Abstract
Ocular toxoplasmosis is a potentially blinding intraocular inflammation. The intent of this study was to investigate the role of Fas-FasL interaction in a murine model of acquired ocular toxoplasmosis induced by intracameral inoculation of Toxoplasma gondii. Intraocular inflammation, Fas and FasL expression on lymphocytes and on ocular tissues, the occurrence of apoptosis, and the frequency of CD8+ and CD4+ T cells in the infected eyes were analyzed in C57BL/6 (B6) mice. Susceptibility to parasite-induced intraocular inflammation was observed in Fas-deficient (B6-lpr) and FasL-deficient (B6-gld) mice. Inoculation of 5,000 T. gondii tachyzoites induced significant intraocular inflammation associated with increase of Fas and FasL expression in the inoculated eyes of wild-type B6 mice. Flow cytometry demonstrated a significant increase of Fas and FasL expression on the splenocytes from naive mice incubated in vitro with the parasite and on the splenocytes harvested from the infected mice at day 8 after parasite inoculation. Apoptosis of inflammatory cells and cells in ocular tissues was seen, and a greater frequency of CD8+ than CD4+ T cells was observed in the infected eyes. The intensity of intraocular inflammation was greater in B6-lpr and B6-gld mice than in wild-type B6 mice (P < 0.05). The results suggest that Fas-FasL interaction associated with apoptosis is involved in the pathogenesis of acquired ocular toxoplasmosis in mice.
Human infection with Toxoplasma gondii, an intracellular parasite, is ubiquitous (18). Although benign in most individuals, infection with T. gondii causes significant disease in two groups, newborns and the immunosuppressed, in particular those with AIDS. The parasite is also a frequent cause of potentially blinding ocular disease (27, 31). Ocular toxoplasmosis causes a necrotizing retinitis or retinochoroiditis (1, 11, 15), which is reportedly immune mediated (28). Although most cases of human eye infection are believed to be congenital in origin, acquired acute toxoplasmic retinochoroiditis may be more prevalent than is now recognized (23, 31, 32). Ocular toxoplasmosis may be a component or the presenting manifestation of human immunodeficiency virus infection or AIDS (29). At present there is no consensus for the best initial treatment for this eye disorder because of the poorly understood pathogenesis (15, 32).
Fas (APO1/CD95) and its ligand (FasL, CD95 ligand) are type I and type II transmembrane proteins and members of the tumor necrosis factor/nerve growth factor receptor and tumor necrosis factor families of proteins, respectively (5, 22, 37). Fas is ubiquitously expressed on lymphoid and nonlymphoid cells, while FasL is restricted to T cells and some nonlymphoid tissues, known to be immune privileged sites, including the eye (10, 24). The roles of these molecules in the regulation of immunity and autoimmune disease have been extensively studied in lpr (for lymphoproliferation) and gld (for general lymphoproliferative disease) mice, which carry autosomal recessive mutations and consequently lack functional expression of Fas and FasL (24, 30). Fas and FasL interaction is closely associated with immune privilege and probably provides a barrier to prevent the interaction of Fas+ inflammatory cells and pathogens from damaging tissues in privileged sites or tissues (10, 24). Recent studies in a herpes simplex virus-induced model of ocular inflammation demonstrated that the Fas-FasL interaction protected the infected eye from the spread of dangerous inflammatory responses (9). We therefore hypothesized that Fas-FasL interaction, as a protective mechanism against infection by the protozoan parasite T. gondii, may be involved in pathophysiological process of the parasite-induced ocular inflammation.
Mice have been shown to be suitable for studying the pathogenesis of this infection (7). A novel murine model of acquired ocular toxoplasmosis was recently developed in our laboratory by intracameral inoculation of strain PLK tachyzoites (12). In this study, we describe the involvement of Fas-FasL interaction in the parasite-induced ocular inflammation in mice.
MATERIALS AND METHODS
Parasite.
T. gondii PLK was used throughout these experiments. This clone of Me49 was produced and is maintained in our laboratory by continuous passage in human fibroblasts in Eagle’s minimum essential medium (MEM; Gibco Laboratories, Grand Island, N.Y.) supplemented with 10% newborn calf serum plus antibiotics. Parasites used in these studies were obtained from tissue culture in human fibroblast cells at passage <50. All parasite challenge experiments were performed with tissue culture-derived tachyzoites at various inoculum levels.
Animals.
Female C57BL/6 (B6), B6-lpr, and B6-gld mice, 6 to 8 weeks of age, were obtained from Jackson Laboratory (Bar Harbor, Maine). For each strain, 10 of each group were used in each experiment. These mice were bred under approved conditions in the Animal Research Facility at Dartmouth Medical School. Control mice of the same parental lineage were age and sex matched.
Intraocular inoculation of parasites.
The mice were intracamerally challenged as previously described in a herpesvirus-induced model of ocular inflammation in mice (12, 35). Mice were anesthetized intraperitoneally with 0.08 ml of a 1:1 mixture of ketamine hydrochloride (Mallinckrodt Veterinary, Inc., Mundelein, Ill.) and xylazine (Phoenix Pharmaceutical, Inc., St. Joseph, Mo.) diluted 1:1 with sterile water. Mice were then placed under the operating microscope, and anterior chamber paracentesis was performed by puncturing the cornea midway between the limbus and central cornea on the right eye, using a glass micropipette. After leaking aqueous humor was blotted, 5 μl of parasite suspension containing 5,000 tachyzoites in MEM was injected into the anterior chamber via a 33-gauge needle attached to a 50-μl Hamilton syringe. In some experiments, 5 μl of MEM was inoculated into the contralateral eye or into eyes of additional mice to exclude injection trauma as a cause of inflammatory reaction.
Clinical observation.
Eyes of all mice were examined under the operating microscope every day following intracameral challenge with T. gondii for conjunctival congestion, corneal edema, pupillary dilatation, iris vessel prominence, anterior chamber clouding, cataract, retinal opacification, loss of red reflex, and scleral reaction. Mice were also monitored for evidence of systemic infection such as behavior, fur ruffling, and moribund status. The presence of inflammation was scored on a scale of 0 (normal) to 5+ as follows: 1+, apparent ciliary congestion around the cornea; 2+, intense ciliary congestion with slight cornea edema and anterior chamber clouding; 3+, obvious intraocular inflammatory reaction such as iris vessel prominence, vitreous, and retinal opacification; 4+, endophthalmitis; 5+, obvious ophthalmia with systemic symptoms and/or death in addition to 4+ ocular inflammation. The final score included findings from histopathological observation.
Histopathology.
At day 8 after parasite challenge, eyes were enucleated after mice were sacrificed by CO2 inhalation and were preserved in 10% buffered formalin. Fixed eyes were embedded in paraffin, serially sectioned at 5 μm, and stained with hematoxylin and eosin. The pathological changes were scored on a scale of 0 (normal) to 5+, similar to the clinical scores: 0, normal histology; 1+, vessel dilation, protein precipitate, and <20 inflammatory cells in anterior chamber and vitreous humor per single section; 2+, obvious inflammatory reaction (more than 20 but fewer than 50 inflammatory cells in the ocular cavity) in the anterior segment and few focal lesions of the inner retina; 3+, >50 inflammatory cells in the anterior chamber and vitreous humor, with moderate necrotic retinitis, uveitis, keratitis, vitreitis, and cataract formation; 4+, intensive retinouveitis or endophthalmitis; 5+, features of the preceding classes with extraocular inflammation including scleritis, conjunctivitis, and blepharitis.
Flow cytometry analysis for Fas and FasL on splenocytes.
To evaluate the influence of T. gondii on Fas and FasL expression, normal splenocytes were isolated and incubated in vitro with T. gondii tachyzoites (splenocyte/parasite ratio of 2:1) for 48 h. Fresh splenocytes of infected mice that had been inoculated intraocularly with 5,000 tachyzoites were isolated from the spleens harvested at day 8 postinfection. The splenocytes were stained with antibodies to CD95 (Jo2) and to CD95L (Kay-10) (PharMingen, San Diego, Calif.) and analyzed by flow cytometry as previously described (20). Normal splenocytes were stained as the control, and the isotype antibodies hamster immunoglobulin G (IgG) and mouse IgG2b were used for staining as controls to CD95 and CD95L, respectively.
Fas and FasL detection by immunohistostaining.
The eyes were harvested at day 8 from mice given 5,000 intraocular tachyzoites and were frozen immediately. Immunohistochemistry on frozen sections were carried out with monoclonal antibodies to Fas (N-18) and to FasL (N-20) (Santa Cruz Biotechnology Inc., Santa Cruz, Calif.) as specified by the manufacturer. Briefly, the sections were exposed at room temperature for 30 min and fixed in cold acetone. Endogenous peroxidase activity was quenched in 0.8% hydrogen peroxide, and nonspecific binding was blocked with 1.5% normal goat serum in phosphate-buffered saline (PBS; pH 7.4). Sections were incubated with antibodies to either Fas or FasL at room temperature for 30 min and then incubated with biotin-conjugated second antibodies. After a series of three washes in PBS and a rinse in 1% Triton X-100–PBS, the sections were incubated in diaminobenzidine solution for 2 min for visible color and counterstained in hematoxylin for contrast.
Detection of apoptosis.
By using a terminal deoxynucleotidyltransferase (TdT) in situ apoptosis detection kit with TACS blue label (Genzyme Diagnostics, Cambridge, Mass.), TdT-mediated dUTP-biotin nick end labeling (TUNEL) (2) was applied to the paraffin-embedded sections of the injected eyes of B6 mice for direct detection of apoptosis according to the manufacturer’s protocol. Briefly, ocular tissues (harvested at 48 h, 96 h, and 8 days after intraocular infection) on slides were deparaffinized at 57°C for 5 min on a slide warmer and then washed three times, once each in 100, 95, and 70% ethanol. Samples were permeabilized with proteinase K (1:50) for 5 min at room temperature, and endogenous peroxidase was quenched in a solution of 2% H2O2. TdT labeling was carried out with the TdT enzyme (1:50) in 1× labeling buffer in the humidity chamber at 37°C for 1 h, followed by detection of TACS blue label for 6 min. After washing with deionized water, counterstaining was performed with Red counterstain C solution for 30 s. Normal B6 eyes were used as controls, and HL-60, ML-1, and WI-L2 cell lines supplied by the manufacturer were used as positive and negative controls of apoptosis.
Determination of CD8+ and CD4+ T cells in the eye.
The frequency of CD8+ and CD4+ T cells in the eye at day 8 postinfection was determined by immunophenotyping as described previously (38). Briefly, 30- to 70-μm sections of fresh enucleated eye were cut with a vibratome (V1000; Technical Products International Inc., St. Louis, Mo.). Fluorescein isothiocyanate-conjugated antibodies (L3T4 for CD4, Ly2 for CD8, and rat IgG2a κ as controls; 2 μg/100 μl; PharMingen) in PBS were added to sections, which were then incubated overnight at 4°C in the dark. Stained sections were visualized in a Bio-Rad MR1000 confocal scanning laser microscope system equipped with a krypton-argon laser. Using this system, we were able to optically section the specimens and build three-dimensional reconstructions of the tissue sections. This allowed accurate determination of the specific relationships among cells. Using Image Space software (Molecular Dynamics), we counted cell numbers in three dimensions (within an area of 240.5 by 240.5 μm) and derived semiquantitative estimations of staining intensities.
Statistical analysis.
Clinical observation, pathological evaluation, and determination of T cells in the eyes by confocal scanning were carried out in a double-blind manner by at least two investigators. Variations were expressed as standard errors of the mean, and statistical comparisons between groups were performed by Student’s t test. P values less than 0.05 were considered statistically significant.
RESULTS
Ocular inflammatory reaction of wild-type B6 mice.
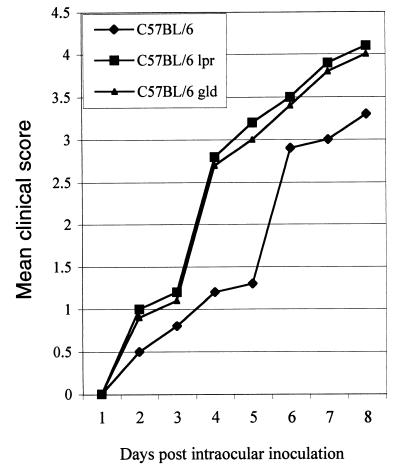
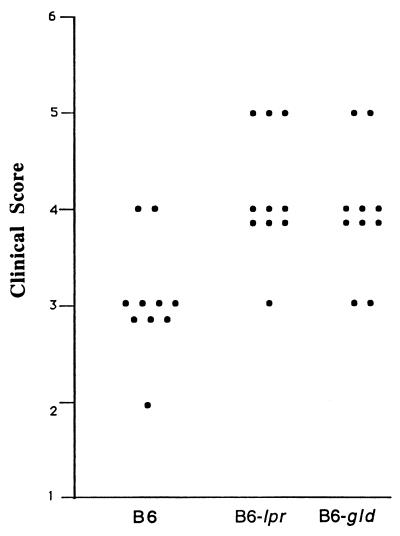
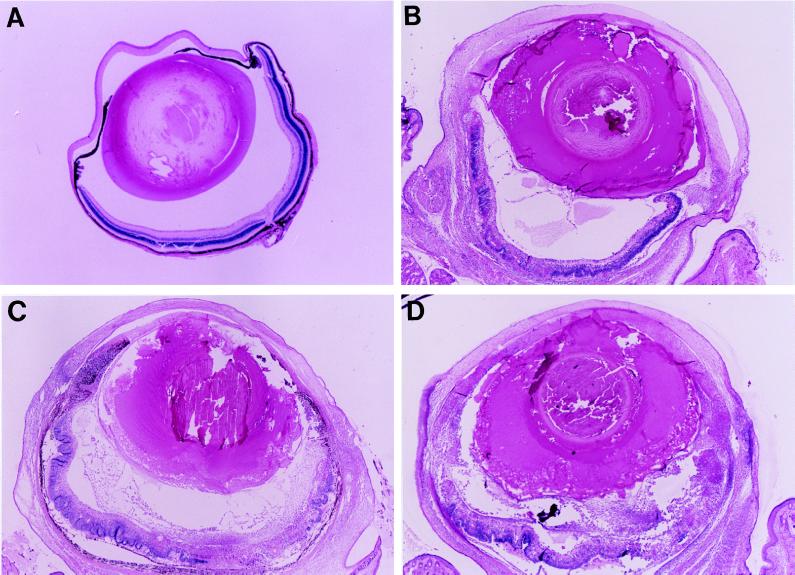
All mice receiving 5,000 parasites developed significant ocular inflammation at day 6 or 7. Inflammation progressed with time as described previously (12). The inflammation score was 3.3 ± 0.7 (n = 10 [Fig. 1 and 2]). Histopathologic analysis was consistent with clinical observation. Intraocular inflammation was obvious (Fig. 3B) and characterized as predominantly mononuclear inflammatory infiltration, accompanied by necrotic retinitis or uveoretinitis.
FIG. 1.
Clinical scores of different groups of mice inoculated with 5,000 strain PLK tachyzoites.
FIG. 2.
Clinical score at day 8 of each group of mice inoculated with 5,000 strain PLK tachyzoites.
FIG. 3.
(A) Uninfected eye; (B to D) ocular inflammation at day 8 of B6 mice (B) and significantly more intensively inflammatory reaction of the eyes of B6-lpr (C) and B6-gld (D) mice intraocularly infected with 5,000 tachyzoites. (Magnification, ×20.)
Ocular inflammation in B6-lpr and B6-gld mice.
Mice with Fas or FasL mutations differed from the parental strain with respect to the induction and severity of the inflammation. The mutant mice were more susceptible than wild-type B6 mice to the parasites. Both Fas and FasL mutant mice that received 5,000 parasites had increased local ocular inflammation, and half exhibited ophthalmia. Obvious systemic reactions such as fur ruffling and/or moribund status were observed in all mice at day 8 postinoculation. The inflammation scores at day 8 after inoculation were 4.1 ± 0.4 (n = 10) and 4.0 ± 0.3 (n = 10) for the B6-lpr and B6-gld mice, respectively. The inflammatory reaction was significantly more severe than that of the wild-type B6 mice (P < 0.05). The inflammation was first noted at day 4 or 5 in the injected eyes of B6-lpr or B6-gld mice (Fig. 1 and 2). Histopathologically, the inflammation was intense and associated with tissue destruction. The cornea became thickened, consisting of a large number of inflammatory cells and neovascularization. The anterior chamber was filled with inflammatory cells and protein, accompanied by necrotic iridocyclitis and cataract formation in all mice. In the posterior segment, necrotic retinitis and uveitis were seen, with striking involvement of vitreous humor and sclera (Fig. 3C and D).
Increased expression of Fas and FasL in infected eyes and splenocytes.
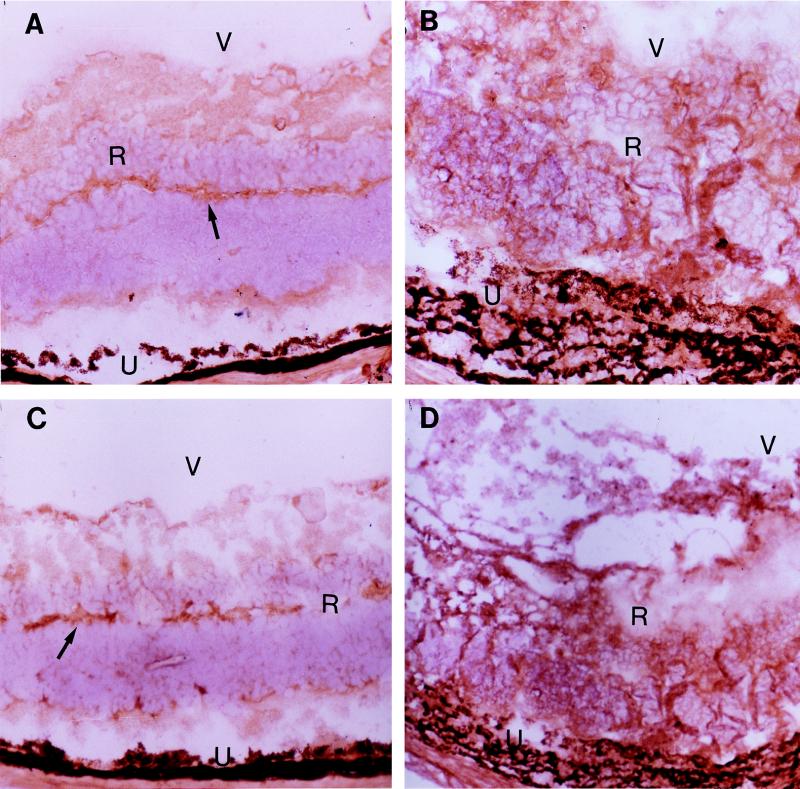
Immunohistostaining showed that normal B6 mouse retina expressed Fas (Fig. 4A) as well as FasL (Fig. 4C), which had been previously demonstrated in mice by Griffith et al. (9, 10). The observation is consistent with the report that Fas and FasL are expressed constitutively in normal human retina (2). The inflamed parasite-inoculated eyes harvested at day 8 postinfection showed a striking increase of both Fas (Fig. 4B) and FasL (Fig. 4D) expression.
FIG. 4.
Fas (A) and FasL (C) expression on normal B6 mouse retina (arrows) and strikingly increased Fas (B) and FasL (D) expression on inflammatory retina of the infected eye at day 8 after intraocular infection with 5,000 parasites. R, retina; U, uvea; V, vitreous humor. (Magnification, ×120.)
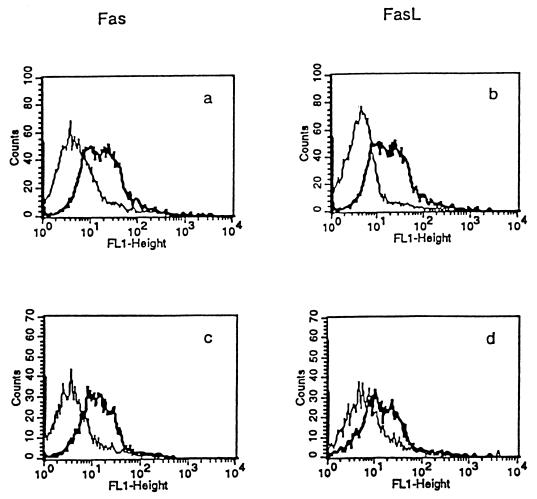
Analysis by flow cytometry showed that T. gondii enhanced expression of Fas and FasL on normal B6 mouse splenocytes incubated with the PLK strain of the parasite (2:1) for 48 h (Fig. 5a and b). Fas and FasL expression on splenocytes of B6 mice infected intraocularly with T. gondii PLK was also notably increased (Fig. 5c and d).
FIG. 5.
Compared with the control isotype antibodies (light line), increased expression (bold line) of Fas and FasL on normal B6 mouse splenocytes incubated for 48 h with T. gondii PLK (2:1) (a and b) and on infected B6 mouse splenocytes and harvested at day 8 after intraocular inoculation with 5,000 tachyzoites (c and d).
Apoptosis in the eye.
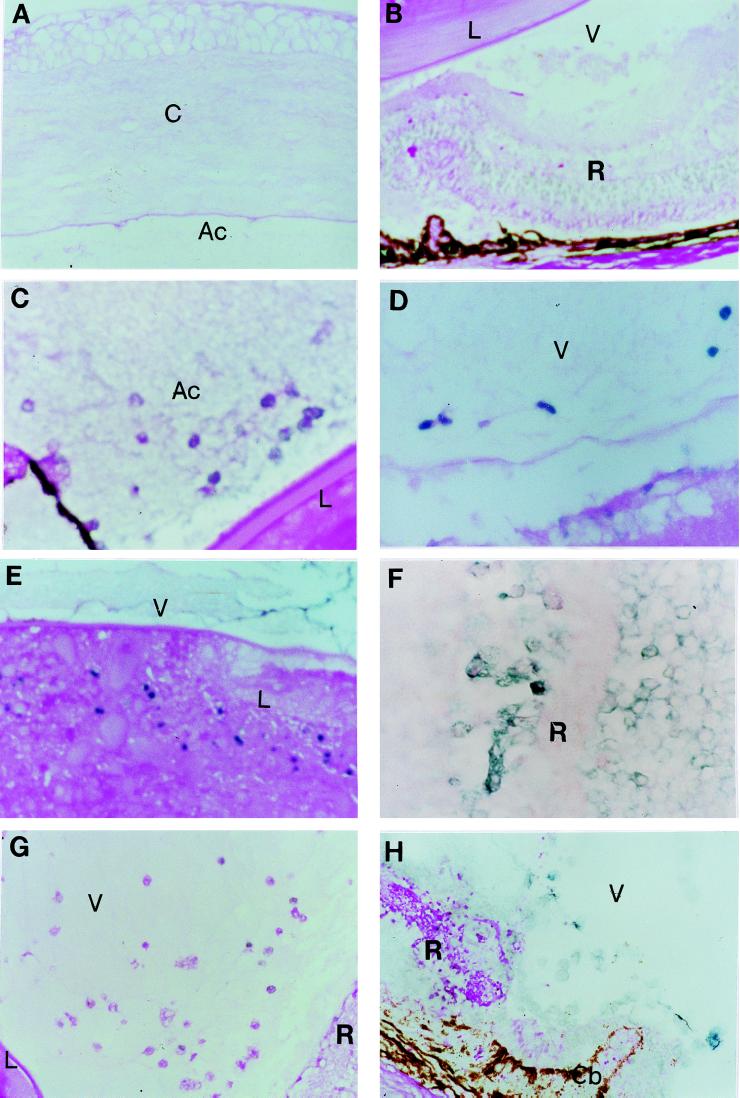
There was no evidence of apoptosis in the normal control B6 eyes (Fig. 6A and B). Apoptosis of infiltrating cells was observed in the anterior chamber 48 h (Fig. 6C) and in the vitreous humor of the injected eyes of the B6 mice 96 h (Fig. 6D) after intraocular infection. These apoptotic changes were demonstrated as well in ocular tissues such as the lens (Fig. 6E) and retina (Fig. 6F), associated with intraocular inflammation at day 8 after infection. In comparison to wild-type B6 mice, B6-lpr and B6-gld mice demonstrated much less apoptosis (Fig. 6G and H) of the infiltrating cells and tissues of the T. gondii-infected eyes.
FIG. 6.
No apoptosis was detected in the anterior chamber and vitreous humor and in tissues such as the cornea, lens and retina of the eye (A and B). Apoptosis (dark blue) of infiltrating cells was seen in the anterior chamber of B6 mice at 48 h (C) and in the vitreous humor at day 4 (D), and apoptotic cells in the lens (E) and retina (F) were detected at day 8 after intraocular inoculation of 5,000 parasites. There were fewer apoptotic cells in the eyes of either B6-lpr (G) or B6-gld (H) mice at day 4 after infection. Ac, anterior chamber; C, cornea; Cb, ciliary body; L, lens; R, retina; U, uvea; V, vitreous humor. (Magnification, ×200.)
Frequency of CD8+ and CD4+ T cells in infected eyes.
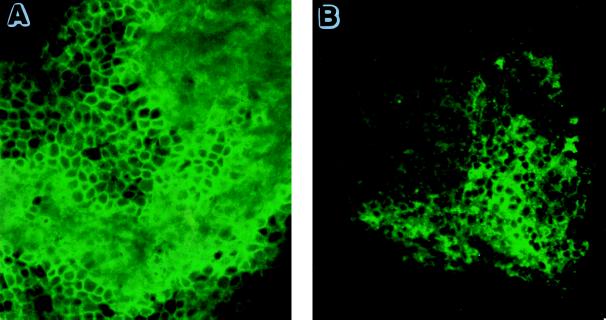
Both CD8+ (Fig. 7A) and CD4+ (Fig. 7B) T cells were evident, with a predominance of CD8+ T cells in the infected eyes at day 8.
FIG. 7.
CD8+ and CD4+ T cells in infected eyes at day 8. A greater frequency of CD8+ (A) than of CD4+ (B) T cells was observed at day 8. (Magnification, ×40.)
DISCUSSION
Ocular toxoplasmosis causes destructive intraocular inflammation targeting the retina and uvea and potentially resulting in blindness (15, 27, 32). Intracameral inoculation of tachyzoites of T. gondii PLK induced ocular inflammation, as we have previously reported (12). Histopathologic analysis of the ocular lesions demonstrated predominantly mononuclear cell infiltration and tissue necrosis within the eye, which resembles features of ocular toxoplasmosis in humans. Using this in vivo model, we have demonstrated that Fas and FasL expression is enhanced on splenocytes as well as in tissues of the eye, accompanied by apoptosis which occurred in early through late stage in this study. Meanwhile, earlier and more severe intraocular inflammation was observed in both B6-lpr and B6-gld mice that lack Fas or FasL compared to parental B6 mice. The results indicate that the Fas-FasL system is involved in the process of parasite-induced ocular inflammation and that apoptosis is associated with the inflammatory reaction in the eye.
The possibility that Fas-FasL interaction plays a role in protection of the eye was suggested by study of a murine model of herpesvirus-induced ocular inflammation (9). Herpesvirus induces uveoretinitis and keratitis which are mediated mainly by CD4+ T cells (16, 26). In our murine model, T. gondii-induced host response and intraocular inflammation are mediated predominantly by CD8+ T cells, as shown in this and a previous (17) study. On the other hand, herpesvirus is a documented inducer of apoptosis (34), while the role of T. gondii in the apoptotic process remains unresolved (20, 21, 25). To clarify the role of Fas-FasL in the process of ocular toxoplasmosis, we have shown for the first time that enhanced Fas-FasL expression was indeed involved and apoptosis occurred in T. gondii-infected eyes.
It is of interest that Fas and FasL expression is significantly increased not only on host splenocytes incubated in vitro with the parasite but also on splenocytes harvested from the intraocularly infected mice. The results are consistent with observations demonstrating induction of Fas on lymphocytes by in vitro coculture with T. gondii tachyzoites (13). Further, Fas and FasL were expressed in the normal mouse eyes, and the level of expression was significantly enhanced upon parasite infection. It is speculated that the increase in Fas and FasL expression of the infected eye could be due directly to parasites, and the increased expression of the apoptotic marker in splenocytes of the infected mice might be elicited directly by the organisms that have migrated to the spleen from the inoculated eye. Details of the mechanisms by which the parasite enhances the expression of Fas and FasL in the eye and how the parasites inoculated in the eye influence the expression of apoptotic molecules on the splenocytes remain to be elucidated.
Our results suggest that a decrease in either Fas or FasL expression may lead to rapid development of severe and destructive ocular inflammation. We hypothesize that apoptosis induced by Fas-FasL interaction may play a role in protection in the anterior chamber in early events after the inoculation of parasites. It is suggested that mice with deficient Fas-FasL function have a decreased capability to limit parasite replication so that inflammation is more intense and destructive than in the parental mice with normal Fas-FasL expression.
Apoptosis is an important mechanism for protection against virus infection in the eye (9, 33). The potential role of T. gondii in apoptotic processes is not entirely understood. Protozoan parasite activation-induced CD4+ T-cell apoptosis has been demonstrated during acute T. gondii infection in mice (20). Gamma interferon-induced, Fas-dependent apoptosis of Peyer’s patch T cells in mice follows peroral infection with T. gondii (21), while T. gondii-infected cells demonstrate resistance to multiple inducers of apoptosis (25). The present study of ocular toxoplasmosis is supportive of the notion that T. gondii can evoke apoptosis. Apoptosis, in contrast to necrosis, characteristically does not elicit an inflammatory response, thereby allowing an organism to safely eliminate unnecessary or potentially harmful cells (36). It seems, however, that enhanced Fas-FasL expression and possible Fas-FasL-induced apoptosis may play dual roles during active toxoplasma replication in the eye. In the early stage apoptosis may be protective, helping to eliminate the relatively small number of tachyzoites. In contrast, overproduction of Fas-FasL is invoked in the pathogenesis of a number of diseases, such as hepatitis, thyroiditis, and uveitis (2, 6). Apoptosis in the retina observed in the late stage of ocular toxoplasmosis may be the result of increased expression of Fas and FasL and may contribute to ocular tissue damage.
An imbalance between the protective and destructive effects of the host immune response in the pathogenesis of ocular toxoplasmosis has been suggested (3). The control of systemic infection is dependent on a strong cell-mediated immune response (4, 14). There is abundant evidence for the crucial role of CD8+ T cells in systemic immunity to this pathogen (4, 14, 19). CD4+ T cells appear to synergize with CD8+ T cells to afford protection against T. gondii (8). Whether these T-cell subsets act in the eye as they do in the systemic infection remains to be illustrated. Further studies are under way to identify in detail the mechanisms by which Fas and/or FasL expression may interact with CD8+ and/or CD4+ T cells and the phenotype of the apoptotic cells that we observed in ocular tissues.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI19613 and K32AI1558.
We thank Karlya Wheeler for assistance with the ocular histopathological sections.
REFERENCES
- 1.Berger B B, Egwuagu C E, Freeman W R, Wiley C A. Miliary toxoplasmic retinitis in acquired immunodeficiency syndrome. Arch Ophthalmol. 1993;111:373–376. doi: 10.1001/archopht.1993.01090030091046. [DOI] [PubMed] [Google Scholar]
- 2.Chan C C, Matteson D M, Li Q, Whitcup S M, Nussenblatt R B. Apoptosis in patients with posterior uveitis. Arch Ophthalmol. 1997;115:1559–1567. doi: 10.1001/archopht.1997.01100160729010. [DOI] [PubMed] [Google Scholar]
- 3.Decoster A, Darcy F, Capron A. Recognition of T. gondii excreted and secreted antigens by human sera from acquired and congenital toxoplasmosis: identification of markers of acute and chronic infection. Clin Exp Immunol. 1988;73:376. [PMC free article] [PubMed] [Google Scholar]
- 4.Denkers E Y, Sher A, Gazzinelli R T. CD8+ T-cell interactions with T. gondii: implications for processing of antigen for class-I-restricted recognition. Res Immunol. 1993;144:51–57. doi: 10.1016/s0923-2494(05)80099-9. [DOI] [PubMed] [Google Scholar]
- 5.Depraetere V, Golstein P. Fas and other cell death signaling pathways. Semin Immunol. 1997;9:93–107. doi: 10.1006/smim.1997.0062. [DOI] [PubMed] [Google Scholar]
- 6.French L E, Tschopp J. Thyroiditis and hepatitis: Fas on the road to disease. Nat Med. 1997;3:387–388. doi: 10.1038/nm0497-387. [DOI] [PubMed] [Google Scholar]
- 7.Gazzinelli R T, Brezin A, Li Q, Nussenblatt R B, Chan C C. Toxoplasma gondii: acquired ocular toxoplasmosis in the murine model, protective role of TNF-α and IFN-γ. Exp Parasitol. 1994;78:217–229. doi: 10.1006/expr.1994.1022. [DOI] [PubMed] [Google Scholar]
- 8.Gazzinelli R T, Hakim F T, Hieny S, Shearer G M, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-γ production and protective immunity induced by an attenuated T. gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- 9.Griffith T S, Brunner T, Fletcher S M, Green D R, Ferguson T A. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 10.Griffith T S, Ferguson T A. The role of FasL-induced apoptosis in immune privilege. Immunol Today. 1997;18:240–244. doi: 10.1016/s0167-5699(97)81663-5. [DOI] [PubMed] [Google Scholar]
- 11.Holland G H, O’Connor G R, Diaz R F, Minasi P, Wara W M. Ocular toxoplasmosis in immunosuppressed nonhuman primates. Investig Ophthalmol Vis Sci. 1988;29:835–842. [PubMed] [Google Scholar]
- 12.Hu M S, Schwartzman J, Channon J Y, Khan I A, Kasper L H. A novel murine model of acute ocular toxoplasmosis by intracameral inoculation. Investig Ophthalmol Vis Sci. 1998;39:S782. . (Abstr. 3618.) [Google Scholar]
- 13.Hundt M, Schmidt R E. Induction of Fas/APO-1 (CD95) on lymphocytes co-culture with T. gondii tachyzoites. Immunol Lett. 1997;56:213. [Google Scholar]
- 14.Innes E A, Panton W R, Sanderson A, Thomson K M, Wastling J M, Maley S, Buxton D. Induction of CD4+ and CD8+ T cell responses in efferent lymph responding to Toxoplasma gondii infection: analysis of phenotype and function. Parasitol Immunol. 1995;17:151–160. doi: 10.1111/j.1365-3024.1995.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 15.Jabs D A, Quinlan P. Ocular toxoplasmosis. In: Ryan S J, Schachat A P, Murphy R B, editors. Retina. C. V. St. Louis, Mo: Mosby; 1994. pp. 1531–1543. [Google Scholar]
- 16.Jayaraman S, Heiligenhaus A, Rodriguez A, Soukiasian S, Dorf M E, Foster C S. Exacerbation of murine herpes simplex virus-mediated stromal keratitis by Th2 type T cells. J Immunol. 1993;151:5777–5789. [PubMed] [Google Scholar]
- 17.Kasper L H, Khan I A, Ely K H, Buelow R, Boothroyd J C. Antigen-specific (p30) mouse CD8+ T cells are cytotoxic against T. gondii-infected peritoneal macrophages. J Immunol. 1992;148:1493–1498. [PubMed] [Google Scholar]
- 18.Kasper L H, Boothroyd J C, Warren K, Agabian N. Toxoplasma gondii. In: Warren K, Agabian N, editors. Immunology and molecular biology of parasitic diseases. Boston, Mass: Blackwell Scientific; 1993. pp. 269–298. [Google Scholar]
- 19.Khan I A, Ely K H, Kasper L H. A purified parasite antigen (p30) mediates CD8+ T cell immunity against fatal T. gondii infection in mice. J Immunol. 1991;147:3501–3506. [PubMed] [Google Scholar]
- 20.Khan I A, Matsuura T, Kasper L H. Activation-mediated CD4+ T cell unresponsiveness during acute T. gondii infection in mice. Int Immunol. 1996;8:887–896. doi: 10.1093/intimm/8.6.887. [DOI] [PubMed] [Google Scholar]
- 21.Liesenfeld O, Kosek J C, Suzuki Y. Gamma interferon induces Fas-dependent apoptosis of Peyer’s patch T cells in mice following peroral infection with Toxoplasma gondii. Infect Immun. 1997;65:4682–4689. doi: 10.1128/iai.65.11.4682-4689.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Memon S A, Hou J, Moreno M B, Zacharchuk C M. Cutting edge: apoptosis induced by a chimeric Fas/FLICE receptor: lack of requirement for Fas- or FADD-binding proteins. J Immunol. 1998;160:2046–2049. [PubMed] [Google Scholar]
- 23.Montoya J C, Remington J S. Toxoplasmic chorioretinitis in the setting of acute acquired toxoplasmosis. Clin Infect Dis. 1996;23:277–282. doi: 10.1093/clinids/23.2.277. [DOI] [PubMed] [Google Scholar]
- 24.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 25.Nash P B, Purner M B, Leon R P, Clarke P, Duke R C, Curiel T J. T. gondii-infected cells are resistant to multiple inducers of apoptosis. J Immunol. 1998;160:1824–1830. [PubMed] [Google Scholar]
- 26.Niemialtowski M G, Rouse B T. Phenotypic and functional studies on ocular T cells during herpetic infections of the eye. J Immunol. 1992;148:1864–1870. [PubMed] [Google Scholar]
- 27.Nussenblatt R B, Palestine A G. Ocular toxoplasmosis. In: Nussenblatt R B, Palestine A G, editors. Uveitis. St. Louis, Mo: Year Book Medical Publisher, Inc.; 1989. pp. 336–354. [Google Scholar]
- 28.Nussenblatt R B, Mittal K K, Fuhrman S, Sharma S D, Palestine A G. Lymphocyte proliferative responses of patients with ocular toxoplasmosis to parasite and retinal antigens. Am J Ophthalmol. 1989;107:632–641. doi: 10.1016/0002-9394(89)90260-2. [DOI] [PubMed] [Google Scholar]
- 29.Pomeroy C, Noble R, McCormick M, Young B. Ocular toxoplasmosis as the presenting manifestation of human immunodeficiency virus infection. Clin Infect Dis. 1996;24:745–746. doi: 10.1093/clind/24.4.745a. [DOI] [PubMed] [Google Scholar]
- 30.Sabelko K A, Kelly K A, Nahm M H, Cross A H, Russell J H. Fas and Fas ligand enhance the pathogenesis of experimental allergic encephalomyelitis, but are not essential for immune privilege in the central nervous system. J Immunol. 1997;159:3096–3099. [PubMed] [Google Scholar]
- 31.Silveira C, Belfort R, Burnier M, Nussenblatt R B. Acquired toxoplasmic infection as the cause of toxoplasmic retinochoroiditis in families. Am J Ophthalmol. 1988;106:362–364. doi: 10.1016/0002-9394(88)90382-0. [DOI] [PubMed] [Google Scholar]
- 32.Stanford M. Acute symptomatic Toxoplasma retinochoroiditis. Eye. 1996;10:3–4. doi: 10.1038/eye.1996.2. [DOI] [PubMed] [Google Scholar]
- 33.Streilein J W. Unraveling immune privilege. Science. 1995;270:1158–1159. doi: 10.1126/science.270.5239.1158. [DOI] [PubMed] [Google Scholar]
- 34.Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 35.Whittum J A, McCulley J P, Niederkorn J Y, Streilein J W. Ocular disease induced in mice by anterior chamber inoculation of herpes simplex virus. Investig Ophthalmol Vis Sci. 1984;25:1065–1073. [PubMed] [Google Scholar]
- 36.Wong B, Park C G, Choi Y. Identifying the molecular control of T-cell death; on the hunt for killer genes. Semin Immunol. 1997;9:7–16. doi: 10.1006/smim.1996.0051. [DOI] [PubMed] [Google Scholar]
- 37.Wu M X, Ao Z, Hegen M, Morimoto C, Schlossman S F. Requirement of Fas (CD95), CD45, and CD11α/CD18 in monocyte-dependent apoptosis of human T cells. J Immunol. 1996;157:707–713. [PubMed] [Google Scholar]
- 38.Yeaman R G, Guyre P M, Fanger M W, Collins J E, White H D, Rathbn W, Orndorff K A, Gonzalez J, Stern J E, Wira C R. Unique CD8+ T cell-rich lymphoid aggregates in human uterine endometrium. J Leukoc Biol. 1997;61:427–435. [PubMed] [Google Scholar]