Abstract
Purpose
Our study analyzes the influence of minimally invasive vs. open surgery on the postoperative need for nursing care in patients with colorectal carcinoma. Colorectal cancer is an age-related disease, and oncologic surgery is increasingly performed in elderly patients. Long-term effects of the procedural choice on patients’ self-sufficiency and autonomy have not been scientifically addressed so far.
Methods
Multivariable logistic regression models based on claims data from a statutory health insurer (AOK, Baden-Württemberg, Germany) were applied to assess potential risk factors for assignment patients to a nursing care level, a German scale to categorize individual need for nursing care, at 12 and 36 months after colorectal cancer surgery.
Results
A total of 3996 patients were eligible to be included in the analysis. At 36 months postoperatively, 44 of 427 (10.3%) patients after minimally invasive colon cancer surgery and 231 of 1287 (17.9%) patients after open procedure were newly graded into a nursing care level (OR = 0.62, 95%CI = 0.44–0.90, p = 0.010). Thirty-four of 251 (13.5%) patients receiving minimally invasive rectal cancer surgery compared to 142 of 602 (23.6%) patients after open approach were newly assigned to a nursing care level (OR = 0.53, 95%CI = 0.34–0.81, p = 0.003).
Conclusions
Laparoscopically assisted resection of colorectal cancer seems to be superior in preserving physical autonomy of elderly patients with colorectal cancer.
Keywords: Nursing care, Claims data analysis, Colorectal cancer, Minimally invasive surgery, Laparoscopic surgery
Introduction
Colorectal cancer is an age-related disease which increasingly requires oncologic surgery in elderly patients [1, 2]. More than 70% of patients diagnosed with colorectal cancer are aged 65 years or older [3]. Consequently, oncological treatment has to be tailored for elderly patients. In particular, treatment strategies should seek to preserve self-sufficiency and functional autonomy, which are cornerstones for quality of life. However, knowledge about the impact of oncological colon cancer surgery on patients’ functional autonomy is scarce and has not been scientifically addressed so far.
Due to well-proven short-term benefits like reduction of postoperative pain and faster recovery, guidelines recommend laparoscopically assisted resection of colorectal cancer as an alternative for open surgical procedures [4–7]. Nevertheless, in clinical routine, open surgery still remains a mainstay in the treatment of colorectal cancer. Downsides of open procedures, such as more pain or prolonged recovery, are often accepted as trade-off for shorter operation time and assumed better oncological outcome, although according to previous RCTs, the oncologic outcome of laparoscopically-assisted surgery seems non-inferior to open surgery [8–14]. Furthermore, the cost-effectiveness of laparoscopic oncological procedures is still being questioned [15].
So far, long-term effects of the procedural choice on patients’ self-sufficiency and functional autonomy have not been scientifically addressed. Consequently, and to our knowledge as a first, this claims data analysis aimed to answer the question whether the choice of surgical approach may have an effect on post-operative nursing care needs in patients with colorectal cancer.
Methods
Study design
A cohort study was conducted. Claims data related to patients treated in German primary care recorded between January 1, 2011 and December 31, and 2017 were supplied by the AOK statutory health insurance company (AOK Baden-Wuerttemberg, Germany). Ethical approval for this analysis was given by the local institutional Ethics Committee of the University Hospital Heidelberg (No. S-460/2020).
Study population and data acquisition
Data were recorded by the AOK statutory health insurance company for reimbursement purposes and continuous evaluation of the HZV program, a comprehensive continuous evaluation program of general practitioner-centered care in German primary care (German: “Hausarztzentrierte Versorgung” (HZV)) [16]. For the analysis, data were supplied by the AOK to the Department of General Practice and Health Services Research at Heidelberg University Hospital. Subjects could not be identified, neither directly or through identifiers linked to the subjects. Data storage and extraction were performed with MySQL Community Server × 64 (Oracle Corporation, Redwood Shores, CA, USA). All national and institutional guidelines concerning data acquisition for retrospective analyses were followed at all times. The obtained dataset comprised age, gender, diagnoses according to ICD-10 coding as well as accounting data on consultations, prescribed medication, and hospital stays. Patient data were included into the analysis if they were aged 65 years or older and underwent non-emergency surgery for non-metastatic colorectal cancer. Cases were identified from the supplied data using the ICD-10 diagnosis (C18, C19, C20) and the German version of the International Classification of Procedures in Medicine (OPS, German: Operationen- und Prozedurenschlüssel) [17].
Outcome parameters
To evaluate the effect of surgery on long-term need for nursing care, the nursing care level was assessed for each patient at 12 and 36 months after surgery. In Germany, the nursing care level is determined via a classification provided by the Medical Control Service (German: “Medizinischer Kontrolldienst”), an independent and governmentally supervised institution for assessment in health services. The evaluation for the nursing care level is usually initiated by patients, relatives, or family physicians, if affected patients need nursing care support. Upon assessment by experts from the Medical Control Service, the current individual need for nursing care and household assistance is categorized on a scale of 1 to 3 reflecting the individual care dependency: minor (I), average daily need of care at least 45 min; moderate (II), average daily need of care at least 120 min; and severe (III), average daily need of care at least 240 min and round-the-clock support, e.g., permanent bed confinement. The classification is based on the degree of personal limitation in daily life. Mobility (e.g., mobility within the domestic environment), cognitive ability (e.g., orientation), communication, psychological problems (e.g., agitation at night), self-care (e.g., independent personal hygiene), dealing with illness-related requirements (e.g., taking medication independently), and the organization of everyday life and social contacts (e.g., organizing daily routines) are assessed.
The following associated factors were determined for the analysis in the multivariable regression model: surgical procedure, age, gender, and morbidity according to Charlson index, a sum score determined according to ICD-10 diagnoses assigned to values between 1 and 6 according to severity to approximate patients’ overall morbidity [18]. Surgical procedure, lymph node metastasis, and metachronous metastasis were identified via ICD-10 coding. Application of neoadjuvant or adjuvant chemotherapy was determined by records of the central pharmaceutical numbers of prescribed medications (“Pharmazentralnummer”, PZN).
Statistical analysis
Statistical analysis was performed using SPSS for Windows (IBM 27 Armonk, NY: IBM Corp). Chi-square test for categorical data and T-test for continuous data were used to assess differences between patients undergoing open or laparoscopically assisted surgical approach. Assignment to a nursing care level was analyzed 12 and 36 months after surgery. Influence of examined factors on these two dependent binary outcomes was determined by multivariable binary logistic regression. To assess the independence among variables, correlation tests were performed using the Phi coefficient for the dichotomic factors, the chi square test for ordinal variables, and the Eta coefficient for continuous parameters. For all analyses, results were considered statistically significant, if the p value was 0.05 or less.
Results
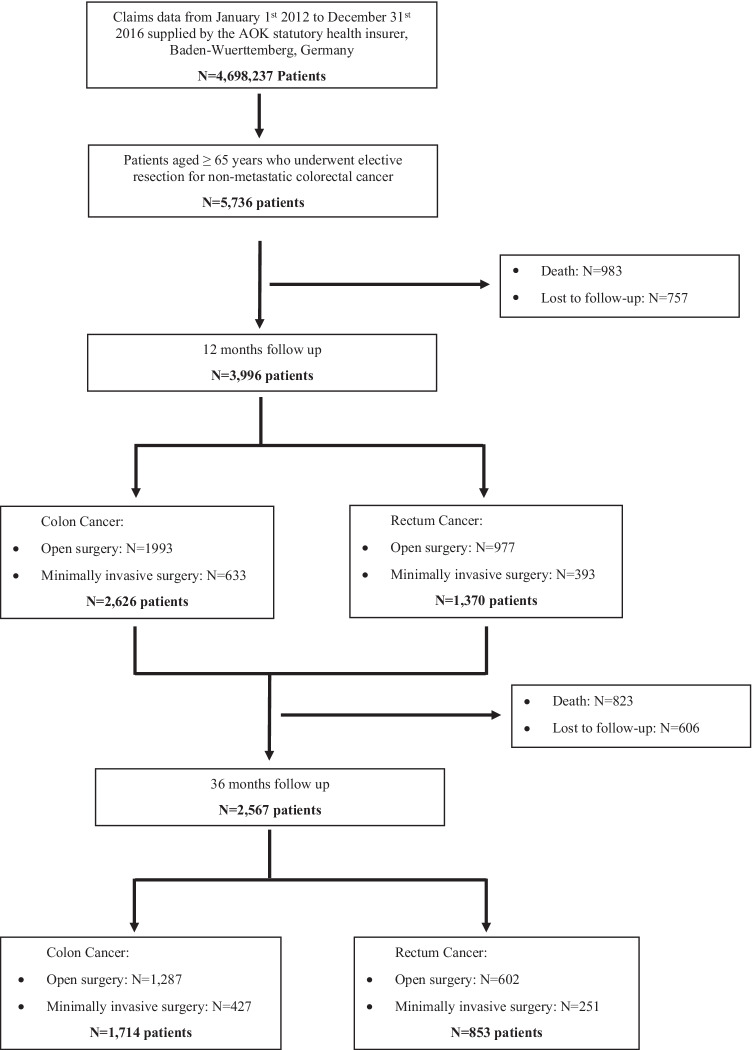
A total of 3996 patients were eligible to be included in the analysis. Figure 1 describes the study population in a flowchart. The baseline characteristics of the included patients are shown in Table 1.
Fig. 1.
The flowchart shows the selection and sorting of claims data cases according to the inclusion and exclusion criteria
Table 1.
Baseline characteristics
| Colon cancer | Rectal cancer | |||||||
|---|---|---|---|---|---|---|---|---|
| Open | Minimal invasive | Open | Minimal invasive | |||||
| (N (%)) | (N (%)) | p value | (N (%)) | (N (%)) | p value | |||
| Patients (N) | 1,993 (75.9) | 633 (24.1) | 977 (71.3) | 393 (28.7) | ||||
| Age (years) | 77.6 ± 6.6 | 76.4 ± 6.2 | < 0.001 | 75.8 ± 6.2 | 75.6 ± 6.5 | 0.557 | ||
| Sex (m) | 986 (49.5) | 330(52.1) | 0.244 | 603 (61.7) | 225 (57.3) | 0.126 | ||
| Charlson Index* | 5.6 ± 2.7 | 5.3 ± 2.7 | 0.351 | 5.9 ± 2.7 | 5.4 ± 2.6 | 0.267 | ||
| Nursing care level† | ||||||||
| None | 1,842 (92.4) | 598 (94.5) | 0.167 | 922 (94.4) | 377 (95.9) | 0.364 | ||
| I | 113 (5.7) | 23 (3.6) | 41(4.2) | 14 (3.6) | ||||
| II | 35 (1.8) | 12 (1.9) | 12 (1.2) | 1 (0.3) | ||||
| III | 3 (0.2) | 0 (0) | 2 (0.2) | 1 (0.3) | ||||
| Surgical procedure | ||||||||
| Ileocecal resection | 24 (1.2) | 9 (1.4) | 0.669 | Anterior resection | 298 (30.5) | 139 (35.4) | 0.080 | |
| Right hemicolectomy | 1,252 (62.9) | 335 (52.9) | < 0.001 | Deep anterior resection | 498 (51.0) | 180 (45.8) | 0.083 | |
| Transverse colectomy | 64 (3.2) | 8 (1.3) | 0.009 | Abdominoperineal resection | 178 (18.2) | 73 (18.6) | 0.878 | |
| Left hemicolectomy | 286 (14.4) | 116 (18.3) | 0.016 | Proctocolectomy | 3 (0.3) | 1 (0.3) | 0.870 | |
| Sigmoid resection | 254 (12.8) | 142 (22.4) | < 0.001 | |||||
| Total colectomy | 100 (5.0) | 10 (1.6) | < 0.001 | |||||
| Segmental resection | 13 (0.7) | 13 (2.1) | 0.002 | |||||
| Stoma placement | ||||||||
| Ileostomy | 15 (0.8) | 2 (0.3) | 0.233 | 2 (0.2) | 1 (0.3) | 0.859 | ||
| Colostomy | 78 (3.9) | 7 (1.1) | 0.001 | 108 (11.0) | 24 (6.1) | 0.005 | ||
| Lymph node metastasis | 96 (4.8) | 42 (6.6) | 0.074 | 62 (6.3) | 23 (5.9) | 0.732 | ||
| Chemotherapy | 516 (25.9) | 146 (23.1) | 0.161 | 362 (37.1) | 135 (34.3) | 0.347 | ||
Continuous values are presented as mean and standard deviation; m male
*The Charlson index is a sum score determined according to ICD-10 diagnoses assigned to values between 1 and 6 according to severity to approximate patients’ overall morbidity[18]
†Nursing care level is a classification determined by independent experts to clarify care insurance claims for individuals in need of care in Germany. Based on special criteria, those in need are assigned to one of three grades. Grade 3 corresponds to the highest need for care
Independence of included factors in the regression models was examined by correlation tests. There was low correlation between the influencing factor “minimal invasive surgery” and influencing factors “age” and “colostomy.” However, both factors do not exceed a correlation value of 0.1. Therefore, the included factors can be considered independent among each other.
Colon cancer
At 12 months postoperatively, 283 of 1993 (14.2%) patients who underwent open surgery and 35 of 633 (5.5%) patients after minimally invasive surgery were newly categorized into a nursing care level. The corresponding odds ratio was significantly lower for patients who underwent minimal invasive surgery compared to open surgery (OR = 0.42, 95%CI = 0.29–0.61, p < 0.001). Rising age (OR = 1.10, 95%CI = 1.08–1.13, p < 0.001), male gender (OR = 1.33, 95%CI = 1.03–1.72, p = 0.030), an increased morbidity index (OR = 1.15, 95% CI = 1.10–1.20, p < 0.001), and the placement of ileostomy (OR = 8.36, 95%CI = 2.87–24.33, p < 0.001) or enterostomy (OR = 3.46, 95%CI = 2.11–5.69, p < 0.001) were associated with a higher risk of being categorized into a nursing care level.
At 36 months postoperatively, 231 of 1287 (17.9%) patients after open surgery and 44 of 427 (10.3%) patients after minimally invasive surgery were newly categorized into a nursing care level. The corresponding odds ratio remains significantly lower for patients who underwent minimal invasive surgery compared to open procedure for colon cancer (OR = 0.62, 95%CI = 0.44–0.90, p = 0.010). Associated risk factors for being assigned to a nursing care level at 36 months were rising age (OR = 1.10, 95%CI = 1.07–1.12, p < 0.001), male gender (OR = 1.58, 95%CI = 1.18–2.11, p = 0.002), increased morbidity index (OR = 1.20, 95%CI = 1.14–1.26, p = 0.004), and placement of an ileostomy (12.36, 95%CI = 2.26–67.52, p = 0.004) or enterostomy (3.22, 95%CI = 1.65–6.30, p = 0.001) (Table 2).
Table 2.
Colon cancer: results of the multivariable analysis of patients graded into a nursing care level within 1 year of follow-up
|
12 months follow-up N = 2626 |
36 months follow-up N = 1714 |
||||||
|---|---|---|---|---|---|---|---|
| OR | (95% CI) | p | OR | 95% CI | p | ||
| Minimal invasive surgery | 0.418 | (0.288, 0.609) | < 0.001 | 0.624 | (0.435, 0.896) | 0.010 | |
| Age | 1.103 | (1.080, 1.127) | < 0.001 | 1.097 | (1.071, 1.124) | < 0.001 | |
| Gender (female) | 1.331 | (1.028, 1.723) | 0.030 | 1.579 | (1.182, 2.110) | 0.002 | |
| Charlson Index* | 1.149 | (1.097, 1.203) | < 0.001 | 1.198 | (1.138, 1.261) | < 0.001 | |
| Ileostomy | 8.357 | (2.871, 24.328) | < 0.001 | 12.356 | (2.261, 67.522) | 0.004 | |
| Colostomy | 3.459 | (2.105, 5.686) | < 0.001 | 3.219 | (1.645, 6.296) | 0.001 | |
| Lymph node metastasis | 0.933 | (0.533, 1.635) | 0.810 | 0.883 | (0.335, 2.332) | 0.802 | |
| Chemotherapy | 0.815 | (0.573, 1.161) | 0.258 | 0.723 | (0.488, 1.071) | 0.723 | |
| Metachronous distant metastasis | 1.370 | (0.931, 2.017) | 0.110 | 1.427 | (0.934, 2.181) | 0.101 | |
Continuous values are presented as mean and standard deviation
OR odds ratio, CI confidence interval
*The Charlson index is a sum score determined according to ICD-10 diagnoses assigned to values between 1 and 6 according to severity to approximate patients’ overall morbidity[18]
Rectal cancer
At 12 months after surgery for rectal cancer, 198 of 977 (20.3%) patients were newly categorized into a nursing care level after open surgery compared to 54 of 393 (13.7%) patients after minimally invasive approach. The corresponding odds ratio was significantly lower for patients who underwent minimal invasive surgery compared to open surgery (OR = 0.68, 95%CI = 0.48–0.96, p = 0.030), Rising age (OR = 1.09, 95%CI = 1,06–1.12, p < 0.001), an increased morbidity index (OR = 1.16, 95%CI = 1.10–1.22, p < 0.001), the placement of colostomy (OR = 2.21, 95%CI = 1.47–3.34, p < 0.001), and adjuvant chemotherapy (OR = 0.62, 95%CI = 0.44–0.87, p = 0.006) were associated risk factors for being assigned to a nursing care level.
At 36 months postoperatively, 142 of 602 (23.6%) patients were newly categorized into a nursing care level after open surgery compared to 34 of 251 (13.5%) patients after minimally invasive approach. The corresponding odds ratio of being categorized into a nursing care level remained lower for minimal invasive surgery compared to open surgery (OR = 0.53, 95%CI = 0.34–0.81, p = 0.003). Associated risk factors for patients being assigned to a nursing care level at 36 months were rising age (OR = 1.08, 95%CI = 1.05–1.15, p < 0.001), p = 0.002), increased morbidity index (OR = 1.15, 95%CI = 1.07–1.23, p < 0.001), placement of colostomy (2.83, 95%CI = 1.63–4.92, p < 0.001), and occurrence of metachronous distant metastasis (1.86, 95%CI = 1.15–3.01, p < 0.001) (Table 3).
Table 3.
Rectal cancer: results of the multivariable analysis of patients graded into a nursing care level within 1 year of follow-up
|
12 months follow-up N = 1370 |
36 months follow-up N = 853 |
||||||
|---|---|---|---|---|---|---|---|
| OR | (95% CI) | p | OR | 95% CI | p | ||
| Minimal invasive surgery | 0.680 | (0.481, 0.962) | 0.030 | 0.527 | (0.343, 0.809) | 0.003 | |
| Age | 1.087 | (1.061, 1.115) | < 0.001 | 1.081 | (1.047, 1.115) | < 0.001 | |
| Gender (female) | 1.039 | (0.766, 1.408) | 0.807 | 1.095 | (0.756, 1.586) | 0.631 | |
| Charlson Index* | 1.159 | (1.097, 1.224) | < 0.001 | 1.150 | (1.074, 1.231) | < 0.001 | |
| Ileostomy | 4.199 | (0.373, 47.259) | 0.245 | n/a | n/a | n/a | |
| Colostomy | 2.214 | (1.470, 3.336) | < 0.001 | 2.830 | (1.629, 4.918) | < 0.001 | |
| Lymph node metastasis | 0.781 | (0.414, 1.475) | 0.447 | 0.781 | (0.351, 2.198) | 0.781 | |
| Chemotherapy | 0.620 | (0.440, 0.874) | 0.006 | 0.841 | (0.557, 1.271) | 0.412 | |
| Metachronous distant metastasis | 1.111 | (0.728, 1.697) | 0.626 | 1.861 | (1.149, 3.013) | 0.012 | |
Continuous values are presented as mean and standard deviation
OR odds ratio, CI confidence interval
*The Charlson index is a sum score determined according to ICD-10 diagnoses assigned to values between 1 and 6 according to severity to approximate patients’ overall morbidity [18]
Discussion
For the first time, the long-term effect of laparoscopic versus open surgery for colorectal cancer on postoperative nursing care needs was evaluated in this present study. The multivariable analysis showed that the minimally invasive approach for colon as well as for rectum cancer is associated with a lower risk of needing nursing care after 12 and 36 months of follow-up.
Since colorectal cancer is a disease with an age-dependent incidence, the results of this analysis are of high relevance for its oncological treatment pathways for elderly patients. In clinical routine, colorectal surgery is still frequently performed by open procedure, particularly in technically challenging resections of locally advanced tumors or operable distant metastasis, although it has been shown that laparoscopically assisted surgery may be performed safely and with comparable oncological outcome [19–22]. According to the results of this analysis, preferring minimally invasive surgery for colorectal cancer in elderly patients may contribute to preserve physical autonomy. While short-term benefits of laparoscopically assisted colorectal surgery, e.g., early mobilization, reduced postoperative pain or early return of bowel function are well-known [4–7], the results of this study indicate that advantages of the minimally invasive approach exceed effects on short-term morbidity in elderly patients and may have lasting healthcare-related effects, which have not been identified so far. The reason for this finding may be hypothesized to lie within the reduced functional reserve of elderly patients to respond to surgical trauma. Frailty is state of multifactorial decline in physiologic function with a prevalence of 25–50% for patients over 80 years and is associated with high vulnerability to sudden severe health status changes [23–26]. In surgery, frailty has been shown to be associated with higher postoperative morbidity and mortality, e.g., after vascular surgery, kidney transplantation, and general surgery or emergency laparotomy [27–30]. Particularly in frail patients, the extent of abdominal incisional trauma may be a decisive factor affecting long-term disability. Severe postoperative pain, slow recovery of bowel function, wound healing disorders, immobilization, and prolonged hospital stay may lead to further morbidity and weakening of general condition, which could tip the scale for patients on the verge of self-sufficiency. These potential effects of open oncologic surgery should be taken into account when choosing an optimal surgical treatment. Postoperative loss of autonomy should be considered as a highly relevant outcome after surgery in elderly patients, particularly with regard to its potential impact on quality of life. The effects of the invasiveness on a postoperative need for nursing care should be clearly explained to patients and incorporated in preoperative counseling and choice of the surgical procedure.
Besides the invasiveness of surgery and unalterable risk factors like patients’ age, morbidity, or gender, the multivariable analysis showed that placement ileostomy or colostomy has a marked effect on the postoperative nursing care needs in colorectal cancer surgery. It is not surprising that stoma management particularly in older patients may require nursing care assistance and therefore contributes to loss of self-sufficiency in many cases, particularly in ileostomy which is known for difficulties in postoperative management due to fluid loss. On the other hand, it has to be mentioned that the descriptive analysis shows a higher rate of colostomy for open surgery of colon as well as rectum. Since stoma is placed particularly in the setting of emergency or complicated surgery which may be associated with a worse outcome, confounding may also be considered in this regard. While cases admitted to hospital as emergency were excluded from the analysis, subacute emergency cases or complicated surgery are not captured by claims data. However, with regard to ostomy and advance of oncologic disease, the results of our analysis suggest that stoma placement in elderly patients should be applied after particularly careful evaluation, and stoma management should best be addressed by comprehensive preoperative instructions to patient and nursing care providers to mitigate postoperative challenges.
To our knowledge, this is the first study evaluating the long-term effects of laparoscopic versus open surgery for colorectal cancer on postoperative need for nursing care. Limitations of this study lie within the nature of claims data. We deliberately chose claims data for this analysis due to high statistical power and its possibilities to facilitate multivariable models. Selection bias regarding the choice of surgical procedure cannot be excluded completely. However, both groups are homogeneous in terms of morbidity. The nursing care level and age differs only modestly for patients with colon cancer. The supplied claims data did not include information regarding tumor stage and frailty. However, all available variables referring to the stage of colorectal cancer have been included in the multivariable analysis by assessing lymph node involvement, occurrence of metastases, and application of adjuvant chemotherapy. Since multimorbidity and age are strong predictors for frailty [25], these factors were included in the regression model.
Conclusion
As a conclusion, this analysis provides evidence that the risk of needing long-term nursing care after colon and rectal cancer surgery is associated with the invasiveness of procedural choice. Laparoscopically assisted resection of colorectal cancer seems superior to preserve the autonomy of patients and may be preferred over open surgery in elderly patients. Ileostomy is associated with a marked effect on the postoperative need for nursing care and should be avoided if possible. To preserve autonomy and quality of life in elderly patients, these procedural risk factors should be taken into account in preoperative counseling and choosing of the surgical approach. Future studies should routinely address the need for nursing care as a relevant outcome after surgery.
Acknowledgements
We would like to thank the statutory health insurance provider AOK Baden-Wuerttemberg (Germany) for providing the pseudonymized data for this analysis.
Authors’ contribution
Study conception and design: J. Senft, G. Laux, B. Brück; acquisition of data: J. Senft, G. Laux; analysis and interpretation of data: J. Senft, B. Brück, G. Laux, T. Bruckner; drafting the manuscript: J. Senft, B. Brück; critical revision: B. Müller-Stich, R. Poß-Doering, J. Szecsenyi; all the authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded solely by institutional means from the University Hospital of Heidelberg.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
Ethical approval for this analysis was given by the local institutional Ethics Committee of the University Hospital Heidelberg (No. S-460/2020).
Competing interests
The authors Jonas D. Senft, Benedikt Brück, Regina Poß-Doering, Thomas Bruckner, Joachim Szecsenyi, Beat P. Müller-Stich, and Gunter Laux have no conflict of interest or financial ties to disclose. Data was supplied by the statutory health insurance AOK Baden-Wuerttemberg, which had no role in analysis, interpretation, or publication of the data.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alley PG. Surgery for colorectal cancer in elderly patients. Lancet. 2000;356:956. doi: 10.1016/s0140-6736(00)02707-0. [DOI] [PubMed] [Google Scholar]
- 2.Hermans E, van Schaik PM, Prins HA, Ernst MF, Dautzenberg PJ, Bosscha K. Outcome of colonic surgery in elderly patients with colon cancer. J Oncol. 2010;2010:1687–8450. doi: 10.1155/2010/865908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 4.Vlug MS, Wind J, Hollmann MW, Ubbink DT, Cense HA, Engel AF, et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study) Ann Surg. 2011;254:868–875. doi: 10.1097/SLA.0b013e31821fd1ce. [DOI] [PubMed] [Google Scholar]
- 5.van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210–218. doi: 10.1016/s1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 6.Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–1726. doi: 10.1016/s0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 7.Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477–484. doi: 10.1016/s1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 8.Kuhry E, Schwenk WF, Gaupset R, Romild U, Bonjer HJ. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev. 2008;2008:Cd003432. doi: 10.1002/14651858.CD003432.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44–52. doi: 10.1016/s1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]
- 10.Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB, et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014;15:767–774. doi: 10.1016/s1470-2045(14)70205-0. [DOI] [PubMed] [Google Scholar]
- 11.Fleshman J, Branda ME, Sargent DJ, Boller AM, George VV, Abbas MA, et al. Disease-free survival and local recurrence for laparoscopic resection compared with open resection of stage II to III rectal cancer: follow-up results of the ACOSOG Z6051 randomized controlled trial. Ann Surg. 2019;269:589–595. doi: 10.1097/sla.0000000000003002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372:1324–1332. doi: 10.1056/NEJMoa1414882. [DOI] [PubMed] [Google Scholar]
- 13.Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW, Jr., et al. (2007) Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg 246:655–62; discussion 62–4. 10.1097/SLA.0b013e318155a762 [DOI] [PubMed]
- 14.Simillis C, Lal N, Thoukididou SN, Kontovounisios C, Smith JJ, Hompes R, et al. Open versus laparoscopic versus robotic versus transanal mesorectal excision for rectal cancer: a systematic review and network meta-analysis. Ann Surg. 2019;270:59–68. doi: 10.1097/sla.0000000000003227. [DOI] [PubMed] [Google Scholar]
- 15.Braga M, Vignali A, Zuliani W, Frasson M, Di Serio C, Di Carlo V (2005) Laparoscopic versus open colorectal surgery: cost-benefit analysis in a single-center randomized trial. Ann Surg 242:890–5, discussion 5–6. 10.1097/01.sla.0000189573.23744.59 [DOI] [PMC free article] [PubMed]
- 16.Laux G, Kaufmann-Kolle P, Bauer E, Goetz K, Stock C, Szecsenyi J. Evaluation of family doctor centred medical care based on AOK routine data in Baden-Württemberg. Z Evid Fortbild Qual Gesundhwes. 2013;107:372–378. doi: 10.1016/j.zefq.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Amtliche Klassifikation für Operationen und Prozeduren. Bundesinstitut für Arzneimittel und Medizinprodukte.
- 18.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57:1288–1294. doi: 10.1016/j.jclinepi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, Kazaryan AM, Kristiansen R, Fagerland MW, et al. Laparoscopic versus open resection for colorectal liver metastases: the OSLO-COMET randomized controlled trial. Ann Surg. 2018;267:199–207. doi: 10.1097/sla.0000000000002353. [DOI] [PubMed] [Google Scholar]
- 20.Ratti F, Fiorentini G, Cipriani F, Catena M, Paganelli M, Aldrighetti L. Laparoscopic vs open surgery for colorectal liver metastases. JAMA Surg. 2018;153:1028–1035. doi: 10.1001/jamasurg.2018.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martínez-Cecilia D, Cipriani F, Shelat V, Ratti F, Tranchart H, Barkhatov L, et al. Laparoscopic versus open liver resection for colorectal metastases in elderly and octogenarian patients: a multicenter propensity score based analysis of short- and long-term outcomes. Ann Surg. 2017;265:1192–1200. doi: 10.1097/sla.0000000000002147. [DOI] [PubMed] [Google Scholar]
- 22.Hida K, Okamura R, Sakai Y, Konishi T, Akagi T, Yamaguchi T, et al. Open versus laparoscopic surgery for advanced low rectal cancer: a large, multicenter, propensity score matched cohort study in Japan. Ann Surg. 2018;268:318–324. doi: 10.1097/sla.0000000000002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–1375. doi: 10.1016/s0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 24.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/s0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 26.Hoogendijk EO, Deeg DJH, de Breij S, Klokgieters SS, Kok AAL, Stringa N, et al. The longitudinal aging study Amsterdam: cohort update 2019 and additional data collections. Eur J Epidemiol. 2020;35:61–74. doi: 10.1007/s10654-019-00541-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houghton JSM, Nickinson ATO, Morton AJ, Nduwayo S, Pepper CJ, Rayt HS, et al. Frailty factors and outcomes in vascular surgery patients: a systematic review and meta-analysis. Ann Surg. 2020;272:266–276. doi: 10.1097/sla.0000000000003642. [DOI] [PubMed] [Google Scholar]
- 28.McAdams-DeMarco MA, King EA, Luo X, Haugen C, DiBrito S, Shaffer A, et al. Frailty, length of stay, and mortality in kidney transplant recipients: a national registry and prospective cohort study. Ann Surg. 2017;266:1084–1090. doi: 10.1097/sla.0000000000002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hewitt J, Moug SJ, Middleton M, Chakrabarti M, Stechman MJ, McCarthy K. Prevalence of frailty and its association with mortality in general surgery. Am J Surg. 2015;209:254–259. doi: 10.1016/j.amjsurg.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Parmar KL, Law J, Carter B, Hewitt J, Boyle JM, Casey P, et al. Frailty in older patients undergoing emergency laparotomy: results from the UK observational emergency laparotomy and frailty (ELF) study. Ann Surg. 2021;273:709–718. doi: 10.1097/sla.0000000000003402. [DOI] [PubMed] [Google Scholar]
- 31.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft DK, AWMF):. S3-Leitlinie Kolorektales Karzinom, Langversion 2.1: AWMF; 2019 [Available from: https://www.awmf.org/uploads/tx_szleitlinien/021-007OLl_S3_Kolorektales-Karzinom-KRK_2019-01.pdf]]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.