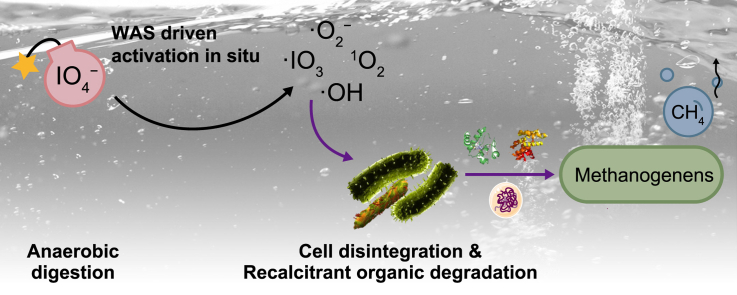
Abstract
The potential of periodate (PI) in sludge anaerobic digestion is not tapped, although it has recently attracted great research interest in organic contaminants removal and pathogens inactivation in wastewater treatment. This is the first work to demonstrate significant improvement in methane generation from waste activated sludge (WAS) with PI pretreatment and to provide underlying mechanisms. Biochemical methane potential tests indicated that methane yield enhanced from 100.2 to 146.3 L per kg VS (VS, volatile solids) with PI dosages from 0 to 100 mg per g TS (TS, total solids). Electron spin resonance showed PI could be activated without extra activator addition, which might be attributed to the native transition metals (e.g., Fe2+) in WAS, thereby generating hydroxyl radical (•OH), superoxide radicals (•O2−), and singlet oxygen (1O2). Further scavenging tests demonstrated all of them synergistically promoted WAS disintegration, and their contributions were in the order of •O2− > •OH > 1O2, leading to the release of substantial biodegradable substances (i.e., proteins and polysaccharides) into the liquid phase for subsequent biotransformation. Moreover, fluorescence and ultraviolet spectroscopy analyses indicated the recalcitrant organics (especially lignocellulose and humus) could be degraded by reducing their aromaticity under oxidative stress of PI, thus readily for methanogenesis. Microbial community analysis revealed some microorganisms participating in hydrolysis, acidogenesis, and acetoclastic methanogenesis were enriched after PI pretreatment. The improved key enzyme activities and up-regulated metabolic pathways further provided direct evidence for enhanced methane production. This research was expected to broaden the application scope of PI and provide more diverse pretreatment choices for energy recovery through anaerobic digestion.
Keywords: Energy recovery, Anaerobic digestion, Recalcitrant organics, Free radicals, Periodate
Graphical abstract
Highlights
-
•
Anaerobic digestion with periodate (PI)-based pretreatment was firstly proposed.
-
•
Waste activated sludge (WAS) could be applied as a nature activator of PI.
-
•
PI pretreatment enhanced the degradation of humus and lignocellulose in WAS.
-
•
PI pretreatment improved hydrolysis rate and biochemical methane potential of WAS.
-
•
Profitable microbial community and metabolic pathways were achieved by PI.
1. Introduction
Waste activated sludge (WAS) has become a serious environmental problem because of its continuous production (predicted yield of up to 103.0 million tons (with 80% water content) per year by 2025) [1] and hazardous substances contained in it, such as pathogens and persistent organic substances [2]. Recovering resources and energy from WAS is gaining enormous interest because it is not merely “waste” but also a vast renewable resource. Methane recovery from WAS is commonly performed through anaerobic digestion (AD), which can effectively achieve several objectives related to waste management policy, environment and energy generation [3]. The low hydrolysis rate and limited methane potential are generally the main limitations of conventional AD, which can be attributed to the limited solubilization degree of WAS and the substantial amounts of recalcitrant substances in WAS, such as humus and lignocellulose [4,5]. So far, most efforts to improve methane generation have been concentrated on enhancing WAS disintegration. The potential inhibitory impact of recalcitrant organics on methane production during anaerobic digestion has been ignored. It was reported that humus and lignocellulose reached 15–28% and 14–44% of the total organics in WAS, respectively [2]. Since these recalcitrant organics contain tightly bound oxygen-containing groups or stable polymers, anaerobic bacteria find it difficult to generate methane from them in conventional AD. Moreover, it has been confirmed that these materials could inhibit methanogens [6]. Thus, AD methane yield might be further improved if part of these recalcitrant organics could be degraded and then used by bacteria. However, scant research effort has focused on this matter.
Recently, sludge pretreatment technology based on the advanced oxidation process (AOP) generating powerful reactive hydroxyl radical (•OH) has been proven to be effective in disintegrating WAS as well as degrading the recalcitrant organics [7,8]. WAS disintegration could release intracellular and/or extracellular substrates for methane production, and the transformation of recalcitrant organics could supply more biodegradable organics or alleviate the inhibitory effects on anaerobic microorganisms [9]. Compared to liquid oxidants (such as hydrogen peroxide), solid oxidants are preferred in some cases because they are relatively stable and safe during transportation, storage, and dosage [10]. Therefore, various solid oxidants have emerged as attractive options for sludge treatment. For example, Wang et al. [2] reported that the reactive radicals (i.e., •OH and superoxide radicals •O2−) generated by 0.14 g per g VSS calcium peroxide (CaO2) increased methane generation by 47% because of the enhanced disintegration of WAS and transformation of recalcitrant organics. Also, Wang et al. [4] found that the strong oxidizing radicals in the sodium percarbonate (SPC) pretreatment system improved the degradation of organics from non-biodegradable to biodegradable materials, thereby promoting methane production. Continuous exploration and development of sludge pretreatment based on advanced oxidation processes are needed.
Periodate (PI, IO4−), a common solid oxidant, has been considered an efficient and promising oxidant for water pollutants destruction and bacterial inactivation [11,12], but there are still very limited applications in sludge treatment. To our knowledge, only one study so far reported the potential of PI on WAS management, in which sludge extracellular polymeric substances (EPS) could be decomposed by PI to improve the dewatering of WAS [13]. Before being used to degrade pollutants, PI was generally required to be activated because the reactivity of PI alone for contaminants oxidation was rather limited [10]. Various activation methods of PI were developed based on alkali [14], transition metals [15], reducing agents [12] and ultrasound [11], producing notably reactive species, such as •OH, •O2−, singlet oxygen (1O2) and iodate radical (•IO3). Among them, iron species (e.g., homogeneous Fe2+, heterogeneous Fe(II), nano zero-valent iron (nZVI)) might be promising options due to their lower costs and higher efficiencies [10]. Iron-based coagulants are usually utilized to promote solid–liquid separation in large-scale WWTPs, leading to the accumulation of Fe species in WAS [16]. According to a survey by Park et al. [17], the Fe content accumulated in WAS was around 2.9–39.7 mg Fe per g TS. Wang et al. [4] found that native irons in WAS (e.g., Fe(Ⅲ)/Fe(II)) could easily activate the SPC, generating reactive radicals such as •OH and •O2−, which improved WAS disintegration and thereby enhanced methane yield in AD.
Based on these findings, it can be hypothesized that WAS could provide natural activators (e.g., transition metals or reducing agents) for activating PI to produce highly reactive species (e.g., 1O2, •O2−, and •OH) in situ. The reactive species could overcome the slow hydrolysis rate and low biochemical methane potential in conventional AD. Meanwhile, these reactive species possessed the capacity to degrade recalcitrant substances (such as humus and lignocellulose) and organic contaminants in WAS [7,18]. If proven effective, PI pretreatment would be economically attractive and environment-friendly for energy recovery from AD, considering the massive quantities of WAS generation.
Therefore, this work aims to elucidate the feasibility of PI pretreatment in improving methane production and its associated mechanisms. Initially, the effects of PI pretreatment on methane production were assessed through biochemical methane potential (BMP) tests combined with model estimation. The synergistic mechanisms were revealed through insights into the biodegradability of pretreated WAS, particularly the conversion of typical recalcitrant humus and lignocellulose, microbial community structure, enzymatic investigations and encoding gene expressions responsible for methanogenic metabolism. Moreover, the identification of reactive species involved in WAS pretreatment and the predominant active radicals was performed by electron spin resonance (ESR) and radical scavenging tests. This study could expand the application of PI and deepen the understanding of advanced oxidation to improve energy recovery from WAS anaerobic digestion.
2. Materials and methods
2.1. Chemicals and sludge
Sodium periodate (PI, 99%, CAS: 7790-28-5) was obtained from Macklin. The WAS and inoculum were collected from the local WWTP's secondary settling tank and anaerobic digester (Tianjin, China). The major characteristics of inoculum were TS = 40.52 ± 0.5 g L−1 and VS = 18.81 ± 0.1 g L−1. The concentration of inoculum met the recommended inoculum conditions (15–20 g VS L−1) for biochemical methane potential (BMP) tests in the standardized protocol [19], which was proved to be adequate for BMP tests. Supplementary Material (Table S1) shows more traits about WAS and inoculum.
2.2. WAS pretreatment procedure using PI
Fermenters of 500 mL were used for WAS pretreatment and each fermenter received 300 mL WAS. Various amounts of PI were added to these fermenters, obtaining the desired PI concentrations of 0, 10, 25, 50, 75, and 100 mg per g TS. All tests were conducted in triplicate. PI could be activated by transition metals existing in WAS; the total Fe content was 14.52 mg per g TS; the concentrations of total Fe and Fe2+ were 288.80 mg L−1 and 103.72 mg L−1, respectively. To study the potential of PI activation in situ, no extra methods or chemicals were employed for activating PI. Pretreatment time was controlled within one day since it was reported that 1–2 days was the optimal time for WAS pretreatment using oxidants [2,20]. After pretreatment, the supernatant was used to perform the biochemical analysis, i.e., soluble chemical oxygen demand (SCOD), soluble proteins and polysaccharides, short-chain fatty acids (SCFAs) and three-dimensional excitation-emission matrix (3D-EEM) fluorescence detection; the remaining sludge was used as the substrate for biochemical methane potential tests. The schematic diagram illustrating the methodology of the entire process is shown in Fig. S1.
2.3. Biochemical methane potential tests and modelling
Methane generation from WAS with and without PI pretreatment was evaluated through BMP tests [21], performed in serum vials with a working volume of 110 mL. Each BMP test vial contained 31 mL WAS and 39 mL inoculum to achieve the ratio of 2.0 (inoculum/WAS) on a dry VS basis, which is the suggested ratio for the standardized BMP tests [19]. Additionally, one blank reactor containing an equivalent volume of Milli-Q water instead of WAS and 39 mL of inoculum was also used to evaluate the methane production from the inoculum. The pH in the reactors was regulated to 7.0 before aeration using high-purity nitrogen. Subsequently, all reactors were hermetically sealed and incubated in a constant temperature incubator shaker (35 ± 1 °C, 120 rpm). All tests were conducted in triplicate. During the BMP tests, the volume and composition of produced biogas (i.e., CH4, H2, CO2) in every reactor were recorded daily over the first week and every 2–5 days afterwards. BMP tests lasted 50 days until the daily methane generation in three consecutive days was <1% of the accumulated methane volume. The generated volume of biogas was first determined based on the pressure increase in the headspace of the BMP test vials using a water-communication container and recorded at standard conditions (1 atm, 25 °C) at the start of each gas sampling event [22]. Subsequently, biogas composition was measured by a gas chromatograph equipped with a thermal conductivity detector (Lunan SP-7980 Plus). The methane volume was calculated by multiplying the biogas volume by the (%) of CH4 in the headspace as determined by GC analysis. The methane production was reported as the volume of methane produced per kilogram of VS added (L CH4 per kg VS) [23]. The one-substrate model was used to estimate the methane production kinetics and potential of WAS [23]. VS destruction (%) of WAS in anaerobic digestion was evaluated according to Wei et al. [24].
2.4. Evaluation of methane generation from the PI-treated lignocellulose and humus
Lignocellulose and humus are the major recalcitrant organics in WAS [5]. PI pretreatment might improve the degradation of these recalcitrant substances. To study whether their degradation products or intermediates could be used for producing methane, batch tests were performed. These tests were divided into three groups, each consisting of three identical 500-mL reactors. In the blank group, each reactor was fed with 300 mL of tap water. The other groups were fed synthetic wastewater, one without PI addition (named here as control) and the other with 447 mg PI. The synthetic wastewater contained commercial alkaline lignin (CAS: 8068-05-1) and humic acid (CAS: 68131-04-04), respectively regarded as model lignocellulose and humus, replacing the real lignocellulose and humus contained in WAS. The contents of alkaline lignin and humic acid accounted for 30% and 20% of the VSS in WAS used in Section 2.2. The concentrations of model lignocellulose and humus were according to Refs. [5,6]. Based on BMP test results, a modest PI dosage of 447 mg was set, equivalent to that added into the 75 mg PI per g TS reactor in Section 2.2. To consider both low and high concentrations of PI, the concentration of 75 mg PI per g TS was used in this study to explore its mechanisms. After 1-day treatment, the fluorescence spectroscopy and ultraviolet (UV) absorption spectrum were employed to analyse these recalcitrant organics' degradation. Subsequently, a 15 mL mixture from each reactor was transferred to the serum vials (110 mL) containing the anaerobically digested sludge (inoculum) of 55 mL for BMP tests. The digestion conditions were the same as in Section 2.3. The assays lasted four days, and methane generation was measured daily. By comparing the methane yield (reported as the volume of methane produced (i.e., mL CH4)) in each reactor, the possibility of methane production from humus and lignocellulose degradation products (or intermediates) due to PI treatment could be evaluated [2].
2.5. Identification of reactive species in the pretreatment system
During the pretreatment phase, PI might be activated in situ by WAS. Hence, the reactive species (e.g., •OH, 1O2, and •O2−) could be major intermediates. To verify this speculation, WAS mixture (1 mL) after 20 min of pretreatment was extracted to perform ESR tests. 2,2,6,6-tetramethyl-4-piperidinol (TEMP) was used to trap 1O2, and 5,5-dimethyl-1-pyrroline (DMPO) was used to trap •O2− and •OH. Measurements of reactive radicals were based on an ESR spectrometer (Bruker EMXnano, Germany), according to Wang et al. [4]. Detailed information is shown in Supplementary Material. To further assess the potential contributions of these reactive species for WAS disintegration, the batch scavenging experiments were performed in the presence of different oxidant scavengers (i.e., phenol, tert-butyl alcohol (TBA), p-benzoquinone (p-BQ), and furfuryl alcohol (FFA)). It was reported that TBA, p-BQ, and FFA were used as •OH (k•OH = 3.8–7.6 × 108 M s−1), •O2− ( = 9.8 × 108 M s−1), and 1O2 (k1O2 = 1.2 × 108 M s−1) scavenger [25], respectively. Besides, phenol has been documented as a scavenger for both iodine radicals (e.g., •IO3 and •IO4) and •OH [26]. More information was presented in Supplementary Material.
2.6. Analysis of microbial community and functional gene prediction
The changes in the microbial communities of the sludge samples from digesters (0 and 75 mg per g TS) after 50-day methanogenic fermentation were analyzed by 16S rRNA sequencing. Two primers were 515FmodF and 806RmodR [27]. To further expand our knowledge of the anaerobic digestion microbiome, the metabolic potential and functional pathways of microorganisms at the genetic level were predicted as complementary information of 16S RNA sequencing through the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) software based on KEGG (Kyoto Encyclopedia of Gene and Genome) pathway annotation [28]. More information was provided in Supplementary Material.
2.7. Analytical methods
SCOD, VS, and TS were determined by standard methods [29]. SCFAs and methane contents were determined based on previous methods using gas chromatography [30]. Details were summarized in Supplementary Material. During the anaerobic digestion process, the solubilization and hydrolysis of sludge flocs occur simultaneously [31]. As previously reported [32], the hydrolysis efficiency could be quantitatively estimated by ΔSCOD/TCOD, where TCOD was the initial total COD of WAS and ΔSCOD was the difference between the initial and terminal COD. The biodegradability of fermentation liquor was analyzed through the percent fluorescence response (Pi,n) of EEM and more details were described in Supplementary Material. The activities of key enzymes at the end of BMP tests were detected by spectrophotometric methods [33]. I2 and I3− were analyzed by a starch colorimetric method [34]. The aromaticity of humus and lignocellulose was evaluated by a UV–Vis spectrophotometer.
2.8. Statistical analysis
Batch experiments in this study were performed in triplicate. The statistical significance of the data was confirmed by analysis of variance (ANOVA) with the LSD post-hoc test using SPSS 25.0; ∗P < 0.05 and ∗∗P < 0.01 were respectively defined as statistically significant and highly significant.
3. Results and discussion
3.1. Methane generation from PI-pretreated WAS in anaerobic digestion
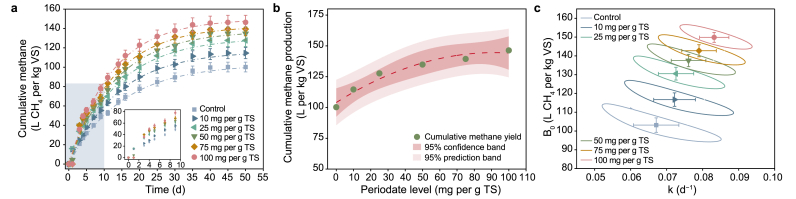
The cumulative methane yield from WAS pretreated with varying PI dosages is shown in Fig. 1a. Stable state in each case was achieved after 50 days, indicating complete anaerobic digestion. For the control (without PI pretreatment), the cumulative methane production was 100.2 L per kg VS, which was lower than that of the PI-pretreatment systems. The maximum methane yield increased non-linearly with increasing PI levels from 0 to 100 mg per g TS (y = 104.01 + 0.88x − 0.005x2, R2 = 0.93, Fig. 1b). The cumulative methane yield reached 146.3 L per kg VS at 100 mg per g TS, showing a significant increase of 46.0% compared to the control. The maximal increase of methane production achieved by PI pretreatment was comparable to that reported previously (47.6% increase under 0.14 g per g VSS CaO2 treatment) [2], indicating a good performance for enhancing methane production through PI pretreatment. Further calculations suggested that VS destruction during anaerobic digestion was enhanced by 46.1% compared to the control (Fig. S2). The improved methane generation and better VS destruction implied a reduction in the amount of WAS, which could be expected to cut down the corresponding sludge disposal and transport costs in WWTPs [35]. From the relationship between the maximum cumulative methane yield and PI levels (Fig. 1b), the increasing trend of maximum methane yield gradually slowed down, implying that superfluous PI might not further lead to methane increase, considering the sensitivity of methanogens. It should be noted that the scope of PI dosages was only instructive and not optimized, given that the major aims of this work were performance verification and mechanism analysis. The profile of biogas production is presented in Fig. S3. The average proportion of methane in biogas from WAS with PI pretreatment was always higher than that in control (Fig. S4), which suggested that PI pretreatment caused more carbon to be biologically converted into methane. The possible reason is that PI pretreatment could improve the activities of hydrogenotrophic methanogens [1], resulting in the improved metabolic reduction of CO2 to CH4. The enhanced activity of coenzyme F420 was the direct evidence for this phenomenon (Table S4).
Fig. 1.
a, Cumulative methane from waste activated sludge (WAS) pretreated by varying periodate (PI) levels during anaerobic digestion. The dotted line represents the results of model simulated. b, The relationship between the maximum cumulative methane yield and PI levels. c, The confidence regions of 95% about the hydrolysis rate (k) and biochemical methane potential (B0) based on the model simulation. Error bars represented standard deviation of triplicate tests.
The one-substrate model was further used to estimate the effect of PI pretreatment on methane production. Results indicated that the proposed kinetic model satisfactorily fitted the measured data (R2 > 0.96) (Fig. 1a). The obtained kinetic parameters based on model analysis could reflect the effects of PI pretreatment on hydrolysis rate (k) and biochemical methane potential (B0) of WAS during anaerobic digestion. Table S2 listed the obtained k and B0 in all cases. Overall, k and B0 rose with increasing PI dosages, which is consistent with the tendency of methane generation. The largest elevations in k and B0 at 100 mg PI per g TSS were around 23.9% (from 0.067 to 0.083 d−1) and 45.3% (from 103.14 to 149.84 L CH4 per kg VS), respectively, to that in the control reactor. The 95% regions of k and B0 also supported such trends (Fig. 1c), which moved to the right and upward after PI pretreatment, indicating a faster hydrolysis rate and greater biomethane potential. Correspondingly, VS destruction potential (Y) increased from 15.4% to 22.5% with increased PI levels (Table S2). To sum up, the above tests revealed that PI pretreatment effectively enhanced methane generation in anaerobic digestion by increasing both the hydrolysis rate and methane production potential of WAS.
3.2. Characteristics of pretreated WAS
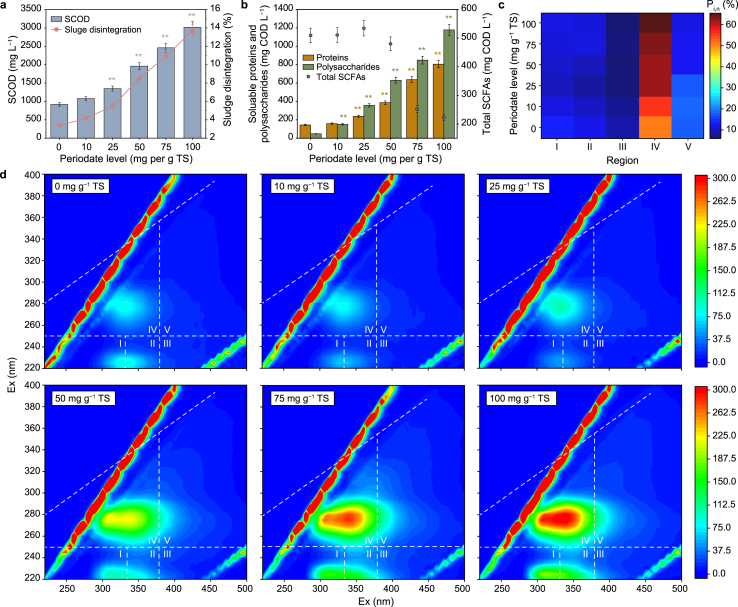
Anaerobic digestion is a multi-step process involving solubilization, hydrolysis, acidification, and methanogenesis. The solubilization of sludge, in which the organics in WAS require to be released to the liquid phase before being utilized, was recognized as the rate-limiting process. Thus, a high-efficient WAS disintegration was the precondition to improve anaerobic digestion performance. Fig. 2a showed that elevated PI concentrations resulted in an increased release of SCOD; the SCOD content after adding 100 mg PI per g TS increased 2.29 times, from 920 mg L−1 in the control to 3020 mg L−1. The 3.06-fold increase of sludge disintegration relative to the control with 100 mg PI per g TS addition further confirmed that PI made WAS cells and EPS become soluble. The soluble substrates in WAS are primarily composed of proteins and polysaccharides [22]. The release of soluble proteins and polysaccharides from WAS exhibited a PI concentration-dependent trend (Fig. 2b). When PI levels were raised from 0 to 100 mg per g TS, soluble proteins and polysaccharides were enhanced by 4.57 and 23.43 times, respectively, in comparison to the control. Since they directly contributed to the hydrolysis process, hydrolysis efficiency remarkably increased after PI pretreatment (Fig. S5). However, SCFAs after the pretreatment stage declined with the increase of PI levels (Fig. 2b), which might be attributed to the inhibitory effect of acidogenic enzymes at higher PI levels [23]. These results showed that the main cause of the improvement of methane production in the methanogenic stage was the increased solubilization and hydrolysis of WAS because of PI pretreatment, leading to increased available substrates (e.g., proteins and polysaccharides) for methanogenesis. Apart from biodegradable substances, a substantial quantity of non-biodegradable substances was also released from WAS solid phase after PI pretreatment [36]. Previous studies reported that oxidation agents (e.g., peroxymonosulfate (PMS) and CaO2) could transform a portion of non-biodegradable organics into biodegradable ones [2,7]. Here, whether PI addition could improve the bioavailability of the released organics was investigated based on the analysis of 3D-EEM fluorescence spectra. The organics were divided into five types, located in five regions (Table S3). Among them, regions I and IV were supposed to be biodegradable organics, and regions III and V were viewed as non-biodegradable organics. The significant increase of fluorescence intensities of EEM peaks proved the remarkable release of soluble organics in WAS after PI pretreatment (Fig. 2c). Moreover, the heatmap of fluorescence response (Pi,n) of regions I–V suggested that the biodegradability of fermentation liquor significantly increased. Specifically, the Pi,n of region IV was 50.25% in the control, increasing to 64.53% with 100 mg PI per g TS addition. This increase in biodegradability was attributed to the release of biodegradable components (i.e., proteins and carbohydrates) and the degradation of non-biodegradable substances. For example, the Pi,n of region III (regarded as fulvic acid-like compounds) and region V (regarded as humic acid-like substances) declined from 7.96% to 17.31% in the control to 5.05% and 10.87%, respectively with the addition of 100 mg PI per g TS. The potential reason for this decrease might be due to the powerful oxidative activity of PI (E0 = +1.60 V vs. standard hydrogen electrode (SHE)) [37], which could destroy carbon double bonds and benzene rings and thereby benefit the degradation of recalcitrant organics [5]. On the other hand, the production of strong oxidizing radicals in PI-pretreatment systems, i.e., •OH, •O2−, and 1O2, might promote the conversion of refractory substances. In the following section, we discuss the potential mechanisms of active radicals. It should be emphasized that the transformation of non-biodegradable substances could not only provide more substrates for subsequent utilization (i.e., methanogenesis) but also alleviate the inhibitory effects on the hydrolysis and methanogenesis during anaerobic digestion [6].
Fig. 2.
a, Soluble chemical oxygen demand (SCOD) release and the degree of sludge disintegration (%). b, Soluble proteins and polysaccharides release, short-chain fatty acids (SCFAs) generation. c, Fluorescence matrix profiles and fluorescence response (Pi,n) percentages of fermentation liquor at the end of 1-day pretreatment with different PI dosages. Asterisks (∗) indicate the significant differences with control (one-way ANOVA). ∗P < 0.05, significant; ∗∗P < 0.01, highly significant.
3.3. Understanding the mechanisms of PI pretreatment
3.3.1. Contribution of the active radicals
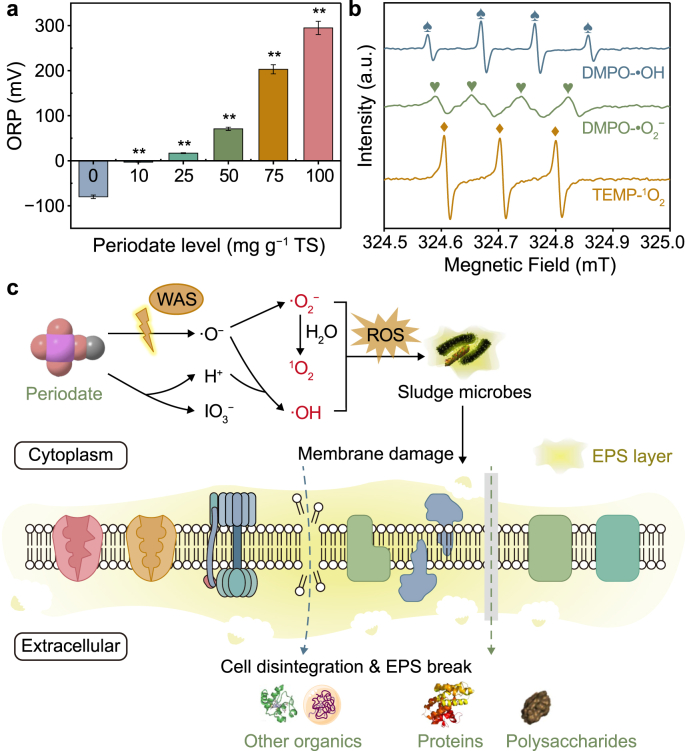
Oxidation-reduction potential (ORP) could intuitively explain the redox characteristics of solution processes [16]. The introduction of PI turned the WAS environment from a reductive (ORP = −80 mV) to an oxidative state (ORP = 295 mV), as shown in Fig. 3a. Owing to its relatively high E0 (+1.60V), PI was expected to increase ORP of WAS. Apart from the strong oxidation potential of PI itself, it hypothesized that it would induce a series of free radical reactions in view of the native activators in WAS (e.g., 14.52 mg per g TS of total Fe).
Fig. 3.
a, Variations of oxidation-reduction potential (ORP) in the pretreatment systems with different periodate (PI) levels. b, ESR spectra of TEMP-1O2 and DMPO-•OH adducts in water, and DMPO-•O2− adduct in methanol. c, The schematic illustration of PI pretreatment. Asterisks (∗) indicate the significant differences with control (one-way ANOVA). ∗P < 0.05, significant; ∗∗P < 0.01, highly significant.
The reactive species existing in the PI-pretreatment system were identified through ESR spectroscopy. As shown in Fig. 3b, the DMPO-1O2 (with an intensity of around 1:1:1 and hyperfine splitting constants of αN = 7.2 G and αH = 4.1 G), DMPO-•O2− (with an intensity of around 1:1:1:1 and hyperfine splitting constants of αN = 14.25 G and αH = 12.45 G), and DMPO-•OH (with an intensity of around 1:2:2:1 and hyperfine splitting constant of αN = 14.25 G and αH = 12.45 G) characteristic peaks in ESR spectra were detected. This indicated that 1O2, •O2−, and •OH were generated by PI under an ordinary WAS fermentation system. The detection of these reactive species directly confirmed the speculations that PI could be activated by WAS, which corroborated the observations by Wang et al. [4], in which SPC was activated by WAS in situ to generate •OH, •O2−, and •CO3−. It is worth noting that the identification result of the Fe2+ accumulated in WAS was 103.72 mg L−1, and the introduction of PI dosage was equivalent to 1490 mg L−1 in the 75 mg per g TS reactor, which was in agreement with the Fe2+ dose and appropriate molar ratio to activate PI reported in previous studies [10,11]. Thus, the “inherent” capacity of activating PI by WAS was reasonable to be ascertained. A plausible process of PI activation by WAS was proposed as follows. The activation of PI might be accompanied by Fe redox transformation. Homogeneous Fe2+ contained in WAS participated in the activation process of PI through an electron transfer mechanism [11], forming •O2− (equation (1)). The precursor •O− was produced by the I–O bond cleavage in PI after activation [26]. The •OH might be generated through the protonation of •O−, followed by the reaction of IO4− and OH− to form •O2−. Furthermore, 1O2 evolved from the reaction between IO4− and •O2− as well as the recombination of •O2− (equation (2)–(5)) [15]. It is worth noting that sludge is a reservoir of various metal ions (e.g., Fe2+, Cu2+, Zn2+, Co2+) [6], which have also been recorded to activate PI [15]. More potential activation pathways need to be further explored.
| Fe2+ + IO4− + H2O → Fe3+ + IO3−+ •O2−+ 2H+ | (1) |
| •O− + H+ → •OH | (2) |
| 3IO4− + 2OH− → 3IO3−+ 2•O2−+ H2O | (3) |
| IO4− + 2•O2− + H2O → IO3−+ 2OH− + 21O2 | (4) |
| 2•O2− + 2H2O→ 1O2+ H2O2 +2OH− | (5) |
Scavenging experiments could further support the observations in ESR tests and evaluate the potential contributions of these reactive species. As illustrated by the results from the scavenging experiments (Fig. S6), TBA inhibited the effect of PI on WAS solubilization, with a 12.5% decrease of SCOD release compared to the reactor with 75 mg PI per g TS treatment, which indicated the important role of •OH in PI pretreatment system. In comparison, phenol inhibited the SCOD release; the SCOD concentration decreased from 2538 mg L−1 to 1692 mg L−1, indirectly showing that iodine radicals might also contribute to WAS disintegration in the PI pretreatment system despite that the conclusive method for the identification of iodine radicals was still unavailable [34]. Besides, adding p-BQ reduced the SCOD release from WAS by 43.1% compared to the 75 mg PI per g TS treatment, suggesting the remarkable role of •O2−. In contrast, the slight inhibition of FAA indicated that 1O2 plays a minor role in the PI pretreatment process. Based on these results, it could be speculated that the contributions of the measured reactive species can be arranged in this order of importance: •O2− > •OH > 1O2.
The use of ESR for the detection of reactive species and the indirect evaluation of potential contributions of these species via specific scavengers indicated that the improvement of WAS disintegration during pretreatment resulted from the synergetic effects of these reactive species generated by native WAS activation. Accordingly, the schematic illustration of WAS disintegration during PI pretreatment is summarized in Fig. 3c. After adding to WAS, PI could be readily decomposed to IO3− and, meanwhile being activated by native transition metals (e.g., Fe2+) in WAS to produce 1O2, •O2−, and •OH. These powerful radicals could attack microbial envelope, leading to cells membrane leakage and intracellular components solubilization [38], which was corroborated by the increased release of soluble COD (Fig. 2a).
3.3.2. Impact of PI pretreatment on recalcitrant substances
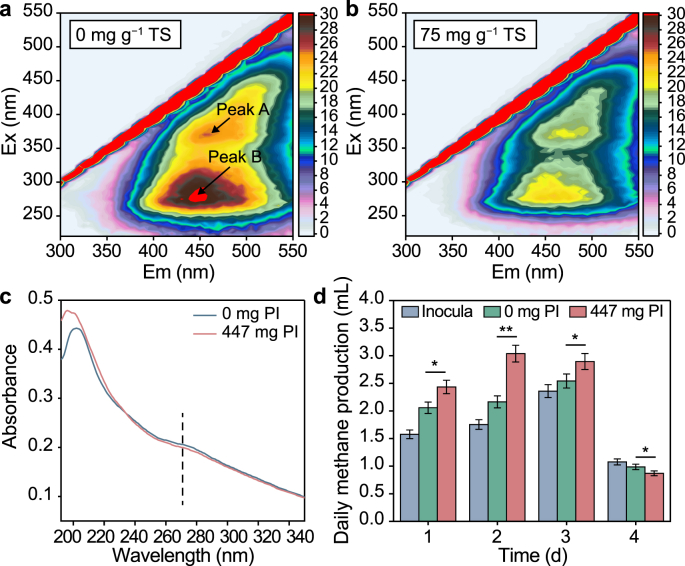
As shown in Fig. 2, apart from the beneficial biodegradable substrates (e.g., polysaccharides and proteins), some aggregated recalcitrant organics in WAS were also released after PI pretreatment due to the disintegration of extracellular polymeric substances matrix [39]. Among these recalcitrant organics, lignocellulose and humus are the key inhibitory substances suppressing both hydrolytic and methanogenic processes [6]. To understand whether PI addition could improve the breakdown of these recalcitrant organics, the degradation mechanism was assessed by model substrates. As shown in Fig. 4a and b, the maximum fluorescence intensities of characteristic fluorescent peaks (Peak A and B) of lignocellulose and humus were greatly reduced from 25.45 a.u. and 30.94 a.u to 20.22 a.u. and 22.26 a.u., respectively, after 1-day PI treatment, showing decreased concentrations of model recalcitrant organics. Yang et al. [7] reported that powerful oxidizable materials could attack the C C bonds in aromatic rings. Xu et al. [5] found that KMnO4 (E0 = +1.68 V vs. SHE) could damage the benzene and aromatic C C bonds in the structure of humus and lignocellulose. A similar observation found that the reactivity of CaO2 (E0 = +1.78 V vs. SHE) caused the rupture of benzene rings in the lignocellulose structure [2]. Since PI (E0 = +1.60V vs. SHE) has a similar redox potential to KMnO4 and CaO2, the degradation of humus and lignocellulose by PI treatment might be because PI effectively destroyed the benzene rings and C C bonds in these recalcitrant organics. The decreased absorbance with PI addition in the UV absorption spectrum at ∼270 nm further confirmed the destruction of aromatic structures (Fig. 4c). The destruction of benzene rings decreased the energy of π→π∗ electronic transition and the obstacle of steric hindrance, thus boosting their conversion [40].
Fig. 4.
a–b, Fluorescence excitation-emission matrix (EEM) spectra of mixed matrix of model lignocellulose and humus without PI (a) and with PI treatment (b). c, UV absorption spectra of mixed matrix of model lignocellulose and humus. d, Daily methane production from model lignocellulose and humus with and without PI treatment. Note: the dosage of PI was equal to the fermenter with 75 mg PI per g TS pretreatment. ∗P < 0.05, significant; ∗∗P < 0.01, highly significant.
The daily methane production with and without PI treatment is displayed in Fig. 4d to reflect whether lignocellulose and humus or their degradation intermediates could serve as substrates for methane generation. It was found that 6.77 mL of methane was generated from the inoculum due to the existence of available substrates in the inoculum [2]. The total methane yield of the reactor supplemented with model lignocellulose and humus (without PI addition) was higher than that from sole inoculum, suggesting methane could be produced by using lignocellulose and humus without extra substrates, which exhibited the adaptation capacity of methanogens. Interestingly, the daily methane production in the reactor with PI addition was slightly lower than that of the control reactor on the fourth day of digestion, implying that the substrate consumption rate and methane production potential were enhanced by PI. This phenomenon was consistent with previous studies [33,41], in which the daily methane production rate was slow when it was close to the maximal methane production potential. Further calculation indicated that total methane yield from model lignocellulose and humus with PI treatment could be enhanced by 1.57 times compared to that without PI treatment. These observations reflected that PI could enhance the direct utilization of recalcitrant organics by anaerobic microbes to generate methane. The transformation from recalcitrant organics to methane corresponded to a further reduction in the amount of sludge [41]. Thus, better solids reduction could be expected under PI pretreatment.
3.4. Effects of PI pretreatment on microbial community succession
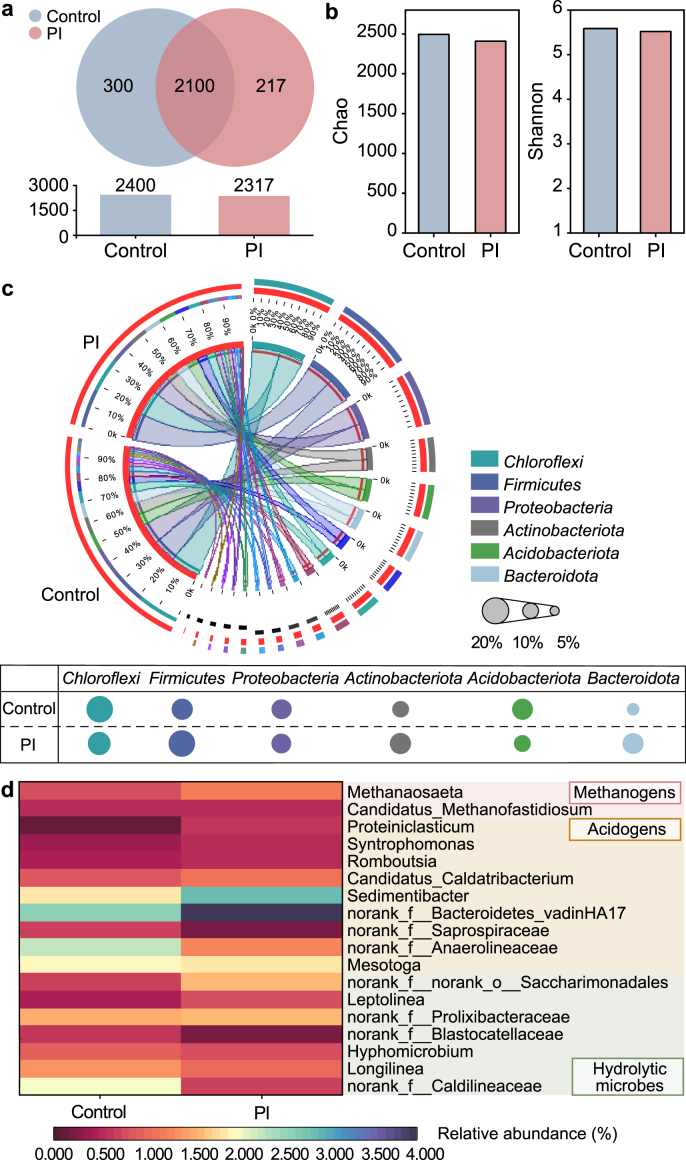
Effects of PI pretreatment on the community succession were elucidated in Fig. 5. Venn analyses indicated that operational taxonomic units (OTUs) present in the experimental digester were slightly smaller than the control (2317 vs. 2400), with 2100 OTUs being shared (Fig. 5a). It suggested that the microbial diversity declined after PI pretreatment. The quantities of individual OTUs in the experimental digester decreased from 300 to 217, suggesting that PI might cause a new type of digestion environment [32]. Alpha diversity results in Fig. 5b showed that the richness index (Chao = 2409.55) and the diversity index (Shannon = 5.52) of the experimental digester were lower than that in the control (Chao = 2494.69 and Shannon = 5.59), which further revealed that the microbial diversity and richness were slightly suppressed by PI. These results were consistent with previous findings that cells of some anaerobes participated in anaerobic digestion might be destroyed or inactivated under oxidization stress [33].
Fig. 5.
Microbial community evaluations of the control and experimental (with 75 mg PI per g TS pretreatment) digesters. a, Venn diagram based on OUTs. b, Alpha diversity comparisons. c, The circos plot of the phylum level. d, The microbial heatmap of the genus level.
The microbial composition at the phylum level was shown in Fig. 5c using Circos. These observed phyla were ubiquitous in anaerobic digesters. The top six populations were Chloroflexi, Firmicutes, Proteobacteria, Actinobacteriota, Acidobacteriota and Bacteroidota, accounting for 68.8% and 70.6% of the entire populations in the experimental and control digesters, respectively. It could be found that Firmicutes and Bacteroidota presented higher abundances in the experimental digester than that in the control (17.4% vs. 15.8% and 7.9% vs. 6.9%). These two phyla are capable of converting organics to SCFAs [27]. The improved biodegradability of WAS could be the important reason for the enrichment of Firmicutes phylum, which tends to utilize readily biodegradable organics (such as soluble polysaccharides and proteins) [42,43]. Additionally, it was reported that the microorganisms belonging to Firmicutes could degrade cellulose, lignin and their intermediate products through secreting hydrolases (such as protease and cellulase) [4]. This further supported the conclusion that PI pretreatment improved the degradation of recalcitrant substances (e.g., lignocellulose) in WAS.
The main generic level heatmap associated with hydrolysis, acidification and methanogenesis was displayed in Fig. 5d. Their relative abundances in the two digesters presented significant differences. Two methanogens (hydrogenotrophic Candidatus_Methanofastidiosum and acetoclastic Methanosaeta) were found in the digesters [30,44]. Compared to hydrogenotrophic methanogens, acetoclastic methanogens (i.e., Methanosaeta) were more affected. Specifically, the abundance of Methanosaeta in the experimental digester (1.22%) had an increase of 54.4% in comparison to the control (0.79%). Obviously, PI pretreatment provided an advantageous condition for the strict acetoclastic methanogens, namely the large increase of acetate kinase activity (see Table S4) and the enrichment of acetate-generated functional microbes (generally affiliated to Firmicutes) [45]. Besides methanogens, some microorganisms associated with hydrolysis and acidification were enriched after PI pretreatment. For example, Longilinea, utilizing carbohydrates for SCFAs production [30], increased by 74.5% relative to the control. The significant increase of polysaccharides (i.e., 23.43 times) (Fig. 2b) via PI pretreatment could cause Longilinea enhancement. Four bacterial genera responsible for SCFAs generation, namely norank_f__Bacteroidetes_vadinHA17, Sedimentibacter, Romboutsia, and Candidatus_Caldatribacterium [4,20], increased by 45.2% relative to the control. In summary, PI pretreatment positively impacted these hydrolytic and acidification-related microorganisms, resulting in adequate substrates for co-existed methanogens (particularly acetoclastic methanogens), echoing the higher available substrates and methane yield in the pretreated reactor and anaerobic digester (Fig. 1, Fig. 2).
3.5. Effects of PI pretreatment on the critical enzymes and genes expressions
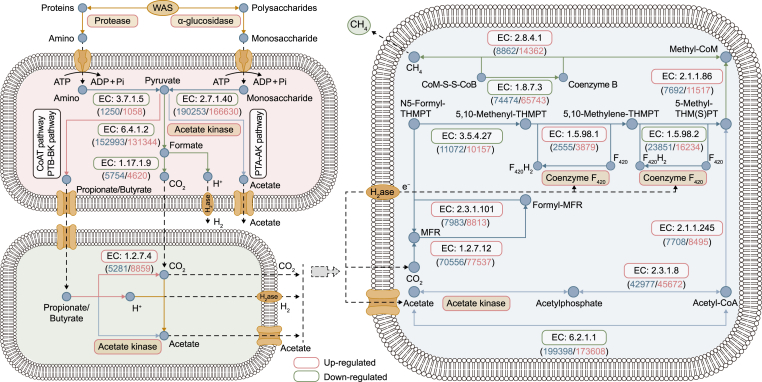
Although the effects of PI pretreatment on the hydrolytic rate of substrates have been clarified by the kinetic model (Fig. 1c), the hydrolytic efficiency also depended on the specific enzyme activities [46]. Protease and α-glucosidase could control soluble proteins and polysaccharides hydrolysis [47]. The improved activities of these two enzymes after PI pretreatment benefited the transformations of proteins and polysaccharides to simple molecules (e.g., amino acid and glucose) for the utilization of acetogens and methanogens (Fig. 6 and Table S4). The enhanced activity of acetate kinase strengthened the conversion of acetyl-CoA to acetic acid, benefiting the acetate-dependent methanogenesis [48]. Further, coenzyme F420, responsible for all methanogenesis pathways, increased in response to PI dosage (Table S4). Although a previous study reported that methanogenic archaea had low tolerance to a strong oxidating environment [48], the dilution effects of inoculum offset the possible inhibition of PI on coenzyme F420. More importantly, the remarkable improvement of biodegradability of pretreated WAS, including the release of biodegradable substrates from the cells and the decrease of refractory organics under the oxidation effects of PI, and the subsequent shifts of functional microbial populations, might play positive roles in stimulating the expressions of these enzymes.
Fig. 6.
Schematic illustration of metabolic pathways for the expressions of key enzymes responsible for hydrolysis-acidogenesis, homoacetogenesis, and methanogenesis. Nodes represent intermediate compounds in the metabolism cycles, boxes represent the specific enzymes information, and blue and pink numbers in the parentheses represent the abundances of the encoding gene of functional enzymes in the control and experimental (with PI pretreatment) samples, while the variations of α-glucosidase, protease, acetate kinase, and F420 coenzyme were directly indicated by their activities (Table S4).
Apart from directly assessing the key critical enzymatic activities discussed above in the methanogenic metabolic pathways, the functional gene abundances of other enzymes were studied based on the KEGG database employing PICRUSt software to predict the impacts of PI on the metabolic pathways in anaerobic digesters. As is known, the metabolic pathways of methanogenesis compose of two types, namely, the utilization of acetate or carbon dioxide with hydrogen. As shown in Fig. 6 and Table S5, the quantities of genes acs coding acetyl-CoA synthetase [EC:6.2.1.1] for acetoclastic reaction slightly declined. Nevertheless, this was not a contradiction. It implied that methane production was related to the multiple mechanisms of action. For the CO2-type pathway, the abundances of gene fwdABCDE, ftr, and mtd, encoding formylmethanofuran dehydrogenase [EC:1.2.7.12], formylmethanofuran:tetrahydromethanopterin formyltransferase [EC:2.3.1.101], and methylenetetrahydromethanopterin dehydrogenase [EC:1.5.98.1], respectively, were higher than that of the control. Moreover, the abundances of mtrDGH and mcr encoding coenzyme M methyltransferase [EC:2.1.1.86] and methyl-coenzyme M reductase [EC:2.8.4.1], respectively, increased, promoting the catalytic action for the terminal methane release [49]. Herein, the visualized gene-level evidence demonstrated that the up-regulated expressions of key functional genes were probably the direct cause of PI pretreatment enhancing methane production. It should be pointed out that the forecasted functional gene abundance was only indicative information at the genetic level; an in-depth investigation into this should be conducted with multi-omics techniques.
3.6. Implications
Sludge pretreatment technologies based on free radicals were highly efficient in boosting methane generation from anaerobic digestion. PI was not yet reported in sludge treatment applications, although it has been used for the oxidation of various organic pollutants in water treatment owing to its great stability and safety during transportation and storage. This is the first study regarding PI pretreatment's role in increasing methane production. It was found that PI pretreatment not only greatly boosted the WAS disintegration but also reduced the restriction of low hydrolysis rate for anaerobic digestion; besides, it increased the conversion of recalcitrant substances, particularly lignocellulose and humus, thus offering more available substrates for methanogenesis. During the pretreatment process, PI could be activated by WAS without any extra activator. The generated active species, especially •O2− and •OH, played important roles in WAS solubilization. Further explorations revealed that the benefic shifts of microbial communities involved hydrolytic and acidification-associated microorganisms, especially acetoclastic methanogens. The up-regulated key enzyme encoding genes helped to explain the improved methane production. These observations could enlarge the application scope of PI and give insights into the potential of WAS as a native activator for oxidants-based pretreatment strategy; moreover, this perspective might not be limited exclusively to PI.
It is worth noting that hazardous iodine species (i.e., I2 and I3−) were not detected in the digestion liquor (Fig. S7) because of the difficulty for PI to convert into low-valence iodine species [34], thereby decreasing the potential environmental risks of PI-based pretreatment. Previous studies have recorded that PI was rapidly consumed with minor residual and stoichiometrically converted to IO3− (nontoxic) in the PI-based treatment system [12,50]. The environment-friendly end product of PI (i.e., IO3−) was also a critically attractive reason for PI-based AOPs [51,52]. A limitation of the present work was the inevitable chemical inputs. BMP tests could be conservatively used to evaluate the potential economic and environmental feasibility of the pretreatment technologies [23,41,53]. Herein, the net benefits was evaluated through desktop scaling-up research on an actual WWTP with a scale of 0.4 million population equivalent (PE) and with a solid retention time (SRT) of 30 days in an anaerobic digester. Based on the results from Fig. 1a, the case of 75 mg PI per g TS pretreatment with a 30-day digestion time, which obtained the highest methane yield, was used to conduct the economic and environmental evaluations. According to the calculation presented in Table S6, compared to the control, PI-based pretreatment could save $11,480 per year in the economy and reduce CO2 emission by 940,800 kg per year in the environment (see SM for details). These net benefits are attributable to the enhanced methane yield resulting in the increased conversion to heat and power, decreased WAS transport and disposal costs, and reduced fossil fuel consumption. Nevertheless, the cost benefits described here should only be considered indicative because they depend on local conditions [23]. An economic comparison with other pretreatment technologies was not performed. Because this process could be impacted by many factors, such as operating conditions and WAS characteristics. Certainly, the relatively high chemical inputs of PI pretreatment might not be competitive for energy recovery in industrial-scale anaerobic digestion of WAS. But it should be noted that the present work focused on revealing the possibility and insights into the underlying mechanisms of PI-based technology. Efforts are still needed to reduce the chemical inputs and further improve methane production efficiency. For instance, the combination with other strategies (e.g., freezing) could be considered. Choi et al. [54] found that the freeze concentration could provide an ice grain boundary for the proton-coupled electron transfer and thereby greatly activate PI to generate more powerful reactive species. Freezing treatment could be easily achieved in the field with cold climates or using electrical refrigeration in warm-temperate climates, while the electrical energy could be generated in situ through combusting methane generated in digesters [55]. Moreover, the IO4−/freezing system had a nonselective degradation capacity, which was used to degrade various pollutants, e.g., pharmaceutical and phenolic compounds [54]. It is known that WAS is a sink of organic pollutants [56] with a high octanol-water partitioning coefficient (log KOW), which restricts their bio-availability and poses environmental and human-health risks [57,58]. The highly nonspecific degradation ability of IO4−/freezing could increase methane production and provide an opportunity for the degradation of organic contaminants accumulated in WAS. However, this is only a reasonable conjecture, and further research is needed. Besides, the in-situ decrease of H2S production in anaerobic WAS fermentation using KMnO4 pretreatment had been confirmed because the oxidative stress deriving from KmnO4 (E0 = +1.68 V, very close to E0(PI) = +1.60 V) inhibited sulfate reduction and sulfur-containing organics hydrolysis [59]. Therefore, the possible side effect of PI-based technology on the reduction of H2S generation during an anaerobic digestion system should be studied because it could enhance the quality of biogas [60].
4. Conclusions
In this study, a novel WAS pretreatment using PI was proposed for the first time to improve methane generation in anaerobic digestion, and we elucidated how it improved methane generation. Results suggested that the maximum methane yield was obtained from WAS with 100 mg PI per g TS pretreatment, showing an increase of 46.0% relative to the ordinary anaerobic digestion. Mechanism analysis revealed that powerful oxidative species (i.e., •OH, •O2−, and 1O2) could be generated by WAS activation without an extra activator, and their contributions were in the sequence of •O2− > •OH > 1O2, greatly increasing the solubilization of WAS and the degradation of refractory substances (particularly lignocellulose and humus) in sludge. The enhanced bio-available substrates not only boosted the hydrolysis rate (with an increase of 23.9% based on model analysis) but also stimulated the enzymatic activities (i.e., protease, α-glucosidase, acetate kinase, and coenzyme F420). Moreover, the positive shifts of microbial community structure involved hydrolysis, acidogenesis and methanogenesis (particularly acetoclastic type) and up-regulated functional genes that participated in methanogenic metabolic pathways could be the direct reasons for the increased methane production. Although the feasibility of PI-based pretreatment on enhancing methane generation from WAS in anaerobic digestion was demonstrated, the potential side effects on pathogens inactivation and organic micropollutants removal in WAS warrants further investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was funded by the National Key R&D Program of China (Grant No. 2018YFE0106400).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ese.2022.100208.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Xu Y., Geng H., Chen R., Liu R., Dai X. Enhancing methanogenic fermentation of waste activated sludge via isoelectric-point pretreatment: insights from interfacial thermodynamics, electron transfer and microbial community. Water Res. 2021;197 doi: 10.1016/j.watres.2021.117072. [DOI] [PubMed] [Google Scholar]
- 2.Wang D., He D., Liu X., Xu Q., Yang Q., Li X., Liu Y., Wang Q., Ni B.J., Li H. The underlying mechanism of calcium peroxide pretreatment enhancing methane production from anaerobic digestion of waste activated sludge. Water Res. 2019;164 doi: 10.1016/j.watres.2019.114934. [DOI] [PubMed] [Google Scholar]
- 3.Adnan A.I., Ong M.Y., Nomanbhay S., Chew K.W., Show P.L. Technologies for biogas upgrading to biomethane: a review. Bioengineering. 2019;6(4) doi: 10.3390/bioengineering6040092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y., Sun P., Guo H., Wang D., Zhu T., Liu Y. Enhancing methane production from anaerobic digestion of waste activated sludge through a novel sodium percarbonate (SPC) pretreatment: reaction kinetics and mechanisms. ACS ES&T Eng. 2022;2:1326–1340. doi: 10.1021/acsestengg.1c00468. [DOI] [Google Scholar]
- 5.Xu Q., Fu Q., Liu X., Wang D., Wu Y., Li Y., Yang J., Yang Q., Wang Y., Li H., Ni B.J. Mechanisms of potassium permanganate pretreatment improving anaerobic fermentation performance of waste activated sludge. Chem. Eng. J. 2021;406 doi: 10.1016/j.cej.2020.126797. [DOI] [Google Scholar]
- 6.Li J., Hao X., van Loosdrecht M.C.M., Liu R. Relieving the inhibition of humic acid on anaerobic digestion of excess sludge by metal ions. Water Res. 2020;188 doi: 10.1016/j.watres.2020.116541. [DOI] [PubMed] [Google Scholar]
- 7.Yang J., Liu X., Wang D., Xu Q., Yang Q., Zeng G., Li X., Liu Y., Gong J., Ye J., Li H. Mechanisms of peroxymonosulfate pretreatment enhancing production of short-chain fatty acids from waste activated sludge. Water Res. 2019;148:239–249. doi: 10.1016/j.watres.2018.10.060. [DOI] [PubMed] [Google Scholar]
- 8.Wang D., Zhang D., Xu Q., Liu Y., Wang Q., Ni B.-J., Yang Q., Li X., Yang F. Calcium peroxide promotes hydrogen production from dark fermentation of waste activated sludge. Chem. Eng. J. 2019;355:22–32. doi: 10.1016/j.cej.2018.07.192. [DOI] [Google Scholar]
- 9.Tian L., Guo H., Wang Y., Su Z., Zhu T., Liu Y. Insights into Fe(Ⅱ)-sulfite-based pretreatment strategy for enhancing short-chain fatty acids (SCFAs) production from waste activated sludge: performance and mechanism. Bioresour. Technol. 2022;353 doi: 10.1016/j.biortech.2022.127143. [DOI] [PubMed] [Google Scholar]
- 10.Zong Y., Shao Y., Zeng Y., Shao B., Xu L., Zhao Z., Liu W., Wu D. Enhanced oxidation of organic contaminants by iron(II)-Activated periodate: the significance of high-valent iron-oxo species. Environ. Sci. Technol. 2021;55:7634–7642. doi: 10.1021/acs.est.1c00375. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q., Zeng H., Liang Y., Cao Y., Xiao Y., Ma J. Degradation of bisphenol AF in water by periodate activation with FeS (mackinawite) and the role of sulfur species in the generation of sulfate radicals. Chem. Eng. J. 2021;407 doi: 10.1016/j.cej.2020.126738. [DOI] [Google Scholar]
- 12.Sun H., He F., Choi W. Production of reactive oxygen species by the reaction of periodate and hydroxylamine for rapid removal of organic pollutants and waterborne bacteria. Environ. Sci. Technol. 2020;54(10):6427–6437. doi: 10.1021/acs.est.0c00817. [DOI] [PubMed] [Google Scholar]
- 13.Lan B., Jin R., Liu G., Dong B., Zhou J., Xing D. Improving waste activated sludge dewaterability with sodium periodate pre-oxidation on extracellular polymeric substances. Water Environ. Res. 2021;93:1680–1689. doi: 10.1002/wer.1553. [DOI] [PubMed] [Google Scholar]
- 14.Bokare A.D., Choi W. Singlet-oxygen generation in alkaline periodate solution. Environ. Sci. Technol. 2015;49(24):14392–14400. doi: 10.1021/acs.est.5b04119. [DOI] [PubMed] [Google Scholar]
- 15.Du J., Tang S., Faheem, Ling H., Zheng H., Xiao G., Luo L., Bao J. Insights into periodate oxidation of bisphenol A mediated by manganese. Chem. Eng. J. 2019;369:1034–1039. doi: 10.1016/j.cej.2019.03.158. [DOI] [Google Scholar]
- 16.Zhan W., Li L., Tian Y., Lei Y., Zuo W., Zhang J., Jin Y., Xie A., Zhang X., Wang P., Li Y., Chen X. Insight into the roles of ferric chloride on short-chain fatty acids production in anaerobic fermentation of waste activated sludge: performance and mechanism. Chem. Eng. J. 2021;420 doi: 10.1016/j.cej.2021.129809. [DOI] [Google Scholar]
- 17.Park C., Muller C.D., Abu-Orf M.M., Novak J.T. The effect of wastewater cations on activated sludge characteristics: effects of aluminum and iron in floc. Water Environ. Res. 2006;78(1):31–40. doi: 10.2175/106143005x84495. [DOI] [PubMed] [Google Scholar]
- 18.Zhang B.T., Kuang L., Teng Y., Fan M., Ma Y. Application of percarbonate and peroxymonocarbonate in decontamination technologies. J. Environ. Sci. (China) 2021;105:100–115. doi: 10.1016/j.jes.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 19.Raposo F., De la Rubia M.A., Fernández-Cegrí V., Borja R. Anaerobic digestion of solid organic substrates in batch mode: an overview relating to methane yields and experimental procedures. Renew. Sustain. Energy Rev. 2012;16(1):861–877. doi: 10.1016/j.rser.2011.09.008. [DOI] [Google Scholar]
- 20.Zheng K., Wang Y., Guo H., Zhu T., Zhao Y., Liu Y. Potassium permanganate pretreatment effectively improves methane production from anaerobic digestion of waste activated sludge: reaction kinetics and mechanisms. Sci. Total Environ. 2022;847 doi: 10.1016/j.scitotenv.2022.157402. [DOI] [PubMed] [Google Scholar]
- 21.Angelidaki I., Alves M., Bolzonella D., Borzacconi L., Campos J.L., Guwy A.J., Kalyuzhnyi S., Jenicek P., van Lier J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: a proposed protocol for batch assays. Water Sci. Technol. 2009;59(5):927–934. doi: 10.2166/wst.2009.040. [DOI] [PubMed] [Google Scholar]
- 22.Wei W., Huang Q.S., Sun J., Wang J.Y., Wu S.L., Ni B.J. Polyvinyl chloride microplastics affect methane production from the anaerobic digestion of waste activated sludge through leaching toxic bisphenol-A. Environ. Sci. Technol. 2019;53(5):2509–2517. doi: 10.1021/acs.est.8b07069. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q., Ye L., Jiang G., Jensen P.D., Batstone D.J., Yuan Z. Free nitrous acid (FNA)-based pretreatment enhances methane production from waste activated sludge. Environ. Sci. Technol. 2013;47(20):11897–11904. doi: 10.1021/es402933b. [DOI] [PubMed] [Google Scholar]
- 24.Wei W., Shi X., Wu L., Liu X., Ni B.-J. Calcium peroxide pre-treatment improved the anaerobic digestion of primary sludge and its co-digestion with waste activated sludge. Sci. Total Environ. 2022;828 doi: 10.1016/j.scitotenv.2022.154404. [DOI] [PubMed] [Google Scholar]
- 25.Kang H., Lee D., Lee K.-M., Kim H.-H., Lee H., Sik Kim M., Lee C. Nonradical activation of peroxymonosulfate by hematite for oxidation of organic compounds: a novel mechanism involving high-valent iron species. Chem. Eng. J. 2021;426 doi: 10.1016/j.cej.2021.130743. [DOI] [Google Scholar]
- 26.Liu F., Li Z., Dong Q., Nie C., Wang S., Zhang B., Han P., Tong M. Catalyst-free periodate activation by solar irradiation for bacterial disinfection: performance and mechanisms. Environ. Sci. Technol. 2022;56:4413–4424. doi: 10.1021/acs.est.1c08268. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Zheng K., Guo H., Tong Y., Zhu T., Liu Y. Unveiling the mechanisms of how vivianite affects anaerobic digestion of waste activated sludge. Bioresour. Technol. 2021;343 doi: 10.1016/j.biortech.2021.126045. [DOI] [PubMed] [Google Scholar]
- 28.Huang Y., Zhang H., Liu X., Ma B., Huang T. Iron-activated carbon systems to enhance aboriginal aerobic denitrifying bacterial consortium for improved treatment of micro-polluted reservoir water: performances, mechanisms, and implications. Environ. Sci. Technol. 2022;56(6):3407–3418. doi: 10.1021/acs.est.1c05254. [DOI] [PubMed] [Google Scholar]
- 29.APHA . 2011. Standard Methods for the Examination of Water and Wastewater. Washington, D.C., USA. [Google Scholar]
- 30.Guo H., Tian L., Wang Y., Zhu T., Tong Y., Liu Y. Improved methane production from the two-phase anaerobic digestion and dewaterability of anaerobically digested sludge by β-cyclodextrin pretreatment. J. Clean. Prod. 2022;363 doi: 10.1016/j.jclepro.2022.132484. [DOI] [Google Scholar]
- 31.Xu Y., Zheng L., Geng H., Liu R., Dai X. Enhancing acidogenic fermentation of waste activated sludge via isoelectric-point pretreatment: insights from physical structure and interfacial thermodynamics. Water Res. 2020;185 doi: 10.1016/j.watres.2020.116237. [DOI] [PubMed] [Google Scholar]
- 32.He D., Zheng S., Xiao J., Ye Y., Liu X., Yin Z., Wang D. Effect of lignin on short-chain fatty acids production from anaerobic fermentation of waste activated sludge. Water Res. 2022;212 doi: 10.1016/j.watres.2022.118082. [DOI] [PubMed] [Google Scholar]
- 33.Guo H., Wang Y., Tian L., Wei W., Zhu T., Liu Y. Unveiling the mechanisms of a novel polyoxometalates (POMs)-based pretreatment technology for enhancing methane production from waste activated sludge. Bioresour. Technol. 2021;342 doi: 10.1016/j.biortech.2021.125934. [DOI] [PubMed] [Google Scholar]
- 34.Zong Y., Zhang H., Shao Y., Ji W., Zeng Y., Xu L., Wu D. Surface-mediated periodate activation by nano zero-valent iron for the enhanced abatement of organic contaminants. J. Hazard Mater. 2021 doi: 10.1016/j.jhazmat.2021.126991. [DOI] [PubMed] [Google Scholar]
- 35.Wei W., Zhou X., Xie G.J., Duan H., Wang Q. A novel free ammonia based pretreatment technology to enhance anaerobic methane production from primary sludge. Biotechnol. Bioeng. 2017;114(10):2245–2252. doi: 10.1002/bit.26348. [DOI] [PubMed] [Google Scholar]
- 36.Guo H., Wang Y., Tian L., Wei W., Zhu T., Liu Y. Insight into the enhancing short-chain fatty acids (SCFAs) production from waste activated sludge via polyoxometalates pretreatment: mechanisms and implications. Sci. Total Environ. 2021;800 doi: 10.1016/j.scitotenv.2021.149392. [DOI] [PubMed] [Google Scholar]
- 37.Du J., Kim K., Min D.W., Choi W. Freeze-thaw cycle-enhanced transformation of iodide to organoiodine compounds in the presence of natural organic matter and Fe(III) Environ. Sci. Technol. 2022;56:1007–1016. doi: 10.1021/acs.est.1c06747. [DOI] [PubMed] [Google Scholar]
- 38.Jiang Z., Wang B., Li Y., Chan H.S., Sun H., Wang T., Li H., Yuan S., Leung M.K.H., Lu A., Wong P.K. Solar-light-driven rapid water disinfection by ultrathin magnesium titanate/carbon nitride hybrid photocatalyst: band structure analysis and role of reactive oxygen species. Appl. Catal. B Environ. 2019;257 doi: 10.1016/j.apcatb.2019.117898. [DOI] [Google Scholar]
- 39.He H., Xin X., Qiu W., Li D., Liu Z., Ma J. Waste sludge disintegration, methanogenesis and final disposal via various pretreatments: comparison of performance and effectiveness. Environ. Sci. Ecotechnol. 2021;8 doi: 10.1016/j.ese.2021.100132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deuss P.J., Scott M., Tran F., Westwood N.J., de Vries J.G., Barta K. Aromatic monomers by in situ conversion of reactive intermediates in the acid-catalyzed depolymerization of lignin. J. Am. Chem. Soc. 2015;137(23):7456–7467. doi: 10.1021/jacs.5b03693. [DOI] [PubMed] [Google Scholar]
- 41.Wei W., Zhou X., Wang D., Sun J., Wang Q. Free ammonia pre-treatment of secondary sludge significantly increases anaerobic methane production. Water Res. 2017;118:12–19. doi: 10.1016/j.watres.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 42.T. Zhu, Z. Su, W. Lai, J. Ding, Y. Wang, Y. Zhao, Y. Liu, Evaluating the impact of sulfamethoxazole on hydrogen production during dark anaerobic sludge fermentation, Front. Environ. Sci. Eng. 17(1) (2023).
- 43.Meng J., Hu Z., Wang Z., Hu S., Liu Y., Guo H., Li J., Yuan Z., Zheng M. Determining factors for nitrite accumulation in an acidic nitrifying system: influent ammonium concentration, operational pH, and ammonia-oxidizing community. Environ. Sci. Technol. 2022;56(16):11578–11588. doi: 10.1021/acs.est.1c07522. [DOI] [PubMed] [Google Scholar]
- 44.Xu Q., Luo T.Y., Wu R.L., Wei W., Sun J., Dai X., Ni B.J. Rhamnolipid pretreatment enhances methane production from two-phase anaerobic digestion of waste activated sludge. Water Res. 2021;194 doi: 10.1016/j.watres.2021.116909. [DOI] [PubMed] [Google Scholar]
- 45.Lim J.W., Chiam J.A., Wang J.Y. Microbial community structure reveals how microaeration improves fermentation during anaerobic co-digestion of brown water and food waste. Bioresour. Technol. 2014;171:132–138. doi: 10.1016/j.biortech.2014.08.050. [DOI] [PubMed] [Google Scholar]
- 46.Li J., Hao X., van Loosdrecht M.C.M., Luo Y., Cao D. Effect of humic acids on batch anaerobic digestion of excess sludge. Water Res. 2019;155:431–443. doi: 10.1016/j.watres.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 47.Liu X., Du M., Lu Q., He D., Song K., Yang Q., Duan A., Wang D. How does chitosan affect methane production in anaerobic digestion? Environ. Sci. Technol. 2021;55:15843–15852. doi: 10.1021/acs.est.1c04693. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y., Sun P., Guo H., Zheng K., Zhu T., Liu Y. Performance and mechanism of sodium percarbonate (SPC) enhancing short-chain fatty acids production from anaerobic waste activated sludge fermentation. J. Environ. Manag. 2022;313 doi: 10.1016/j.jenvman.2022.115025. [DOI] [PubMed] [Google Scholar]
- 49.Wang J., Ma D., Feng K., Lou Y., Zhou H., Liu B., Xie G., Ren N., Xing D. Polystyrene nanoplastics shape microbiome and functional metabolism in anaerobic digestion. Water Res. 2022;219 doi: 10.1016/j.watres.2022.118606. [DOI] [PubMed] [Google Scholar]
- 50.Allard S., Nottle C.E., Chan A., Joll C., von Gunten U. Ozonation of iodide-containing waters: selective oxidation of iodide to iodate with simultaneous minimization of bromate and I-THMs. Water Res. 2013;47(6):1953–1960. doi: 10.1016/j.watres.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Chen T., Sun Y., Dong H., Chen J., Yu Y., Ao Z., Guan X. Understanding the importance of periodate species in the pH-dependent degradation of organic contaminants in the H2O2/periodate process. Environ. Sci. Technol. 2022 doi: 10.1021/acs.est.2c02446. [DOI] [PubMed] [Google Scholar]
- 52.Chia L., Tang X., Weavers L.K. Kinetics and mechanism of photoactivated periodate reaction with 4-chlorophenol in acidic solution. Environ. Sci. Technol. 2004;38:6875–6880. doi: 10.1021/es049155n. [DOI] [PubMed] [Google Scholar]
- 53.Liu X., Huang X., Wu Y., Xu Q., Du M., Wang D., Yang Q., Liu Y., Ni B.J., Yang G., Yang F., Wang Q. Activation of nitrite by freezing process for anaerobic digestion enhancement of waste activated sludge: performance and mechanisms. Chem. Eng. J. 2020;387 doi: 10.1016/j.cej.2020.124147. [DOI] [Google Scholar]
- 54.Choi Y., Yoon H.I., Lee C., Vetrakova L., Heger D., Kim K., Kim J. Activation of periodate by freezing for the degradation of aqueous organic pollutants. Environ. Sci. Technol. 2018;52(9):5378–5385. doi: 10.1021/acs.est.8b00281. [DOI] [PubMed] [Google Scholar]
- 55.Sun F., Xiao K., Zhu W., Withanage N., Zhou Y. Enhanced sludge solubilization and dewaterability by synergistic effects of nitrite and freezing. Water Res. 2018;130:208–214. doi: 10.1016/j.watres.2017.11.066. [DOI] [PubMed] [Google Scholar]
- 56.Wang J., Lou Y., Feng K., Zhou H., Liu B., Xie G., Xing D. Enhancing the decomposition of extracellular polymeric substances and the recovery of short-chain fatty acids from waste activated sludge: analysis of the performance and mechanism of co-treatment by free nitrous acid and calcium peroxide. J. Hazard Mater. 2021;423(Pt A) doi: 10.1016/j.jhazmat.2021.127022. [DOI] [PubMed] [Google Scholar]
- 57.Sun S., Hou Y., Wei W., Sharif H.M., Huang C., Ni B.J., Li H., Song Y., Lu C., Han Y., Guo J. Perturbation of clopyralid on bio-denitrification and nitrite accumulation: long-term performance and biological mechanism. Environ. Sci. Ecotechnol. 2022;9 doi: 10.1016/j.ese.2021.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang A.-J., Wang H.-C., Cheng H.-Y., Liang B., Liu W.-Z., Han J.-L., Zhang B., Wang S.-S. Electrochemistry-stimulated environmental bioremediation: development of applicable modular electrode and system scale-up. Environ. Sci. Ecotechnol. 2020;3 doi: 10.1016/j.ese.2020.100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fu Q., Xu Q., Liu Z., Wang D., Liu X., He D., He Y., Li Y., Yang J., Duan A. Insights into potassium permanganate reducing H2S generation from anaerobic fermentation of sludge. Chem. Eng. J. 2021;430 doi: 10.1016/j.cej.2021.133150. [DOI] [Google Scholar]
- 60.Uddin M.N., Siddiki S.Y.A., Mofijur M., Djavanroodi F., Hazrat M.A., Show P.L., Ahmed S.F., Chu Y.-M. Prospects of bioenergy production from organic waste using anaerobic digestion technology: a mini review. Front. Energy Res. 2021;9 doi: 10.3389/fenrg.2021.627093. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.