Abstract
The α-Klotho is an anti-aging protein that, when overexpressed, extends the life span in humans and mice. It has an anti-inflammatory and protective action on renal cells by inhibiting NF-κB activation and production of inflammatory cytokines in response to TNF-α. Furthermore, studies have shown the neuroprotective effect of α-Klotho against neuroinflammation on different conditions, such as aging, animal models of neurodegenerative diseases, and ischemic brain injury. This work aimed to evaluate the effects of α-Klotho protein on primary glial cell culture against the proinflammatory challenge with LPS and how this could interfere with neuronal health. Cortical mixed glial cells and purified astrocytes were pretreated with α- α-Klotho and stimulated with LPS followed by TNFα, IL-1β, IL-6, IFN-γ levels, and NF-κB activity analysis. Conditioned medium from cortical mixed glia culture treated with LPS (glia conditioned medium (GCM) was used to induce neuronal death of primary cortical neuronal culture and evaluate if GCM-KL (medium from glia culture pretreated α-Klotho followed by LPS stimulation) or GCM + LPS in the presence of KL can reverse the effect. LPS treatment in glial cells induced an increase in proinflammatory mediators such as TNF-α, IL-1β, IL-6, and IFN-γ, and activation of astrocyte NF-κB. GCM treated-cortical neuronal culture induced a concentration-dependent neuronal death. Pretreatment with α-Klotho decreased TNF-α and IL-6 production, reverted NF-κB activation, and decreased neuronal death induced by GCM. In addition, KL incubation together with GCM + LPS completely reverts the neuronal toxicity induced by low concentration of GCM-LPS. These data suggest an anti-inflammatory and neuroprotective effect of α-Klotho protein in the CNS. This work demonstrated the therapeutic potential of α-Klotho in pathological processes which involves a neuroinflammatory component.
Subject terms: Biochemistry, Neuroscience
Introduction
α-Klotho protein gene was discovered randomly in 1997 by Kuro-o and colleagues1. The α-Klotho protein can be found in two forms, a transmembrane and a soluble one, the latter composed of both the cleaved α-Klotho and the secreted α-Klotho acting in the central nervous system (CNS)1–3. The transmembrane α-Klotho has a short intracellular domain, and two extracellular domains, known as KL1 and KL2, and the secreted α-Klotho is a result of alternative splicing composed of only KL11. These extracellular domains can be cleaved by the proteases disintegrin A and metalloproteinase 10 (ADAM10), disintegrin A and metalloproteinase 17 (ADAM17), and β-secretase 1 (BACE1). These cleavage fragments are present in the blood, urine, and cerebrospinal fluid, acting as a humoral factor4–6.
Evidence suggests that α-Klotho expression is predominantly circumscribed to the kidneys and the CNS1. This protein could be found in the choroid plexus7, neurons, oligodendrocytes, cortical layers, hippocampal formation8, and Purkinje cells9. The membrane-bound α-Klotho complexes with several fibroblast growth factor (FGF) receptors isoforms1, modulating kidney phosphate reabsorption and 1,25-dihydroxycholicalciferol (vitamin D) production for systemic regulation of phosphate homeostasis10,11. The soluble forms of α-Klotho act as a humoral factor and can be found in extracellular fluids such as blood, urine, and cerebral spinal fluid (CSF)4,12,13.
Physiological and pathological processes influence the expression of α-Klotho. Rats with spontaneous hypertension, 5/6 nephrectomized, and type 1 diabetes had their α-Klotho mRNA levels decreased14, and endogenous factors such as insulin and glutamate modulate α-Klotho expression in mouse neurons15. The α-Klotho’s expression increases significantly after birth and adulthood2,8, and there is a decrease during aging16–18.
In aging, there is a low-grade chronic systemic inflammation called inflammaging19. Deregulation of inflammation in the brain is associated not only with cognitive deficits related to aging20 but also with the pathogenesis and progression of neurodegenerative diseases21.
Lipopolysaccharides (LPS)-treated glial cells (a model of neuroinflammation) activates pathways involving TLR4 and the nuclear transcription factor kappa B (NF-kB). The NF-κB activity plays an important role in modulating proteins and cytokines22. This nuclear factor is constitutively expressed in the cytoplasm, which is bound to the inhibitor κB (IκB) protein which masks its nuclear localization signal, thus retaining it in the cytoplasm23. Cytokines and other pro-inflammatory mediators are involved in hippocampal neuronal functions24, but they can also cause damage to hippocampal working memory consolidation and LTP24–26. The present study investigated the role of α-Klotho and the activity of NF-kB in glial cells challenged with LPS and determined the ability of this protein to revert the neurotoxicity on neuronal culture cells caused by the GCM.
Materials and Methods
Chemicals and kits
Cell culture reagents were purchased from Thermo Fisher Scientific (Waltham, MA, U.S.A.). Protein assay kit was purchased from Bio-Rad (Hercules, CA, U.S.A.). TNF-α, IL-10, and IL-1β immunoassay kits were purchased from eBioscience (San Diego, CA, U.S.A.). The kits were used according to the manufacturer´s instructions. Routine reagents and LPS from Escherichia coli (O111:B4, L2630) were purchased from Sigma Chemicals (St. Louis, MO, U.S.A.), and recombinant α-Klotho from R&D Systems (1819-KL-050, Minnesota, MN, U.S.A.). All solutions were prepared immediately before use.
Primary cell culture
Primary mixed cortical glia culture and cortical neuron culture were prepared from C57BL/6J mice obtained at the Biomedical Sciences Institute of the University of São Paulo, São Paulo, São Paulo State, Brazil. The newborn animals were euthanized by decapitation, and cortical tissue was removed and used for both cell culture preparation (glial cells and neurons). The procedure was conducted following ARRIVE guidelines (https://arriveguidelines.org) which are similar to the Ethical Principle in Animal Research adopted by the Brazilian College of Animal Experimentation (CONCEA) and were approved by the Ethical Committee for Animal Research (CEEA) of the Biomedical Sciences Institute of the Universidade de São Paulo, São Paulo, São Paulo State, Brazil. The protocol was registered under number 12/2016 CEEA of animals used for experimentation. All methods were performed in accordance with the relevant guidelines and regulations of the standard Scientific Reports editorial criteria.
Primary glial cells were prepared as previously described22. Briefly, cortex from newborn C57BL/6J mice (postnatal days 1–4) was dissected in ice-cold Hanks’ balanced salt solution (HBSS) under a microscope and their meninges were removed. Small pieces of cortices were incubated in a trypsin solution (GIBCO) for dissociation at 37 °C for 20 min. DMEM (complemented with glutamine, 10% Hyclone FetalLOne III serum, GE Healthcare, and 1% Penicillin/ Streptomycin) was added to the solution to inhibit trypsin action, and cells were dissociated with a Pasteur pipette. The cells were passed through Cell Strainer, and cells were counted in a Neubauer chamber, and each Flask T75 (Sarstedt) was plated with 1 × 106 cells. The medium was changed every three days and kept for 10–14 days. The culture was plated in a 6-well plate or 24-well plate for experiments. The culture used for the experiments was considered an astroglial-enriched culture based on previous data showing 91,2% astrocytes labeled with GFAP22. To obtain astrocyte-pure cell cultures (> 98%) for NF-kB experiments, flasks were incubated in an orbital shaker at 37 °C, 180 r/min, for 15 h as previously described15.
The primary cortical neuronal culture was prepared from both male and female postnatal (P1-3) mice C57BL/6 (Mus musculus). The meninges were separated from the brain, and the cortex was cut into small pieces and incubated in trypsin solution (2 mg/mL) for 20 min in a 37 °C, 5% CO2 incubator. After removal of trypsin solution, tissue was washed twice with HBSS. Tissue was dissociated in HBSS containing 0.1 mg/mL DNAse by mechanical trituration with a glass pipette. Cells were counted and plated (1 × 106 cells) in polyethylene (Sigma-Aldrich) pre-coated dishes. Neurons were maintained for two weeks in a Neurobasal medium (GIBCO) supplement with B27 (GIBCO), 2 mM L-glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin, and 0.25 mg/mL amphotericin B.
α-Klotho and LPS treatment
On the 15th day, the cells were treated with only DMEM without fetal bovine serum (FBS), LPS, α-Klotho (AA 35–982), or LPS + α-Klotho for 24 h. A dose–response of recombinant α-Klotho and LPS treatments were made with different concentrations (α-Klotho 0.1–4.0 nM) and LPS (0.01–100 μg/mL) for 24 h to observe cell viability. After this first screening, only α-Klotho concentrations (0.1–2.0 nM) were tested at different time points (1, 4, and 24 h) to evaluate the α-Klotho effect in LPS (1 µg/mL/8 h)-induced changes in TNF-α levels. Based on these data, the concentration of 1 nM of α-Klotho and 1 µg/mL to LPS for 4 and 8 h was used to evaluate changes in NF-kB activity and IL-1β, IL-6, and IFN-γ levels, respectively.
For the experiments involving Glial conditioned Medium (GCM), on the 15th day, cell culture was pretreated with DMEM without FBS in the absence (control) or the presence of α-Klotho (1 nM) for 24 h and then challenged with 1 µg/mL LPS for 8 h. The media were collected and named GCM or GCM-KL. Primary neuronal culture cells were challenged on the 10th day by changing the normal conditioned medium by 25% or 50% of GCM or GCM-KL for 24 h. The neuronal cultures were also challenged on the 10th day by changing the normal conditioned medium by 25% or 50% of GCM (challenged with 1 µg/mL LPS for 8 h) in the absence (control) or the presence of α-Klotho (1 nM).
Cell viability by LDH and MTT
Cell viability was estimated by Cytotox 96 non-radioactive assay (Promega). The assay was performed according to the Manufacturer's instructions. Lactate dehydrogenase (LDH) release was assayed after 24 h of treatment with α-Klotho or LPS by removing 50 μl supernatant from each well into a 96-well plate and incubating with Cytotox 96 reagents for 30 min covered with aluminum foil at room temperature. The stop solution was added, and the absorbance was measured at 490 nm. The percentage of LDH activity was measured by the ratio: (Absorbance of the sample/Absorbance of maximum activity) × 100%.
The cells were incubated with filtered MTT in DMEM without FBS at 37 °C for 2 h and 30 min. The supernatant was removed from the plate, and DMSO was added. The new supernatant was plated on a 96-well plate and measured at 570 nm. MTT % related to control was calculated by the ratio: (absorbance of the sample–absorbance of DMSO)/ (absorbance of the control–absorbance of DMSO) × 100%.
Multiplex analysis of cytokines and chemokines
Concentrations of TNF-α, IL-1β, IL-6, IL-18, and INF-γ were simultaneously measured in 25 μL of medium from homogenized cells using a Milliplex M.A.P. kit Mice Cytokine/Chemokine Magnetic Bead Panel (Millipore, Billerica, MA) by following manufacturer’s instructions. Antibody immobilized beads were detected on a Luminex 100 xMAP technology machine (Austin, TX). Standard curves were generated for each cytokine/chemokine using standards included in the kit for serum samples. The median fluorescence intensity for each analyte was calculated using a five-point logistic parameter curve and normalized to the amount of protein in each sample.
Immunofluorescence
To evaluate the effects of α-Klotho on NF-κB activation by LPS, after purification, astrocytes were treated for 24 h with vehicle (control) (PBS) or 1 nM α-Klotho. Inflammatory stimulation with LPS 1 μg/ml was then performed and the cells were fixed with methanol (10 min) 4 h later and washed with PBS three times for 5 min. The fixed cells were incubated with serum blocking (5% normal donkey serum in triton X-100 0.01%) for one hour and incubated overnight with primary antibodies GFAP (1:300) (3670; Cell Signaling, and RelA (p65 (1:100) (ab7970; ABCAM, USA). The primary antibody was removed, and the plate was washed with serum blocking three times for 10 min. The cells were incubated with secondary antibody (rabbit or mouse anti-donkey- Alexa 594 or 488, Thermo Fischer Scientific, 1:1000), diluted in PBS with Triton X-100 0.01% for 2 h, and protected from the light. The coverslips were washed five times with PBS for 5 min and incubated with DAPI (4'-6-Diamidino-2-phenylindole; Sigma) for 1 min in dilution 1:10.000. Slices were transferred to glass slides and analyzed in Fluorescence microscope Nikon Eclipse 80i (Nikon, Tokyo, Japan) with a camera system, Nikon Digital Camera DXM 1200C.
Protein extraction—nucleus and cytoplasm
Culture media were removed from the 6-well plate, and the cells were scraped in cold PBS with 0.5 mM PMSF and centrifuged at 4 °C for 2 min at 13,000g. Pellet was resuspended in lysis buffer (10 mM HEPES pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 0.5 mM PMSF, 0.1 mM EDTA, 2 μg/mL leupeptin, 2 μg/mL antipain, 30 mM NaF, 3 mM sodium orthovanadate, 20 mM sodium pyrophosphate and 5 mM BG-P) and incubated on ice for 15 min. NP-40 was added, and the samples were homogenized and centrifuged for the 30 s at 13,000g at 4 °C. Supernatants were used for Western blotting assay and pellets were resuspended in extraction buffer (1.5 mM MgCl2, 20 mM HEPES, pH 7.9, 25% glycerol, 300 mM NaCl, 0.5 mM PMSF, 0.25 mM EDTA, 2 μg/mL leupeptin, 2 μg/mL antipain, 3 mM sodium orthovanadate, 30 mM NaF, 20 mM sodium pyrophosphate and 5 mM BG-P) and kept on ice for 20 min. Samples were centrifuged for 20 min at 13,000g at 4 °C and supernatants were aliquoted as nuclear extract used for EMSA assay. Protein concentration was determined using the Bradford protein reagent (BioRad).
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts from control or treated cells were prepared as previously described22. A double-stranded oligonucleotide containing the NF-κB consensus sequence from Promega (5′-AGTTGAGGGGACTTTCCCAGGC-3′) was end labeled using T4 polynucleotide kinase (Promega) in the presence of γ-32P dATP. Nuclear extracts (2.5 μg) were incubated with a 32P-labeled NF-κB probe. The binding reaction was performed at room temperature for 30 min in a reaction buffer containing 50 mM Tris–HCl pH 7.5, 250 mM NaCl, 5 mM MgCl2, 2.5 mM EDTA, 20% glycerol, 0.25 μg/μL of poly (dI–dC) and 2.5 mM dithiothreitol. DNA protein complexes were separated by electrophoresis through a 6% acrylamide:bis-acrylamide (37.5:1) gel in TBE (45 mM Tris, 45 mM boric acid, 0.5 mM EDTA) for 2 h at 150 V. Gels were vacuum dried for 1 h at 80 °C and exposed to X-ray film at − 80 °C. For competition assays, the nuclear extract was incubated with a specific competitor (unlabelled double-stranded NF-κB consensus oligonucleotide) or a non-specific competitor (unlabelled transcription initiation factor IID [TFIID]). For the supershift assay, antibodies against subunits of NF-κB (p50 and p65, 1:20) (Santa Cruz Biotechnology) were added to the binding reactions. Autoradiographs were visualized using a photo documentation system DP-001-FDC and quantified with ImageJ (NIH) software 70.
Western Blotting
Electrophoresis was performed using 10% polyacrylamide gel and the Bio-Rad mini-Protean III apparatus (Bio-Rad, Hercules, CA, U.S.A.). In brief, the proteins present in cytosolic and nuclear fractions were size-separated in 10% SDS-PAGE (90 V). The immunoblotting was performed as described previously27. The proteins were blotted onto a nitrocellulose membrane (Bio-Rad, Hercules, CA, U.S.A.) and incubated with the specific antibody: RelA (p65) (1:1000, sc-0372; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and β-actin (1:2000 (58169; Cell Signaling, U.S.A.) and after with secondary antibody (Rabbit). Proteins recognized by antibodies were revealed by an electrochemiluminescence (ECL) technique, following the Manufacturer's instructions (Amersham Biosciences, Amersham, U.K.). To standardize and quantify the immunoblots, we used the photo documentation system DP-001-FDC (VilberLourmat, Torcy, France) and N.I.H. ImageJ software (http://rsb.info.nih.gov/ij). Several exposure times were analyzed to ensure the linearity of the band intensities.
Statistical analysis
Results are expressed as mean ± S.E.M. of the indicated number of experiments. Statistical comparisons for α-Klotho-induced changes in cytokines, Western blotting, and cell viability were performed by one-way analysis of variance (ANOVA), followed by the Tukey post-test. All analyses were performed using a Prism 9 software package (GraphPad Software, San Diego, CA, U.S.A.). P-values < 0.05 were considered to reflect a statistically significant difference.
Ethical approval and consent to participate
Both cell culture preparation (glial cells and neurons) was conducted under The Ethical Principle in Animal Research adopted by the Brazilian College of Animal Experimentation (CONCEA) and were approved by the Ethical Committee for Animal Research (CEEA) of the Biomedical Sciences Institute of the Universidade de São Paulo, São Paulo, São Paulo State, Brazil. The protocol was registered under number 12/2016 CEEA of animals used for experimentation.
Results
Effect of α-Klotho and LPS treatments on cell viability and cytotoxicity of primary glia culture
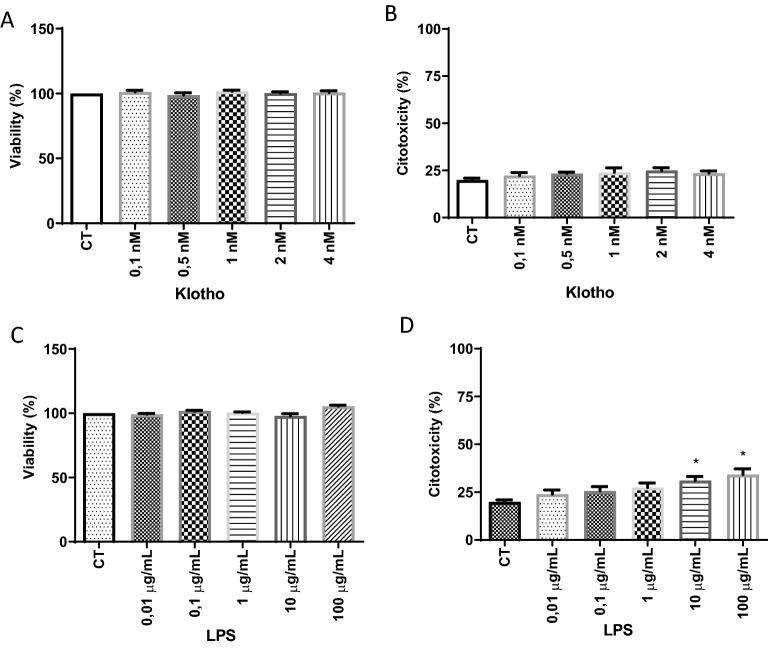
The different α-Klotho concentrations (0.1–4 nM) did not alter cell viability or show toxicity to glial cells in both MTT and LDH assays (Fig. 1A,B). Regarding the LPS challenge, concentrations between 0.01 and 100 ug/mL caused no effect in the MTT assay, but in the LDH assay, only concentrations of 10 and 100 ug/mL showed toxicity to glial cells when compared to the control group (Fig. 1C,D). Based on these data, the concentration of 1 µg/mL of LPS was chosen for the subsequent experiments.
Figure 1.
Effect of α-Klotho (A,B) and LPS (C,D) treatment on cell viability (A,C) and cytotoxicity (B,D) of mouse glial cells. The primary culture of glial cells was subjected to 24-h treatment with LPS in different concentrations. Cell viability and cytotoxicity were assessed by MTT and LDH assays, respectively. One-way ANOVA analysis, followed by Tukey's post-test, *p < 0.05. Results are presented as mean ± SEM of 7 independent experiments.
Effect of α-Klotho on LPS-induced cytokines secretion in primary mouse glia culture
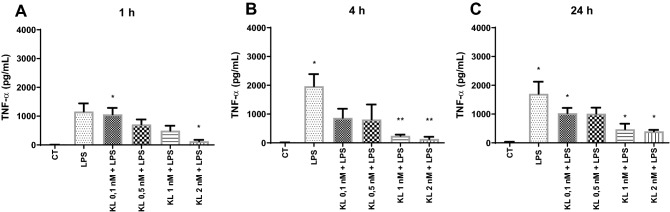
After analyzing cell viability and toxicity of different concentrations of α-Klotho in primary glia culture, time-course and concentration of α-Klotho effects on LPS-induced TNF-α secretion was evaluated. This cytokine was chosen based on previous studies in glial cells showing an increase of this cytokine after LPS treatment28. Thus, concentrations ranging from 0.1 to 2 nM of α-Klotho and different time points of treatment (1, 4 and 24 h) were used, followed by a LPS challenge (1 µg /mL for 8 h) and TNF-α levels were determined by ELISA. Data showed that LPS -induced an increase in TNF-α levels compared to the control group, and this effect was blocked by α-Klotho protein pretreatment at concentrations of 2 nM for 1 h, and 1–2 nM for 4 and 24 h (Fig. 2A–C).
Figure 2.
Effect of α-Klotho on LPS-induced TNF-α secretion in mouse glial cells. The glial culture was pre-treated with serum-free medium (control) or with α-Klotho protein in different concentrations (0.1, 0.5, 1, and 2 nM) and times 1 (A), 4 (B), and 24 (C) hours, and then challenged with 1 µg/mL/mL LPS for 8 h. The supernatant was collected to measure TNF-α levels. One-way ANOVA analysis, followed by Tukey's post-test, *p < 0.05, **p < 0.01. Results are presented as mean ± SEM of 7 independent experiments.
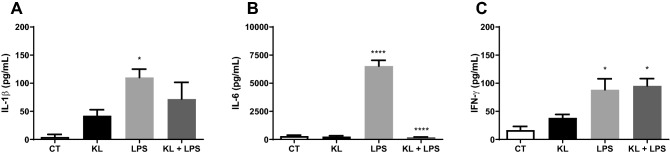
Based on these data, the concentration of 1 nM for 24 h was chosen for evaluating the influence of α-Klotho on LPS (1 µg/mL for 8 h) induced change in proinflammatory cytokines. In addition to TNF-α, LPS increased the production and secretion of other pro-inflammatory cytokines, such as IL-1β, IL6, and IFN-γ (Fig. 3A–C). However, α-Klotho treatment (1 nM for 24 h) only reversed the LPS -induced increase of IL-6 levels (Fig. 3B) but not of IL-1β and IFN-γ (Fig. 3A,C).
Figure 3.
Effect of α-Klotho on IL-1β, IL-6, and IFN-γ levels in mouse cell glia challenged with LPS. The glial culture was pretreated with serum-free medium (control) or with 1 nM α-Klotho for 24 h, and then challenged with 1 µg/mL/mL LPS for 8 h. The supernatant was collected to measure the levels of IL-1β (A), IL-6 (B), and IFN-γ (C). One-way ANOVA analysis, followed by Tukey's post-test, *p < 0.05, **p < 0.01, **** < 0.0001. Results are presented as mean ± SEM of 7 independent experiments.
Effect of α-Klotho on LPS-induced NF-κB activation in astrocytes primary mouse glia culture
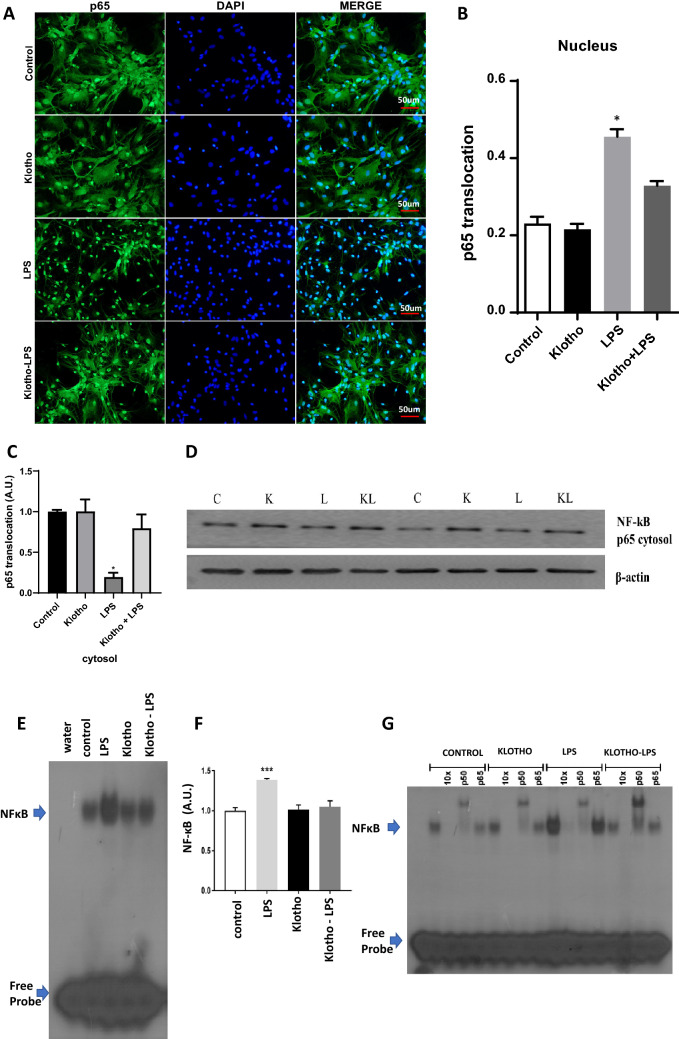
To evaluate the influence of α-Klotho on NF-κB signaling we performed experiments in astrocyte primary culture based on previous evidence that secreted neuronal α-Klotho modulates astrocytic metabolic activity15. For immunofluorescence experiments, we used staining of RelA (p65), which is an NF-kB subunit that is activated during LPS-induced inflammation (Fig. 4). Pre-treatment of cells with concentrations of 1 nM α-Klotho for 24 h, followed by challenge with LPS at 4 h (Fig. 4A, B) confirmed increased RelA nuclear translocation by LPS vs. control. RelA nuclear translocation induced by LPS was completely reverted by recombinant α-Klotho treatment (Fig. 4A, B). For a quantitative assessment, cells extracted from cytosolic and nuclear fractions were used to evaluate the RelA (p65) content in each compartment by Western Blotting (Fig. 4C). Results confirmed immunofluorescence data as the RelA (p65) subunit translocation subunit induced by LPS is inhibited by recombinant α-Klotho (Fig. 4C). Finally, these groups were submitted to an EMSA assay to more precisely detect whether the NF-kB that translocated to the nucleus was active and bound to its specific sequence in DNA (Fig. 4D, E). EMSA data confirmed both previous data as the binding of the nuclear extract to the 32P-labeled probe was much higher in LPS-treated astrocytes, and pre-treatment with α-Klotho was able to inhibit the activation of this transcription factor. A super-shift assay was also performed (Fig. 4F) to clarify that NF-kB subunits are involved in this activation. Data confirmed that RelA (p65) and p50 subunits are involved, which typically occurs following activation by LPS29.
Figure 4.
α-Klotho rescue the NF-κB activation induced by LPS in the nuclear fraction. (A) Purified astrocytes from cortical glial cells were treated for 24 h with vehicle (control) (PBS) or 1 nM α-Klotho. Inflammatory stimulation with LPS 1 μg/ml was then performed. (B) The RelA (p65)-positive nuclei were counted and divided by the total number of nuclei, and the graph shows the comparison between the LPS group and for α-Klotho + LPS group expressed by the ratio in arbitrary units of RelA (p65) translocated to the nucleus over the total amount of RelA (p65) (n = 5). One-way ANOVA analysis, followed by Tukey's post-test, **p < 0,01; ***p < 0,001; ****p < 0,0001. (C,D) Effect of α-Klotho on p65 subunit NFkB translocation in astrocytes cells. Cytosol (20 mg) proteins were extracted from primary cultured cells: (C) Representative Western blotting autoradiographs of RELA (p65) cytosolic and β-actin; (D) Densitometric analysis (arbitrary units, A.U.) of p65 cytolosic/β-actin ratios of groups presented in the panel (n = 5). One-way ANOVA analysis, followed by Tukey's post-test, **p < 0,01; ***p < 0,001; ****p < 0,0001. (E) Nuclear fraction was used to perform the EMSA assay to measure NF-kB activity. (F) Densitometric analysis comparing NF-κB activity of control, α-Klotho, LPS, and α-Klotho–LPS groups (n = 5). One-way ANOVA analysis, followed by Tukey's post-test,***p < 0,0001. (G) A super-shift was also performed to show which NF-κB subunits are involved in this phenomenon.
Effects of α-Klotho on GCM -induced cytotoxicity of mouse primary neuronal culture
Primary glia culture was pretreated with serum-free medium (GCM) or with 1 nM α-Klotho (GCM-KL) for 24 h and then challenged with 1 µg/mL LPS for 24 h. Initially, it was necessary to determine the amount of GCM coming from the LPS-challenged glial cell (GCM group) which caused neuronal death. Thus, we replaced 10, 25, and 50% of the cultured medium of the neuronal culture with GCM. The results showed that when the concentration of 25 and 50% of the neuronal culture medium was switched to GCM, there was an increase in neuronal toxicity when compared to the control group (Fig. 5).
Figure 5.
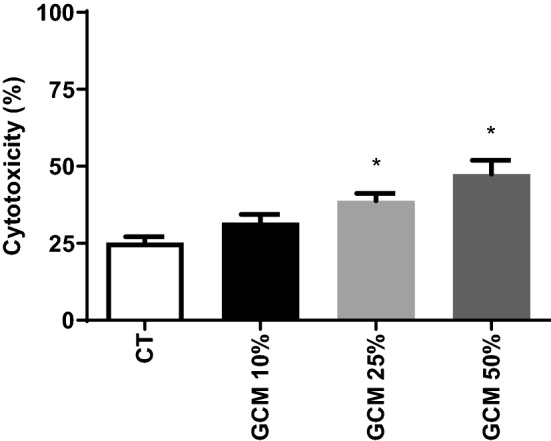
Effects of GCM -induced cytotoxicity of mouse neurons. The primary embryonic culture of neurons was subjected to 24-h treatment with GCM in different concentrations (25% and 50%). Cytotoxicity was assessed by the LDH assay. One-way ANOVA analysis, followed by Tukey's post-test, *p < 0.05. Results are presented as mean ± SEM of 5 independent experiments.
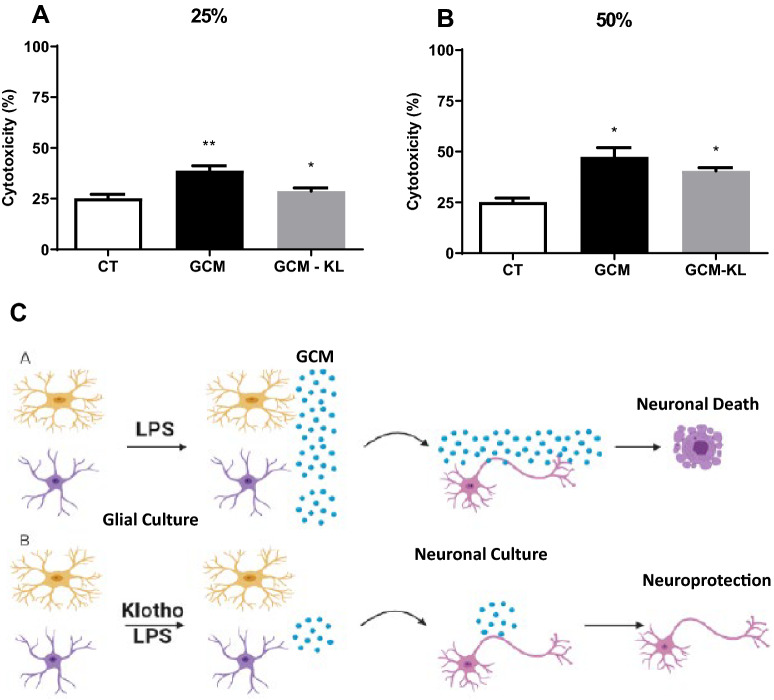
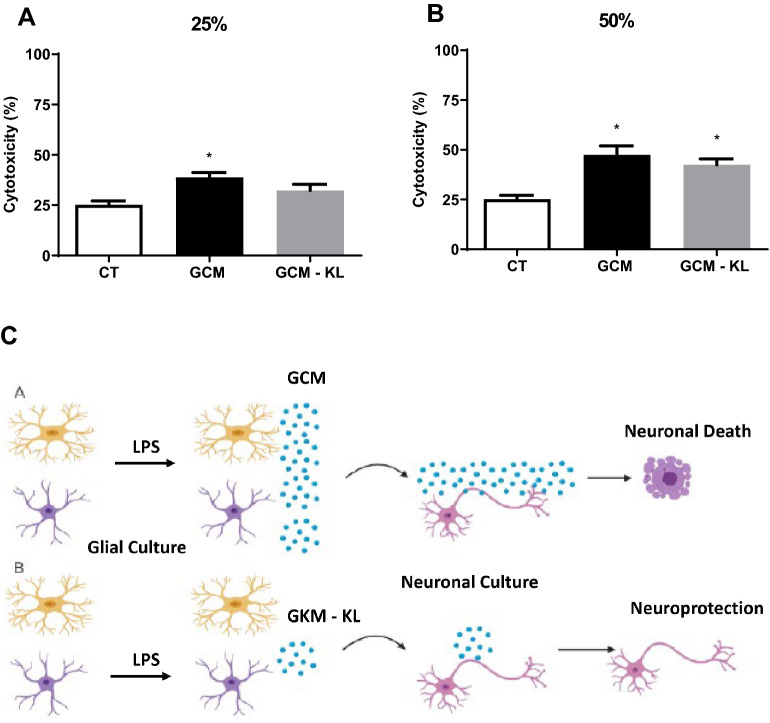
After determining the concentration of GCM that induced neurotoxicity, we investigated whether the GCM from α-Klotho pretreated glial culture (1 nM) and stimulated with LPS (GCM-KL) would be able to decrease neuronal death when compared to GCM at both 25 and 50% concentrations (Fig. 6). The results showed that 25% of GCM-KL reversed the increase in neuronal death caused by GCM (Fig. 6A), but α-Klotho pretreatment was not able to induce any effect to a high concentration (50% of GCM-KL) (Fig. 6B). We also evaluate the neuroprotective effect of KL on GCM -induced neurotoxicity of primary cortical mouse neurons. The primary embryonic culture of neurons was submitted to 24-h treatment with GCM (stimulated or not with LPS) in different concentrations, 25% (A) and 50% (B) in the presence or absence of KL. Cytotoxicity was assessed by the LDH assay (Fig. 7). The results confirmed KL neuroprotective action since the presence of this protein in GCM -challenge incubation to neurons was able to revert the increase in neuronal death caused by 25% of GCM (Fig. 7A), but not at 50% of GCM (Fig. 7B).
Figure 6.
Effects of GCM and GCM-KL -induced cytotoxicity of primary cortical mouse neurons. The primary embryonic culture of neurons was submitted to 24-h treatment with GCM or GCM-KL in different concentrations, 25% (A) and 50% (B). Cytotoxicity was assessed by the LDH assay. One-way ANOVA analysis, followed by Tukey's post-test, *p < 0.05, **p < 0.01. Results are presented as mean ± SEM of 5 independent experiments. (C) Representative schedule of the anti-inflammatory and neuroprotective effect of α-Klotho protein. LPS induces GCM to produce pro-inflammatory mediators that can lead to neuronal death (A). α-Klotho protein decreases the production of pro-inflammatory mediators induced by LPS in GCM (B), and it can have a protective effect on neurons from the neurotoxic effects of LPS induced by GCM. The figure was “Created with BioRender.com—Agreement number FH248VCXX8 to Scientific Reports”.
Figure 7.
Effects of Klotho on GCM -induced cytotoxicity of primary cortical mouse neurons. The primary embryonic culture of neurons was submitted to 24-h treatment with GCM in different concentrations, 25% (A) and 50% (B) in the presence or absence of KL. Cytotoxicity was assessed by the LDH assay. One-way ANOVA analysis, followed by Tukey's post-test, *p < 0.05, Results are presented as mean ± SEM of 5 independent experiments. (C) Representative schedule of the neuroprotective effect of α-Klotho protein on GMP-induced cytotoxicity of neurons. LPS induces GCM to produce pro-inflammatory mediators that can lead to neuronal death (A). α-Klotho protein decreases the production of pro-inflammatory mediators induced by LPS in GCM (B), and it can have a protective effect on neurons from the neurotoxic effects of LPS induced by GCM. The figure was “Created with BioRender.com—Agreement number FH248VCXX8 to Scientific Reports”.
Discussion
Neuroinflammation is a key feature of the aging process, neurodegenerative diseases, and injuries that affect the CNS, and is characterized by the activation of microglia and astrocytes. These cells have a fundamental role in the regulation of neuroinflammation. Depending on the nature of their activation, they can lead to the production of pro and/or anti-inflammatory mediators and have both beneficial and detrimental effects on neurons30,31. In this work, we sought to evaluate the role of α-Klotho in the modulation of inflammatory processes induced by LPS in glial cells. It is well known that activation by LPS in those cells leads to increased production and secretion of pro-inflammatory cytokines (including TNF-α, IL-6, IL-1, and IL-12). In addition, LPS increases the expression of other pro-inflammatory mediators, such as chemokines (like CXCL8, CCL5, and CCL2), complement system proteins (like C3, C3aR, C5a5, and factor B), and enzymes (like cyclooxygenase type 2 (COX-2) and induced nitric oxide synthase (iNOS)32,33. Our data confirmed these data since LPS increased the secretion of pro-inflammatory cytokines, such as TNF-α, IL-6, IL-1β, and IFN-γ.
Pro-inflammatory mediators, such as TNF-α, IL-1β, and IL-6, when produced in excess and/or in a chronic manner by glial cells, can lead to neuronal death34,35. Studies have already shown that the application of GCM of microglia and mixed glial culture activated by LPS in neurons leads to neuronal death28,36–38. According to these data, our data showed that the application of GCM from LPS-activated GCM in neurons led to a concentration-dependent increase in neuronal death. As the GCM was used, it is not possible to say which molecule is leading to neuronal death. Probably not just one, but a set of mediators, including the proinflammatory cytokines that were elevated after LPS stimulation such as TNF-α, as we previously demonstrated28.
The activation of glial cells by LPS leads to an increase in the production of proinflammatory mediators in glial cells and these mediators are capable of inducing neuronal death39. Therefore, we investigated whether α-Klotho would be able to decrease the effects induced by LPS in glial cells since the protective and anti-inflammatory activity of α-Klotho protein has already been seen in the renal, vascular and pulmonary systems40. However, the protective effect of α-Klotho protein in neuroinflammation has been poorly studied. α-Klotho has been shown to decrease NF-κB activation and reduce the production of pro-inflammatory cytokines, such as TNF-α, IL-6, IL-8, and IL-1β, in vivo and in vitro in models of cardiac inflammation41,42, kidney disease43 and lung disease44,45. For example, in human kidney embryonic cells (HEK293), Zhao et al. demonstrated that pretreatment with 200 pM of α-Klotho for 45 min was able to decrease NF-κB activation by approximately 70% and reduce the expression of IL-8, MCP-1, RANTES, and IL-6 after the addition of TNF-α43. In HUVECs cells, the same pretreatment for 6 h has been shown to decrease NF-κB activation and expression of adhesion molecules, intercellular adhesion molecule 1 (ICAM-1), and adhesion molecule of vascular cell 1 (VCAM-1), induced by TNF-α46.
Recent studies have demonstrated the protective and anti-inflammatory effect of α-Klotho in the CNS. α-Klotho overexpression in the mouse choroid plexus improved behavioral deficit and increased the number of live neurons after cerebral hypoperfusion, accompanied by a decrease in translocation p65 from the cytoplasm to the nucleus, production of proinflammatory cytokines, and activation of astrocytes and microglia47. A recently published study showed that α-Klotho's systemic overexpression in an experimental model of amyotrophic sclerosis (mouse transgenic for superoxide dismutase 1) led to later onset and progression of the disease and increased survival of these animals48. Still, it was observed that α-Klotho decreased the expression of inflammatory markers and prevented neuronal death. In addition, a reduction in the secretion of TNF-α, IL-6, and the expression of iNOS and COX-2 induced by LPS/IFN-γ in mouse microglia culture that overexpressed α-Klotho48.
In our study, α-Klotho was able to decrease the secretion of pro-inflammatory cytokines, TNF-α and IL-6. Pretreatment with 1 nM α-Klotho for 4 and 24 h and 2 nM for 1, 4, and 24 h decreased LPS-induced TNF-α secretion in glial cells. Also, pretreatment with 1 nM α-Klotho for 24 h reversed the increase in IL-6 secretion induced by LPS.
Interestingly, experiments in astrocytes purified glial cells reinforce the anti-inflammatory action of α-Klotho as pretreatment with this recombinant protein significantly reduces nuclear translocation of Rel A (p65) subunit of NF-κB induced by LPS as revealed by immunofluorescence and Westering blotting experiments. In addition, EMSA data confirmed that α-Klotho was able to revert the p65/p50 NF-κB activation induced by LPS suggesting a putative neuroprotective action of this protein. The present data reinforce the importance of understanding the roles of astrocytes and microglia in neurodegenerative diseases to develop effective therapies for neurodegenerative diseases49. Considering that insulin and glutamate-induced neuronal secreted α-Klotho play an important role in brain metabolism and neuroinflammation by modulating neuron-astrocyte coupling15 it would be important to consider it an important player in the complex activated glial cells during degenerative diseases.
When the conditioned medium of glial cells was preincubated with α-Klotho (1 nM) for 24 h before they were challenged with 1 µg/mL LPS for 8 h (GCM-KL), the neuronal death caused by the GCM of glial cells treated only with LPS was rescued in a lower concentration of GCM (25%), reinforcing, the therapeutic potential of α-Klotho in the CNS shown in other studies. In addition, KL (1 nM) incubation for 24 h together with GCM of glial cells treated with LPS for 8 h completely revert the neuronal to a lower concentration of GCM (25%), confirming the neuroprotective action of α-Klotho.
It is unclear which mediators were leading to neuronal death, as well as if α-Klotho is the only mediator involved in the neuroprotective effect. It may have been due to the decrease in TNF-α and IL-6 levels, which were observed in this study, as well as the decrease in other inflammatory mediators not evaluated or by inducing an adaptive response linked to an increase in protective factors, such as BDNF, or GNFT. In addition, α-Klotho may have had this protective effect due to the secretion of anti-inflammatory mediators and/or neuroprotective factors. Studies that have demonstrated an anti-inflammatory effect of α-Klotho observed a decrease in NF-κB activation43,46. This modulation of NF-κB activation was also observed in the CNS47. Our data confirmed that at least part of the mechanism by which α-Klotho could be leading to this anti-inflammatory and neuroprotective effect is mediated by NF-κB. The pretreatment with α-Klotho led to a decrease in the activation of NF-κB in astrocytes and, consequently, resulted in a decrease in the production and secretion of proinflammatory cytokines. Interestingly, previous studies from our laboratory showed that α-Klotho has a strong influence on the astrocytic metabolism stimulating aerobic glycolysis and lactate release mediated by FGFR1 and Erk1/2 activation15. The ability of α-Klotho to modulate the FGFR and NF-κB signaling in astrocytes is consistent with results in other cell types50 and suggests that in CNS α-Klotho can modulate neuroinflammation by neuron-glia coupling action.
Conclusions
In conclusion, our work demonstrated for the first time in primary cortical neuronal culture an anti-inflammatory and neuroprotective effect of the α-Klotho protein as the cytotoxicity of GCM could be rescued by α-Klotho pre-treatment (GCM-KL), as well as, by α-Klotho + GCM challenged with LPS -induced neurotoxicity. This neuroprotective α-Klotho effect is at least partially mediated by astrocyte/glia NF-κB modulation of neuroinflammation. These data suggest that α-Klotho can act not just in the metabolic coupling between neurons and astrocytes15, but it is also an important player in modulating glia neuroinflammation which is important to integrate insulin and glutamate action in neurons. Thus, α-Klotho's therapeutic potential is evidenced in pathological processes that have a neuroinflammatory component.
Supplementary Information
Acknowledgements
VWN and PSM are a Master students sponsored by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). CHYM (FAPESP 2013/10787-8) is a Ph.D. and PFK is a post-doctoral fellow from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP – 2018/14289-6). LSL is supported by grants from University of São Paulo. CS and EMK are research fellows of the National Council for Scientific and Technological Development (CNPq) (Grant No. 4050892018-0).
Abbreviations
- ADAM10
Metalloproteinase 10
- ADAM17
Metalloproteinase 17
- BACE1
β-Secretase 1
- BDNF
Brain-derived neurotrophic factor
- CNS
Central Nervous System
- COX-2
Cyclooxygenase-2
- CSF
Cerebral Spinal Fluid
- CXCL
C-X-C Motif Chemokine Ligand
- DMEM
Dulbecco´s Modified Eagle Medium
- EMSA
Electrophoretic mobility shift assay
- Erk1/2
Extracellular signal-regulated protein kinase
- FBS
Fetal bovine serum
- FGF
Fibroblast Growth Factor
- GCM
Glia conditioned medium
- GCM-KL
GCM pretreated with α- α-Klotho followed by LPS
- GFAP
Glial fibrillary acidic protein
- IFN-γ
Interferon-γ
- IL-1β
Interleukin 1 beta
- IL-6
Interleukin-6
- iNOS
Inducible nitric oxide synthase
- LDH
Lactate dehydrogenase
- LPS
Lipopolysaccharides
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
- NF-κB
Nuclear factor kappa B
- p50
NF-κB-subunit
- RelA (p65)
NF-κB- subunit
- TLR4
Toll-like receptor 4
- TNF-α
Tumor necrosis factor-alpha
- α-Klotho
Klotho
Author contributions
Conceived and designed the experiments: V.W.N., C.H.Y.M., E.M.K., and C.S. Performed the experiments: V.W.N., C.H.Y.M., N.M.S.P., P.S.M., P.F.K., J.A.L., and L.S.L. Analyzed the data: V.W.N., C.H.Y.M., L.S.L., P.F.K., E.M.K., and C.S. Contributed reagents/materials/analysis tools: V.W.N., C.H.Y.M., L.S.L., P.F.K., and C.S. Wrote the paper: V.W.N., C.H.Y.M., P.F.K., E.M.K., and C.S. All authors reviewed the manuscript. The results/data/figures in this manuscript have not been published elsewhere, nor are they under consideration by another publisher. The corresponding author declares that he has read the BMC journal policies on author responsibilities and submits this manuscript in accordance with those policies.
Funding
This publication was made possible by grants from FAPESP to CS (2016/07427-8) and EMK (2016/22996-9; 2019/12974-6); CNPq 405089/2018-0; CAPES – STINT program 88887.125409/2016-00 (Joint Brazilian-Swedish Research Collaboration) and USP Neuroscience Research Support Centres (NAPNA) to CS.
Data availability
"The datasets used and/or analysed during the current study available from the corresponding author on reasonable request".
Competing interests
The authors report no biomedical financial interests or potential conflicts of interest in accordance with the BMC journal policies on author responsibilities of interest policy. We declare that the authors have no competing interests as defined by BMC, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Vinicius Wanatable Nakao and Caio Henrique Yokowama Mazucanti.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-21132-4.
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 2.Ohyama Y, Kurabayashi M, Masuda H, Nakamura T, Aihara Y, Kaname T, Suga T, Arai M, Aizawa H, Matsumura Y, et al. Molecular cloning of rat klotho cDNA: Markedly decreased expression of klotho by acute inflammatory stress. Biochem. Biophys. Res. Commun. 1998;251:920–925. doi: 10.1006/bbrc.1998.9576. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Sun Z. Current understanding of klotho. Ageing Res. Rev. 2009;8:43–51. doi: 10.1016/j.arr.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted Klotho protein in sera and CSF: Implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 5.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc. Natl. Acad. Sci. USA. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-o M, Kaether C. Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett. 2009;583:3221–3224. doi: 10.1016/j.febslet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nabeshima Y. Klotho: A fundamental regulator of aging. Ageing Res. Rev. 2002;1:627–638. doi: 10.1016/s1568-1637(02)00027-2. [DOI] [PubMed] [Google Scholar]
- 8.Clinton SM, Glover ME, Maltare A, Laszczyk AM, Mehi SJ, Simmons RK, King GD. Expression of klotho mRNA and protein in rat brain parenchyma from early postnatal development into adulthood. Brain Res. 2013;1527:1–14. doi: 10.1016/j.brainres.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.German DC, Khobahy I, Pastor J, Kuro OM, Liu X. Nuclear localization of Klotho in brain: An anti-aging protein. Neurobiol. Aging. 2012;33(1483):e1425–1430. doi: 10.1016/j.neurobiolaging.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Razzaque MS. The FGF23-Klotho axis: Endocrine regulation of phosphate homeostasis. Nat. Rev. Endocrinol. 2009;5:611–619. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erben RG. Update on FGF23 and Klotho signaling. Mol. Cell. Endocrinol. 2016;432:56–65. doi: 10.1016/j.mce.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Akimoto T, Yoshizawa H, Watanabe Y, Numata A, Yamazaki T, Takeshima E, Iwazu K, Komada T, Otani N, Morishita Y, et al. Characteristics of urinary and serum soluble Klotho protein in patients with different degrees of chronic kidney disease. BMC Nephrol. 2012;13:155. doi: 10.1186/1471-2369-13-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li SA, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell. Struct. Funct. 2004;29:91–99. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- 14.Aizawa H, Saito Y, Nakamura T, Inoue M, Imanari T, Ohyama Y, Matsumura Y, Masuda H, Oba S, Mise N, et al. Downregulation of the Klotho gene in the kidney under sustained circulatory stress in rats. Biochem. Biophys. Res. Commun. 1998;249:865–871. doi: 10.1006/bbrc.1998.9246. [DOI] [PubMed] [Google Scholar]
- 15.Mazucanti CH, Kawamoto EM, Mattson MP, Scavone C, Camandola S. Activity-dependent neuronal Klotho enhances astrocytic aerobic glycolysis. J. Cereb. Blood Flow Metab. 2019;39:1544–1556. doi: 10.1177/0271678X18762700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao NM, Zhang YM, Zheng Q, Gu J. Klotho is a serum factor related to human aging. Chin. Med. J. (Engl.) 2004;117:742–747. [PubMed] [Google Scholar]
- 17.Duce JA, Podvin S, Hollander W, Kipling D, Rosene DL, Abraham CR. Gene profile analysis implicates Klotho as an important contributor to aging changes in brain white matter of the rhesus monkey. Glia. 2008;56:106–117. doi: 10.1002/glia.20593. [DOI] [PubMed] [Google Scholar]
- 18.King GD, Rosene DL, Abraham CR. Promoter methylation and age-related downregulation of Klotho in rhesus monkey. Age (Dordr.) 2012;34:1405–1419. doi: 10.1007/s11357-011-9315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 20.Ownby RL. Neuroinflammation and cognitive aging. Curr. Psychiatry Rep. 2010;12:39–45. doi: 10.1007/s11920-009-0082-1. [DOI] [PubMed] [Google Scholar]
- 21.Kempuraj D, Thangavel R, Natteru PA, Selvakumar GP, Saeed D, Zahoor H, Zaheer S, Iyer SS, Zaheer A. Neuroinflammation induces neurodegeneration. J. Neurol. Neurosurg. Spine. 2016;1:1003. [PMC free article] [PubMed] [Google Scholar]
- 22.Kinoshita PF, Yshii LM, Orellana AMM, Paixão AG, Vasconcelos AR, Lima LDS, Kawamoto EM, Scavone C. Alpha 2 Na+, K+-ATPase silencing induces loss of inflammatory response and ouabain protection in glial cells. Sci. Rep. 2017;7:4894. doi: 10.1038/s41598-017-05075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 24.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat. Rev. Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Wu Z, Hayashi Y, Nakanishi H. Age-dependent neuroinflammatory responses and deficits in long-term potentiation in the hippocampus during systemic inflammation. Neuroscience. 2012;216:133–142. doi: 10.1016/j.neuroscience.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 26.Thomson LM, Sutherland RJ. Systemic administration of lipopolysaccharide and interleukin-1beta have different effects on memory consolidation. Brain Res. Bull. 2005;67:24–29. doi: 10.1016/j.brainresbull.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Kawamoto EM, Lepsch LB, Boaventura MF, Munhoz CD, Lima LS, Yshii LM, Avellar MC, Curi R, Mattson MP, Scavone C. Amyloid beta-peptide activates nuclear factor-kappaB through an N-methyl-D-aspartate signaling pathway in cultured cerebellar cells. J. Neurosci. Res. 2008;86:845–860. doi: 10.1002/jnr.21548. [DOI] [PubMed] [Google Scholar]
- 28.Yshii LM, Denadai-Souza A, Vasconcelos AR, Avellar MC, Scavone C. Suppression of MAPK attenuates neuronal cell death induced by activated glia-conditioned medium in alpha-synuclein overexpressing SH-SY5Y cells. J. Neuroinflamm. 2015;12:193. doi: 10.1186/s12974-015-0412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glezer I, Munhoz CD, Kawamoto EM, Marcourakis T, Avellar MC, Scavone C. MK-801 and 7-Ni attenuate the activation of brain NF-kappa B induced by LPS. Neuropharmacology. 2003;45:1120–1129. doi: 10.1016/s0028-3908(03)00279-x. [DOI] [PubMed] [Google Scholar]
- 30.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 31.Kotas ME, Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell. 2015;160:816–827. doi: 10.1016/j.cell.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nazem A, Sankowski R, Bacher M, Al-Abed Y. Rodent models of neuroinflammation for Alzheimer's disease. J Neuroinflamm. 2015;12:74. doi: 10.1186/s12974-015-0291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivest S. Regulation of innate immune responses in the brain. Nat. Rev. Immunol. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- 34.Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat. Rev. Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- 35.Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014;14:463–477. doi: 10.1038/nri3705. [DOI] [PubMed] [Google Scholar]
- 36.Guadagno J, Xu X, Karajgikar M, Brown A, Cregan SP. Microglia-derived TNFα induces apoptosis in neural precursor cells via transcriptional activation of the Bcl-2 family member Puma. Cell Death Dis. 2013;4:e538. doi: 10.1038/cddis.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun L, Shen R, Agnihotri SK, Chen Y, Huang Z, Büeler H. Lack of PINK1 alters glia innate immune responses and enhances inflammation-induced, nitric oxide-mediated neuron death. Sci. Rep. 2018;8:383. doi: 10.1038/s41598-017-18786-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dai D, Yuan J, Wang Y, Xu J, Mao C, Xiao Y. Peli1 controls the survival of dopaminergic neurons through modulating microglia-mediated neuroinflammation. Sci. Rep. 2019;9:8034. doi: 10.1038/s41598-019-44573-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D'Angelo B, Astarita C, Boffo S, Massaro-Giordano M, Antonella Ianuzzi C, Caporaso A, Macaluso M, Giordano A. LPS-induced inflammatory response triggers cell cycle reactivation in murine neuronal cells through retinoblastoma proteins induction. Cell Cycle. 2017;16:2330–2336. doi: 10.1080/15384101.2017.1363943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuro-o M. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 2019;15:27–44. doi: 10.1038/s41581-018-0078-3. [DOI] [PubMed] [Google Scholar]
- 41.Hui H, Zhai Y, Ao L, Cleveland JC, Jr, Liu H, Fullerton DA, Meng X. Klotho suppresses the inflammatory responses and ameliorates cardiac dysfunction in aging endotoxemic mice. Oncotarget. 2017;8:15663–15676. doi: 10.18632/oncotarget.14933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo Y, Zhuang X, Huang Z, Zou J, Yang D, Hu X, Du Z, Wang L, Liao X. Klotho protects the heart from hyperglycemia-induced injury by inactivating ROS and NF-κB-mediated inflammation both in vitro and in vivo. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:238–251. doi: 10.1016/j.bbadis.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y, Banerjee S, Dey N, LeJeune WS, Sarkar PS, Brobey R, Rosenblatt KP, Tilton RG, Choudhary S. Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine)536 phosphorylation. Diabetes. 2011;60:1907–1916. doi: 10.2337/db10-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li L, Wang Y, Gao W, Yuan C, Zhang S, Zhou H, Huang M, Yao X. Klotho reduction in alveolar macrophages contributes to cigarette smoke extract-induced inflammation in chronic obstructive pulmonary disease. J. Biol. Chem. 2015;290:27890–27900. doi: 10.1074/jbc.M115.655431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krick S, Baumlin N, Aller SP, Aguiar C, Grabner A, Sailland J, Mendes E, Schmid A, Qi L, David NV, et al. Klotho Inhibits Interleukin-8 Secretion from Cystic Fibrosis Airway Epithelia. Sci. Rep. 2017;7:14388. doi: 10.1038/s41598-017-14811-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maekawa Y, Ishikawa K, Yasuda O, Oguro R, Hanasaki H, Kida I, Takemura Y, Ohishi M, Katsuya T, Rakugi H. Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine. 2009;35:341–346. doi: 10.1007/s12020-009-9181-3. [DOI] [PubMed] [Google Scholar]
- 47.Zhou HJ, Li H, Shi MQ, Mao XN, Liu DL, Chang YR, Gan YM, Kuang X, Du JR. Protective effect of klotho against ischemic brain injury is associated with inhibition of RIG-I/NF-κB signaling. Front. Pharmacol. 2017;8:950. doi: 10.3389/fphar.2017.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeldich E, Chen CD, Boden E, Howat B, Nasse JS, Zeldich D, Lambert AG, Yuste A, Cherry JD, Mathias RM, et al. Klotho Is neuroprotective in the superoxide dismutase (SOD1(G93A)) mouse model of ALS. J. Mol. Neurosci. 2019;69:264–285. doi: 10.1007/s12031-019-01356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwon HS, Koh S-H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020;9:42. doi: 10.1186/s40035-020-00221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang C, Liu Z, Ke Y, Wang F: Intrinsic FGFR2 and Ectopic FGFR1 Signaling in the Prostate and Prostate Cancer. Frontiers in Genetics 2019, 10. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
"The datasets used and/or analysed during the current study available from the corresponding author on reasonable request".