Abstract
Protected areas that restrict human activities can enhance wildlife habitat quality. Efficacy of protected areas can be improved with increased protection from illegal activities and presence of buffer protected areas that surround a core protected area. Habitat value of protected areas also can be affected by seasonal variation in anthropogenic pressures. We examined seasonal space use by African lions (Panthera leo) within a core protected area, Serengeti National Park, Tanzania, and surrounding buffer protected areas with varying protection strengths. We used lion locations in logistic regression models during wet and dry seasons to estimate probability of use in relation to protection strength, distance to protected area edge, human and livestock density, distance to roads and rivers, and land cover. Lions used strongly protected buffer areas over the core protected area and unprotected areas, and moved away from protected area boundaries toward the core protected area when buffer protected areas had less protection. Lions avoided high livestock density in the wet season and high human density in the dry season. Increased strength of protection can decrease edge effects on buffer areas and help maintain habitat quality of core protected areas for lions and other wildlife species.
Subject terms: Animal behaviour, Behavioural ecology, Conservation biology
Introduction
Many mammal species are increasingly experiencing range contractions, population declines, and extirpations due to anthropogenic destruction of habitat, increased persecution, and overexploitation1–3. Large carnivores are especially affected because their extensive ranges and prey requirements often make them more susceptible to human-wildlife conflicts4,5, which have contributed to their population declines and range contractions during the past two centuries2,4,6. Protected areas that restrict human activities such as hunting, livestock grazing, logging, or land conversion are crucial for large carnivore conservation, as they can mediate anthropogenic pressures on wildlife because they conserve habitat7 and reduce human-wildlife conflicts8, and thus extinction risk9.
Carnivores experience higher levels of human-wildlife conflicts close to the edges of protected areas8,10. However, these “edge effects” can be mediated through the presence of buffer protected areas that surround a core protected area and buffer the core protected area from anthropogenic impacts, therefore enhancing the conservation value of the core protected area11–14. Though buffer areas sometimes permit hunting, livestock grazing, and other sustainable resource uses11,12, they simultaneously allow carnivores to reduce interactions with humans by moving further from populated edges into better quality core habitat11,13,15. However, fewer restrictions on resource use, inadequate law enforcement against illegal activities, and limited community-based benefit sharing (i.e., protected areas provide services or direct payments to surrounding communities) can lead to weaker protection of protected areas, and therefore may allow increased human and livestock incursions and poaching16,17. These human activities can reduce habitat quality and decrease effectiveness of protected areas for carnivores16,17.
Effectiveness of protected areas for wildlife may also vary seasonally due to variation of anthropogenic pressures or prey distribution18–20. To mediate anthropogenic pressures, carnivores within protected areas can alter their habitat use seasonally to avoid interactions with humans21. Carnivores may avoid areas closer to roads or with higher human populations during periods of higher tourism, legal hunting, or poaching20. During times of year with reduced forage, there may be increased livestock incursions into protected areas, where there is better forage18,19, which may in turn shift carnivores away from protected area edges, especially in protected areas with limited law enforcement22. Alternatively, during times of resource scarcity, carnivores may be attracted to protected area edges due to availability of livestock as alternative prey, which can increase human-carnivore conflicts23,24. Non-anthropogenic factors such as water availability and land cover can influence prey distribution seasonally, which in turn can alter carnivore habitat use18,25. During periods of resource scarcity, prey congregate closer to limited resources18,25, and carnivore habitat use shifts to follow prey distribution20,26,27.
African lions (Panthera leo) are especially susceptible to human-wildlife conflicts due to their large home ranges and potential threat to humans and their livelihoods28. Lions within protected areas experience a gradient of seasonal negative interactions with humans, ranging from human and livestock incursions to poaching and bushmeat hunting, due in part to varying protection among protected areas29. Because of lions’ susceptibility to human-wildlife conflicts and the seasonal variation in this susceptibility, it is important to determine the impact that varying protection strength and presence of buffer protected areas have on lion space use, while incorporating a gradient of potential human interactions, water availability, and land cover types.
The Serengeti ecosystem is in northwest Tanzania and is comprised of Serengeti National Park (SNP) and a surrounding network of game reserves and conservation areas, which together create a protected area complex (Fig. 1)19,30. The surrounding game reserves and conservation areas act as a buffer (“buffer protected areas”) to reduce cross-boundary human pressures on the core protected area (SNP)12,19,29–31. Because the surrounding buffer areas have varying regulations and amounts of funding, law enforcement, and community-based benefit sharing12,19,32, this protected area complex is an ideal system to examine variation in effectiveness of buffer protected areas for lions. Though much is known about lion habitat use in the Serengeti ecosystem27,33,34, there has been little research on how buffer protected areas and their protection strength influence lion space use35.
Figure 1.
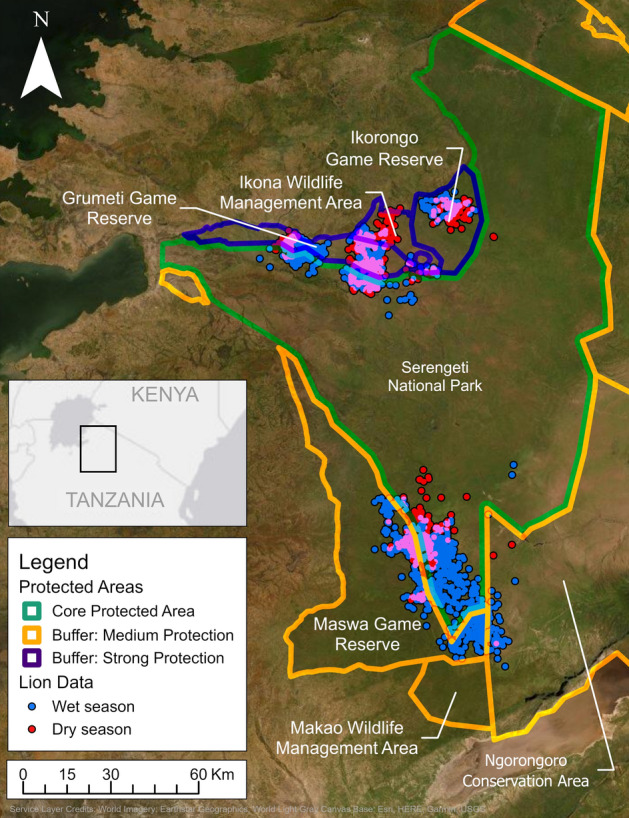
Locations of GPS-collared lions during the wet (November–May) and dry (June–October) seasons in the northern and southern study areas in the protected area complex, with protected area classification (core protected area, buffer protected areas with medium or strong protection)38, Serengeti ecosystem, Tanzania, 2018–2019. Map was created using ESRI ArcGIS Pro Version 2.9 using World Imagery basemap, 2022, Esri Inc [https://www.arcgis.com/home/item.html?id=10df2279f9684e4a9f6a7f08febac2a9].
Our purpose was to determine how strength of protection of protected areas, presence of buffer areas, human and livestock presence, roads, water sources, and land cover affect lion seasonal space use. We predicted that lions would disproportionately use areas with stronger protection and further toward the interior of the protected area complex, and their habitat use would vary seasonally due to differences in prey distribution and human activities. We also predicted that lions would select for areas with lower human population and livestock density and distant from roads, and that these effects would be greater in the dry season. Because prey congregate closer to water and move into shaded areas with thicker, denser forage during periods of resource scarcity18,25, we predicted that lions would disproportionately use these areas during the dry season.
Methods
Study area
This study was conducted in northern and southern portions of the western corridor of SNP, Tanzania, and surrounding protected areas which together comprise a "protected area complex" (2° S, 35° W, 40,000 km2; Fig. 1). Serengeti National Park has the highest conservation priority within this protected area complex and therefore we refer to it as the core protected area in the Serengeti ecosystem29–31. We defined buffer areas as areas within the protected area complex that surround the core protected area of SNP, though we recognize that some of the protected areas we classified as buffer areas could be considered core protected areas19.
The northern study area (3500 km2) included parts of northern SNP and buffer protected areas of Grumeti Game Reserve (GGR), Ikona Wildlife Management Area (IWMA), and Ikorongo Game Reserve (IGR), along with adjacent unprotected areas (Fig. 1). The southern study area (3300 km2) included parts of southern SNP, and buffer areas Ngorongoro Conservation Area (NCA; designated World Heritage Site) and Maswa Game Reserve (MGR; designated IUCN category IV [habitat/species management area])30. Makao Wildlife Management Area is also part of the protected area complex, but lions in our study did not use this protected area and therefore we excluded it from analysis. There are no anthropogenic barriers in the protected area complex except for a 30-km fence along a portion of the northern border of GGR that is unlikely to restrict lion movement36. Serengeti National Park and surrounding game reserves and wildlife management areas prohibit livestock grazing and agriculture, and largely prohibit human settlement, while NCA allows human settlement, sustainable resource extraction, and regulated livestock grazing32,37. Lion harvest is prohibited in Tanzania except in game reserves and wildlife management areas, but GGR, IWMA, and IGR prohibit hunting31. Harvest of lions is allowed in MGR during 1 July–31 December, but no legal harvest of lions has occurred in MGR since 201335. The majority of tourism activity occurs in the Serengeti protected area complex during May–September39.
Annual rainfall varies along a southeast (500 mm) to northwest (1100 mm) gradient, with rain typically occurring from November to May40. The study area contains sparse woodland-grassland with patches of dense woodland, as well as some cultivated areas19,41. There is a seasonal migration of about 1.3 million wildebeest (Connochaetes taurinus) across the ecosystem40,43. During most of the wet season (December–April), wildebeest occur throughout the southern part of our study area before migrating north through the western corridor during the early dry season, and crossing through the northern portion of our study area during August–November43 (Supplementary Fig. S1).
Data collection
We captured 16 lions (12 female, 4 male) from 11 prides during March–November 2018 using broadcasted vocalizations44 and rifle-fired (Palmer CapChur SS cartridge-fired rifle; Cap-Chur Equipment, Powder Springs, Georgia, USA) darts (Pneudart Type U Remote Delivery Devices; Pneudart Inc., Williamsport, PA, USA) from vehicles45. We equipped lions with global positioning system (GPS) collars (Model IR-SAT, African Wildlife Tracking, South Africa) programmed to obtain hourly locations. Animal capture and handling protocols for conducting darting and collaring were approved by State University of New York College of Environmental Science and Forestry (180502) Institutional Animal Care and Use Committee. Conduct of research was approved and permitted by the Tanzania Commission for Science and Technology (2018-6-ER-2016-125) and the Tanzanian Wildlife Research Institute. This study was carried out in compliance with ARRIVE guidelines and all methods were carried out in accordance with relevant guidelines and regulations.
We used previously published strength of border control to represent strength of protection for buffer protected areas19. The classification was based qualitatively on amount of funding, consistency of border patrols and other law enforcement, prevalence of illegal activity, and level of community-based benefit sharing (Fig. 1; Supplementary Methods)12,19,29,31. Grumeti Game Reserve, IGR, and IWMA were categorized as strongly protected buffer areas, while MGR and NCA were categorized as having medium protection (Fig. 1)38. Grumeti Game Reserve, IGR, and IWMA are managed jointly by the government of Tanzania and Singita Grumeti Limited, a private company which strictly limits trophy hunting31, and provides law enforcement resources19,46–49 and increased community-based benefit sharing31,49–52. Thus, GGR, IGR, and IWMA experience less poaching12,46,53, livestock incursion19,46, and illegal crop cultivation19,54 and timber harvest46 as compared to MGR and NCA19,29,55.
To account for influences of land cover on lion space use, we used data from Copernicus Global Land Operations level-1 land cover classification, combining open and closed forest land cover types42. Our final land cover categories were cultivated land, herbaceous vegetation, shrublands, and forest42. We calculated distance from roads and rivers using data from Serengeti GIS and Data Centre, removing rivers classified as ephemeral56,57. We obtained gridded 2018 human population density (people/hectare) from Worldpop58. We summed number of cattle, sheep and goats from the 2010 Gridded Livestock of the World Database (9.26 km2 resolution) to estimate total livestock density (individuals/9.26 km2)59–62.
Analysis
We removed GPS location data for five days following capture to account for potential capture effects. We randomly thinned hourly lion GPS locations to one location each day per lion (“used” points) to reduce spatial and temporal autocorrelation63. We separated lion GPS locations between the north and south study areas and created separate 95% kernel density estimates around used points in each area using the R package “adehabitat”64–66. We then generated an equal number of random available locations within each 95% kernel and combined points across the two areas for our complete dataset.
We extracted distance inside protected area complex (points outside the complex had a negative value), protected area classification (buffer area with strong or medium protection, core protected area, or unprotected area), human and cattle densities, distance to nearest road, distance to nearest river, and land cover classification for each used and available point using program R66. We normalized continuous variables (distance-based metrics and human and cattle densities), then calculated pairwise Pearson product-moment correlations, finding no strong correlations (|r|< 0.7)67. We separated data into wet (November–May) and dry (June–October) seasons33, then used logistic regression models for each season to determine the effects of level of protection, distance to protected area edges, human and livestock density, distance to rivers and roads, and land cover on lion probability of use68. Because lion used and available points in the northern study area were within buffer protected areas with strong protection, the core protected area, and unprotected areas, whereas lion points in the southern area were within buffer protected areas with medium protection and the core protected area, we included an interaction factor between area (north and south) and distance to protected area edge to test the effect of buffers while accounting for the differences in protected area strength. To determine the effects of seasonality on lion probability of use, we fit separate models for wet and dry seasons.
We tested the predictive accuracy of our models with k-fold cross-validation using five folds to calculate area under the receiver operating characteristic curve (AUC)69. We used the statistical significance of individual covariate effects using Wald tests with α = 0.05 to determine which variables contributed to lion probability of use for each season70,71. We compared directionality and effect size of each significant (p < 0.05) variable between seasons to determine which variables most affected lion probability of use. We also computed estimated marginal means for both models to determine significant differences between the effects of protected area classification and land cover type using R package “emmeans”72. We used program R for all analyses66.
Results
Our final dataset included 3612 locations from 16 lions (12 female, 4 male) with an average of 226 locations per lion (range = 79–400, Supplementary Table S1). The sex ratio of collared lions was a consequence of lion availability during captures, but broadly represented the adult sex structure in the population73. We obtained 2418 locations during the wet season and 1194 locations during the dry season. Both seasonal models had adequate fit based on K-fold cross validation (wet season AUC = 0.64, dry season AUC = 0.76).
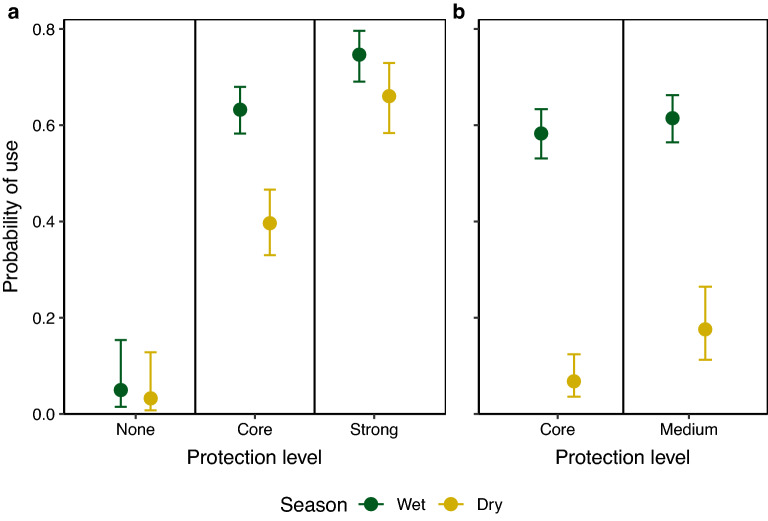
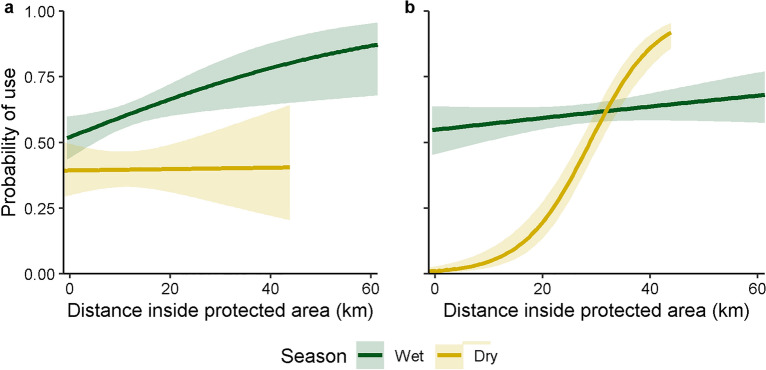
In the northern study area, lion probability of use of buffer protected areas with strong protection and the core protected area was greater than probability of use of unprotected areas, particularly during the wet season (Table 1, Fig. 2, Supplementary Table S2). Lion probability of use was similar in the core protected area and buffer areas with medium protection in the southern study area during the wet season, but in the dry season probability of use of buffer areas with medium protection was greater than probability of use of the core protected area. Lion probability of use increased with increasing distance inside the protected area complex in the wet season in both study areas, but during the dry season, lion probability of use increased with increasing distance inside protected area complex borders only in the southern study area (Fig. 3).
Table 1.
Parameter estimates, standard errors (SE), and p-values for lion probability of use during the wet (November–May) and dry (June–October) seasons, Serengeti ecosystem, Tanzania, 2018–2019. Values for categorical variables are as compared to reference categories of cultivated (land cover), no protection (protected area status), female (sex), and the northern study area. Significant predictors (p < 0.05) are marked in bold.
| Parameter | Wet season | Dry season | ||||
|---|---|---|---|---|---|---|
| Estimate | SE | p-value | Estimate | SE | p-value | |
| Land cover | ||||||
| Herbaceous | − 0.651 | 0.076 | < 0.001 | − 0.712 | 0.121 | < 0.001 |
| Shrublands | − 0.048 | 0.080 | 0.547 | − 0.043 | 0.132 | 0.744 |
| Forest | − 0.402 | 0.138 | 0.004 | 0.333 | 0.199 | 0.095 |
| Population density (people/hectare) | 0.220 | 0.045 | < 0.001 | − 0.171 | 0.071 | 0.016 |
| Livestock density (livestock/9.26 km2) | − 0.248 | 0.051 | < 0.001 | − 0.139 | 0.081 | 0.087 |
| Distance from roads (km) | − 0.281 | 0.035 | < 0.001 | − 0.276 | 0.055 | < 0.001 |
| Distance from rivers (km) | − 0.059 | 0.034 | 0.085 | − 0.577 | 0.06 | < 0.001 |
| Distance inside protected areas (km) | 0.311 | 0.122 | 0.011 | 0.011 | 0.142 | 0.939 |
| Protected area status | ||||||
| Core | 3.490 | 0.629 | < 0.001 | 2.971 | 0.753 | < 0.001 |
| Medium | 3.622 | 0.637 | < 0.001 | 4.050 | 0.783 | < 0.001 |
| Strong | 4.029 | 0.615 | < 0.001 | 4.058 | 0.731 | < 0.001 |
| Sex | ||||||
| Male | 0.121 | 0.079 | 0.124 | 0.030 | 0.117 | 0.798 |
| Study area | ||||||
| South | − 0.208 | 0.135 | 0.122 | − 2.201 | 0.340 | < 0.001 |
| Area × distance inside protected areas | ||||||
| South × distance (km) | − 0.216 | 0.135 | 0.110 | 1.576 | 0.225 | < 0.001 |
Figure 2.
Lion probability of use and 95% confidence intervals in areas without protected status (“none”), core protected areas (“core”), buffer protected areas with medium protection (“medium”), and buffer protected areas with strong protection ("strong”)38 during wet (November–May) and dry (June–October) seasons in the northern (a) and southern (b) study areas, holding other continuous variables constant at mean values and using categorical variables of female sex and cultivated land, Serengeti ecosystem, Tanzania, 2018–2019.
Figure 3.
Lion probability of use and 95% confidence intervals relative to distance from edge of the protected area complex (km) in northern (a) and southern (b) study areas during wet (November–May) and dry (June–October) seasons, holding other continuous variables constant at mean values and using categorical variables of female sex, core protection, and cultivated land, Serengeti ecosystem, Tanzania, 2018–2019.
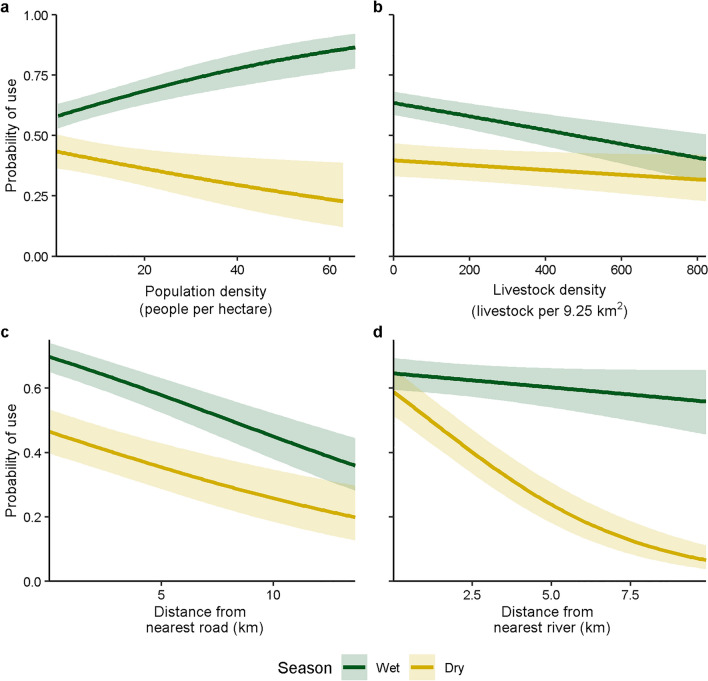
During the wet season, lion probability of use increased with increasing human population density and decreased with increasing livestock density and distance from roads (Table 1, Fig. 4). During the dry season, lion probability of use decreased with increasing human population density, distance from roads, and distance from rivers. Lion probability of use during the wet season was greatest in cultivated areas and shrublands, intermediate in forest, and lowest in herbaceous vegetation (Fig. 5; Supplementary Table S3). Lion probability of use during the dry season was greatest in forest land cover, intermediate in cultivated areas and shrublands, and lowest in herbaceous vegetation.
Figure 4.
Lion probability of use and 95% confidence intervals relative to (a) population density (people/hectare), (b) livestock density (livestock/9.26 km2), (c) distance from nearest road (km), and (d) distance from nearest river (km) during wet (November–May) and dry (June–October) seasons, holding all other continuous variables constant at mean values, and using categorical variables of female sex, northern area, core protection, and cultivated land cover, Serengeti ecosystem, Tanzania, 2018–2019.
Figure 5.
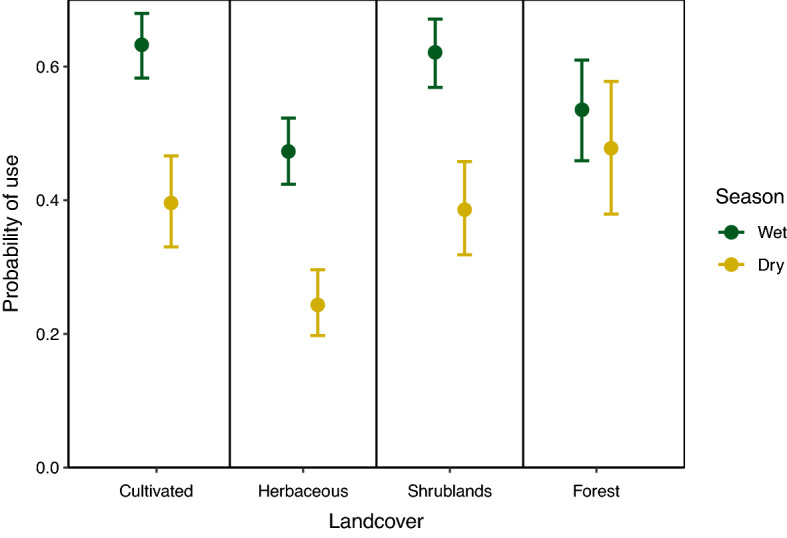
Lion probability of use and 95% confidence intervals in cultivated lands, herbaceous vegetation, shrublands, and forest during wet (November–May) and dry (June–October) seasons, holding other continuous variables at mean values and using categorical variables of female sex, northern area, and core protection, Serengeti ecosystem, Tanzania, 2018–2019.
Discussion
Lions demonstrated lower probability of use of unprotected areas as compared to core protected areas and strongly protected buffer areas, which confirms the importance of protected areas to conserve lion habitat17,74. In the northern study area, lions selected for strongly protected buffer areas (GGR, IWMA, and IGR) over non-protected and core protected areas. In the southern study area, lions used buffer areas with medium protection and the core protected area similarly in the wet season, whereas in the dry season, lions had higher probability of use in buffer areas with medium protection as compared to the core protected area.
Though lion probability of use increased similarly in the north and the south with increasing distance to protected area edges in the wet season, lions avoided protected area edges in the dry season only in the southern study area, where buffer areas have medium protection. In the dry season human and livestock incursions into protected areas increase because forage quality is better18,19,75,76 and water is more plentiful31 inside protected areas. Additionally, there is increased poaching in the dry season due to food scarcity77. That lions avoided protected area edges in the dry season only in buffer areas with medium protection rather than strong protection indicates that increased protection strength decreases impacts of edge effects on lions, especially during periods of resource scarcity.
Strongly protected buffer areas have better law enforcement and increased community-based benefit sharing which decrease prevalence of illegal grazing and poaching29,37,78. These measures can result in decreased edge effects19 including human disturbance21 and increased prey availability17,79, resulting in higher lion survival80 and abundance17. Buffer areas with stronger protection may provide high quality habitat within their borders and therefore may function as core protected areas19,81. We suggest that because buffer protected areas with weaker protection experience increased edge effects19, their edges provide lower quality habitat than edges of strongly protected buffer areas. However, we found that toward the interior of the protected area complex, buffer areas with medium protection provided similar habitat value for lions as compared to core protected areas, while allowing lions to move away from protected area complex edges and increasing habitat value of the core protected area35.
Though seasonal avoidance of protected area complex edges is likely due to greater anthropogenic pressures19,35,82, lion avoidance of edges may also have been influenced by seasonal prey distributions26,43. During the dry season in the northern study area, increased lion use of strongly protected buffer areas over the core protected area was more pronounced and lions did not avoid protected area edges, as compared to the wet season. This pattern may be due to wildebeest migrating through the northern strongly protected areas during August–October (dry season; Supplementary Fig. S1); this increased prey availability in the strongly protected areas and closer to the edges of the protected area complex likely attracts lions into those areas43. In contrast, lions in the southern study area continued to avoid protected area edges when wildebeest were present (in the wet season), though wildebeest occur throughout the buffer protected areas, providing increased prey accessability39,43. Additionally, when wildebeest were not present (dry season in the south; wet season in the north), lions demonstrated greater avoidance of protected area edges in the less strongly protected southern study area as compared to the more strongly protected northern study area. Our findings indicate that while wildebeest presence influences lion habitat use33, strength of protection for buffer protected areas also contributes to lion habitat use.
We found that lions disproportionately used forested areas more than other land cover types in the dry season, while in the wet season they used cultivated areas and shrublands more than herbaceous land cover or forest. While wild ungulates are widely dispersed across the landscape in the wet season, they frequently congregate near permanent water in the dry season18,25. Permanent water sources not only provide water, but the associated woodlands provide a foraging refuge as well as shade22,23,25,26. Therefore, that lions had increased probability of use of forested land cover and closer to permanent water sources in the dry season was likely due to location of prey as well as water availability22,83,84, whereas increased probability of use of cultivated areas and shrublands in the wet season may have been due to more dispersed prey18,20,26.
Lion probability of use decreased with increasing distance to roads, potentially because roads facilitate stealth predation27 or because lions, like other large carnivores85, use roads to facilitate travel86. Additionally, that lions did not avoid roads in either season indicates that photographic tourism may not strongly influence lion space use in this ecosystem because lions in this area are habituated to vehicles17,87. Lion probability of use was negatively related to livestock density during the wet season. Increased livestock presence can reduce effectiveness of protected areas for lions because livestock are associated with increased human presence and therefore human-wildlife conflicts17. Livestock also may compete for resources with wild ungulates, potentially reducing prey availability for lions11,18. That lions only avoided livestock during the wet season, and that lion habitat use was influenced by distance to nearest river only in the dry season, suggests that decreased availability of water during the dry season concentrates humans and livestock at permanent water sources used by lions and their prey23. Therefore, water scarcity could lead to increased predation of livestock and increased human-wildlife conflicts23,88.
Consistent with our predictions, lion probability of use decreased with increasing human population density, but only in the dry season. Lions may have avoided areas of higher human population density especially during the dry season because more tourism, legal hunting, and poaching occur during this time20,39,46,48. In contrast to previous research22,26,89, we found that lion probability of use increased with increasing human population density in the wet season. We suggest that because prey are more widely distributed in the wet season18,22,33, lions may have used areas with higher human population due to difficulty of hunting wild prey33. Alternatively, because we were unable to account for seasonal variation in human population density, the areas lions used may have only appeared to have higher human population density, when in actuality the areas were sparsely inhabited. Human population density varies seasonally in the Serengeti ecosystem90, due to pastoral practices22 and increased use of tourist camps in the dry season39. We suggest that future research on lion habitat use considers seasonal variation in spatial distribution of humans.
Due to a lack of reliable data, we were unable to account for wild prey abundance or distribution. Together with anthropogenic activity91, prey availability is a major determinant of lion space use20,26,27. Therefore, increased lion probability of use of areas with stronger protection was likely driven not only by the wildebeest migration, but also non-migratory prey availability in these areas, as both lions and their prey benefit from lower human disturbance provided by protected areas17. Our findings on lion use of buffer areas were influenced by migrating wildebeest, but by separating northern and southern study areas and modeling seasons separately we were able to incorporate the effects of wildebeest migration on lion space use43. Additionally, though we collared lions from 11 prides, lion space use could have been influenced by territoriality of surrounding lion prides, and increased lion use of buffer protected areas with medium protection as compared to core protected areas may be due to territoriality of lion prides within the core protected area34. Lion lack of use of Makao Wildlife Management Area may have been due to lion territoriality or where lions were captured. Similarly, we only used data from lions collared in buffer protected areas, so our results are applicable only to lions that primarily use these areas.
An additional caveat is that the classification of strength of protection in protected areas we used is inherently qualitative. Many factors can influence the effectiveness of protected areas for wildlife including adjacent human density7,19, community support of protected areas7, extent of community involvement92, corruption in protected area management29, amount and efficacy of law enforcement10, and economic conditions of the surrounding communities92 and country10. The protected areas in this study had varying combinations of these factors31,32,46, and therefore their efficacy for protecting wildlife likely varied within categories. However, the categorization of buffer protected areas into areas with medium and strong protection is supported quantitatively19,46, with areas categorized as medium protection having more livestock incursions, illegal agriculture, poaching, and timber harvest than areas that were strongly protected (Supplementary Methods)19,46,47. Improved quantification of law enforcement, community-based benefit sharing, and protected area funding would be beneficial to determine which factors of protected area strength are most influential for lion habitat use and would contribute to protected area management.
Creation of effective protected areas for large carnivore conservation requires an understanding of seasonal factors that affect their habitat use21,89. Our findings on seasonal lion habitat use in relation to human and livestock density, distance to roads and rivers, and land cover were broadly consistent with previous research that demonstrated that lions balance seasonal patterns of prey availability22,33 with avoiding humans20,93. We also quantified the importance of buffer protected areas and their strength of protection for lions. Buffer protected areas likely increase habitat value of core protected areas for large carnivores by allowing them to move away from human disturbance along the edges of buffer protected areas into core protected areas35. Additionally, buffer protected areas that provide increased funding and alternative employment for neighboring communities and have increased enforcement against illegal activity not only enhance integrity of core protected areas, but also themselves may provide high quality habitat. While strongly protected buffer areas may increase suitable habitat, those with less protection can reduce edge effects for core protected areas and therefore maintain quality of wildlife habitat within core protected areas.
Supplementary Information
Acknowledgements
Funding was provided by Safari Club International Foundation and Camp Fire Conservation Fund. Field project support was provided by C. Comer and J. Goergen. I. Mkasanga, N. Ngowe, R. Hastings, E. Kisega, J. Pallanyo, B. Lubengo and A. Mbwambo, while P. Kessy provided field assistance. T. Petroelje and B. Orning aided in conceptualization and editing.
Author contributions
S.L.S., S.P.F., N.L.F., K.F.K., A.L.L., J.P., A.Z.P., M.V.D.B., H.M.B., M.G.G., J.E.H., T.M.K., N.H.W., and J.L.B. conceptualized the work. L.M.M., S.B.M., R.F., and J.L.B. created capture methodology and completed field work. S.L.S., N.L.F., and A.L.L. compiled data. S.L.S., N.L.F., and K.F.K. devised analysis methodology and S.L.S. completed formal analyses and created figures and tables. S.L.S. wrote the original manuscript text with contributions from S.P.F., J.P., A.Z.P., and M.V.D.B. All authors reviewed the manuscript and S.L.S. revised the manuscript. R.F. and J.L.B. acquired funding and supervised project completion.
Data availability
Raw data on animal locations are unavailable to ensure the well-being of the animals. All other datasets used during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-22053-y.
References
- 1.Pacifici M, Di Marco M, Watson JEM. Protected areas are now the last strongholds for many imperiled mammal species. Conserv. Lett. 2020;13:1–7. [Google Scholar]
- 2.Cardillo M, et al. Human population density and extinction risk in the world’s carnivores. PLoS Biol. 2004;2:909–914. doi: 10.1371/journal.pbio.0020197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steffen W, Broadgate W, Deutsch L, Gaffney O, Ludwig C. The trajectory of the anthropocene: the great acceleration. Anthr. Rev. 2015;2:81–98. [Google Scholar]
- 4.Wolf C, Ripple WJ. Range contractions of the world’s large carnivores. R. Soc. Open Sci. 2017;4:170052. doi: 10.1098/rsos.170052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf C, Ripple WJ. Prey depletion as a threat to the world’s large carnivores. R. Soc. Open Sci. 2016;3:160252. doi: 10.1098/rsos.160252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ripple WJ, et al. Status and ecological effects of the world’s largest carnivores. Science. 2014;343:1241484. doi: 10.1126/science.1241484. [DOI] [PubMed] [Google Scholar]
- 7.Geldmann J, et al. Effectiveness of terrestrial protected areas in reducing habitat loss and population declines. Biol. Conserv. 2013;161:230–238. [Google Scholar]
- 8.Benítez-López A, et al. The impact of hunting on tropical mammal and bird populations. Science. 2017;356:180–183. doi: 10.1126/science.aaj1891. [DOI] [PubMed] [Google Scholar]
- 9.Di Marco M, Ferrier S, Harwood TD, Hoskins AJ, Watson JEM. Wilderness areas halve the extinction risk of terrestrial biodiversity. Nature. 2019;573:582–585. doi: 10.1038/s41586-019-1567-7. [DOI] [PubMed] [Google Scholar]
- 10.Rija AA, Critchlow R, Thomas CD, Beale CM. Global extent and drivers of mammal population declines in protected areas under illegal hunting pressure. PLoS One. 2020;15:1–14. doi: 10.1371/journal.pone.0227163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bamford AJ, Ferrol-Schulte D, Wathan J. Human and wildlife usage of a protected area buffer zone in an area of high immigration. Oryx. 2014;48:504–513. [Google Scholar]
- 12.Snyder KD, Mneney PB, Wittemyer G. Predicting the risk of illegal activity and evaluating law enforcement interventions in the western Serengeti. Conserv. Sci. Pract. 2019;1:1–13. [Google Scholar]
- 13.Woodroffe R, Ginsberg JR. Edge effects and the extinction of populations inside protected areas. Science. 1998;280:2126–2128. doi: 10.1126/science.280.5372.2126. [DOI] [PubMed] [Google Scholar]
- 14.Lynagh FM, Urich PB. A critical review of buffer zone theory and practice: A Philippine case study. Soc. Nat. Resour. 2002;15:129–145. [Google Scholar]
- 15.Paolino, R. M. et al. Buffer zone use by mammals in a Cerrado protected area. Biota Neotrop.16, (2016).
- 16.Mills KL, et al. Comparable space use by lions between hunting concessions and national parks in West Africa. J. Appl. Ecol. 2020;57:975–984. [Google Scholar]
- 17.Lindsey PA, et al. The performance of African protected areas for lions and their prey. Biol. Conserv. 2017;209:137–149. [Google Scholar]
- 18.Tyrrell P, Russell S, Western D. Seasonal movements of wildlife and livestock in a heterogenous pastoral landscape: Implications for coexistence and community based conservation. Glob. Ecol. Conserv. 2017;12:59–72. [Google Scholar]
- 19.Veldhuis MP, et al. Cross-boundary human impacts compromise the Serengeti-Mara ecosystem. Science. 2019;363:1424–1428. doi: 10.1126/science.aav0564. [DOI] [PubMed] [Google Scholar]
- 20.Everatt KT, Andresen L, Somers MJ. The influence of prey, pastoralism and poaching on the hierarchical use of habitat by an apex predator. Afr. J. Wildl. Res. 2015;45:187–196. [Google Scholar]
- 21.Oriol-Cotterill A, Macdonald DW, Valeix M, Ekwanga S, Frank LG. Spatiotemporal patterns of lion space use in a human-dominated landscape. Anim. Behav. 2015;101:27–39. [Google Scholar]
- 22.Schuette P, Creel S, Christianson D. Coexistence of African lions, livestock, and people in a landscape with variable human land use and seasonal movements. Biol. Conserv. 2013;157:148–154. [Google Scholar]
- 23.Beattie K, Olson ER, Kissui B, Kirschbaum A, Kiffner C. Predicting livestock depredation risk by African lions (Panthera leo) in a multi-use area of northern Tanzania. Eur. J. Wildl. Res. 2020;66:1–4. [Google Scholar]
- 24.Loveridge, A. J., Hemson, G., Davidson, Z. & Macdonald, D. W. African lions on the edge: Reserve boundaries as ‘attractive sinks’. In Biology and Conservation of Wild Felids (eds. Macdonald, D. W. & Loveridge, A. J.) 283–304 (Oxford University Press, 2010).
- 25.Boyers M, Parrini F, Owen-Smith N, Erasmus BFN, Hetem RS. How free-ranging ungulates with differing water dependencies cope with seasonal variation in temperature and aridity. Conserv. Physiol. 2019;7:1–12. doi: 10.1093/conphys/coz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abade L, et al. The relative effects of prey availability, anthropogenic pressure and environmental variables on lion (Panthera leo) site use in Tanzania’s Ruaha landscape during the dry season. J. Zool. 2020;310:135–144. [Google Scholar]
- 27.Hopcraft JGC, Sinclair ARE, Packer C. Planning for success: Serengeti lions seek prey accessibility rather than abundance. J. Anim. Ecol. 2005;74:559–566. [Google Scholar]
- 28.Treves A, Karanth KU. Human-carnivore conflict and perspectives on carnivore management worldwide. Conserv. Biol. 2003;17:1491–1499. [Google Scholar]
- 29.Kisingo, A. W. Governance of Protected Areas in the Serengeti Ecosystem, Tanzania (University of Victoria, 2013).
- 30.UNEP-WCMC & IUCN. Protected planet: the world database on protected areas (WDPA). www.protectedplanet.net (2020).
- 31.Zella AY. The management of protected areas in Serengeti ecosystem: A case study of Ikorongo and Grumeti Game Reserves (IGGRs) Int. J. Eng. Sci. 2016;6:22–50. [Google Scholar]
- 32.IUCN. Ngorongoro Conservation Area conservation outlook assessment. The IUCN World Heritage Outlookhttps://worldheritageoutlook.iucn.org/explore-sites/wdpaid/2010 (2020).
- 33.Kittle AM, Bukombe JK, Sinclair ARE, Mduma SAR, Fryxell JM. Landscape-level movement patterns by lions in western Serengeti: Comparing the influence of inter-specific competitors, habitat attributes and prey availability. Mov. Ecol. 2016;4:1–18. doi: 10.1186/s40462-016-0082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Packer C, et al. Ecological change, group territoriality, and population dynamics in Serengeti lions. Science. 2005;307:390–393. doi: 10.1126/science.1105122. [DOI] [PubMed] [Google Scholar]
- 35.Mwampeta SB, et al. Lion and spotted hyena distributions within a buffer area of the Serengeti-Mara ecosystem. Sci. Rep. 2021;11:1–8. doi: 10.1038/s41598-021-01518-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grumeti Fund. Protecting wildlife and human lives in the western corridor of the Serengeti. https://www.grumetifund.org/blog/updates/protecting-wildlife-and-human-lives-in-the-western-corridor-of-the-serengeti/ (2020).
- 37.IUCN. Serengeti National Park conservation outlook assessment. The IUCN World Heritage Outlookhttps://worldheritageoutlook.iucn.org/explore-sites/wdpaid/2575 (2017).
- 38.Veldhuis, M. P. et al. Data from: Cross-boundary human impacts compromise the Serengeti-Mara ecosystem. Dryad 10.5061/dryad.b303788 (2021). [DOI] [PubMed]
- 39.Larsen F, et al. Wildebeest migration drives tourism demand in the Serengeti. Biol. Conserv. 2020;248:108688. [Google Scholar]
- 40.Norton-Griffiths M, Herlocker D, Pennycuick L. The patterns of rainfall in the Serengeti Ecosystem, Tanzania. Afr. J. Ecol. 1975;13:347–374. [Google Scholar]
- 41.McNaughton SJ. Serengeti grassland ecology: The role of composite environmental factors and contingency in community organization. Ecol. Monogr. 1983;53:291–320. [Google Scholar]
- 42.Buchhorn, M. et al. Copernicus global land service: land cover 100m: version 3 globe 2015-2019. Copernicus Global Land Operations. Zenodo. 10.5281/zenodo.3938963.
- 43.Boone RB, Thirgood SJ, Hopcraft JGC. Serengeti wildebeest migratory patterns modeled from rainfall and new vegetation growth. Ecology. 2006;87:1987–1994. doi: 10.1890/0012-9658(2006)87[1987:swmpmf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 44.Ogutu JO, Dublin HT. The response of lions and spotted hyaenas to sound playbacks as a technique for estimating population size. Afr. J. Ecol. 1998;36:83–95. [Google Scholar]
- 45.Fyumagwa RD, et al. Comparison of anaesthesia and cost of two immobilization protocols in free-ranging lions. S. Afr. J. Wildl. Res. 2012;42:67–70. [Google Scholar]
- 46.Rija, A. A. Spatial Pattern of Illegal Activities and the Impact on Wildlife Populations in Protected Areas in the Serengeti Ecosystem. (University of York, 2017).
- 47.Kideghesho, J. R. Wildlife Conservation and Local Land Use Conflicts in Western Serengeti Corridor, Tanzania (Norwegian University of Science and Technology, 2006).
- 48.Holmern T, Muya J, Røskaft E. Local law enforcement and illegal bushmeat hunting outside the Serengeti National Park, Tanzania. Environ. Conserv. 2007;34:55–63. [Google Scholar]
- 49.Schmitt, J. A. Improving Conservation Efforts in the Serengeti Ecosystem, Tanzania: An Examination of Knowledge, Benefits, Costs, and Attitudes (University of Minnesota, 2010).
- 50.Kaaya E, Chapman M. Micro-credit and community wildlife management: Complementary strategies to improve conservation outcomes in Serengeti National Park, Tanzania. Environ. Manag. 2017;60:464–475. doi: 10.1007/s00267-017-0856-x. [DOI] [PubMed] [Google Scholar]
- 51.Kideghesho JR, Røskaft E, Kaltenborn BP. Factors influencing conservation attitudes of local people in Western Serengeti, Tanzania. Biodivers. Conserv. 2007;16:2213–2230. [Google Scholar]
- 52.Kegamba JJ, Sangha KK, Wurm P, Garnett ST. A review of conservation-related benefit-sharing mechanisms in Tanzania. Glob. Ecol. Conserv. 2022;33:e01955. [Google Scholar]
- 53.Rija, A. A. & Kideghesho, J. R. Poachers’ strategies to surmount anti-poaching efforts in Western Serengeti, Tanzania. In Protected Areas in Northern Tanzania (eds. Durrant, J. O. et al.) 91–112 (Springer Nature Switzerland AG, 2020).
- 54.Mfunda IM, Røskaft E. Wildlife or crop production: The dilemma of conservation and human livelihoods in Serengeti, Tanzania. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2011;7:39–49. [Google Scholar]
- 55.Kideghesho JR. Reversing the trend of wildlife crime in Tanzania: Challenges and opportunities. Biodivers. Conserv. 2016;25:427–449. [Google Scholar]
- 56.Sisya, E., Frankfurt Zoological Society & Tanzania National Parks Authority. Serengeti Park Roads. Serengeti GIS and Data Centre and ArcGIS Online. ArcGIS online https://www.arcgis.com/home/item.html?id=f8d9e2cb6ab24b92bd6d645a0d659963. (2018).
- 57.Maliti, H., von Hagen, C., Frankfurt Zoological Society, Tanzania National Parks Authority & Hopcraft, J. G. C. Serengeti Park rivers. https://serengetidata.weebly.com/rivers-and-lakes.html (2008).
- 58.Worldpop & Center for International Earth Science Information Network. The spatial distribution of population density in 2018, Tanzania. 10.5258/SOTON/WP00674 (2018).
- 59.Gilbert, M. et al. Global cattle distribution in 2010 (5 minutes of arc). Harvard Dataverse, Version 3.10.7910/DVN/GIVQ7 (2018).
- 60.Gilbert, M. et al. [dataset] Global goat distribution in 2010 (5 minutes of arc). Harvard Dataverse, Version 3. 10.7910/DVN/OCPH42 (2018).
- 61.Gilbert, M. et al. Global sheep distribution in 2010 (5 minutes of arc). Harvard Dataverse, Version 3. 10.7910/DVN/BLWPZN (2018).
- 62.Gilbert M, et al. Global distribution data for cattle, buffaloes, horses, sheep, goats, pigs, chickens and ducks in 2010. Sci. Data. 2018;5:1–11. doi: 10.1038/sdata.2018.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Swihart RK, Slade NA. Testing for independence of observations in animal movements. Ecology. 1985;66:1176–1184. [Google Scholar]
- 64.Seaman DE, Powell RA. An evaluation of the accuracy of kernel density estimators for home range analysis. Ecology. 1996;77:2075–2085. [Google Scholar]
- 65.Calenge C. The package adehabitat for the R software: Tool for the analysis of space and habitat use by animals. Ecol. Model. 2006;197:516–519. [Google Scholar]
- 66.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Version 4.0.4. https://www.r-project.org/ (2021).
- 67.Dormann CF, et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography (Cop.) 2013;36:27–46. [Google Scholar]
- 68.Thomas DL, Taylor EJ. Study designs and tests for comparing resource use and availability II. J. Wildl. Manag. 2006;70:324–336. [Google Scholar]
- 69.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 70.Sommer S, Huggins RM. Variables selection using the Wald test and a robust CP. J. R. Stat. Soc. 1996;45:15–29. [Google Scholar]
- 71.Burnham, K. P. & Anderson, D. D. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (Springer, 2002).
- 72.Lenth, R. emmeans: Estimated Marginal Means, aka Least-Squares Means. (2020).
- 73.Ogutu JO, Dublin HT. Demography of lions in relation to prey and habitat in the Maasai Mara National Reserve, Kenya. Afr. J. Ecol. 2002;40:120–129. [Google Scholar]
- 74.Henschel P, et al. Determinants of distribution patterns and management needs in a critically endangered lion (Panthera leo) population. Front. Ecol. Evol. 2016;4:1–14. [Google Scholar]
- 75.Melubo K, Lovelock B. Living inside a UNESCO World Heritage Site: The perspective of the Maasai community in Tanzania. Tour. Plan. Dev. 2019;16:197–216. [Google Scholar]
- 76.Makupa, E. E. Conservation Efforts and Local Livelihoods in Western Serengeti, Tanzania: Experiences from Ikona Community Wildlife Management Area (University of Victoria, 2013).
- 77.Ndibalema VG, Songorwa AN. Illegal meat hunting in serengeti: Dynamics in consumption and preferences. Afr. J. Ecol. 2008;46:311–319. [Google Scholar]
- 78.Geldmann J, Joppa LN, Burgess ND. Mapping change in human pressure globally on land and within protected areas. Conserv. Biol. 2014;28:1604–1616. doi: 10.1111/cobi.12332. [DOI] [PubMed] [Google Scholar]
- 79.Tuqa JH, et al. Impact of severe climate variability on lion home range and movement patterns in the Amboseli ecosystem, Kenya. Glob. Ecol. Conserv. 2014;2:1–10. [Google Scholar]
- 80.Blackburn S, Hopcraft JGC, Ogutu JO, Matthiopoulos J, Frank L. Human–wildlife conflict, benefit sharing and the survival of lions in pastoralist community-based conservancies. J. Appl. Ecol. 2016;53:1195–1205. [Google Scholar]
- 81.Thirgood S, et al. Can parks protect migratory ungulates? The case of the Serengeti wildebeest. Anim. Conserv. 2004;7:113–120. [Google Scholar]
- 82.Wittemyer G, Elsen P, Bean WT, Burton ACO, Brashares JS. Accelerated human population growth at protected area edges. Science. 2008;321:123–126. doi: 10.1126/science.1158900. [DOI] [PubMed] [Google Scholar]
- 83.Hayward MW, Kerley GIH. Prey preferences and dietary overlap amongst Africa’s large predators. S. Afr. J. Wildl. Res. 2008;38:93–108. [Google Scholar]
- 84.Mkonyi FJ, Estes AB, Lichtenfeld LL, Durant SM. Large carnivore distribution in relationship to environmental and anthropogenic factors in a multiple-use landscape of northern Tanzania. Afr. J. Ecol. 2018;56:972–983. [Google Scholar]
- 85.Hill JE, De Vault TL, Belant JL. A review of ecological factors promoting road use by mammals. Mamm. Rev. 2021;51:214–227. [Google Scholar]
- 86.Hägerling HG, Ebersole JJ. Roads as travel corridors for mammals and ground birds in Tarangire National Park, Tanzania. Afr. J. Ecol. 2017;55:701–704. [Google Scholar]
- 87.Bateman PW, Fleming PA. Are negative effects of tourist activities on wildlife over-reported? A review of assessment methods and empirical results. Biol. Conserv. 2017;211:10–19. [Google Scholar]
- 88.de Boer WF, et al. Spatial distribution of lion kills determined by the water dependency of prey species. J. Mammal. 2010;91:1280–1286. [Google Scholar]
- 89.Loveridge AJ, Valeix M, Elliot NB, Macdonald DW. The landscape of anthropogenic mortality: How African lions respond to spatial variation in risk. J. Appl. Ecol. 2017;54:815–825. [Google Scholar]
- 90.Suraci JP, et al. Behavior-specific habitat selection by African lions may promote their persistence in a human-dominated landscape. Ecology. 2019;100:1–11. doi: 10.1002/ecy.2644. [DOI] [PubMed] [Google Scholar]
- 91.Snyman A, Raynor E, Chizinski C, Powell L, Carroll J. African lion (Panthera leo) space use in the Greater Mapungubwe Transfrontier Conservation Area. Afr. J. Wildl. Res. 2018;48:023001. [Google Scholar]
- 92.Mwakaje AG, et al. Community-based conservation, income governance, and poverty alleviation in Tanzania: The case of the Serengeti Ecosystem. J. Environ. Dev. 2013;22:51–73. [Google Scholar]
- 93.Everatt KT, Moore JF, Kerley GIH. Africa’s apex predator, the lion, is limited by interference and exploitative competition with humans. Glob. Ecol. Conserv. 2019;20:e00758. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data on animal locations are unavailable to ensure the well-being of the animals. All other datasets used during the current study are available from the corresponding author on reasonable request.