors.
Capillary density
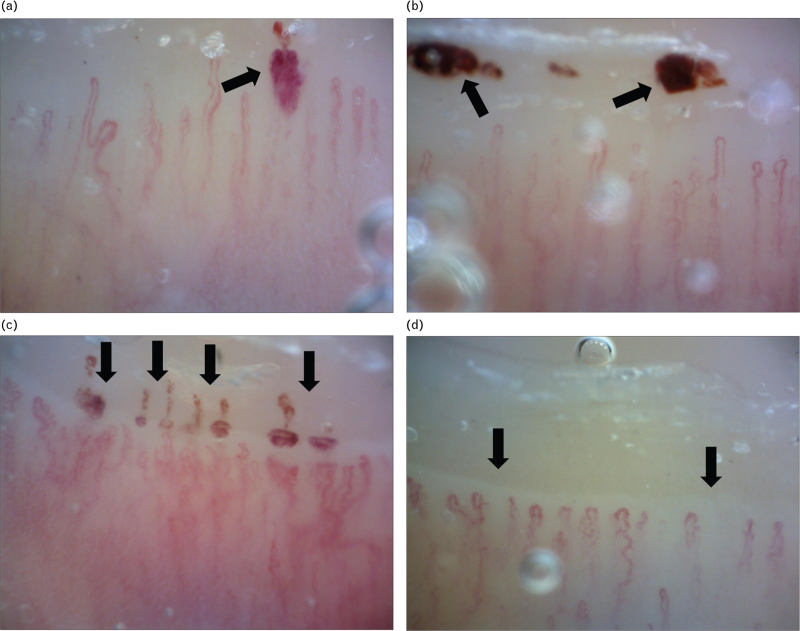
A significant reduction of BCD in the dorsum of the finger was observed after 3 months from the acute infection (Student's paired t test: P = 0.0039, Wilcoxon test: P = 0.009) (Fig. 1). Interestingly enough, patients with COVID-19 showed in the nailfold capillaries, skin microvascular complications such as thrombosis (32%), microhaemorrhages (36.36%) and neoangiogenesis (27.27%), which were not detected at the follow-up visit, after 3 months from the discharge (Fig. 2).
FIGURE 1.
Dorsal capillary density during acute severe acute respiratory syndrome coronavirus 2 infection (acute infection) and after 3 months from discharge (recovery). Student's paired t test: P = 0.0039, Wilcoxon test: P = 0.009.
FIGURE 2.
Nailfold capillaroscopy abnormalities found in patient with acute coronavirus disease 2019 (a–c arrows) and after 3 months from acute infection (d) (200× magnification) (arrow). (a) Recent capillary microbleeding with an irregularly enlarged loop in a patient with SarsCov2 acute infection. (b) Microbleeding of the cuticle, which suggests previous occlusion of capillaries (capillary thrombosis) or a solution of continuity of the vascular wall (capillary haemorrhage). (c) Capillaroscopic pattern indicative of previous and synchronous episode of multiple capillary thrombosis. The alignment of the microbleeding on the cuticle is a typical pattern of capillary thrombosis. Elongated and tortuous capillaries are signs of vascular neoformation. (d) Morphological and architectural derangement with capillary loss, irregularly enlarged loops and neoformation of capillaries. A vascular area in the right-hand part of the image is present, where it is possible to see the deep microvascular network.
Correlation between capillary density and marker of inflammation/severe acute respiratory syndrome coronavirus 2 infections
A significant inverse correlation between Max CRP and BCD was observed in patients with acute SarsCov2 infection (r = 0.44, P = 0.04) (Fig. 3a). A direct correlation between BCD and lymphocyte count during recovery was detected (r = 0.49, P = 0.03) (Fig. 3b). No significant correlation was observed between dorsal BCD and D-dimer levels. A significant inverse relation, although not a close one, was detected between dorsal BCD and max ferritin median levels (P = 0.043) (Fig. 4).
FIGURE 3.
(a) Inverse correlation between max C-reactive protein level and basal dorsal capillary. (b) Direct correlation between basal capillary density and lymphocyte count. CRP, C-reactive protein.
FIGURE 4.
Relationship between dorsal basal capillary density (capillary density during the acute phase) and max ferritin (P = 0.043).
Correlation between capillary density and population features
Taking into account dorsal BCD, a significant correlation was observed with microvascular complications such as thrombosis, microhaemorrhages and neoangiogenesis (r = 0.48, P = 0.03). No significant correlations were detected between dorsal BCD or periungueal BCD and thrombotic events (such as stroke, pulmonary embolism), presence of diabetes, use of antagonist of renin–angiotensin system, smoking habits, age and BMI (data not shown).
No other statistically significant correlation between capillary density and other parameters reported in Table 1 (clinical characteristics or laboratory parameters during acute infection) was observed.
DISCUSSION
This is the first in-vivo evidence of skin microvascular complications in patients with acute SarsCov2 infection and their disappearance after 3 months of discharge; this supports the presence of endothelial dysfunction, inflammation and thrombosis during COVID-19 disease. Recently, the presence of microvascular alterations evaluated by nailfold-video capillaroscopy has been demonstrated in COVID-19 patients; the alterations observed were the following: pericapillary oedema, enlarged capillaries, sludge flow, meandering capillaries and also a higher prevalence of hemosiderin deposits as a result of microhaemorrhages and microthrombosis, sludge flow and pericapillary oedema [32]. However, in this study, the authors enrolled 82 patients of whom 28 during the hospitalization and 54 after recovery and hospital discharge, thus they evaluated two different populations, while in our study we examined the same patients during the acute phase of SarsCov2 infection and after 3 months of discharge.
Capillary alterations may reflect systemic vascular effects of viral infection. Surprisingly, a reduction of BCD was observed after 3 months from acute infection probably because of acute inflammation and hypoxia occurred during acute SarsCov2 infection, which might have induced both vasodilation and angiogenesis.
Interestingly enough, we observed, in COVID-19 patients, relevant skin microvascular complications such as microthrombosis, microhaemorrhages and neoangiogenesis, which disappeared at the follow-up visit. It is well known that the innate immune response and thrombotic state are closely linked. The cytokine storm caused by SarsCov2 infection consists in an excessive and uncontrolled release of pro-inflammatory cytokines, a condition that can be found not only in the course of infectious but also rheumatological pathologies and during anticancer immunotherapy. This process is triggered at the level of the organ primarily affected (in the case of COVID-19 disease, the lung) but extends rapidly to the whole organism by introducing inflammatory mediators into the bloodstream, thus generating a systemic inflammatory condition that configures the clinical picture of multiorgan failure. Huang et al. measured plasma cytokines in 41 patients hospitalized for COVID-19 at the dedicated Wuhan hospital (13 of them were admitted to an ICU while 28 patients were hospitalized in other wards) observing high concentrations of interleukin (IL)-1B, IL-6, IL-1RA, IL-7, IL-8, IL-9, IL-10, fibroblast growth factor (FGF), macrophage and granulocyte colony-stimulating factor (GM-CSF), interferon (IFN)γ, granulocyte colony-stimulating factor (G-CSF), interferon-γ inducible protein (IP10), monocyte chemotactic protein (MCP1), inflammatory macrophage protein 1 alpha (MIP1A), platelet-derived growth factor (PDGF), tumour necrosis factor alpha (TNFα) and vascular endothelial growth factor (VEGF) [33]. In a multicentre retrospective cohort study conducted by Zhou et al.[34], an increased circulating IL-6 levels was observed in the sample of patients who died with COVID-19 compared with that of survivors; the same finding emerged in patients suffering from severe forms of COVID-19 compared with patients with mild symptoms [35]. All these biomarkers and acute-phase reactants are associated with adverse clinical outcomes, increased severity of infection and may contribute to COVID-19-associated hypercoagulability state [36–38].
In our study, microthrombosis and microhaemorrhages were seen in patients with hypertension suggesting that COVID-19-associated endothelial dysfunction may worsen preexisting hypertension-linked microvascular alterations. In these patients, the extent of inflammation, in terms of circulating CRP and ferritin levels, was mild, and no thrombocytopenia was present. One patient that showed very pronounced microthrombosis was also affected by rheumatoid arthritis and chronic cerebral ischaemia. No association between microthrombosis and severe hypoxia was observed; in fact only two patients with evidence of microthrombosis were treated with no invasive ventilation, suggesting that capillary alterations are present also in patients with mild symptoms of COVID-19. Two patients with microthrombosis had also systemic thrombotic complications such as acute cerebral ischemia and pulmonary embolism. The presence of microthrombosis and microhaemorrhages is not surprising as it is consistent with previous findings of diffuse microvascular thrombosis in the lungs of COVID-19 patients at postmortem examinations [16,17].
The disappearance of capillary alterations after 3 months from the discharge is of clinical interest, suggesting an acute and reversible effect of hypoxia and inflammation. In addition to microthrombosis and microhaemorrhages, neoangiogenesis was observed in four patients with elevated inflammatory profile and thrombocytosis. In particular, vascular angiogenesis seems to be a distinctive feature of SarsCov 2 infection. A recent autoptic study has demonstrated that, in the lung of patients who died from COVID-19, the amount of vessel growth was 2.7 times higher than that in the lungs from patients who died from ARDS secondary to a flu-syndrome [39] with unexpected intussusceptive angiogenesis. It was also recently demonstrated that higher VEGF levels were present in COVID-19 patients compared with healthy controls [33]. The anti-VEGF drug bevacizumab, added to standard therapy, was demonstrated to be beneficial for patients with severe COVID-19, improving oxygen-support in 92% of patients during a 28-day follow-up, shortening the duration of oxygen therapy and reducing mortality to nil compared with the control cohort [40]. In this trial, in addition to an improvement of the respiratory pattern, a rapid disappearance of fever, an increase of peripheral blood lymphocyte counts and a more than 12-fold decrease of CRP levels were observed in a bevacizumab-treated patient, suggesting that VEGF plays a crucial role in SarsCov2 infection-associated acute inflammation. On the other hand, VEGF blockade with consequent NO inhibition could result in vascular abnormalities. Mourad et al.[41] in a study of 2007, showed that bevacizumab treatment resulted in endothelial dysfunction and capillary rarefaction with consequent rise in blood pressure levels.
SarsCov2 infects target cells by interacting with the converting enzyme of angiotensin 2 (ACE2), which is expressed in various organs, including the lung, the heart, the kidney and the gut. ACE2 receptors are also expressed in endothelial cells [42,43] within which the presence of viral included bodies has been shown. This may determine the accumulation of inflammatory cells and the recruitment of immune cells, both through direct viral invasion of the endothelium and through an immune-mediated mechanism, promoting widespread endothelial dysfunction associated to apoptosis.
In fact, another interesting finding in the blood of COVID-19 patients is a reduction in circulating lymphocytes [44,45], with an increase in total leukocytes and in the neutrophil-to-lymphocyte ratio. In our study, all patients showed the presence of lymphocytopenia, no more present at follow-up and a positive correlation between BCD and lymphocyte count during recovery was detected.
Another interesting aspect of our study is the observation of a significant reduction of BCD in the dorsum of the finger after 3 months from the acute infection. In this capillary district, the absolute number of perfused vessels contributes to the total vascular resistance. Vascular rarefaction is defined either as a functional rarefaction (when vessels are temporarily not perfused) or anatomical rarefaction (when vessels are permanently absent) [46,47]. In our study, we did not observe a real rarefaction because we measured only BCD and not total capillary density. The BCD reduction seen in the postacute viral infection state could be the result of an increase of capillary recruitment as a consequence of neoangiogenesis, inflammation and hypoxia during acute viral phase. As mentioned, it is well accepted that a low inflammation condition associated to hypertension is associated to capillary rarefaction [26,28,29,30]. It also has been demonstrated that, in chronic and systemic inflammatory conditions, such as in rheumatoid arthritis, microvascular and macrovascular damage is present [48–50]. COVID-19 represents a model of acute and pronounced inflammation with consequent microvascular complication, such as transient thrombosis and neoangiogenesis. Recently, the role of effects of COVID-19-related capillary damage and preexisting microvascular alterations has been analysed; the presence of a vicious cycle was postulated as SarsCov2 infection and consequent inflammation and hypoxia could determine capillary dysfunction, which, in turn, could accelerate hypoxia-related inflammation and tissue damage [51].
The crucial role of microcirculation on integrity of tissue and organ function is well evidenced by Levy et al.[52] in their review of 2008. In this study, they demonstrated that impaired tissue perfusion because of abnormality of the microvascular system is a common hallmark among cardiovascular risk factors and diseases. In our study, we postulated that microvascular changes, in particular capillary alteration could represent a mechanism underlying COVID-19 complications.
In our study, an inverse relationship between dorsal capillary density and circulating CRP or ferritin was observed, whereas a direct relationship was observed with circulating lymphocyte count. There is no clear explanation for such an observation; however, it is possible that angiogenesis, with increased number of capillaries may occur during COVID-19, but the extent of the angiogenetic process is more pronounced in those patients with a less severe disease (less inflammation, more circulating lymphocytes), although this should be confirmed in larger populations.
However, there are some important limitations of the present study that are to be taken into account. First of all, the sample size is relatively small: the critical clinical situation that was faced in that period limited the chances of clinical research, in terms of number of patients that could be enrolled or preventing us from the possibility to perform venous congestion during the capillaroscopic investigation and, thus, to measure total capillary density. Second, we could not measure BCD before the viral infection, and finally, there is a dispersion of some of the data related to thrombosis and inflammation that may have influenced results.
In conclusion, this is the first in-vivo evidence of skin capillary thrombosis, microhaemorrhages and angiogenesis in patients with acute SarsCov2 infection, which disappeared after 3 months from acute infection, supporting the presence of potentially reversible endothelial dysfunction and inflammation. Capillary alteration might reflect systemic vascular effects of viral infection. Whether SarsCov2-associated microvascular alterations and/or related complications remain long-term is unclear. In this study, a reduction in BCD was observed after 3 months after acute viral infection, although major capillary complications, such as thrombosis or neoangiogenesis were no more present.
Therefore, further studies are needed in order to confirm these preliminary evidences also after a longer follow-up. Video-capillaroscopy could be a useful, noninvasive method in order to detect early signs of microthrombosis, microhaemorrhages and other capillary alterations that may represent a relevant therapeutic target, with the aim of protecting capillary function and stimulating capillary repair.
ACKNOWLEDGEMENTS
Conflicts of interest
There are no conflicts of interest.
Footnotes
Abbreviations: ACE2, angiotensin-converting enzyme 2; ALI, acute lung injury; ARDS, acute respiratory distress syndrome; BCD, basal capillaries’ density; BPC, basal periungueal capillaries; COVID-19, corona virus disease-19; CRP, C-reactive protein; FGF, fibroblast growth factor; G-CSF, granulocyte colony-stimulating factor; GM-CSF, macrophage and granulocyte colony-stimulating factor; IFNγ, interferon γ; IL, interleukin; IP10, interferon-γ-inducible protein; MCP1, monocyte chemotactic protein; MIP1-A, inflammatory macrophage protein 1 alpha; MLR, media–internal lumen ratio; NK, lymphocyte natural killer; PDGF, platelet-derived growth factor; SarsCov2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; Th17, lymphocyte T helper 17; TNFα, tumour necrosis factor alpha; VEGF, vascular endothelial growth factor; WLR, wall–internal lumen ratio
REFERENCES
- 1.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 2017; 39:529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol 2017; 39:517–528. [DOI] [PubMed] [Google Scholar]
- 3.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endothelitis in COVID-19. Lancet 2020; 395:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flammer AJ, Anderson T, Celermajer DS, et al. The assessment of endothelial function: from research into clinical practice. Circulation 2012; 126:753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction - a marker of atherosclerotic risk. Arterioscl Throm Vas 2003; 23:168–175. [DOI] [PubMed] [Google Scholar]
- 6.Wichmann D. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med 2020; 173:1030. [DOI] [PubMed] [Google Scholar]
- 7.Xu X, Chang XN, Pan HX, Su H, Huang B, Yang M, et al. Pathological changes of the spleen in ten patients with coronavirus disease 2019 (COVID-19) by postmortem needle autopsy. Zhonghua Bing Li Xue Za Zhi 2020; 49:576–582. [DOI] [PubMed] [Google Scholar]
- 8.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res 2020; 191:148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanff TC, Mohareb AM, Giri J, Cohen JB, Chirinos JA. Thrombosis in COVID-19. Am J Hematol 2020; 95:1578–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McFadyen JD, Stevens H, Peter K. The emerging threat of (micro)thrombosis in COVID-19 and its therapeutic implications. Circ Res 2020; 127:571–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-vessel stroke as a presenting feature of covid-19 in the young. N Engl J Med 2020; 382:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh B, Aly R, Kaur P, Gupta S, Vasudev R, Virk HS, et al. COVID-19 infection and arterial thrombosis: report of three cases. Ann Vasc Surg 2020; 395:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choudry FA, Hamshere SM, Rathod KS, Akhtar MM, Archbold RA, Guttmann OP, et al. High thrombus burden in patients with COVID-19 presenting with ST-segment elevation myocardial infarction. J Am Coll Cardiol 2020; 76:1168–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang ZW, Zhang LJ, Zhang SJ, Meng X, Li JQ, Song CZ, et al. A clinicopathological study of three cases of severe acute respiratory syndrome (SARS). Pathology 2003; 35:526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med 2020; 8:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, et al. Pulmonary postmortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis 2020; 20:P1135–P1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, da Silva LFF, de Oliveira EP, Saldiva PHN, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost 2020; 18:1517–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Ciuceis C, Agabiti Rosei C, Caletti S, Trapletti V, Coschignano MA, Tiberio GAM, et al. Comparison between invasive and noninvasive techniques of evaluation of microvascular structural alterations. J Hypertens 2018; 36:1154–1163. [DOI] [PubMed] [Google Scholar]
- 19.Rizzoni D, Agabiti Rosei C, De Ciuceis C, Semeraro F, Rizzoni M, Docchio F. New methods to study the microcirculation. Am J Hypertens 2018; 31:265–273. [DOI] [PubMed] [Google Scholar]
- 20.Koch E, Rosenbaum D, Brolly A, Sahel JA, Chaumet-Riffaud F, Girerd X, et al. Morphometric analysis of small arteries in the human retina using adaptive optics imaging: relationship with blood pressure and focal vascular changes. J Hypertens 2014; 32:890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenbaum D, Kachenoura N, Koch E, Paques M, Cluzel P, Redheuil A, et al. Relationships between retinal arteriole anatomy and aortic geometry and function and peripheral resistance in hypertensives. Hypertens Res 2016; 39:536–542. [DOI] [PubMed] [Google Scholar]
- 22.Gallo A, Dietenbeck T, Giron A, Paques M, Kachenoura N, Girerd X. Noninvasive evaluation of retinal vascular remodeling and hypertrophy in humans: intricate effect of ageing, blood pressure and glycaemia. Clin Res Cardiol 2021; 110:959–970. [DOI] [PubMed] [Google Scholar]
- 23.Rosenbaum D, Mattina A, Koch E, Rossant F, Gallo A, Kachenoura N, et al. Effects of age, blood pressure and antihypertensive treatments on retinal arterioles remodeling assessed by adaptive optics. J Hypertens 2016; 34:1115–1122. [DOI] [PubMed] [Google Scholar]
- 24.De Ciuceis C, Salvetti M, Paini A, Rossini C, Muiesan ML, Duse S, et al. Comparison of lercanidipine plus hydrochlorothiazide vs. lercanidipine plus enalapril on micro and macrocirculation in patients with mild essential hypertension. Intern Emerg Med 2017; 12:963–974. [DOI] [PubMed] [Google Scholar]
- 25.Bohlen HG. Localization of vascular resistance changes during hypertension. Hypertension 1986; 8:181–183. [DOI] [PubMed] [Google Scholar]
- 26.Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Structural skin capillary rarefaction in essential hypertension. Hypertension 1999; 33:998–1001. [DOI] [PubMed] [Google Scholar]
- 27.Debbabi H, Uzan L, Mourad JJ, Safar M, Levy B, Tibiriçà E. Increased skin capillary density in treated essential hypertensive patients. Am J Hypertens 2006; 19:477–483. [DOI] [PubMed] [Google Scholar]
- 28.Schiffrin EL. Reactivity of small blood vessels in hypertension: relation with structural changes. State of the art lecture. Hypertension 1992; 19: (2 Suppl): II1–II9. [DOI] [PubMed] [Google Scholar]
- 29.Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in borderline essential hypertension suggests an early structural abnormality. Hypertension 1999; 34 (4 Pt 1):655–658. [DOI] [PubMed] [Google Scholar]
- 30.Paiardi S, Rodella LF, De Ciuceis C, Porteri E, Boari GEM, Rezzani R, et al. Immunohistochemical evaluation of microvascular rarefaction in hypertensive humans and in spontaneously hypertensive rats. Clin Hemorheol Microcirc 2009; 42:259–268. [DOI] [PubMed] [Google Scholar]
- 31.Boari GEM, Chiarini G, Bonetti S, Malerba P, Bianco G, Faustini C, et al. Prognostic factors and predictors of outcome in patients with COVID-19 and related pneumonia: a retrospective cohort study. Biosci Rep 2020; 40:BSR20203455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Natalello G, De Luca G, Gigante L, Campochiaro C, De Lorenzisa E, Verardi L, et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: Broadening the spectrum of COVID-19 microvascular involvement. Microvasc Res 2021; 133:104071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). MedRxiv 2020; 2020.02.10.20021832. [Google Scholar]
- 36.Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol 2020; 127:104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maier CL, Truong AD, Auld SC, Polly DM, Tanksley CL, Duncan A. COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? Lancet 2020; 395:1758–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell. Cell Host Microbe 2020; 27:992.e3–1000.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020; 383:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pang J, Xu F, Aondio G, Li Y, Fumagalli A, Lu M, et al. Efficacy and tolerability of bevacizumab in patients with severe Covid-19. Clinical Trial Nat Commun 2021; 12:814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mourad JJ, Des Guetz G, Debbabi H, Levy BI. Blood pressure rise following angiogenesis inhibition by bevacizumab. A crucial role for microcirculation. Ann Oncol 2008; 19:927–934. [DOI] [PubMed] [Google Scholar]
- 42.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005; 111:2605–2610. [DOI] [PubMed] [Google Scholar]
- 43.Monteil VKH, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, Leopoldi A, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 2020; 181:905.e7–913.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, et al. Coronavirus infections and immune responses. J Med Virol 2020; 92:424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Health Commission of the People's Republic of China, The Diagnosis and treatment protocol for novel coronavirus Pneumonia (Trial Version 7). Chin Med J (Engl) 2020; 133:1087–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agabiti-Rosei E, Rizzoni D. Microvascular structure as a prognostically relevant endpoint. J Hypertens 2017; 35:914–921. [DOI] [PubMed] [Google Scholar]
- 47.Virdis A, Savoia C, Grassi G, Lembo G, Vecchione C, Seravalle G, et al. Evaluation of microvascular structure in humans: a 'state-of-the-art’ document of the Working Group on Macrovascular and Microvascular Alterations of the Italian Society of Arterial Hypertension. J Hypertens 2014; 32:2120–2129. [DOI] [PubMed] [Google Scholar]
- 48.Dimitroulas T, Hodson J, Sandoo A, Smith J, Kitas GD. Endothelial injury in rheumatoid arthritis: a crosstalk between dimethylargi-nines and systemic inflammation. Arthritis Res Ther 2017; 19:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foster W, Carruthers D, Lip GYH, Blann AD. Inflammation and microvascular and macrovascular endothelial dysfunction in rheumatoid arthritis: effect of treatment. J Rheumatol 2010; 37:711–716. [DOI] [PubMed] [Google Scholar]
- 50.Anyfanti P, Gkaliagkousi E, Triantafyllou A, Zabulis X, Dolgyras P, Galanopoulou V, et al. Dermal capillary rarefaction as a marker of microvascular damage in patients with rheumatoid arthritis: association with inflammation and disorders of the macrocirculation. Microcirculation 2018; 25:e12451. [DOI] [PubMed] [Google Scholar]
- 51.Østergaard L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol Rep 2021; 9:e14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, Struijker-Boudier HA. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation 2008; 118:968–976. [DOI] [PubMed] [Google Scholar]