Background:
Morbidity from undiagnosed atrial fibrillation (AF) may be preventable with early detection. Many consumer wearables contain optical photoplethysmography (PPG) sensors to measure pulse rate. PPG-based software algorithms that detect irregular heart rhythms may identify undiagnosed AF in large populations using wearables, but minimizing false-positive detections is essential.
Methods:
We performed a prospective remote clinical trial to examine a novel PPG-based algorithm for detecting undiagnosed AF from a range of wrist-worn devices. Adults aged ≥22 years in the United States without AF, using compatible wearable Fitbit devices and Android or iOS smartphones, were included. PPG data were analyzed using a novel algorithm that examines overlapping 5-minute pulse windows (tachograms). Eligible participants with an irregular heart rhythm detection (IHRD), defined as 11 consecutive irregular tachograms, were invited to schedule a telehealth visit and were mailed a 1-week ambulatory ECG patch monitor. The primary outcome was the positive predictive value of the first IHRD during ECG patch monitoring for concurrent AF.
Results:
A total of 455 699 participants enrolled (median age 47 years, 71% female, 73% White) between May 6 and October 1, 2020. IHRDs occurred for 4728 (1%) participants, and 2070 (4%) participants aged ≥65 years during a median of 122 (interquartile range, 110–134) days at risk for an IHRD. Among 1057 participants with an IHRD notification and subsequent analyzable ECG patch monitor, AF was present in 340 (32.2%). Of the 225 participants with another IHRD during ECG patch monitoring, 221 had concurrent AF on the ECG and 4 did not, resulting in an IHRD positive predictive value of 98.2% (95% CI, 95.5%–99.5%). For participants aged ≥65 years, the IHRD positive predictive value was 97.0% (95% CI, 91.4%–99.4%).
Conclusions:
A novel PPG software algorithm for wearable Fitbit devices exhibited a high positive predictive value for concurrent AF and identified participants likely to have AF on subsequent ECG patch monitoring. Wearable devices may facilitate identifying individuals with undiagnosed AF.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT04380415.
Keywords: atrial fibrillation, diagnostic screening programs, digital technology, photoplethysmography, wearable electronic devices
Clinical Perspective.
What Is New?
A novel photoplethysmography algorithm for wearable devices requires at least 30 continuous minutes of an irregular rhythm to generate an irregular heart rhythm detection during periods of inactivity (to minimize motion artifact).
The novel algorithm can enable large-scale identification of undiagnosed atrial fibrillation with a high positive predictive value during periods of inactivity.
What Are the Clinical Implications?
The Fitbit wearable-based irregular heart rhythm algorithm may be useful for early detection of undiagnosed atrial fibrillation.
Individuals with a Fitbit wearable-based irregular heart rhythm detection have a substantial likelihood of having atrial fibrillation confirmed on a subsequent ECG patch monitor and considerable burden of atrial fibrillation.
Because wearable-based irregular heart rhythm detections using photoplethysmography sensors operate during periods of inactivity, wearing devices at night may maximize the sensitivity.
Detection of atrial fibrillation during periods of active motion remains a challenge.
Atrial fibrillation (AF) and flutter are associated with increased risks of stroke, heart failure, and other adverse outcomes.1 Strokes caused by AF are preventable with thromboembolism prophylaxis.2 Lifestyle interventions targeting physical activity, alcohol cessation, and weight loss can also minimize morbidity from AF.3,4 Because AF can be paroxysmal and asymptomatic, wearable devices may help identify individuals with undiagnosed AF.
Newer smartwatch and fitness tracker models typically include an optical photoplethysmography (PPG) sensor to measure pulse rate. Algorithms can analyze for pulse irregularity from the PPG data and infer the presence of AF. Optimizing these algorithms for use in consumer wearable devices at scale is critical, because false positives might lead to anxiety, increased use of health care resources, and treatment side effects.
The reported clinical trial and regulatory metric for evaluating algorithm performance has been the positive predictive value (PPV) for AF during simultaneous ambulatory electrocardiographic monitoring in 2 previous large-scale validation studies of wrist-worn wearable device–based algorithms that relied on intermittent PPG data.5,6 For example, the Apple Heart Study included >400 000 Apple Watch users and found that, among those receiving an irregular rhythm notification, 34% had AF >30 seconds identified on subsequent ECG patch monitoring with an 84% PPV of the algorithm for concurrent AF on the ECG patch. The algorithm was subsequently cleared in the United States and received a CE mark in Europe for over-the-counter use.7,8
We designed a novel algorithm to use more frequent sampling of PPG data and stricter criteria for irregular rhythm detection. We conducted a large-scale single-arm remote clinical trial to test whether the algorithm would achieve a high PPV for detecting undiagnosed AF in users of an array of wrist-worn smartwatches and fitness trackers from a single manufacturer. We additionally assessed the probability of a confirmed diagnosis of new AF on a subsequent 1-week ECG patch monitor after detection of an irregular heart rhythm by the algorithm.
Methods
Individual-level data from the study will not be made available because of participant confidentiality and privacy, and company policy regarding user data, as well.
Study Design and Participants
The study design has been described previously.9 This was a single-arm remote clinical trial. We invited Fitbit device users aged at least 22 years to enroll between May 6 and October 1, 2020. Participants were eligible if they were US residents and used a compatible Fitbit device (Ionic, Charge 3, Charge 4, Versa, Versa Lite, Versa 2, Versa 3, Sense, Inspire HR, or Inspire 2) with a paired Fitbit account and an Android or Apple iOS smartphone with installed Fitbit app. A valid phone number and email address were required for participation. All participants were required to confirm that they met eligibility criteria for the trial, including that they had not been diagnosed with AF, used oral anticoagulants, or had a pacemaker or defibrillator. Participants were invited to participate through email, Fitbit app notifications, social media, and other marketing channels. All participants provided informed consent through smartphone or web. The protocol was approved by the Advarra Institutional Review Board (Columbia, MD). The protocol is available in the Supplemental Material.
Procedures
The novel algorithm ran centrally on a server using routinely collected PPG data after device syncing through the smartphone. The algorithm was unlocked centrally only for users who consented to the study. The algorithm analyzed PPG data continuously during periods in which participants were stationary (as determined by device accelerometers), to minimize motion artifact and thereby reduce the likelihood of false-positive AF detections. The PPG data were analyzed as 5-minute pulse tachograms acquired every 2.5 minutes (ie, overlapping by 50%). If 11 consecutive tachograms were classified as irregular, then an irregular heart rhythm detection (IHRD) was generated (Figure S1). The algorithm therefore required at least 30 minutes of a sensed irregular heart rhythm to generate an IHRD. The algorithm was developed by Fitbit, was prespecified before study initiation, and was not altered in any way after study initiation. During the study, we encouraged participants to wear their devices through the night to maximize periods of stationary time.
Participants with an IHRD were notified through the Fitbit app and instructed to schedule a telehealth visit with a PlushCare (San Francisco, CA) physician through the app. From the Fitbit app, participants were transferred to the PlushCare site to register for an account, and the Fitbit and PlushCare accounts were linked. Participants received a link to download the PlushCare app to schedule and conduct their telehealth video visit, and they also had the option to conduct the visit through a web browser or by telephone. The telehealth physician collected medical history, assessed for symptoms and adverse events, and confirmed eligibility. Participants were then mailed a single-lead ECG patch monitor (ePatch, BioTelemetry, Inc.) and instructed to apply and wear the monitor for 1 week before returning it by mail using prepaid packaging. The final day to receive ECG patches for processing was January 23, 2021. After the ECG patch was processed, participants were notified through PlushCare that results were available through a PlushCare portal and instructed to schedule a second telehealth visit to discuss. Participants with abnormal findings were encouraged to follow up with their personal health care providers or given a referral to a physician in their local area. Participants received up to a $50 incentive for completing each telehealth visit.
A survey was administered at 90 days after an IHRD notification, which assessed whether participants interacted with health care providers or received other diagnostic testing or treatments. All participants were invited to complete an end-of-study survey that assessed for new AF diagnoses or related clinical management either after their second telehealth visit for those that wore and returned an ECG patch monitor or on February 13, 2021. The last end-of-study survey was completed by March 9, 2021.
Outcomes
The primary outcome was the PPV of the first IHRD during ambulatory ECG patch monitoring, defined as the proportion of subjects with an IHRD who also had AF confirmed by independent cardiologist review of concurrent ECG tracings. Because AF occurring at any time during the first IHRD during ECG patch monitoring would satisfy the primary outcome, the secondary outcome was the proportion of 5-minute pulse tachograms within the first IHRD that corresponded to concurrent AF confirmed on the ECG. This secondary outcome assessed how much of the first IHRD was coincident with ECG-detected AF. Tertiary outcomes included the proportion of participants with an IHRD notification with AF on the subsequent ECG patch monitor. Additional outcomes are the subject of ongoing analysis.
Outcome Adjudication
All ECG patch monitors were processed by technicians using standard protocols, and the clinical reports were overread by 1 of 4 board-certified cardiologists at BioTelemetry using a single-reviewer adjudication process. For the primary and secondary outcomes, BioTelemetry cardiologists adjudicated the cardiac rhythm during each 5-minute ECG interval corresponding to the 11 pulse tachograms comprising the first IHRD, including a 1-minute buffer before and after each tachogram. For tertiary and exploratory outcomes, the clinical ECG patch monitor report was used, in which the presence of AF was determined by a standardized BioTelemetry process involving semiautomated AF detection with technician overreading and cardiologist confirmation. For all outcomes, AF was considered present on the ECG patch monitor when it lasted at least 30 seconds. Participants with prespecified emergent rhythm abnormalities detected on the ECG patch monitors were contacted by PlushCare as described in the study protocol (see Supplemental Material).
Statistical Analysis
The analytic approach is detailed in the Statistical Analysis Plan (see Supplemental Material). Participants were at risk for an IHRD notification between the time Fitbit data were available (up to 30 days before study enrollment) and the first occurrence of either an IHRD notification or end of active data monitoring by the algorithm (October 26, 2020). We estimated Fitbit wear time, and sleep and awake time classifications, using Fitbit device software metrics (Supplemental Material). We estimated the cumulative incidence of an IHRD at 90 days among participants who contributed at least 1 hour of wear time and stratified the analysis by those who contributed retrospective data after consent and those who contributed prospective data only. For cumulative incidence analyses, person time was defined as the IHRD at-risk time described earlier.
The primary outcome analysis was based on participants who started wearing an ECG patch within 45 days of the IHRD notification and whose patch contained analyzable data (Figure 1). To be considered analyzable, the ECG patch had to contain at least 1 hour of readable ECG data that were simultaneous with analyzable Fitbit PPG data. If an ECG patch did not contain analyzable data, a replacement patch was sent to the participant. Overall, 18 ECG patches were returned with no data (possibly because of an error in activating the patch) and 32 patches were not readable by BioTel systems (attributable to either corrupted data or device damage). The target sample size for the number of participants with an IHRD during ECG patch wear was 155, which provided 80% power to test the hypothesis that the true PPV was >70% on the basis of on a 1-sided, 1-sample test of proportion, a type-1 error rate of 2.5%, and an expected PPV of 80%. We further characterized the PPV by reporting the primary end point analysis stratified by sex, age (<65 and ≥65 years), and CHA2DS2-VASc score (0, 1, or ≥2 in men, and 1, 2, or ≥3 in women).10 CHA2DS2-VASc is a stroke prediction score for patients with AF in which 1 point is allocated for congestive heart failure, hypertension, age ≥65 years, diabetes, vascular disease, and female sex; an additional point is allocated for age ≥75 years, and 2 points are allocated for previous stroke or transient ischemic attack.
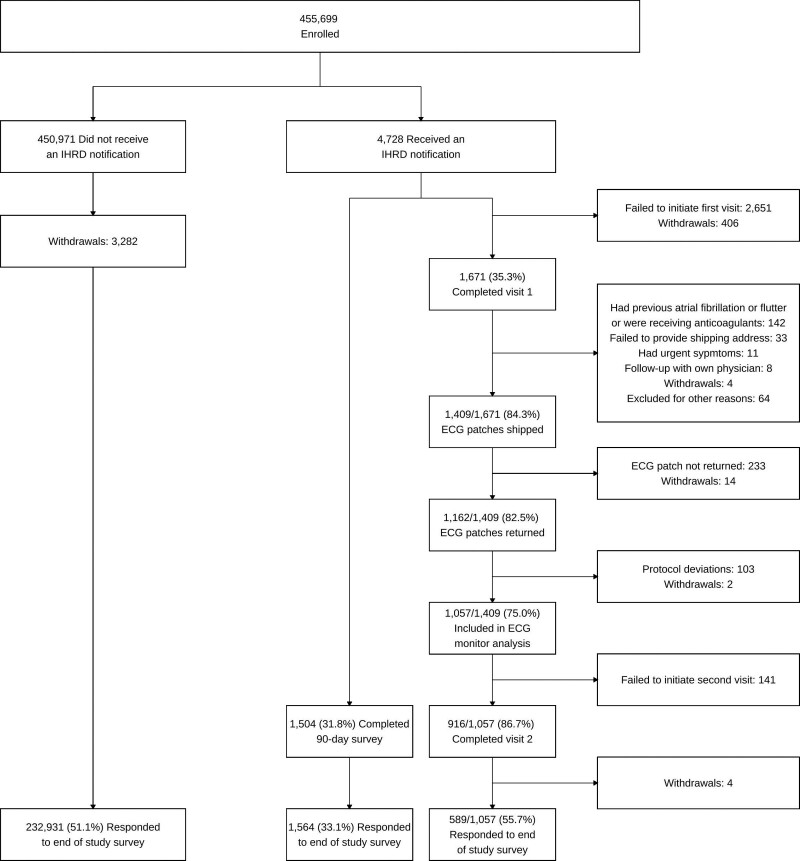
Figure 1.
Participant flow through the trial. IHRD indicates irregular heart rhythm detection.
We tabulated analyzable pulse tachogram data and the frequency of first IHRDs during ECG patch monitoring by sleep and awake time. AF burden and episode duration were based on the ECG patch clinical report. In post hoc exploratory analyses we tabulated the sensitivity and specificity of the first IHRD for AF during the ECG patch monitoring using the ECG patch clinical report. All algorithm test characteristics, including the primary and secondary outcomes, were reported as point estimates and 2-sided 95% Clopper-Pearson exact intervals. Analyses were conducted using Python 3.7 and R 4.1.0.
Role of the Funding Source
Fitbit funded the study. The study was designed by Fitbit in collaboration with Massachusetts General Hospital investigators. Trial conduct was monitored routinely by executive and steering committees that included coauthors from Fitbit. Statistical analyses in this report were performed by the Massachusetts General Hospital investigators. Coauthors employed by Fitbit were involved in the data collection, interpretation, and review of the manuscript in collaboration with the academic coauthors. All authors had full access to the data and had final responsibility for the decision to submit for publication.
Data Sharing
Individual participant data will not be made available because of the sensitive and confidential nature of the personal health information contained. The study protocol and statistical analysis plan are available in the Supplemental Material). Additional reasonable requests for study documentation may be made to the corresponding author.
Results
A total of 455 699 participants enrolled (Figure 1). The median age was 47 (interquartile range [IQR], 35–58) years, 12% were aged ≥65 years, 71% were female, and 73% White (Table 1). At enrollment, 254 430 (56%) participants used smartwatches and 199 895 (44%) used fitness trackers.
Table 1.
Characteristics of Participants
The median number of days at risk for an IHRD was 122 (IQR, 110–134). The median hours of Fitbit device wear time per day was 23 (IQR, 22–24). The fraction of days at risk with ≥18 hours of Fitbit device wear time was 85% (IQR, 54%–96%; Table S2).
IHRD notifications occurred for 4728 (1.0%) participants (Figure 2A). In general, participants with IHRD notifications were older, more likely to be male, and report a family history of AF, personal history of cardiovascular disease, and sleep apnea (Table 1). Among participants aged ≥65 years, IHRD notifications occurred for 2070 (3.6%), and among participants aged <65 years IHRD notifications occurred for 2658 (0.7%). IHRD notifications were based on pulse tachogram data available the 30 days before study entry for 2422 (51.2%) participants, with the remainder based on prospectively acquired pulse tachogram data. Among participants who contributed at least 1 hour of wear time, the cumulative incidence of an IHRD at 90 days was 0.94% (95% CI, 0.91%–0.97%) and was similar among participants who contributed prospective pulse tachogram data only (n=14 746; 0.87% [95% CI, 0.73%–1.03%]).
Figure 2.
Irregular heart rhythm detection frequency and confirmed atrial fibrillation or flutter. Plots demonstrating the fraction of participants receiving an irregular heart rhythm detection (IHRD) notification (A), fraction of participants with an IHRD notification with confirmed atrial fibrillation (AF) or flutter on a subsequent ECG patch monitor (B), and IHRD positive predictive value (PPV) for AF, as confirmed on a concurrent ECG patch monitor (C).
Of participants who received IHRD notifications, 1671 (35.3%) completed a telehealth visit (Figure 1). The proportions of participants who completed a first telehealth visit (34.7% versus 36.0%) and wore and returned an ECG patch monitor (24.2% versus 25.0%) were comparable among those who were notified of an IHRD on the basis of retrospective data versus those notified on the basis of prospective data, respectively. Characteristics of participants who did not initiate a first telehealth visit compared with those who completed the first telehealth visit are provided in Table S3. On average, individuals who initiated a telehealth visit were slightly younger, more likely to be female and White, and had fewer cardiovascular comorbidities, although the differences were modest. Of the 1671 participants who initiated a telehealth visit, 142 (8%) were excluded because of a previous diagnosis of AF confirmed at the time of the telehealth visit or because they were receiving oral anticoagulation (Figure 1).
The ECG patch monitoring set included 1057 participants who returned a monitor with analyzable data (Table 1). The median time from IHRD notification to the start of ECG patch monitoring was 18.6 (IQR, 10.9–30.7) days. As anticipated, participants who received an IHRD notification on the basis of retrospectively collected data received ECG patch monitors later than those who received an IHRD notification on the basis of prospectively collected data (mean±SD: 32±19 versus 16±13 days, respectively). The median wear time of the ECG patch monitor was 7.0 (IQR, 6.2–7.0) days. The proportion of participants with an IHRD notification with confirmed AF on the subsequent ECG patch monitor report was 32.2% (340/1057; Figure 2B).
In total, 241 of the 1057 participants in the ECG analysis set had at least 1 IHRD during ECG patch monitoring, of whom 16 were excluded from the primary and secondary outcome analyses because the cardiologist adjudicated all ECG tracings corresponding to the first IHRD as “unable to evaluate.” Thus, 225 participants were included in the primary and secondary outcome analyses. For the primary outcome, the PPV of the first IHRD during ECG monitoring for concurrent AF was 98.2% (221/225 [95% CI, 95.5%–99.5%]) and was similar for men and women (Figure 2C). On the basis of this interval, we conclude that the PPV is statistically greater than the hypothesized 70%. Among participants aged ≥65 years, the PPV was 97.0% (95% CI, 91.4%–99.4%). Corresponding rhythms among the 4 participants with an IHRD during ECG patch monitoring but without confirmed AF are summarized in Table S4. For the secondary outcome, the proportion of 5-minute tachograms comprising the first IHRD during ECG monitoring corresponding to AF was 98.1% (95% CI, 96.5%–99.9%). The results of sensitivity analyses stratified by CHA2DS2-VASc score are displayed in Table S5.
Acquired Fitbit pulse tachogram data during ECG patch monitoring were analyzable for a median of 8 (IQR, 7–9) hours per day and were primarily available during sleep (Figure S2). The proportion of first IHRDs during ECG patch monitoring that occurred during sleep was 75.9% (95% CI, 70.0%–81.2%). Among participants with AF during ECG patch monitoring, 242 (71.2%) had AF during awake and sleep periods, 23 (6.8%) had AF during sleep periods only, and 75 (22.1%) had AF during awake periods only.
In an exploratory analysis we used the ECG patch monitor report as the reference for confirmed AF. The sensitivity (67.6%) and specificity (98.4%) of the IHRD during the 1-week ECG patch monitoring period are provided in Table 2 and Table S6. In this analysis, 11 participants who had an IHRD during the ECG monitoring period did not have AF ≥30 seconds on the ECG patch report (reflecting a false-positive rate of 1.5%).
Table 2.
End-of-Study Survey Data
The median AF burden was 7% (IQR, 2%–29%) among the 340 participants with AF on the ECG patch (Figure 3) and 14% (IQR, 5%–50%) among the subset of 241 participants who had an IHRD during the ECG patch monitoring period. The median duration of the longest AF episode was 7 (IQR, 2–22) hours among participants with AF on the ECG patch (Table S7) and 11 (IQR, 5–61) hours among the subset of participants who had an IHRD during the ECG patch monitoring period.
Figure 3.
Burden and duration of atrial fibrillation or flutter among 340 participants with confirmed arrhythmia during ECG patch monitoring. Plots demonstrating the burden of atrial fibrillation (AF) or flutter among participants with confirmed arrhythmia on the ECG patch monitor (A) and the duration of the longest AF episode during ECG patch monitoring (B).
Of the 4728 participants who received an IHRD notification, 1504 (31.8%) returned the 90-day postnotification survey. Participants who were notified of an IHRD on the basis of retrospective data were less likely to complete a 90-day postnotification survey than those notified on the basis of prospective data (29.0% versus 34.7%). Postnotification surveys were completed by 916 of the 1671 (54.8%) participants who attended an initial telehealth visit and 588 of the 3057 (19.3%) participants who did not (P<0.001). Among postnotification survey respondents who did not attend an initial telehealth visit, the most common reason cited (n=139, 23.6%) for not attending was that they discussed the notification with their own doctor instead of the study telehealth provider.
Of the 455 699 participants enrolled, 234 532 (51.5%) returned an end-of-study survey. A new diagnosis of AF was reported by 256 of the 773 (34.2%) respondents who received an IHRD notification and attended an initial telehealth visit, and 198 of the 791 (26.9%) respondents who received an IHRD but did not attend a telehealth visit. In contrast, among the 450 971 respondents who did not receive an IHRD notification, 2363 (1%) reported a new diagnosis of AF (Table 3).
Table 3.
Test Characteristics of the Initial Irregular Heart Rhythm Detection During ECG Patch Monitoring, With Reference to the Clinical Report
Of the 2732 adverse events reported in 1275 (0.28%) participants, none was serious (Table S8). Skin irritation from a Fitbit device occurred in 977 (0.21%) participants and from the ECG patch monitor in 615 (0.13%). Stress or anxiety resulting from participating in the study was reported by 1124 (0.25%).
Discussion
In this remote trial comprising 455 699 wearable Fitbit device users, we examined a novel software algorithm to identify undiagnosed AF occurring during periods of inactivity. The primary outcome showed a 98% PPV for the algorithm during ECG patch wear among 1057 participants who had previously received an IHRD notification and then wore and returned an ECG patch monitor. The predictive value of the algorithm was similar across age, sex, and CHA2DS2-VASc score strata. Approximately one-third of participants who received an IHRD notification had AF on the subsequent 1-week ECG monitor, similar to the “diagnostic yield” reported in the Apple Heart Study.5
Our findings indicate that wearable devices using the algorithm studied may enable large-scale identification of undiagnosed AF. In the Apple Heart Study,5 the overall PPV was 84% (72/86) during ECG patch wear, and 78% among participants aged ≥65 years. In the Huawei Heart Study,6 the patient-level PPV was 87% (227/262) using various clinical follow-up approaches. The PPVs we observed are greater than those previously reported, although formal comparisons of algorithm performance cannot be clearly drawn from the study given the potential differences in study participant composition. Nevertheless, features of our algorithm may minimize false-positive notifications. By comparison, our algorithm uses a more continuous pulse data–sampling approach and a strict requirement that all 11 tachograms within a classification window be irregular to generate an IHRD (Table S9). The high device wear time in our study enabled prolonged analyzable periods of inactivity, including during sleep, to detect irregular rhythms. It is also possible that differences in study composition and design account for differences in observed PPVs.
We note that, as with previous studies,5,6 the PPV of the IHRD algorithm was estimated in participants with a previous IHRD. Patients with a previous IHRD are enriched for having AF, and, because a greater prevalence of disease can increase the PPV, the PPV estimates reported in our and previous studies5,6 may be higher than would be expected for an initial IHRD that occurs in the general population. Therefore, test characteristics of the algorithm in the general population warrant prospective evaluation.
Ours is the largest remote study of wearable devices and was conducted for only 5 months during a global pandemic, underscoring the potential for remote direct-to-participant recruitment for efficient clinical trial enrollment. Our study also included a range of smartwatches and fitness trackers compatible with both Apple iOS and Android smart device operating systems. More older participants enrolled in our study than in previous studies, an important subset given the increased risks of stroke among older individuals with AF.11 We also enrolled a greater proportion of women, extending the generalizability of PPG-software algorithms for detecting AF.
A key issue is that, although 98% of those with an IHRD during ECG patch monitoring had concurrent AF on the ECG, two-thirds of those that received an initial IHRD notification and returned a subsequent 1-week ECG patch did not have AF confirmed. Given the paroxysmal nature of AF, episodes of AF may not have coincided with the 1-week ECG patch monitor, so the fact that 32% had confirmed AF on the ECG patch likely underestimates the true fraction with AF. The optimal duration of monitoring and management of individuals with an IHRD who do not have AF confirmed on an ambulatory ECG patch monitor are unknown and warrant further evaluation. Nevertheless, our results indicate that IHRDs enrich for individuals likely to have AF on subsequent ECG patch monitoring and who have an increased AF burden. Previous reports of 2-week ECG patch monitoring in adults without any PPG-based prescreening for irregular rhythms have demonstrated AF in only up to ≈5% of participants12–14 and a median AF burden of up to 2% in studies of individuals at elevated AF risk.12,13 In contrast, the median AF burden after IHRD notification was 7% in our study. The enrichment for higher-burden AF observed in our study may be clinically relevant because recent data highlight the important prognostic value of longer episodes and higher AF burden for stroke risk.15–18
The current Fitbit algorithm was designed to be specific and to minimize false-positive detections. Given the facts that the algorithm requires at least 30 minutes of an irregular pulse to detect AF, and only operates during sedentary periods, the algorithm is not expected to have perfect sensitivity for episodes of AF. Indeed, we observed that the sensitivity of an IHRD for an episode of AF occurring during the 1-week ECG patch monitoring period was ≈68%, and the specificity was 98%. The imperfect sensitivity of the current IHRD algorithm highlights the challenge of algorithms that use PPG data to assess for irregular rhythm during periods of motion, limits AF burden assessment, and may selectively detect vagally mediated AF.
Our study highlights important challenges related to study retention in remote clinical trials. We observed attrition of participants who received an IHRD notification, an observation that was similarly observed in 2 other clinical trials examining PPG-based software algorithms for AF detection.5,6 Participant attrition broadly represents a challenge to remote digital health studies.19 In our study, many participants who received an IHRD notification contacted their own health care providers in lieu of study physicians. We observed limited survey response rates and the missingness may be informative. We submit that integration of existing care pathways with trial protocols and engagement with telehealth providers during study enrollment may facilitate participant retention in future remote clinical trials. Generation of high-quality evidence supporting methods for participant engagement is needed.
Our study included existing consumers who may have been concerned about heart rhythm abnormalities to begin with. Despite the relative geographic and racial diversity of participants, future assessment of the software algorithm in more diverse groups, including individuals who may not be able to afford a wearable device, is necessary.20 Adjudication of the ambulatory ECG patch monitors involved single reviewers only, which may result in misclassification. The algorithm operates centrally using data transmitted to a server and is not capable of real-time notification. The pairing of PPG technology with single-lead ECG algorithms, which are increasingly available in wearable devices, requires prospective evaluation. Although we administered surveys, we did not have access to clinical records to measure the associations between IHRD notifications and health care outcomes or utilization. The clinical- and cost-effectiveness of screening for AF remains uncertain and future studies are needed to assess the downstream benefits and potential adverse events resulting from detecting AF through a wearable device, in particular, given the increasing access to consumer-based technology enabling AF detection.21 Major professional society guidelines warrant specific guidance for clinicians regarding the management of consumer-based AF detections.
In conclusion, we found that detection of irregular heart rhythms by using a novel PPG-based software algorithm in smartwatches and fitness trackers is highly concordant with undiagnosed AF. Irregular heart rhythm notifications from the software algorithm are associated with a substantial enrichment for confirmed AF during subsequent ambulatory ECG patch monitoring.
Article Information
Acknowledgments
The authors are grateful to the participants who enrolled in the trial.
Sources of Funding
The study was funded by Fitbit.
Disclosures
Dr Lubitz previously received sponsored research support from Fitbit, Bristol Myers Squibb/Pfizer, Bayer AG, Boehringer Ingelheim, and IBM, and has consulted for Bristol Myers Squibb/Pfizer, Bayer AG, Blackstone Life Sciences, and Invitae. Dr Lubitz is a full-time employee of Novartis Institutes for BioMedical Research as of July 18, 2022. He previously received support from National Institutes of Health (NIH) grants R01HL139731 and R01HL157635, and American Heart Association 18SFRN34250007. Dr Faranesh receives sponsored research support from Fitbit and Bristol Myers Squibb, is supported by NIH grants R01GM127862, OT2HL161841 and U01HL123009 as principal or co-principal investigator, and serves as an expert witness for Round Table Group. Dr Atlas has consulted for Fitbit and Bristol Myers Squibb/Pfizer and receives sponsored research support from Bristol Myers Squibb/Pfizer. Dr Atlas is supported by an American Heart Association grant (18SFRN34250007). Dr McManus has consulted for and received personal fees and nonfinancial support from Fitbit, is supported by NIH grants U54HL143541, R01HL137734, R01HL155343, R01HL137794, R01HL141434, and R61HL158541 as primary or multi-primary investigator and grants and personal fees from Bristol Myers Squibb, Pfizer, Flexcon, and Heart Rhythm Society; grants from Boehringer Ingelheim and Philips; nonfinancial support from Apple; personal fees and nonfinancial support from Samsung; personal fees from Avania and Rose Consulting. Dr Singer has consulted for Fitbit, Bristol Myers Squibb, and Pfizer, and receives research funding from Bristol Myers Squibb and the Eliot B. and Edith C. Shoolman Fund of the Massachusetts General Hospital (Boston, MA). Dr Pagoto has consulted for Fitbit and is a scientific advisor for WW (formerly Weight Watchers). Drs Faranesh, McConnell, and Pantelopoulos are employees of Fitbit (part of Google). The other authors report no conflicts.
Supplemental Material
Supplemental Methods
Figures S1 and S2
Tables S1–S9
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AF
- atrial fibrillation
- IHRD
- irregular heart rhythm detection
- IQR
- interquartile range
- PPG
- photoplethysmography
- PPV
- positive predictive value
Circulation is available at www.ahajournals.org/journal/circ
Supplemental Material, the podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.122.060291.
For Sources of Funding and Disclosures, see page 1424.
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
Contributor Information
Anthony Z. Faranesh, Email: tfaranesh@google.com.
Caitlin Selvaggi, Email: CSELVAGGI@mgh.harvard.edu.
Steven J. Atlas, Email: SATLAS@mgh.harvard.edu.
David D. McManus, Email: David.McManus@umassmed.edu.
Daniel E. Singer, Email: desinger@mgh.harvard.edu.
Sherry Pagoto, Email: sherry.pagoto@uconn.edu.
Michael V. McConnell, Email: mcconnell@stanford.edu.
Alexandros Pantelopoulos, Email: alekos@google.com.
Andrea S. Foulkes, Email: afoulkes@mgh.harvard.edu.
References
- 1.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 2.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007 [DOI] [PubMed] [Google Scholar]
- 3.Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, Lorimer MF, Lau DH, Antic NA, Brooks AG, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310:2050–2060. doi: 10.1001/jama.2013.280521 [DOI] [PubMed] [Google Scholar]
- 4.Voskoboinik A, Kalman JM, De Silva A, Nicholls T, Costello B, Nanayakkara S, Prabhu S, Stub D, Azzopardi S, Vizi D, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382:20–28. doi: 10.1056/NEJMoa1817591 [DOI] [PubMed] [Google Scholar]
- 5.Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, Balasubramanian V, Russo AM, Rajmane A, Cheung L, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381:1909–1917. doi: 10.1056/NEJMoa1901183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo Y, Wang H, Zhang H, Liu T, Liang Z, Xia Y, Yan L, Xing Y, Shi H, Li S, et al. Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol. 2019;74:2365–2375. doi: 10.1016/j.jacc.2019.08.019 [DOI] [PubMed] [Google Scholar]
- 7.De Novo Classification Request for Irregular Rhythm Notification Feature. Food and Drug Administration. Accessed August 8, 2018. https://www.accessdata.fda.gov/cdrh_docs/reviews/DEN180042.pdf.
- 8.Irregular rhythm notification instructions for use (IFU). Apple Inc. Accessed March 24, 2022. https://www.apple.com/legal/ifu/irnf/ [Google Scholar]
- 9.Lubitz SA, Faranesh AZ, Atlas SJ, McManus DD, Singer DE, Pagoto S, Pantelopoulos A, Foulkes AS. Rationale and design of a large population study to validate software for the assessment of atrial fibrillation from data acquired by a consumer tracker or smartwatch: the Fitbit heart study. Am Heart J. 2021;238:16–26. doi: 10.1016/j.ahj.2021.04.003 [DOI] [PubMed] [Google Scholar]
- 10.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 11.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–1457. [PubMed] [Google Scholar]
- 12.Steinhubl SR, Waalen J, Edwards AM, Ariniello LM, Mehta RR, Ebner GS, Carter C, Baca-Motes K, Felicione E, Sarich T, et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA. 2018;320:146–155. doi: 10.1001/jama.2018.8102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gladstone DJ, Wachter R, Schmalstieg-Bahr K, Quinn FR, Hummers E, Ivers N, Marsden T, Thornton A, Djuric A, Suerbaum J, et al. Screening for atrial fibrillation in the older population: a randomized clinical trial. JAMA Cardiol. 2021;6:558–567. doi: 10.1001/jamacardio.2021.0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rooney MR, Soliman EZ, Lutsey PL, Norby FL, Loehr LR, Mosley TH, Zhang M, Gottesman RF, Coresh J, Folsom AR, et al. Prevalence and characteristics of subclinical atrial fibrillation in a community-dwelling elderly population: the ARIC study. Circ Arrhythm Electrophysiol. 2019;12:e007390. doi: 10.1161/CIRCEP.119.007390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turakhia MP, Ziegler PD, Schmitt SK, Chang Y, Fan J, Than CT, Keung EK, Singer DE. Atrial fibrillation burden and short-term risk of stroke: case-crossover analysis of continuously recorded heart rhythm from cardiac electronic implanted devices. Circ Arrhythm Electrophysiol. 2015;8:1040–1047. doi: 10.1161/CIRCEP.114.003057 [DOI] [PubMed] [Google Scholar]
- 16.Van Gelder IC, Healey JS, Crijns HJGM, Wang J, Hohnloser SH, Gold MR, Capucci A, Lau C-P, Morillo CA, Hobbelt AH, et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38:1339–1344. doi: 10.1093/eurheartj/ehx042 [DOI] [PubMed] [Google Scholar]
- 17.Go AS, Reynolds K, Yang J, Gupta N, Lenane J, Sung SH, Harrison TN, Liu TI, Solomon MD. Association of burden of atrial fibrillation with risk of ischemic stroke in adults with paroxysmal atrial fibrillation: the KP-RHYTHM study. JAMA Cardiol. 2018;3:601–608. doi: 10.1001/jamacardio.2018.1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan RM, Koehler J, Ziegler PD, Sarkar S, Zweibel S, Passman RS. Stroke risk as a function of atrial fibrillation duration and CHA2DS2-VASc score. Circulation. 2019;140:1639–1646. doi: 10.1161/CIRCULATIONAHA.119.041303 [DOI] [PubMed] [Google Scholar]
- 19.Pratap A, Neto EC, Snyder P, Stepnowsky C, Elhadad N, Grant D, Mohebbi MH, Mooney S, Suver C, Wilbanks J, et al. Indicators of retention in remote digital health studies: a cross-study evaluation of 100,000 participants. NPJ Digit Med. 2020;3:21. doi: 10.1038/s41746-020-0224-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Editors; Rubin E. Striving for diversity in research studies. N Engl J Med. 2021;385:1429–1430. doi: 10.1056/NEJMe2114651 [DOI] [PubMed] [Google Scholar]
- 21.A study to investigate if early atrial fibrillation (AF) diagnosis reduces risk of events like stroke in the real-world. ClinicalTrials.gov Accessed November 25, 2021. https://clinicaltrials.gov/ct2/show/NCT04276441.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.