Abstract
The acquisition of Burkholderia cepacia in some cystic fibrosis patients is associated with symptoms of acute pulmonary inflammation that may be life threatening. The ability of lipopolysaccharide (LPS) from B. cepacia to prime a monocyte cell line for enhanced superoxide anion generation was investigated and compared with the priming activities of LPSs from Pseudomonas aeruginosa, Stenotrophomonas maltophilia, and Escherichia coli. The human monocyte cell line MonoMac-6 (MM6) was primed overnight with different LPSs (100 ng/ml), and the respiratory burst was triggered by exposure to opsonized zymosan (125 μg/ml). Superoxide generation was detected by enhanced chemiluminescence with Lucigenin. B. cepacia LPS was found to prime MM6 cells to produce more superoxide anion than P. aeruginosa or S. maltophilia LPS, and this priming response was CD14 dependent. In addition, the inhibition of respiratory burst responses in monocytes by a bacterial melanin-like pigment purified from an epidemic B. cepacia strain was investigated. The melanin-like pigment was isolated from tyrosine-enriched media on which B. cepacia had been grown and was purified by gel filtration, anion ion-exchange chromatography, and ethanol precipitation. The scavenging potential of the melanin-like pigment for superoxide anion radical (•O2−) generated during the respiratory burst was confirmed with superoxide produced from a cell-free system with xanthine-xanthine oxidase and detected by electron paramagnetic resonance spectroscopy with the spin trap 5-diethoxyphosphoryl-5-methyl-1-pyrroline-n-oxide. The addition of melanin during the LPS priming stage had no effect on the subsequent triggering of the respiratory burst, but melanin inhibited •O2− detection when added at the triggering stage of the respiratory burst. We conclude that melanin-producing B. cepacia may derive protection from the free-radical-scavenging properties of this pigment.
In recent years, Burkholderia cepacia infection in cystic fibrosis (CF) patients has become a clinical issue of increasing concern. B. cepacia strains are intrinsically resistant to many antibiotics (13, 26), and some strains are highly transmissible from person to person (14, 21). Once acquired, B. cepacia infection is rarely eradicated from the lungs of patients with CF. In addition, a proportion of patients who acquire B. cepacia infection develop a rapidly fatal pneumonia and sepsis with a high mortality rate, the so-called “cepacia syndrome” (16, 20). However, the pathogenic mechanisms involved in this syndrome remain unclear. Recent work done in our laboratory and by other groups has demonstrated that lipopolysaccharide (LPS) from B. cepacia can stimulate larger amounts of tumor necrosis factor alpha (TNF-α) than LPSs from other gram-negative pathogens of the lungs of CF patients, such as Pseudomonas aeruginosa and Stenotrophomonas maltophilia (30, 34). Raised TNF-α levels are associated with local and systemic inflammation (17).
LPS-mediated macrophage stimulation may play an important role in the development of lung pathology, and the differences in the stimulatory activities of LPSs from B. cepacia, P. aeruginosa, and S. maltophilia could explain the different pathogenic sequelae seen with lung infections caused by these three organisms in CF patients. S. maltophilia, in particular, shows some similarities to B. cepacia in certain aspects, such as patient-to-patient transmission (9), resistance to many antibiotics, and a lack of important exotoxin production. However, despite numerous investigations, there have been no reports of acute pulmonary inflammation following S. maltophilia acquisition, unlike the data for B. cepacia (10).
The respiratory burst is an important mechanism of defense against invading pathogens (5, 8, 19), and this oxidative activity is necessary for effective microbicidal action (4, 27). Macrophages and monocytes produce superoxide anion in the respiratory burst in response to engulfed bacteria or bacterial products. The generated reactive oxygen radicals which help to kill phagocytosed bacteria are transformed to water by host detoxifying enzymes (3). However, excess production of reactive oxygen radicals can cause tissue damage, which may be particularly important in the lungs (6). Moreover, some pathogens can evade the bactericidal effect of the respiratory burst and may thus persist at the site of infection. LPS, while not triggering the respiratory burst directly, has been shown to upregulate this oxidative response to other stimuli and therefore to act as a priming agent. Hughes et al. (18) described the potent priming activity of B. cepacia LPS for neutrophils; they suggested that the increased neutrophil recruitment and respiratory burst responses in the lungs may contribute to inflammation by the release of tissue-damaging proteolytic enzymes and reactive oxygen species. It has been suggested that B. cepacia LPS may play a considerable role in CF lung inflammation.
Certain epidemic strains of B. cepacia produce a melanin-like pigment, and similar pigments from other bacterial species, such as Proteus mirabilis, have been shown to act as free-radical scavengers (1). In the present investigation, we attempted to determine if the melanin-like pigment purified from an epidemic B. cepacia strain could protect the bacteria from the bactericidal effect of superoxide anion and thus be a virulence factor aiding infection in the lungs of CF patients.
MATERIALS AND METHODS
Reagents.
The human monocytic cell line MonoMac-6 (MM6) was obtained from the Deutsche Sammlung von Mikroorganismen, Braunschweig, Germany. All reagents were obtained from Sigma Chemical Co., Poole, United Kingdom, unless otherwise stated. Phorbol myristate acetate (PMA) was prepared as a 1 μM stock solution in dimethyl sulfoxide. Opsonised zymosan was prepared as a 2.5-mg/ml stock solution by a method described previously (3). Lucigenin (10,10′-dimethyl-9,9′-biacridinium dinitrate) (10 mM) was made in dimethyl sulfoxide, stored in the dark at 4°C, diluted in pyrogen-free water, and used at a final concentration of 25 μM (2). The standard buffer used in the chemiluminescence assay to suspend the cells was prepared with 4.58 mM KH2PO4, 8.03 mM Na2HPO4, 0.76% NaCl, 0.033% KCl, 0.1% glucose, 0.1% endotoxin-free albumin, 0.5 mM MgCl2, and 0.45 mM CaCl2 (pH 7.3). Superoxide dismutase (SOD) enzyme was used at 400 U in a final reaction volume of 200 μl. A 10 mM stock solution of xanthine was prepared by dissolving 17.4 mg in 10 ml of 0.05 M potassium phosphate buffer (pH 7.8) and boiling the mixture in a water bath until the xanthine dissolved completely. Xanthine oxidase was prepared at 1.0 U/ml, and ascorbic acid was prepared as a 25 mM stock solution in 0.05 M potassium phosphate buffer (pH 7.8). The spin trap 5-diethoxyphosphoryl-5-methyl-1-pyrroline-n-oxide (DEPMPO) was obtained from Oxis International Inc. and prepared at 1 mM in K2HPO4 buffer. An anti-CD14 (MY4) monoclonal antibody (Coulter Corporation, Miami, Fla.) was used at 200 μg/106 cells. The LPSs used in this study were purified in our laboratory from clinical bacterial isolates (eight B. cepacia [Table 1], four Pseudomonas aeruginosa, and four Stenotrophomonas maltophilia) by the phenol-water extraction method adapted from Galanos et al. (12). Escherichia coli O111:B4 LPS was obtained from Sigma.
TABLE 1.
Sources and genomovars of clinical B. cepacia strains used in this study
| Straina | Source | Genomovar |
|---|---|---|
| Cardiff epidemic (A1) | CF, clinical | II |
| Cardiff epidemic (P1)b | CF, clinical (ET12 lineage) | III |
| Cardiff epidemic (A3) | CF, clinical | II |
| Cardiff epidemic (A1) | CF, clinical | II |
| Cardiff epidemic (P2) | CF, clinical | II |
| B. cepacia epidemic (J2315) | ET12 lineage | III |
| B. cepacia NCTC 10661 | Clinical | I |
| B. cepacia 03 | Unknown | I |
A and P are the PCR types of B. cepacia strains isolated from adult (A) and pediatric (P) CF patients.
The Cardiff epidemic strain P1 is identical to the United Kingdom epidemic strain.
Table 1 shows the B. cepacia strains used in this investigation. The Cardiff epidemic strain of B. cepacia (P1) is identical to the United Kingdom epidemic strain (ET12 lineage). Epidemic strain B. cepacia J2315 (a kind gift from J. R. W. Govan, Medical Microbiology, University of Edinburgh Medical School, Scotland, United Kingdom) is closely related to the Cardiff epidemic strain but has a different PCR ribotype (29). B. cepacia in this manuscript refers to the B. cepacia genomovars listed in Table 1. The term “genomovar” is used to denote phenotypically similar but genotypically distinct groups of strains.
Melanin purification.
A melanin-producing strain of B. cepacia (Cardiff epidemic strain P1) was grown on melanin-enhancing medium (24) at 37°C for 48 h. Much of the bacteria were removed from the surface of the agar, which was then cut into 1-cm blocks, frozen, and thawed to extract the melanin-like pigment. The extract was filtered through a 0.45-μm-pore-size filter, and the filtrate was divided into 25-ml aliquots and stored at −20°C until further purification.
The extract (25 ml) was separated on a column of Sephacryl S100 (80 by 2.6 cm) (Amersham Pharmacia Biotech, St. Albans, United Kingdom) with 25 mM Tris-HCl buffer (pH 8.6) containing 50 mM sodium chloride. The melanin-like pigment eluted in a large peak monitored at an absorbance of 340 nm, and the fractions containing the melanin were collected and pooled. The melanin was estimated to have a molecular weight of 10,000 by comparison to known proteins.
The pooled fractions were applied to a column of DEAE-Sepharose (1.6 by 20 cm) (Amersham Pharmacia Biotech) that had been equilibrated with 25 mM Tris-HCl buffer (pH 8.6) containing 50 mM sodium chloride. Following washing to remove all unbound material, the column was developed stepwise with 25 mM Tris-HCl buffer (pH 8.6) containing 100 mM sodium chloride, 25 mM Tris-HCl buffer (pH 6.6) containing 1 M sodium chloride, and finally 25 mM Tris-HCl buffer (pH 6.6) containing 2 M sodium chloride. The fractions eluting between 100 mM NaCl and 1 M NaCl were pooled. Ethanol was added to a final concentration of 66%, and the mixture was allowed to stand for 30 min at 4°C. The precipitate was removed by centrifugation at 3,600 × g for 10 min. The supernatant was adjusted to a final ethanol concentration of 80% and allowed to stand overnight at 4°C. The precipitate was collected by centrifugation, placed in water, freeze-dried, and stored at −20°C. Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) of the purified material stained with silver stain revealed the presence of one band that coincided with the brown color of melanin on the gel prior to staining. No protein bands which indicated the purity of the melanin-like pigment preparation were detected.
Cellular respiratory burst activity.
MM6 cells were grown in culture medium consisting of RPMI 1640 containing 10% fetal calf serum, l-glutamine, nonessential amino acids, sodium pyruvate, and penicillin and streptomycin. The cells were tested for viability (>95%) by a trypan blue exclusion assay, counted, washed, and resuspended in 5 ml of culture medium. To prime the respiratory burst, the cells were adjusted to 2 × 106/ml, transferred to a small tissue culture flask, and incubated with 100 ng of LPS per ml overnight at 37°C under 5% CO2. Unprimed cells were incubated in the same way but without LPS. The primed cells were washed twice with culture medium and resuspended in 5 ml of the standard buffer at 2 × 106/ml. The chemiluminescence probe Lucigenin was added to the cell suspension (25 μl/ml of cells from a 1.0 mM stock solution) in Bijoux bottles. Aliquots (150 μl) of the mixture were transferred into quadruplicate wells of a white 96-well plate (FluoroNunc-PolySorp; Gibco, Paisley, United Kingdom). Chemiluminescence was measured in relative light units (a measure of the number of photons generated by the reaction at each time point). Chemiluminescence was measured with a luminometer (Molecular Light Technology, Cardiff, United Kingdom), and an initial reading of the plate was taken prior to triggering of the respiratory burst. To trigger the respiratory burst, cells were stimulated with either 50 μl of PMA (1 μM) or 50 μl of opsonized zymosan (500 μg/ml). The plate was read immediately and then at 5-min intervals for 200 min.
Scavenging of superoxide radical.
Experiments were carried out on the same cell preparation as that described above. Melanin was either added just prior to triggering of the respiratory burst with PMA or zymosan or at the peak of the chemiluminescence reaction (typically after 50 min) at final concentrations ranging from 10 to 100 μg/200 μl of cell suspension (∼50 to ∼500 μM). In order to investigate if melanin interfered with the respiratory burst at the cellular level, melanin was incubated overnight with primed cells as well as unprimed cells, and the respiratory burst response was measured. Classical superoxide anion scavengers such as ascorbic acid (25 to 400 μM) and SOD (400 U/ml) were used in the assay for comparison with the scavenging ability of purified melanin.
To study the scavenging ability of the purified melanin-like pigment in a cell-free system, superoxide anions were generated by the xanthine-xanthine oxidase reaction as follows. Lucigenin was diluted in K2HPO4 buffer at a 25 μM final concentration, and 50 μl was dispensed into quadruplicate wells of a white 96-well plate. Fifty microliters of 10 mM xanthine was added, and the reaction was triggered by the addition of 10 μl of xanthine oxidase (1.0 U). Melanin or ascorbic acid (10 μl) was added at different doses at either the start or the peak of the reaction. Chemiluminescence was monitored for 60 min.
To confirm that the melanin-like pigment was directly scavenging superoxide anions, electron paramagnetic resonance (EPR) spectroscopy of the cell-free system with xanthine-xanthine oxidase was used. Fifty microliters of 10 mM xanthine was placed to an Eppendorf tube containing 50 μl of K2HPO4 buffer. Fifty microliters of the spin trap DEPMPO (1.0 mM) was added. Superoxide anion generation was triggered by the addition of 50 μl of xanthine oxidase (1.0 U/ml). The purified melanin-like pigment was then added at various final concentrations ranging from 50 to 2,500 μM. The reaction mixture was transferred to a sealed glass pipette for EPR spectroscopy. The classical superoxide anion scavenger SOD at a final concentration of 400 U was used instead of melanin as a control.
Effect of melanin on TNF-α production.
Melanin (∼50 and ∼250 μM) was incubated with 106 cells overnight in the presence of 100 ng of LPS per ml and 50 ng of PMA per ml. Unstimulated cells were incubated with melanin and PMA but not LPS. TNF-α production from stimulated and unstimulated cells was measured by an enzyme-linked immunosorbent assay (R&D, Abingdon, United Kingdom) as described elsewhere (7).
Role of CD14 in LPS priming of the monocyte respiratory burst.
In order to determine if LPS priming was CD14 dependent, an anti-CD14 monoclonal antibody (MY4) was used to block CD14 receptors prior to the LPS priming step. MY4 was incubated with the cells at a final concentration of 200 μg/106 cells for 30 min at 37°C. The cells were washed gently once, and LPS was added and incubated overnight at 37°C. The respiratory burst response was measured as described above.
EPR spectroscopy.
EPR spectroscopy was performed with a Varian E-104 EPR spectrometer operating at 9 GHz with 100-kHz field modulation. Typical EPR spectrometer settings were as follows: 3,200-G magnetic field, 100-G scan range, 5 × 103 receiver gain, 1-G modulation amplitude, and 10 mW of power. EPR spectra were collected sequentially for 15 min with a digital data acquisition system linked to a personal computer.
Statistical analysis.
The mean of the quadruplicates and the standard error for each sample were calculated with Sigma-plot computer software (Jandel Scientific). Unless otherwise stated, samples from four separate experiments were analyzed in quadruplicate. P values were obtained by nonparametric Mann-Whitney U analysis with Minitab release 9.0 software.
RESULTS
Respiratory burst responses in monocytes primed by different LPSs.
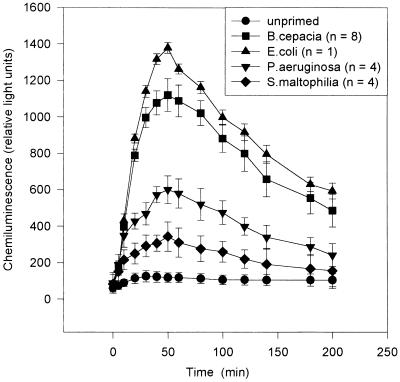
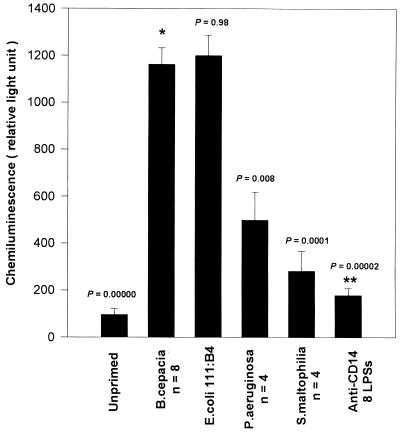
MM6 cells incubated overnight in the presence of the different LPSs generated superoxide radical anions when triggered with opsonized zymosan (Fig. 1). Similar respiratory burst responses were obtained when PMA was used as a triggering agent (results not shown). On a weight-for-weight basis, monocytes primed with B. cepacia LPS produced amounts of superoxide anion similar to those produced by cells primed with E. coli LPS and significantly larger than those produced by cells primed with P. aeruginosa LPS (P, 0.008) or S. maltophilia LPS (P, 0.0001). Moreover, cells incubated in the absence of LPS (unprimed) did not produce any significant amounts of superoxide anion when stimulated with opsonized zymosan (Fig. 2). There were no significant differences in priming activity between any of the isolated LPSs from the various clinical B. cepacia strains used in this study (data not shown), but LPSs from all of these strains showed significantly higher priming activity for the respiratory burst than P. aeruginosa or S. maltophilia LPS (Fig. 2). No correlation was found between the priming capability of B. cepacia LPS and the various genomvars used in this study.
FIG. 1.
Superoxide anion production by MM6 cells primed with LPS and triggered with opsonized zymosan, as measured by enhanced chemiluminescence. Error bars indicate the standard error of the mean for chemiluminescence in priming experiments performed in quadruplicate with each different LPS. n, number of strains of each organism.
FIG. 2.
Superoxide anion production by MM6 cells primed with LPS, as measured by enhanced chemiluminescence. Bars represent the means for eight B. cepacia LPSs, four P. aeruginosa LPSs, and four S. maltophilia LPSs. Chemiluminescence in priming experiments with each LPS was measured in quadruplicate, and error bars indicate the standard error of the mean. P values were obtained against B. cepacia LPS activity (asterisk). The double asterisks indicate cells primed with B. cepacia LPS in the presence of anti-CD14 antibody.
Role of CD14.
The priming of monocytes by LPS was found to be CD14 dependent. When cells were treated with anti-CD14 monoclonal antibody prior to priming with B. cepacia LPS, the respiratory burst response was significantly inhibited (Fig. 2). Anti-CD14 antibody also inhibited priming by LPSs from E. coli, P. aeruginosa, and S. maltophilia (data not shown), indicating that CD14 is required for LPS priming of the monocyte respiratory burst.
A melanin-like pigment attenuates the respiratory burst response.
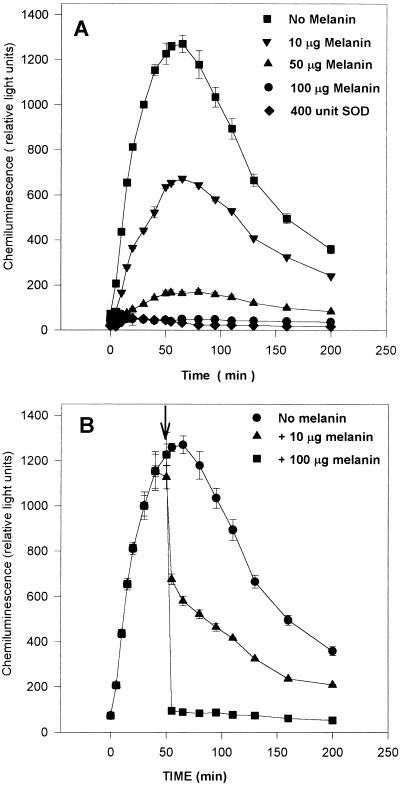
A purified melanin-like pigment from an epidemic B. cepacia strain was added to the monocytes at the start of the chemiluminescence reaction. Melanin was found to inhibit superoxide anion in a dose-dependent manner, suggesting the scavenging ability of this pigment (Fig. 3A). Even when melanin was added at the peak of superoxide anion production, it was able to scavenge the produced superoxide anion in a dose-dependent manner (Fig. 3B). However, when melanin was incubated with the cells alone or with LPS during monocyte priming overnight, it had no effect on the subsequent triggering of the respiratory burst and did not inhibit superoxide anion production (data not shown). This finding suggests that melanin does not inhibit the respiratory burst at the cellular level but scavenges the produced superoxide anion. In addition, melanin had no effect on other biological responses of monocytes. When cells were stimulated with LPS in the presence of either high (100 μg/ml) or low (10 μg/ml) doses of melanin and TNF-α production was measured by an enzyme-linked immunosorbent assay, no difference was found between cells incubated with the high or low doses of melanin (data not shown).
FIG. 3.
(A) Superoxide anion production in MM6 cells primed with LPS from the B. cepacia Cardiff epidemic strain (P1), as measured by chemiluminescence. Different doses of melanin added with opsonized zymosan at the triggering stage of the respiratory burst scavenged superoxide anion. For comparison, 400 U of SOD was added at the triggering stage. (B) Like panel A, except that melanin was added at the peak of superoxide anion production (55 min [arrow]).
Xanthine-xanthine oxidase chemiluminescence activity.
A cell-free system with xanthine-xanthine oxidase was used to measure the scavenging ability of the melanin-like pigment in comparison with other superoxide scavengers, such as SOD and ascorbic acid. The IC50 (concentration that scavenged 50% of superoxide anion) for the melanin-like pigment was found to be approximately 50 μM; for ascorbic acid, the IC50 was 35 μM, and for SOD the IC50 was approximately 0.01 μM under the conditions.
EPR studies.
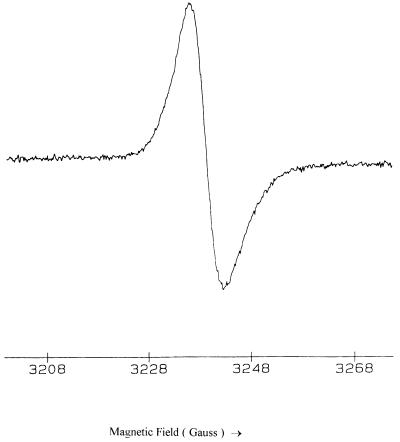
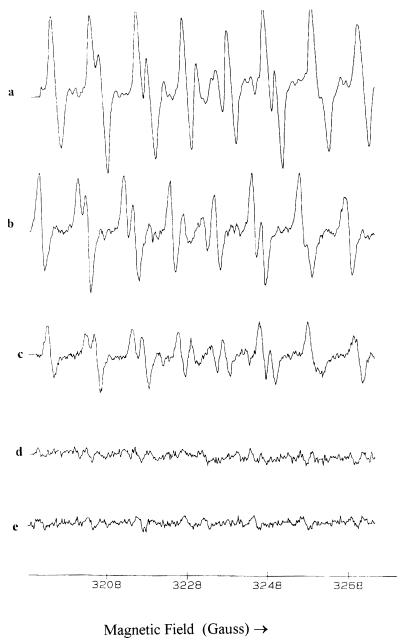
The purified bacterial melanin was found to be paramagnetic, as demonstrated by EPR spectroscopy (Fig. 4), confirming its identity with other melanins (1, 31). EPR studies were performed to investigate the scavenging potential of the purified melanin-like pigment for superoxide anion. The EPR spectra generated by the reaction of xanthine with xanthine oxidase in the presence of the spin trap DEPMPO are shown in Fig. 5. Figure 5a shows a typical spectrum of the superoxide anion radical adduct of DEPMPO and confirms that superoxide is generated in this system (28). The addition of the melanin-like pigment inhibited the formation of this EPR signal in a dose-dependent manner (Fig. 5b, c, and d), indicating that the melanin-like pigment competed with the spin trap for the superoxide anion radical. Similar results were obtained when the generated superoxide anion radical was scavenged by SOD in place of the melanin-like pigment (Fig. 5e).
FIG. 4.
EPR spectrum of the purified melanin-like pigment powder.
FIG. 5.
EPR spectra of superoxide anions detected by the spin trap DEPMPO. The melanin-like pigment scavenged superoxide radicals in a dose-dependent manner. (a) Xanthine-xanthine oxidase plus DEPMPO after 5 min of reaction. (b) As in panel a but with 50 μM melanin. (c) As in panel a but with 250 μM melanin. (d) As in panel a but with 2,500 μM melanin. (e) As in panel a but with 400 U of SOD.
DISCUSSION
This study has shown that LPSs from clinical isolates of B. cepacia are powerful priming agents for the respiratory burst in monocytes and that a melanin-like pigment purified from an epidemic strain of B. cepacia is an efficient scavenger of superoxide radicals produced in the respiratory burst.
We found that monocytes primed with B. cepacia LPS and those primed with E. coli LPS produced similar respiratory burst responses; these responses were significantly greater than that produced with LPS from P. aeruginosa or S. maltophilia. These findings are in agreement with a recent study (18) which found that neutrophils primed with B. cepacia LPS were able to produce a greater respiratory burst response than those primed with P. aeruginosa LPS. In the present study, priming of MM6 monocytes with B. cepacia LPS led to increased superoxide radical production when the respiratory burst was triggered with opsonized zymosan. Increased superoxide anion production in vivo could overcome antioxidant defenses and result in tissue damage in the lungs, leading to a loss of function (6). This idea suggests that B. cepacia LPS may play a considerable role in tissue damage and lung pathogenesis in CF patients infected with that bacterium. In addition, our previous work (34) showed that on a weight-for-weight basis, LPSs from clinical B. cepacia strains stimulated monocytes to produce significantly larger amounts of TNF-α than LPSs from other CF lung pathogens, such as P. aeruginosa and S. maltophilia, in agreement with other work (30). Our previous work also showed that whole cells and LPSs from clinical B. cepacia isolates stimulated larger amounts of TNF-α than whole cells and LPSs from environmental isolates (34).
Recent studies in our laboratory suggested that LPS priming of the respiratory burst in MM6 cells is dependent on protein kinase C activity (25). We found that LPS priming of monocytes for both the respiratory burst and cytokine production was CD14 dependent. When the CD14 receptor was blocked with antibody, the subsequent respiratory burst was significantly inhibited, as was cytokine secretion.
Previous studies demonstrated that epidemic B. cepacia strains have putative transmissibility markers. Some epidemic strains, such as the highly transmissible ET12 strain, possess cable pili which enhance bacterial adherence to mucin and respiratory epithelium (32). Enhanced binding to cell surface glycolipid receptors (33) and the genomic marker termed the Burkholderia cepacia epidemic strain marker are also putative transmissibility markers (22). In addition, B. cepacia genomvar III was found to be associated with severe pulmonary disease in CF patients (15). However, other factors may facilitate the colonization and consequently the transmission of this bacterium. The highly transmissible United Kingdom epidemic strain of B. cepacia (23) produces a melanin-like pigment. Previous studies on a brown pigment from Proteus mirabilis showed that the pigment was a melanin which was capable of scavenging superoxide radicals (1, 31). In this study, we investigated the possible role of an epidemic B. cepacia melanin-like pigment as a virulence factor which scavenges superoxide radicals and thereby attenuates the respiratory burst response. Our results indicated that this purified highly soluble B. cepacia pigment is able to scavenge superoxide radicals (Fig. 5). The IC50 of the purified melanin-like pigment for superoxide was found to be ∼50 μM, which is comparable to the ascorbic acid IC50 of 35 μM. This result suggests that melanin-producing B. cepacia may derive protection from its free-radical-scavenging properties, which would aid colonization and infection by this organism.
Although the pathogenic mechanisms reported here may appear contradictory, they may be regarded as consistent with the complex etiology of B. cepacia infection in CF patients. In some patients, the acquisition of B. cepacia appears to lead to acute pneumonitis and death (11). We suggest that B. cepacia LPS contributes to underlying inflammation by stimulation of cytokine production and increased levels of reactive oxygen species in the pulmonary tissue. However, in the majority of patients, it is not clear to what extent the acquisition of B. cepacia contributes to the underlying pathology of lung disease. Factors that protect B. cepacia strains from host immune responses, including the leukocyte respiratory burst, will contribute to the persistence of the organism, with consequent pathological effects.
The results of our investigation suggest that melanin-producing strains of B. cepacia may survive phagocytosis through the superoxide-quenching properties of the melanin. Such strains would persist in the lungs, where the LPSs derived from these bacteria would prime pulmonary macrophages and recruit neutrophils for enhanced oxidative and inflammatory reactions. The accumulation of such primed and activated inflammatory leukocytes would contribute to tissue damage and potent inflammatory lung disease, which in some cases might lead to cepacia syndrome. The melanin-producing strain (Cardiff epidemic strain P1, a genomovar III strain which is identical to the United Kingdom epidemic strain of the ET12 lineage) used here represents 50% of B. cepacia carriage by patients attending the Cardiff Paediatric CF clinic. (A total of 9 of 16 patients known to carry B. cepacia carry the melanin-producing strain.) Furthermore, it has been reported that the United Kingdom epidemic strain is also a melanin-producing strain (23). Consequently, melanin production may be yet another factor that aids in the colonization and transmission of certain B. cepacia strains.
REFERENCES
- 1.Agodi A, Stefani S, Corsaro C, Campanile F, Gribaldo S, Sichel G. Study of melanic pigment of Proteus mirabilis. Res Microbiol. 1996;147:167–174. doi: 10.1016/0923-2508(96)80216-6. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht D, Jungi T W. Luminol-enhanced chemiluminescence induced in peripheral blood-derived human phagocytes: obligatory requirement of myeloperoxidase exocytosis by monocytes. J Leukocyte Biol. 1993;54:300–306. doi: 10.1002/jlb.54.4.300. [DOI] [PubMed] [Google Scholar]
- 3.Allen R C. Phagocytic leukocyte oxygenation activities and chemiluminescence: a kinetic approach to analysis. Methods Enzymol. 1986;133:449–493. doi: 10.1016/0076-6879(86)33085-4. [DOI] [PubMed] [Google Scholar]
- 4.Allen R C, Stjernholm R L, Steele R H. Evidence for the generation of (an) electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972;47:679. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- 5.Baehner R L, Nathan D G. Leukocyte oxidase: defective activity in chronic granulomatous disease. Science. 1967;155:835–836. doi: 10.1126/science.155.3764.835. [DOI] [PubMed] [Google Scholar]
- 6.Brown R K, Kelly F J. Role of free radicals in the pathogenesis of cystic fibrosis. Thorax. 1994;49:738–742. doi: 10.1136/thx.49.8.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darke B M, Jackson S K, Hanna S M, Fox J D. Detection of human TNFα mRNA by NASBA. J Immunol Methods. 1998;212:19–28. doi: 10.1016/s0022-1759(97)00211-1. [DOI] [PubMed] [Google Scholar]
- 8.DeChatelet L R, Shirley P S. Evaluation of chronic granulomatous disease by a chemiluminescence assay of microlitre quantities of whole blood. Clin Chem. 1981;27:1739–1741. [PubMed] [Google Scholar]
- 9.Demko C A, Stern R C, Doershuk C F. Stenotrophomonas maltophilia in cystic fibrosis: incidence and prevalence. Pediatr Pulmonol. 1998;25:304–308. doi: 10.1002/(sici)1099-0496(199805)25:5<304::aid-ppul3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Denton M. Stenotrophomonas maltophilia: an emerging problem in cystic fibrosis patients. Rev Med Microbiol. 1997;8:15–19. [Google Scholar]
- 11.Elborn J S, Dodd M, Maddison J, Nixon L E, Nelson J, Govan J, Webb A K, Shale D. Clinical and inflammatory responses in CF patients infected with Pseudomonas aeruginosa and Pseudomonas cepacia. Pediatr Pulmonol Suppl. 1994;10:278. [Google Scholar]
- 12.Galanos C, Luderitz O, Westphal O. A new method for the extraction of R-lipopolysaccharide. Eur J Biochem. 1969;9:245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 13.Goldman D A, Klinger J D. Pseudomonas cepacia: biology, mechanisms of virulence, epidemiology. J Pediatr. 1986;108:806–812. doi: 10.1016/s0022-3476(86)80749-1. [DOI] [PubMed] [Google Scholar]
- 14.Govan J R W, Brown P H, Maddison J, et al. Evidence of transmission of Pseudomonas cepacia by social contact in cystic fibrosis. Lancet. 1993;342:15–19. doi: 10.1016/0140-6736(93)91881-l. [DOI] [PubMed] [Google Scholar]
- 15.Govan J R W, Hughes J E, Vandamme P. Burkholderia cepacia: medical, taxonomic and ecological issues. J Med Microbiol. 1996;45:395–407. doi: 10.1099/00222615-45-6-395. [DOI] [PubMed] [Google Scholar]
- 16.Govan J R W, Nelson J W. Microbiology of lung infection in cystic fibrosis. Br Med Bull. 1992;48:912–930. doi: 10.1093/oxfordjournals.bmb.a072585. [DOI] [PubMed] [Google Scholar]
- 17.Greally P, Hussein M J, Cook A J, Sampson A P, Piper P J, Price J F. Sputum tumor necrosis factor-α and leukotriene concentrations in cystic fibrosis. Arch Dis Child. 1993;68:389–392. doi: 10.1136/adc.68.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes J E, Stewart J, Barclay G R, Govan J R W. Priming of neutrophil respiratory burst activity by lipopolysaccharide from Burkholderia cepacia. Infect Immun. 1997;65:4281–4287. doi: 10.1128/iai.65.10.4281-4287.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonston R B, Keele B B, Jr, Misra H P, Jr, Lehmeyer J E, Werb L S, Baehner R L, Rajagopalan K V. The role of superoxide anion generation in phagocytic bactericidal activity. J Clin Investig. 1975;55:1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lancet. Pseudomonas cepacia—more than a harmless commensal? Lancet. 1992;339:1385–1386. . (Editorial.) [PubMed] [Google Scholar]
- 21.Lipuma J J, Dasen S E, Neilson D W, Stern R C, Stull T L. Person-to-person transmission of Pseudomonas cepacia between patients with cystic fibrosis. Lancet. 1990;336:527–532. doi: 10.1016/0140-6736(90)92571-x. [DOI] [PubMed] [Google Scholar]
- 22.Mahenthiralingam E, Simpson D A, Speert D P. Identification and characterization of a novel DNA marker associated with epidemic Burkholderia cepacia strains recovered from patients with cystic fibrosis. J Clin Microbiol. 1997;35:808–816. doi: 10.1128/jcm.35.4.808-816.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson J W, Butler S L, Krieg D, Govan J R W. Virulence factors of Burkholderia cepacia. FEMS Immunol Med Microbiol. 1994;8:89–98. doi: 10.1111/j.1574-695X.1994.tb00430.x. [DOI] [PubMed] [Google Scholar]
- 24.Ogunnariwo J, Hamilton-Millar J M T. Brown- and red-pigmented Pseudomonas aeruginosa: differentiation between melanin and pyrorubrin. J Med Microbiol. 1975;8:199–203. doi: 10.1099/00222615-8-1-199. [DOI] [PubMed] [Google Scholar]
- 25.Parton J, Darke B M, Jackson S K. Abstract for the 5th Conference of the International Endotoxin Society, Santa Fe, N. Mex. 1998. Priming of the respiratory burst in monocytes: the role of lipopolysaccharide structures and protein kinase C. [Google Scholar]
- 26.Prince A. Antibiotic resistance of Pseudomonas species. J Pediatr. 1986;108:830–834. doi: 10.1016/s0022-3476(86)80753-3. [DOI] [PubMed] [Google Scholar]
- 27.Rossi F, Bellavite P, Dobrina A, Dri P, Zabucchi G. Oxidative metabolism of mononuclear phagocytes. In: van Furth R, editor. Mononuclear phagocytes: functional aspects. The Hague, The Netherlands: Martinus Nijoff; 1980. pp. 1187–1213. [Google Scholar]
- 28.Roubaud V, Sankarapandi S, Kuppusamy P, Tordo P, Zweier J. Quantitative measurement of superoxide generation using the spin trap 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide. Anal Biochem. 1997;247:404–411. doi: 10.1006/abio.1997.2067. [DOI] [PubMed] [Google Scholar]
- 29.Ryley H C, Millar-Jones L, Paull A, Weeks J. Characterisation of Burkholderia cepacia from cystic fibrosis patients living in Wales by PCR ribotyping. J Med Microbiol. 1995;43:436–441. doi: 10.1099/00222615-43-6-436. [DOI] [PubMed] [Google Scholar]
- 30.Shaw D, Poxton I R, Govan J R W. Biological activity of Burkholderia (Pseudomonas) cepacia lipopolysaccharide. FEMS Immunol Med Microbiol. 1995;11:99–106. doi: 10.1111/j.1574-695X.1995.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 31.Sichel G, Corsaro C, Scalia M, Di Bilio A J, Bonomo R P. In vitro scavenger activity of some flavonoids and melanins against O2•−. Free Rad Biol Med. 1991;11:1–8. doi: 10.1016/0891-5849(91)90181-2. [DOI] [PubMed] [Google Scholar]
- 32.Sun L, Jiang R-Z, Steinbach S, Holmes A, Campanelli C, Forstner J, Tan Y, Riley M, Goldstein R. The emergence of a highly transmissible lineage of cable+ Pseudomonas (Burkholderia) cepacia causing CF center epidemics in North America and Britain. Nat Med. 1996;1:661–666. doi: 10.1038/nm0795-661. [DOI] [PubMed] [Google Scholar]
- 33.Sylvester F A, Sajjan U S, Forstner J F. Burkholderia (basonym Pseudomonas) cepacia binding to lipid receptors. Infect Immun. 1996;64:1420–1425. doi: 10.1128/iai.64.4.1420-1425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zughaier, S. M., H. C. Ryley, and S. K. Jackson. Lipopolysaccharide (LPS) from Burkholderia cepacia is more active than LPS from Pseudomonas aeruginosa and Stenotrophomonas maltophilia in stimulating tumor necrosis factor alpha from human monocytes. Infect. Immun., in press. [DOI] [PMC free article] [PubMed]