Abstract
Remodelling is a fundamental biological process involved in the maintenance of bone physiology and function. We know that a range of health and lifestyle factors can impact this process in living and past societies, but there is a notable gap in bone remodelling data for populations from the Pacific Islands. We conducted the first examination of femoral cortical histology in 69 individuals from ca. 440–150 BP Taumako in Solomon Islands, a remote ‘Polynesian Outlier’ island in Melanesia. We tested whether bone remodelling indicators differed between age groups, and biological sex validated using ancient DNA. Bone vascular canal and osteon size, vascular porosity, and localised osteon densities, corrected by femoral robusticity indices were examined. Females had statistically significantly higher vascular porosities when compared to males, but osteon densities and ratios of canal-osteon (~ 8%) did not differ between the sexes. Our results indicate that, compared to males, localised femoral bone tissue of the Taumako females did not drastically decline with age, contrary to what is often observed in modern populations. However, our results match findings in other archaeological samples—a testament to past female bone physiology resilience, also now observed in the Pacific region.
Subject terms: Biological anthropology, Anthropology, Bone
Introduction
Peak bone mass attainment in modern humans occurs around the third life decade and is marked by a striking sex-specific difference whereby biological females (hereafter ‘females’) typically accrue less bone than biological males (hereafter ‘males’)1–3. Bone density becomes further compromised around the fifth to sixth life decade when females experience menopause and a significant reduction in the osteoclast inhibiting estrogen4–6. The physiological maintenance of bone throughout the life-course is executed by remodelling, a process sensitive to a range of internal and external stimuli7. Bioarchaeological research on human skeletal remains with well-preserved bone microstructure has provided data on bone remodelling under a range of cultural and environmental conditions8–12. However, there is a notable gap in data for past populations from across the Pacific Islands, except for two recent studies that used small samples sizes quantifying bone vascular porosity in eight individuals from Tonga13, and comparing bone histology between the femur, rib, and humerus in one individual from the Marshall Islands14. Here, we report the first adult human femur quantitative bone histology data for an archaeological ‘Polynesian Outlier’ skeletal assemblage from a ca. 440–150 BP site on Taumako, Southeast Solomon Islands15–17 (Fig. 1).
Figure 1.
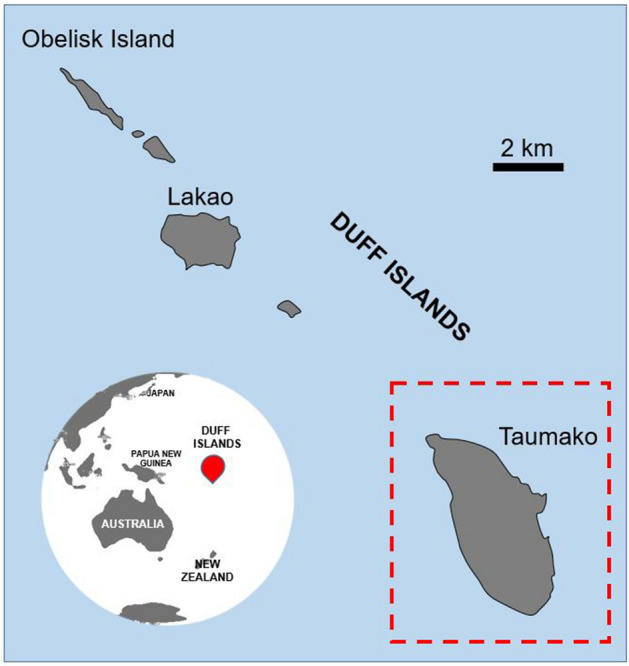
Location of Taumako (red dashed outline), part of the Duff Islands (red marker) complex in Melanesia. Map was drawn by first author (JJM) using Microsoft Office 365 PowerPoint (version 2207) https://www.microsoft.com/en-au/microsoft-365.
There are several reasons why bone remodelling in the past inhabitants of Taumako is worth investigating. The Solomon Islands are part of Oceania, which is a region with complex migration histories18. Taumako Island, despite being part of the ‘Melanesian’ Solomon Islands, is known as a ‘Polynesian Outlier’ (i.e. part of Polynesia) due to a purported blow-back migration of populations from Polynesia during the mid-second millennium AD, and where Polynesian is the main language today19. Human mobility between different regions, including islands from Melanesia, Micronesia, and Polynesia, facilitated an exchange of cultural practices but also encouraged spread of diseases19,20. A notoriously high incidence of metabolic and infectious conditions is widespread across the Pacific Islands, particularly in Near Oceania, evidence for which has come from modern epidemiological research and studies of disease in archaeological human remains21,22. Although Taumako lies directly east of the boundary with Near Oceania, the archaeological remains from Taumako display a high prevalence of skeletal lesions indicative of endemic yaws and iron-deficiency anaemia (potentially exacerbated by high malaria pathogen loads)23–25. This is in addition to large variation in stature, age- and sex-specific dietary practices relating to social status17,20,26, and tendency for males to die younger than females at Taumako when compared with neighbouring Tonga in western Polynesia23,24. All of these findings suggest experiences of population-wide physiological stress at Taumako. As we do not yet have bone remodelling data for this archaeological sample, we do not understand how, and if, bone growth varied across this population, or whether it was influenced by these experiences of physiological stress. The aim of our study is to report baseline bone remodelling data for Taumako, which will add new insights into the current limited knowledge about past human bone physiology across Pacific Island habitats. Our data will expand understanding of bone growth dynamics within spatially and temporally distributed archaeological populations, and might be of interest to the International Osteoporosis Foundation, which is currently mapping the occurrence of fractures across the Asia–Pacific region27.
Bone remodelling through human life-course
Based on bone mineral density (BMD) and fracture incidence data, it is established that significant bone loss occurs with age28,29. Bone building capacities in early adulthood play a key role in determining the rate at which bone metabolic activity becomes out of balance later in life2. While early life skeletal mass accrual is largely genetically determined, other factors such as physical activity, diet, and lifestyle habits, can also impact bone metabolism12,30. Generally, there are three key areas that characterise bone mass change in modern humans—peak bone mass accrual in the third life decade, drastic bone loss after menopause in females, and significant bone loss in both sexes in old age2. The first three life decades are spent creating a ‘bone bank’ that is used for the remainder of the life-course31. The female preponderance of bone loss is due to life-course variability in estrogen levels, which inhibit prolonged bone resorption4. The effect of menopause on bone health can be mediated through lifestyle factors and calcium supplements available to women today. Modern clinical techniques can diagnose osteoporotic bone from BMD T-scores32 and bone remodelling histological markers to check whether osteoclast-mediated bone resorption outweighs bone deposition by osteoblasts.
While BMD has been previously examined in some archaeological samples (see reviews in8–12,33), histological characteristics of cortical remodelling assessed from thin sections by histomorphometric and histomorphological methods have also been successfully evaluated34,35. Cortical bone not only experiences metabolic turnover events that ensure suitable calcium reservoirs, it also responds to biomechanical stimuli that drive bone cell activity7. As teams of osteoblasts and osteoclasts execute bone remodelling as part of Bone Multicellular Units (BMUs) that travel through the cortex, they leave behind remodelling products of circular structure—secondary osteons (hereafter ‘osteons’)—that can be studied histologically, and thus offer an insight into bone remodelling activity in an individual36. The area of osteons and Haversian canals within these can aid in determining whether a typical BMU formed over relatively longer or shorter periods of time, whereby larger osteons simply fill more space in bone (though this depends on the ratio of lamellar bone to Haversian canal in individual osteons)37–40. Osteons are also replaced by subsequent generations of osteons, creating a total population of remodelled bone per given region40, whereas the densities of vascular pores (Haversian canals, Volkmann’s canals, primary/simple vessels) reflect the complex interconnected network cortical bone uses to circulate blood and interstitial fluid containing oxygen and nutrients important for bone homeostasis41,42.
Bone remodelling in archaeological humans
In cases of archaeological human bone that is well preserved microstructurally, studies have been able to reconstruct bone remodelling capacities and link them to aspects such as gender division of labour and sex-specific bone remodelling43, changes in subsistence strategies through time44, or medieval lifestyles associated with socio-economic disparities45. For example, Mulhern and Van Gerven43 found higher osteon densities in femoral cross-sections of males than females from Medieval Sudanese Nubia, but no sex differences in Haversian canal dimensions, suggesting sex-specific activities with physically strenuous tasks of males contributing to the observed remodelling patterns. Miszkiewicz et al.13 found severely porous Haversian bone in adult females compared to denser bone samples of males from 2650 BP Tonga, indicating experiences of abnormal bone loss likely related to both age and activity. However, bioarchaeological studies where bone remodelling has been investigated through histological means have also cautioned that we do not yet fully understand the spectrum of bone histology parameters manifested in archaeological samples46, and that relying on very specific interpretations (e.g. behaviour) made from histological data is clouded by multiple other confounding variables47 such as health, nutrition, ancestry and individual or population-based variations in metabolic activity. Therefore, interpretations of archaeological human bone histology data are usually context specific. However, with an increasing number of sites/collections reported, we may be able to start building a better understanding of possible changes in bone remodelling through time and space in recent humans. For example, one prior analysis comparing tibial and femoral bone histology between Pleistocene specimens (including Broken Hill, Shanidar 2, 3, 4, 5, 6, Tabun 1, and Skhul 3, 6, 7) and a pre‐Columbian Pecos human sample, reported similar levels of bone remodelling characterising the two48, but smaller size of osteonal structures in the Pleistocene sample49.
As bone histology research using archaeological samples gathers increasing amounts of data, it is apparent that a significant gap remains for populations from across the Asia–Pacific region. While access to large samples of human remains is limited in the remote areas of the Pacific, excavation on Taumako Island in the southeast Solomon Islands produced one of the largest well-preserved skeletal samples in the region15. Study of this skeletal assemblage presents an excellent opportunity for bone remodelling research.
Modern Pacific Island nations, particularly in Polynesia, are impacted by widespread metabolic syndrome related conditions, including type 2 diabetes and obesity21. Archaeological evidence demonstrates the occurrence of gout and diffuse idiopathic skeletal hyperostosis, as well as infectious and nutritional conditions affecting health from the time of first settlement ca 3000 BP in Remote Oceania (the islands east of the main Solomon Islands chain)21,50–52. Island environments are associated with food shortages, climate and environmental instability, affecting health in the past and today21,50,53. Long-term exposure to pathogens, and population admixture prior to, and crossing-over with, the European contact in the sixteenth to seventeenth centuries could be reflected in community-specific bone remodelling capacities as an adaption to endemic disease and society specific structures that determine diet and society roles. For example, one prior study of 61 Taumako individuals recorded cortical bone indices of the metacarpal and femur, in addition to femur length, to find that no distinct stress or functional adaptation signal could be detected specifically as a result of island conditions54. However, this study did not collect microscopic bone data—a gap which our study will fill.
Given the significance of archaeological human samples in improving modern bone biology research, the Pacific Island gap in our knowledge relating to archaeological bone remodelling, and the island environmental context of the Solomon Islands, this study tested whether (1) femur bone histology from archaeological Taumako males and females showed differences in remodelling and tissue organisation indicators, and (2) to what extent these bone microstructure features changed with age. Our total sample size was 69 (33 males and 36 females). We selected the posterior midshaft femur because of its biomechanical versatility reflecting sex-specific lifestyles and sexual dimorphism, which we first evaluated in this sample through basic gross measures of femoral size (midshaft circumference, cortical width, maximum length54) and robusticity indices55 computed from these values. Next, we created thin sections from which we measured standard static histological variables (vascular canal and osteon area to compute canal-osteon ratios56, and localised osteon density40) as proxies for bone remodelling activity, and vascular porosity as a proxy for bone blood supply and reflecting bone tissue organisation41. Histology was examined in intra-cortical, and combined (including periosteal and endosteal areas) cortical regions of interests (ROIs) of the thin sections (Fig. 2). The femoral size data were then used to adjust histology data to account for microscopic-macroscopic scaling issues, and within-sample variation stemming from inherent bone size differences between males and females. We then compared the data between male and age groups.
Figure 2.
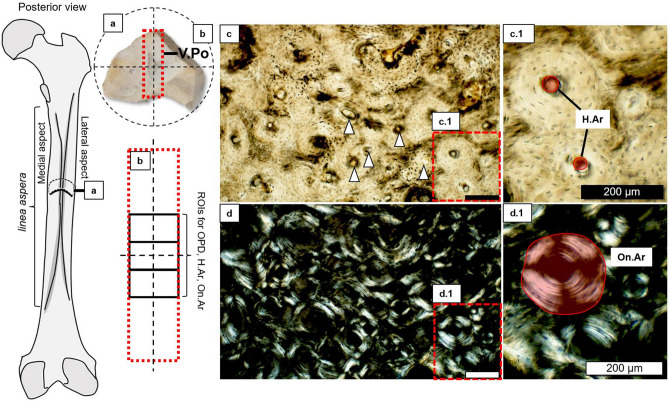
Summary of histomorphometric techniques used in this study. From left sketch of right posterior human femur: (a) posterior cortical bone quadrant showing a strip (red dashed lines) of bone surface examined histologically from which vascular porosity (V.Po) was collected; (b) three intra-cortical regions of interest (black rectangles) contained within the larger strip examined for osteon population density (OPD), Haversian canal area (H.Ar), and secondary osteon area (On.Ar); (c) bone histology under transmitted light showing Haversian canals counted for V.Po (white triangle markers) and measured for area (c.1); (d) bone histology under linearly polarised light showing secondary osteon area (d.1). Scale bars in (c,d) are 200 μm.
We hypothesised indicators of higher bone resorption over formation should be evident in females when compared with males, and in older individuals when compared with those of younger age. Our age and sex estimates are based upon standard gross anatomy methods, which assess age-progressive and sexually dimorphic skeletal landmarks of the skull, teeth, and the pelvis57,58. Our sex estimates were validated by determining XY or XX karyotypes via ancient DNA (aDNA), yielding 88% of successful sex matching through these two methodological approaches. This study can only treat sex as a biological trait and cannot consider gender identity which is unknown for these Taumako individuals. We present analyses based on skeletal sexual dimorphism and genetic information within the limits of our sample and available context, but we recognise that many biological traits associated with sex are not binary and exist on a spectrum59.
Results
Sexual dimorphism manifested in size variation across the Taumako femora. The Taumako males had (p < 0.001, Tables 1, 2, 3) larger femoral midshafts and thicker posterior cortical walls (average circumference = 95.79 mm, average cortical width = 10.77 mm) compared to females (average circumference = 89.81 mm, average cortical width = 8.77 mm). Females also had slightly shorter femora than males, though this difference was not tested statistically due to a small sub-sample size (n = 23) of the individuals with intact femora. Robusticity indices (unitless values) calculated based upon midshaft circumference and cortical width were greater in males (average circumference robusticity = 22.85; average cortical width robusticity = 2.58) when compared to females (average circumference robusticity = 20.66, average cortical width robusticity = 2.08), but could not be validated using statistical tests either because they were based on the limited femoral length data. We could not apply age related inferential statistical tests to the gross morphometric femoral data, except for circumference and cortical width, which did not change statistically across any of the age classes in the whole sample (p > 0.05, Table 1). Within females and males, there was no age effect on midshaft circumference or cortical width either. Given that some of the gross femoral measurements varied with sex (likely because males have larger femora than females in our sample) adjustments of bone histology data by femoral size were necessary60,61.
Table 1.
Descriptive summary of gross femoral data sub-divided by estimated sex and age-at-death. SD standard deviation, MAX. maximum, MIN. minimum, Ct.W_RI robusticity index (RI) calculated using cortical width (Ct.W) data, Circ_RI robusticity index (RI) calculated using midshaft circumference (Circ). The RI variables are unitless.
| Gross femoral measures | |||||
|---|---|---|---|---|---|
| Sub-divided by sex and age-at-death | N | Min | Max | Mean | SD |
| Female | |||||
| Femur max length (cm) | 6 | 40.60 | 44.50 | 42.42 | 15.41 |
| Circ (mm) | 36 | 69.00 | 106.00 | 89.81 | 7.86 |
| Ct.W (mm) | 36 | 3.95 | 13.23 | 8.77 | 1.96 |
| Circ_RI | 6 | 17.93 | 22.33 | 20.66 | 1.91 |
| Ct_W_RI | 6 | 1.47 | 2.57 | 2.08 | 0.46 |
| Male | |||||
| Femur max length (cm) | 17 | 30.40 | 47.00 | 42.92 | 4.70 |
| Circ (mm) | 33 | 85.00 | 106.00 | 95.79 | 5.53 |
| Ct.W (mm) | 33 | 6.56 | 15.27 | 10.77 | 1.65 |
| Circ_RI | 17 | 19.43 | 34.21 | 22.85 | 3.84 |
| Ct_W_RI | 17 | 1.75 | 4.16 | 2.58 | 0.56 |
| Young adult | |||||
| Femur max length (cm) | 16 | 304.00 | 477.00 | 426.81 | 48.13 |
| Circ (mm) | 34 | 69.00 | 106.00 | 91.29 | 8.05 |
| Ct.W (mm) | 34 | 3.95 | 15.27 | 9.61 | 2.21 |
| Circ_RI | 16 | 17.93 | 34.21 | 22.40 | 4.18 |
| Ct_W_RI | 16 | 1.47 | 4.16 | 2.45 | 0.67 |
| Middle-aged adult | |||||
| Femur max length (cm) | 2 | 406.00 | 421.00 | 413.50 | 10.61 |
| Circ (mm) | 13 | 81.00 | 106.00 | 93.15 | 7.54 |
| Ct.W (mm) | 13 | 6.64 | 13.23 | 9.42 | 2.07 |
| Circ_RI | 2 | 21.43 | 22.33 | 21.88 | 0.64 |
| Ct_W_RI | 2 | 2.43 | 2.57 | 2.50 | 0.10 |
| Old adult | |||||
| Femur max length (cm) | 5 | 420.00 | 458.00 | 437.00 | 15.31 |
| Circ (mm) | 22 | 83.00 | 104.00 | 94.50 | 6.15 |
| Ct.W (mm) | 22 | 6.73 | 13.03 | 10.07 | 1.86 |
| Circ_RI | 5 | 19.43 | 23.80 | 22.05 | 1.78 |
| Ct_W_RI | 5 | 2.21 | 2.78 | 2.44 | 0.25 |
Table 2.
Descriptive summary of histology data sub-divided by estimated sex and age-at-death groups. SD standard deviation, MAX. maximum, MIN. minimum, V.Po density of canals/pores per mm2, H.Ar/On.Ar ratio of Haversian canal to osteon area in µm2, OPD osteon population density per mm2, Ct.W_RI robusticity index (RI) calculated using cortical width (Ct.W) data, Circ_RI robusticity index (RI) calculated using midshaft circumference (Circ). All variables are unitless.
| Femur histology measures | |||||
|---|---|---|---|---|---|
| Sub-divided by sex and age-at-death | N | Min | Max | Mean | SD |
| Female | |||||
| V.Po/Ct.W | 36 | 1.29 | 4.42 | 2.34 | 0.77 |
| V.Po/Circ | 36 | 12.86 | 39.05 | 22.02 | 5.80 |
| H.Ar/On.Ar | 9 | 6.50 | 10.40 | 8.31 | 1.36 |
| OPD/Ct.W_RI | 4 | 6.34 | 6.84 | 6.62 | 0.24 |
| OPD/Circ_RI | 4 | 5.53 | 7.86 | 6.76 | 0.53 |
| Male | |||||
| V.Po/Ct.W | 32 | 0.83 | 3.16 | 1.64 | 0.49 |
| V.Po/Circ | 32 | 10.46 | 33.41 | 18.34 | 4.49 |
| H.Ar/On.Ar | 12 | 4.57 | 9.46 | 7.61 | 1.52 |
| OPD/Ct.W_RI | 8 | 3.40 | 6.69 | 5.31 | 1.20 |
| OPD/Circ_RI | 9 | 4.78 | 7.66 | 6.34 | 1.08 |
| Young adult | |||||
| V.Po/Ct.W | 33 | 1.07 | 4.42 | 2.02 | 0.74 |
| V.Po/Circ | 33 | 12.77 | 29.11 | 20.37 | 4.27 |
| H.Ar/On.Ar | 13 | 4.57 | 9.85 | 7.83 | 1.40 |
| OPD/Ct.W_RI | 8 | 3.40 | 6.69 | 5.75 | 1.16 |
| OPD/Circ_RI | 9 | 4.90 | 7.66 | 6.44 | 0.96 |
| Middle-aged adult | |||||
| V.Po/Ct.W | 13 | 1.29 | 3.40 | 2.22 | 0.69 |
| V.Po/Circ | 13 | 13.98 | 28.19 | 21.53 | 4.75 |
| H.Ar/On.Ar | 4 | 6.50 | 10.40 | 8.17 | 1.83 |
| OPD/Ct.W_RI | 1 | 6.84 | 6.84 | 6.84 | n/a |
| OPD/Circ_RI | 1 | 7.86 | 7.86 | 7.86 | n/a |
| Old adult | |||||
| V.Po/Ct.W | 22 | 0.83 | 3.33 | 1.88 | 0.76 |
| V.Po/Circ | 22 | 10.46 | 39.05 | 19.43 | 7.37 |
| H.Ar/On.Ar | 4 | 5.42 | 9.36 | 7.92 | 1.73 |
| OPD/Ct.W_RI | 3 | 4.04 | 6.81 | 5.36 | 1.39 |
| OPD/Circ_RI | 3 | 4.78 | 7.39 | 6.10 | 1.30 |
Table 3.
All results of inferential analyses comparing femoral size, and statistically significant results of bone histological markers compared between the sex and age groups. V.Po density of canals/pores per mm2, H.Ar/On.Ar ratio of Haversian canal to osteon area in µm2, OPD osteon population density per mm2, t independent samples t test, U Mann Whitney U test, H Kruskal–Wallis test, **p < 0.01, ***p < 0.001.
| Comparisons | Test statistic | n | p |
|---|---|---|---|
| Males vs. females | |||
| Circ (mm) | t = 3.626 | F = 36, M = 33 | < 0.001*** |
| Ct.W (mm) | t = 4.577 | F = 36, M = 33 | < 0.001*** |
| Bone histology markers compared between males and females | |||
| V.Po/Ct.W (unitless) | U = 249 | F = 36, M = 32 | < 0.0001*** |
| V.Po/Circ (unitless) | t = 2.905 | F = 36, M = 32 | 0.005** |
| H.Ar/On.Ar (unitless) | U = 40 | F = 9, M = 12 | 0.345 |
| Bone histology markers compared between age groups | |||
| V.Po/Ct.W (unitless) | H = 2.4 | Y = 33, MA = 13, O = 22 | 0.301 |
| V.Po/Circ (unitless) | H = 3.278 | Y = 33, MA = 13, O = 22 | 0.194 |
Femoral vascular porosity and bone remodelling indicators at Taumako
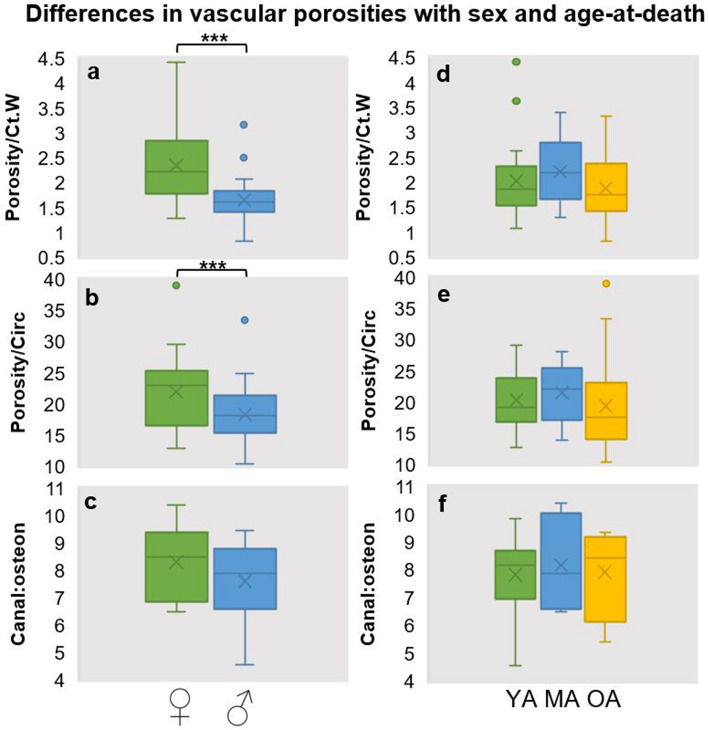
Looking at descriptive statistics only, vascular porosity, canal-osteon ratios, and osteon densities were greater in females when compared to males (Table 2). When applying inferential statistical tests, out of all three variables, vascular porosity in females adjusted (unitless values) by both cortical width (average vascular porosity adjusted by cortical width = 2.34) and midshaft circumference (average vascular porosity adjusted by midshaft circumference = 22.02) were statistically significantly higher (p < 0.0001) than in males (average vascular porosity adjusted by cortical width = 1.64, average vascular porosity adjusted by midshaft circumference = 18.34) (Table 3, Fig. 3). However, the vascular canal-osteon ratios did not differ statistically between the sexes (p > 0.05, Tables 2, 3), with both sub-groups bordering an approximate 8% (averages of 7.61% in males and 8.31% in females). We did not attempt an inferential statistical comparison of the osteon density data in the whole sample, or on further sub-divisions by age, due to inadequately small sample sizes (Table 2).
Figure 3.
Simple boxplots illustrating differences in vascular porosities adjusted by different measures of femoral bone size (Circ circumference of midshaft, Ct.W cortical width), and ratio of Haversian canal area to osteon area, compared between the sexes (boxplots a–c), and age-at-death categories (d–f; where YA young adults, MA middle-aged adults, OA older adults). ***p < 0.001 using Mann Whitey U test (see Table 3).
Secondly, there was a clear change in the descriptive statistics of bone histology values from young to old individuals whereby all peaked in the middle-age category (Tables 2, 3, Fig. 3). While all the histology data were lower in the young or old age sub-groups when compared to the middle-age sub-group, the old individuals showed the lowest values across the entire sample with the exception of canal-osteon ratios which were slightly higher. However, none of these changes with age, apparent when considering the data means, were statistically significant (p > 0.05) (Table 3). As above, we did not attempt an inferential statistical comparison of the osteon density data in the whole sample, or on further sub-divisions by sex due to inadequately small sample sizes (Table 2). Collectively, our results partly support our hypothesised expectations.
Discussion
Our age and sex analyses of the Taumako bone histology data revealed that the Taumako females had higher vascular porosity of their femoral cortical bone compared to males, while intra-cortical variables of osteon densities and canal-osteon area ratios did not differ statistically significantly between the sexes. This occurred despite males and females having sexually dimorphic femora at Taumako. A possible isometric effect of larger male femur size on bone histology can be excluded as underlying these results as our data were corrected by femoral midshaft size measures and robusticity indices60,61. Acknowledging small sample size in some of the age and sex sub-groups, and with the data at hand, we will discuss possible implications of our results for adult femur bone physiology at Taumako.
Sex and cortical bone histology at Taumako
The femoral samples of Taumako females were more vascularised than those of males. We will not link this to bone remodelling only42, because our data for the vascular porosity are made up of Haversian canals with the possibility of including some primary vessels (see “Materials and methods”). This is a result of us accounting for localised diagenesis apparent in the thin sections. Therefore, this measure is that of an accumulation of vascular cavities up until the point of death, rather than just reflecting recent remodelling events, and we cannot be sure which canals had been replaced in the first few life decades in these individuals. Further, the vascular porosity data stem from the posterior femur ‘strip’ region overlapping an entirety of compact bone, so the porosity counts reflect our inclusion of both the periosteal and endosteal bone regions where there might have been region-specific variation in pore counts. Our main interpretation is that the higher densities of vascular pores in females suggest their bones received greater blood and nutrient supply than that of males42. Male frailty due to endemic disease, inferred from their younger mortality compared to females at Taumako16,26, may have contributed to this bone characteristic, which we discuss further below. Despite the greater density of cortical pores in females, neither the osteon population density nor the geometric properties of secondary osteons differed statistically between the sexes. The ratio relationship between Haversian canals and osteon area was almost the same when comparing the sexes (approximately 8%). We expected higher Haversian canal area in females than males indicating prolonged osteoclast-mediated bone resorption. This suggests that the intra-cortical midshaft femoral bone in Taumako males and females experienced similar remodelling events.
Prior bioarchaeological research reported inconsistencies in osteon morphometry when comparing the sexes similar to those we present for Taumako. For example, data for males in the Medieval Sudanese sample, mentioned in our Introduction, showed higher osteon densities than in females, but females had larger osteons than males43. However, similar to us, Mulhern and Van Gerven43 reported a lack of statistically significant differences in the geometric properties of Haversian canals between the sexes. Similarly, fourteenth to nineteenth centuries Pecos females (New Mexico) had relatively large secondary osteons, but with smaller Haversian canals when compared to males62. Burr et al.62 observed a lack of distinct bone loss in the Pecos females, citing a physically active lifestyle as a possible factor driving the maintenance of good bone density. In the 700 BC to nineteenth century Canadian Baffin Island male and female skeletons, no significant differences were noted when considering the density of Haversian canals and the area of secondary osteons63. As noted by Pfeiffer46, there is a clear variability in how bone histology is expressed in archaeological populations, as illustrated by the above examples, complicating inter-population comparisons. A Taumako-specific approach is needed to contextualise our results.
Outside of a genetic basis to the morphology of adult compact bone, the Taumako femoral bone histology could reflect a combination of the following population-specific factors: the socio-economic make-up and diet of the community, and the effect of physiological stress and disease on skeletal development. Having studied grave goods (including shell money, bobbles, and Tridacna shell ‘tavi’ neck ornaments) from across the Taumako burials, archaeologists have previously determined that this community was stratified into status groups on the basis of wealth and inherited rank15. Leach and Davidson15 quantified the value of grave goods finding that the Tridacna breast pendants were the most prestigious. Kinaston et al.17 used this information to test for status-related access to food in 99 of Taumako individuals. They17 analysed carbon, nitrogen, and sulfur stable isotopes in bone collagen to confirm that wealthy Taumako individuals ate high status foods consisting of high trophic level animals such as pigs, fish, and turtles. This was the case for both high status males and females. However, all wealthy individuals, and all males, overall, had elevated levels of nitrogen when compared to low status females. In our study, at least 15 (~ 22%) individuals were of very high status (Leach and Davidson15 used a ‘wealth index’), including nine males and six females, with seven males and five females being buried with the prestigious Tridacna breast pendants. The combination of mixed-sex individuals who regularly fed on high protein foods, and others who fed on lower ranking foods or experienced nutritional stress (see below), could have resulted in the balanced osteon density and canal-osteon areas across the sexes. This mirrors prior research comparing high and low social status human bone histology in medieval England where upper-class foods were associated with higher osteon densities in the femur45,64.
In addition, the lack of intra-cortical remodelling differences between the sexes, but lower vascular porosity in males, could be explained through Taumako male frailty. Kinaston and Buckley16 used carbon and nitrogen stable isotopes in bone collagen and tooth dentine to infer that nutritional stress led to early deaths of some adolescent and young males at Taumako. This was also found by Stantis et al.26 who examined nitrogen stable isotopes in tooth dentine in this sample. Combined with the long history of malaria and yaws exposure at Taumako, experiences of inconsistent dietary intake in lower ranking adolescents might have led to poor bone maintenance later in life65 (males are considered to be more susceptible to physiological stress than females because sex-steroids regulate immune response66,67). Also, in some indigenous Solomon Island populations today, growth and nutritional status of females is reported to be much better than that of young males68. Even though no sex-specific differences in gross anatomical markers of stress, such as linear enamel hypoplasia or lesions indicative of yaws, have been previously noted in the Taumako assemblage24,69, our bone histology data offer a microscopic perspective which is a proxy for repetitive, longer-term bone physiological cycles. Thus, we infer that Taumako females might have been equipped with dense intra-cortical femoral bone to buffer excessive bone loss. We know from experimental research that loss of calcium is compensated for by increasing remodelling in lactating females, which ultimately restores compromised bone tissue during reproduction70,71.
The effect of age on bone histology at Taumako
Two key areas of concern to life-long human bone building capacities are the third and fifth-sixth decades reflecting peak bone mass accrual and female menopause, respectively2. Bone mass in modern humans through the life-course is easier to map than in past populations as we cannot observe life-long change to bone mass accrual in the archaeological record. However, our sample size is large enough to begin unravelling Taumako bone remodelling differences across the three anthropological age categories. The entire sample followed an expected trend in secondary bone change with age, whereby both osteons and vascular porosity increased through the lifespan in mature individuals72,73. Further, all the bone histology data appeared to peak at the middle-age category, which mirrors the expectation based on modern bone health through the life-course paradigms. We acknowledge such comparison cannot be exact given the broad age anthropological categories, but the end of the young, and the start of the middle-age age category, overlaps with the peak age for bone mass accrual in living humans2. When considering intra-cortical osteon densities and canal-osteon ratios, it becomes apparent that Taumako females maintain similar localised amounts of bone as males across different age categories. This aligns with the same age-related observation in 1250–1450 AD Sudanese Kulubnarti, Nubia, where no statistically significant changes in the geometric parameters of osteons were observed43. Our data also match some of the findings reported by Burr et al.62 for the Pecos females who appeared to maintain good bone well into adulthood, and any age-related reduction in bone quality was that of marrow cavity expansion in both males and females. Generally, a smaller secondary osteon size has been previously noted to occur with age in both males and females today73, which applies to our results, and is similar to previous reports for the Pecos males62.
A preliminary and cautious comment about bone histology in our old males and females can also be made. Although osteons were only measured in four well-preserved histology samples, representing four different individuals, all had densely remodelled Haversian bone. There was no indication of early stages of osteoporosis, including cortical bone trabecularisation or the presence of ‘giant’ coalescing pores13. Further, osteon density data in the one old female matched osteon density data for the female middle-age category, and were higher than the combined osteon density data in all three old male samples. We cannot exclude the effect of osteon population density asymptote on the data in the old category, whereby the evidence of pre-existing secondary osteons may have been erased by subsequent generations of remodeled bone74. However, we can build a hypothesis, worth testing in future bioarchaeological research, whether bone histology from old archaeological females exhibits severe porosity (in the sense of trabecularisation, not just vascular counts) intra-cortically75. This would help in validating to what extent, and at what age, past human female long bone cortex develops osteoporosis in the fifth life decades or later. While most anthropological methods of age estimation do not provide specific chronological ages, or decades, some studies have recognised it might be possible to separate individuals aged 50 + years old into further age classes76,77. Histological sampling in such instances could contribute to this vein of research wherein older individuals could be gradually examined decade by decade (similar to modern efforts, e.g.75). Although, a detailed contextual information and burial/population background78, along with permissions for destructive sampling, would be needed.
Our study highlights the significance of combining gross anatomical and microscopic approaches to understanding bone biology in archaeological contexts. Robb et al.54 reported some effect of age on metacarpal cortical bone indices, and femoral length, in the Taumako sample without accessing microstructural indicators of cortical bone remodelling. Our robusticity indices were calculated for femora instead of just reporting length, and followed a robusticity methodological recommendation based on a published thorough technical evaluation of different robusticity measures55. It will be important for future bioarchaeological studies to combine macro- and microscopic technical approaches as limb bone size and shape determined through ontogenetic modelling completes after the first two life decades79. While modelling declines for the remainder of the lifespan, and re-activates in extreme biomechanical situations, bone remodelling information can be only accessed microscopically.
Remarks on temporal and spatial bone histology data
We acknowledge that bone histology interpretations in archaeological settings need to be conducted at a population level, but given our study presents the first osteon remodelling data for the Pacific region, we can establish that the Taumako data fall into a global range of secondary osteon parameters for archaeological humans38,43,45,62,63. Some examples include: the Taumako male and female combined average osteon area (28,433 μm2) data are similar to 27,303 μm2 reported for medieval Canterbury, England38,45; the male and female combined area of Haversian canals in Taumako is 2221 μm2 which compares closely to 2100 μm2 in medieval (1250–1450 AD) Sudanese Kulubnarti, Nubia43, 2336 μm2 in fourteenth to nineteenth centuries Pecos, New Mexico62, and 2334 μm2 in medieval Canterbury, England38,45. Similarities can also be noted in raw osteon density data, whereby the Taumako data of 13.64/mm2 are close to 11.78/mm2 in Sudanese Nubia43. We acknowledge the above studies used slightly different region of interest (ROI) selection techniques, but all considered femoral midshaft cortical bone. Future bone histology research on archaeological specimens spanning other geographical regions will expand this range.
Limitations
We cannot exclude a series of confounding factors that have impacted our results and interpretations. The estimates of age and sex for a portion of the sample at Taumako rely on anthropological standards, as such they are probability scores. However, the aDNA validation of the bulk of the sex estimates in this study overcame some of the uncertainty of gross methods. Age assessments were validated as much as possible by ensuring that each individual’s histology profile generally matched its age status established from the gross anatomical methods (e.g. thin sections were inspected for possible presence of primary bone in samples from older adults). Unfortunately, we cannot overcome the inconsistencies in sample size in each age and sex sub-group either, and do not have access to better preserved bone histology. It must be said that some of the statistically insignificant results could simply be a result of sampling given the specimens available to us, which is an issue for all bioarchaeological studies. Finally, we only use two-dimensional methods of thin sectioning, but a wider volumetric dataset providing three-dimensional perspectives on vascularity connectedness, in combination with mineral density information, would provide a more in-depth picture of bone building and remodelling capacities in the Taumako sample.
Conclusions
The Pacific islands are yet to be thoroughly studied for past human bone histological variation. Our study forms the first, largest sample size based, report of archaeological human intra-cortical secondary osteon, and cortical vascular porosity, data in this part of the world. We found that archaeological females at Taumako show highly vascularised femoral midshaft bone, but also have localised areas of intra-cortical bone that remodels similarly to that of males. This finding mirrors bone remodelling data from other archaeological sites from across North America, Europe, and Africa, but does not conform entirely to our modern understanding of bone loss through the life-course. These new data fall in the range of bone histology archaeological variability reported globally, extending currently available bone histology data by this site from the Pacific Islands. The lower vascular porosity in males might reflect their higher frailty in the cultural and environmental context of Taumako, and the balanced remodelling indicators between the sexes could be a result of socially stratified dietary practices. Ongoing efforts examining bone histology in Asia–Pacific will further our understanding of ancient human bone remodelling capacities in this region, contributing to modern efforts investigating the conditions under which humans experience significant bone loss.
Materials and methods
Taumako is one of the remote Duff Islands, which lie northeast of Santa Cruz Islands in the far southeast Solomon Islands15 (Fig. 1). While the island is located within the Melanesian geographical boundary, Taumako is known as a ‘Polynesian Outlier’ representing a probable ‘blow-back’ migration of populations from Polynesia around the mid-second millennium AD15,80,81. The modern inhabitants of Taumako speak a Polynesian language, but as a result of admixture with established populations, share similar cultural traditions to nearby Melanesian islands in the Duff and Santa Cruz groups82.
The Namu burial mound, an archaeological site dated to ca. 440–150 BP20, yielded a significant number of human remains and associated grave goods that have been since examined to reconstruct the lives of the past inhabitants of Taumako. This has included social status stratification15,17 reflected in dietary and child feeding practices reconstructed from bone and tooth stable isotope data16,17,26,83; abnormalities of the alveolar bone suggesting possible experiences of periodontitis84; evidence for interpersonal violence and warfare inferred from skeletal patterns of trauma85; a high prevalence of yaws (Treponema pertenue)24; and, more recently, gender specific migration patterns from neighbouring islands20. The site is known for archaeological evidence of using shell money and ornamentation practices that include a tavi—a neck ornament thought to represent high status (see15). Another study also analysed Taumako femur length and metacarpal and femur cortical indices and noted a lack of distinct bone functional adaptation in remote Pacific Island environments54.
With permissions from, and in collaboration with, the Solomon Islands National Museum, n = 69 Taumako adults were sampled in the present study. There were 19 left and 50 right femora. Both sides were pooled due to no statistically significant bilateral differences in the recorded data (Supplementary Information Tables 1, 2). Following standard methods recommended by Buikstra and Ubelaker57, and Brickley and McKinley58, each individual was thoroughly examined for skeletal markers of sex, and those that change with age, to arrive at morphologically informed biological sex and age-at-death (we use ‘age’ in the main article) estimates. The age categories follow these standard recommendations whereby individuals are assigned into ‘young’ (20–35 years old), ‘middle-aged’ (36–50 years old) and old (50 + years old) age-at-death classes. As is good practice in bioarchaeology, for each individual, as many techniques of examination were applied as possible to increase the accuracy of the estimates. These methods involve a gross anatomical examination of the following: dental wear of permanent teeth with higher degrees of wear progressing with age; obliteration degree of cranial sutures which progressively close with age; texture and general morphology changes of the pelvic auricular surface and the pubic symphysis, which disintegrate with age. Each skeletal technique gives an independent age range, which are then compiled into common ranges that can be placed into the major age-at-death classes. We have no way of corroborating the anthropological estimates, which are necessarily broad, with actual chronological age of these individuals, which is unknown.
The biological sex estimation was based upon examining the skull and pelvic anatomical landmarks which are known to be sexually dimorphic. A non-exhaustive list of these features includes: the robusticity level of the mastoid process; the nuchal crest of the occipital bone; the shape of the eye orbits and the thickness of the orbital roof; the prominence of the glabella; and the mental eminence of the mandible; the angle of the pelvic sciatic notch; the presence or absence of the pelvic ventral arc, subpubic concavity, and a medial ridge in the pubis region.
Skeletal morphology is more robust in the male skeletal remains, though we acknowledge this is a generalisation. Thus, unlike with our age-at-death estimates, with permission from the Solomon Islands Museum, we were able to validate the sex estimates through aDNA obtained from genome-wide data that had been produced for a subset of samples investigated histologically. Individuals were sampled for DNA by drilling the petrous part of the temporal bone86 (see dataset87). DNA was extracted from the sampled powder and prepared for next-generation sequencing by producing a double-stranded DNA library following established protocols88–90. Deaminated cytosines were enzymatically partially removed and retained only in the terminal positions as described in91. All libraries were directly shotgun sequenced on an Illumina HiSeq 4000 platform (1 × 75 + 8 + 8 cycles). The sequenced reads were mapped to the human genome reference hg19 using EAGER92. The retained damage was excluded from the analysis by masking the two terminal positions of each read93. The genetic sex was inferred using two independent methods:
The number of reads covering each position was counted across a total of around 1.24 million genome-wide SNP positions94–96 and subsequently averaged for each sex chromosome and all autosomal ones. The Y- and the X-chromosome average coverages were normalized by the average autosomal coverage and compared to determine the sex assignment97.
An approach specifically designed for low-covered shotgun genomes in which the ratio between the average coverage across the entire X-chromosome and the coverage averaged across the autosomes was calculated as in98 (see Supplementary Information for extended aDNA methods). This was possible for n = 48 individuals. There was an 88% success rate (42/48) in corroborating the macroscopic and aDNA sex results, with only six individuals misclassified by the gross methodologies (see Supplementary Information Table 3). Therefore, the presented sex classification can be treated as fairly reliable. We acknowledge we do not attempt to classify these as ‘gender’, but treat them as a biological entity in relation to bone metabolic processes. As a result, this study comprised 34 young adults, 13 middle-aged adults, 22 old adults, and 36 females and 33 males. Further sub-division by age-at-death within each sex group can be seen in the dataset87.
Prior to histological analyses we recorded a series of femur morphometric measurements to characterise the size of each femoral midshaft and calculate femoral robusticity indices where possible55,60. Three variables were included: midshaft circumference (Circ) in mm, posterior cortical width (Ct.W) in mm, and femur maximum length in cm. These were measured using standard osteological laboratory equipment composed of an osteometric board, digital calipers, and a soft measuring tape. Two robusticity indices were computed using the Stock and Shaw55 recommendation and following prior methods combining femoral bone histology and robusticity measures45: femoral robusticity index based on Circ where the circumference values are divided by femoral length, and a femoral robusticity index based on Ct.W where cortical width values are divided by femoral length and multiplied by 100. The latter included multiplication by 100 to increase decimals in the resulting robusticity index values for the ease of our statistical analysis. Only 23 femora were of a suitable preservation for measuring the maximum length, and so only these were used in the robusticity index calculations.
Next, posterior cortical bone samples from the midshaft of each femur were extracted using a Dremel tool with a rotary blade, resulting in approximately 1 cm thick cortical quadrants (see45). The posterior femur is of interest to our study because it overlaps the linea aspera, a rich leg muscle site insertion anatomical landmark. Bone remodelling detected there should capture stimulation resulting from lifestyle99,100, which will strengthen our analyses of age and sex. Our minimal invasive approach ensured the femora remained as intact as possible, limiting the amount of archaeological bone being taken for the histological analysis101. Standard histological methods relevant to archaeological human remains were then followed to produce ~ 100 μm thin sections34. Each sample was embedded in Buehler epoxy resin, cut using a Kemet MICRACUT precision cutter equipped with a diamond blade, glued to a microscope slide, further reduced, ground, and polished to obtain a clear view of bone histology. The thin sections were examined using an Olympus BX53 microscope with a DP74 camera using transmitted and linearly polarised light at a magnification of 10 × (100 × total magnification).
Once histology slides were prepared, it became apparent that not all microstructures could be measured in all sections. Well preserved ROIs where cement lines of secondary osteons were easily identifiable were the case for only 21 individuals, but 68 individuals had consistently and suitably preserved Haversian canals. A diagenetically obscured band that ran along the outer posterior and endosteal layers of bone samples was also observed in the thin sections. However, the intra-cortical regions of bone were of an almost pristine preservation, which allowed us to focus on intra-cortical remodelling activity away from the immediately sub-periosteal and sub-endosteal regions of cortical bone. As such, we designed the ROI selection procedure so that data can be collected from the mid-portion of each sample by scanning a full cortical strip down the midline and then capturing three ROIs within its centre (Fig. 2). The examination of cortical strips as ROIs, and intra-cortical bone regions generally, were successful in prior archaeological studies44,102.
We used the Olympus cellSens software (“Standard” version 2018, https://www.olympus-lifescience.com/en/software/cellsens/), which allows to automatically stitch images in live scanning mode. This function was used to record each ROI ‘strip’. A thin section was placed on the microscope stage so that the mid-point of the periosteal border was in the field of view. The stage was then slowly moved forward (away from the observer) until the endosteal end of the border was reached. The area of the ROI strips ranged from 6.76 to 25.84 mm2 in our sample given variation in cortical wall thickness (mean = 14 mm2, standard deviation = 3.67 mm2). From within the strip, the first ROI was located at the mid-point (by dividing the length of the entire strip by two), and then one ROI was taken either side of this midpoint, ensuring no overlap in histology shown in the field of view (Fig. 2). Using FIJI/ImageJ tools that included the “Multi-Point Count” and “Polygon” selections, three histomorphometric variables indicative of cortical bone remodelling events were measured (we use bone histomorphometry nomenclature recommended by Dempster et al.103 Fig. 2):
Vascular porosity (V.Po) per mm2 (e.g.13,62,104,105): total number of intact Haversian and primary canals across a full strip ROI of bone measured from the posterior to the endosteal borders of the section, and divided by the strip area in mm2. Volkmann’s canals were excluded because they were rarely visible in the sample. Because we worked with archaeological specimens and 2D histology sections, true vascular porosity, including other minute capillaries is not possible to obtain. In instances where cement lines of osteons were not visible, we cannot be entirely confident that a counted canal derives from a secondary osteon structure. As such, we use V.Po to represent all major vascular canals seen in the ROI strip.
Osteon population density (OPD) per mm2 (e.g.43,45): sum of intact osteon and fragmentary osteon numbers counted from three intra-cortical ROIs of 2.05 mm2 area each (totalling 6.15 mm2). Each sum was divided by the ROI area in mm2.
Haversian canal:osteon area ratio (H.Ar/On.Ar), measured in μm2 separately, and then converted to unitless (dimensionless variable (DV)) ratio values (e.g.45,56,60): H.Ar is the average area (total area/total number of measured canals) of intact Haversian canals measured from three intra-cortical ROIs of 2.05 mm2 area each (totalling 6.15 mm2); On.Ar is the secondary osteon area in μm2 created from average area (total area/total number of measured secondary osteons) of intact secondary osteons with complete cement lines measured from three intra-cortical ROIs of 2.05 mm2 area each (totalling 6.15 mm2). Secondary osteons cut off by an image border were excluded. The average values of H.Ar are then divided by the average values of On.Ar and multiplied by 100 to indicate percentage of canal to osteon area.
Recommended standards for reporting of bone histomorphometric data stipulate a minimum of 25 osteons examined per thin section40. Our study meets those standards by examining a minimum of 47 and maximum of 126 secondary osteons across the samples for the purposes of osteon density calculations, and minimum 25 and maximum 50 for the purpose of ratio calculations from area measurements of osteon units. The V.Po and OPD data are used in our study as products of bone remodelling events that indicate the amount of bone produced and remodeled per mm2 intra-cortically45. The area of Haversian canals and secondary osteons can be used as indicators of the stage of a BMU travelling through the cortex36. Larger areas of osteons and canals can be associated with longer periods of BMU activity, and smaller areas would indicate a shorter-term BMU activity, particularly if it is strain-suppressed38,106.
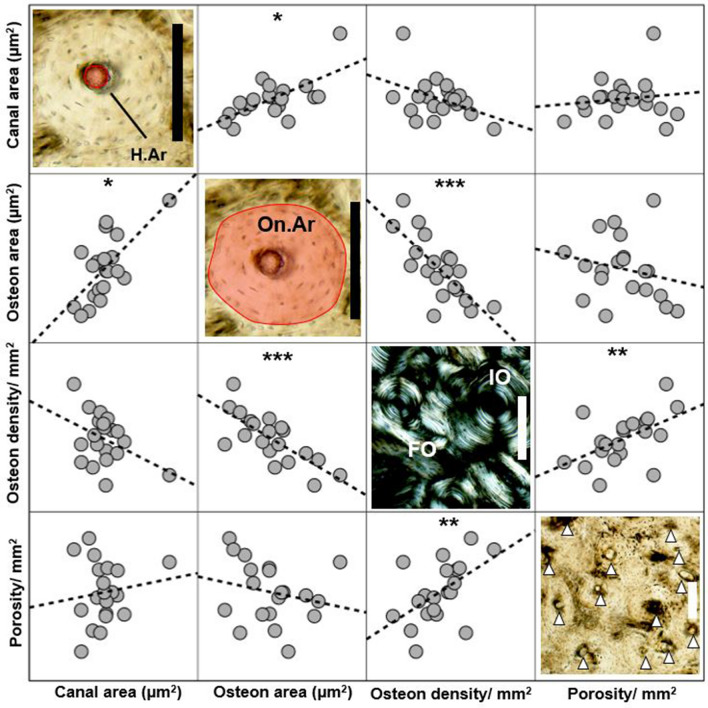
Prior to addressing the main questions of our study, we ran non-parametric Spearman’s Rho tests (due to sample size smaller than 30 in at least one sub-group that was being included in the correlations) correlating all the histology variables to check whether porosities, densities, and area measures increased or decreased in values when considered alongside each other. This step was necessary as we have different histology data from two different types of ROIs (the ‘strip’ and three localised ROIs within), so we wanted to check that each variable can be treated independently in our interpretation. Statistically significant relationships, and those of Rho > 0.35107, were taken to indicate that the variables reflected expected relationships such as allometry between osteon and canal area, and the density variables60. The histology correlations returned three statistically significant and strong relationships (Fig. 4; Supplementary Information Table 4) for the area of osteons and their Haversian canals (positive correlation), osteon area and population density (negative correlation), and vascular porosity and osteon population density (positive correlation). The area of osteons increased as the area of canals increased, higher osteon densities were associated with smaller osteons (which is expected for a strained posterior femur), and higher vascular porosities corresponded to higher osteon densities. This information means that despite collecting different histology data from different ROIs, all related to one another statistically allowing us to interpret them all in the comparisons with age and sex.
Figure 4.
Montage combining simple correlations between the key histomorphometric variables examined in this study. We do not show y and x axis values as this figure is intended as a simple illustrative overview of how well the variables agree with each other. *p < 0.05, **p < 0.01, ***p < 0.001 using Spearman’s Rho tests (see Supplementary Information Table 4).
Next, the V.Po and OPD variables were adjusted by the previously measured midshaft variables and calculated RIs to account for a possible isometric relationship between femur size and the underlying histological structures61. It is possible that larger femora could simply show higher values of canals and osteons as a result of inherent size variation across the sample. This was also important as previous research indicated that sexually dimorphic bones may still build bone tissue of similar quality108.
The H.Ar/On.Ar ratio variable did not require adjustments as it is in itself already a quantitative relation between two histology measures of size. The V.Po variable was adjusted by raw Circ and Ct.W (creating V.Po/Circ, and V.Po/Ct.W), whereas OPD was adjusted by robusticity index (Circ) and robusticity index (Ct.W) where the femoral maximum length was available for robusticity index calculations.
A brief descriptive analysis summarising data using mean, minimum, maximum, and standard deviation (SD) values was conducted in first instance. The quantitative variables in n > 30 (Circ, Ct.W, V.Po, V.Po/Ct.W, V.Po/Circ) were tested for normality using the Kolmogorov–Smirnov test. Parametric tests were then selected for normally distributed variables (Circ, Ct.W, V.Po, V.Po/Circ), and non-parametric tests were applied to V.Po/Ct.W where data were not normally distributed. For data in sub-groups of n < 30, non-parametric inferential tests were selected without normality tests given the sample size. As a result, Mann–Whitney U tests or t tests were applied when comparing bone macro- and microstructure between the sexes. When comparing the three age-at-death groups, we used a non-parametric Kruskal–Wallis test with a post-hoc pairwise comparison. For the gross femoral analyses we report significant results only, whereas for the histology analyses we show all results because they are interpreted to answer our research questions. We did not run statistical analyses on the OPD data, and age-at-death and sex sub-divisions due to inadequate sample sizes in the sub-groups.
Ethics approval
Approval to conduct this research was obtained from the Solomon Islands National Museum. The analysis and release of data were in consultation with, and co-authorship by, community representative (Lawrence Kiko), with whom also a report summarising the findings was filed prior to discussing publication. The thin sections will be repatriated to the Solomon Islands National Museum upon the completion of this project. All research followed ethical guidelines of the American Association of Biological Anthropologists and the Australasian Society for Human Biology.
Supplementary Information
Acknowledgements
We are indebted to the Solomon Islands National Museum for approval to conduct this research, support, consultation, and collaboration on the project. Research funding was from the Australian Research Council (DE190100068 to JJM). Travel to the Solomon Islands was funded by the Max Planck Institute for the Science of Human History (to RLK). The College of Arts and Social Sciences at the Australian National University funded microscopy equipment used in the preparation of the histology samples (to JJM). David McGregor offered technical assistance with microscopy. Ancient genetic data generation was funded by the European Research Council Starting Grant ‘Waves’ (ERC758967) awarded to AP. We thank Johannes Krause for his advice and for the use of the ancient DNA facilities of the archaeogenetic department at the Max Planck Institute for the Science of Human History. We thank Rita Radzeviciute for assistance with the aDNA lab processing.
Author contributions
J.J.M. led project and consultation, secured funding, carried out histology lab work, data analysis, wrote first draft of manuscript; H.B. supervised project, interpreted data; L.K. assisted with osteology, led research permissions and consultation, interpreted data; M.F. performed aDNA analysis; S.C. performed initial in-silico screening for aDNA; K.N. and E.B. assisted with aDNA lab work; N.R.D.G. and M.M.W. assisted with histology lab work; A.P. secured funding and coordinated the sample collection; C.P. supervised the aDNA data generation and aDNA analysis; R.L.K. supervised project, secured funding, conducted osteology, collected samples, interpreted data, organised research permissions and consultation. All edited the manuscript and gave approval for publication.
Data availability
Data are available open access from Figshare (Miszkiewicz et al. 202287) 10.6084/m9.figshare.16815295.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-23171-3.
References
- 1.Gordon CM, et al. The determinants of peak bone mass. J. Paediatr. 2017;180:261–269. doi: 10.1016/j.jpeds.2016.09.056. [DOI] [PubMed] [Google Scholar]
- 2.Heaney RP, et al. Peak bone mass. Osteoporos. Int. 2000;11:985. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- 3.Seeman E. Reduced bone density in women with fractures: Contribution of low peak bone density and rapid bone loss. Osteoporos. Int. 1994;4:S15–S25. doi: 10.1007/BF01623430. [DOI] [PubMed] [Google Scholar]
- 4.Kanis JA. Estrogens, the menopause, and osteoporosis. Bone. 1996;19:185S–190S. doi: 10.1016/s8756-3282(96)90163-5. [DOI] [PubMed] [Google Scholar]
- 5.Lindsay R. The menopause and osteoporosis. Obstet. Gynecol. 1996;87:16S–19S. doi: 10.1016/0029-7844(95)00430-0. [DOI] [PubMed] [Google Scholar]
- 6.Riggs BL. The mechanisms of estrogen regulation of bone resorption. J. Clin. Investig. 2000;106:1203–1204. doi: 10.1172/JCI11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu. Rev. Biomed. Eng. 2006;8:455–498. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal SC. Bone morphologies and histories: Life course approaches in bioarchaeology. Am. J. Phys. Anthropol. 2016;159:130–149. doi: 10.1002/ajpa.22905. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal SC, Grynpas MD. Bone quantity and quality in past populations. Anat. Rec. 1996;246:423–432. doi: 10.1002/(SICI)1097-0185(199612)246:4<423::AID-AR1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal SC, Stout SD. Bone Loss and Osteoporosis: An Anthropological Perspective. Springer; 2003. [Google Scholar]
- 11.Miszkiewicz JJ, Cooke KM. Socio-economic determinants of bone health from past to present. Clin. Rev. Bone Miner. Metab. 2019;17:109–122. [Google Scholar]
- 12.Miszkiewicz JJ, Brennan-Olsen S, Riancho JA. Bone Health: A Reflection of The Social Mosaic. Springer; 2019. [Google Scholar]
- 13.Miszkiewicz JJ, et al. Bone loss markers in the earliest Pacific Islanders. Sci. Rep. 2021;11:1–6. doi: 10.1038/s41598-021-83264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miszkiewicz JJ, Matisoo-Smith EA, Weisler MI. Behavior and intra-skeletal remodeling in an adult male from 1720 BP Ebon Atoll, Marshall Islands, eastern Micronesia. J. Isl. Coast. Archaeol. 2022;17:445–459. [Google Scholar]
- 15.Leach, F. & Davidson, J. The Archaeology on Taumako: A Polynesian Outlier in the Eastern Solomon Islands. Dunedin: N. Z. J. Archaeol. (2008).
- 16.Kinaston RL, Buckley HR. Isotopic insights into diet and health at the site of Namu, Taumako Island, Southeast Solomon Islands. Archaeol. Anthropol. Sci. 2017;9:1405–1420. [Google Scholar]
- 17.Kinaston RL, Buckley HR, Gray A. Diet and social status on Taumako, a Polynesian outlier in the Southeastern Solomon Islands. Am. J. Phys. Anthropol. 2013;151:589–603. doi: 10.1002/ajpa.22314. [DOI] [PubMed] [Google Scholar]
- 18.Gosling AL, Matisoo-Smith EA. The evolutionary history and human settlement of Australia and the Pacific. Curr. Opin. Genet. Dev. 2018;53:53–59. doi: 10.1016/j.gde.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Davidson JM. Polynesian outliers and the problem of cultural replacement in small populations. In: Green RC, Kelly M, editors. Studies in Oceanic Culture History Volume I, Pacific Anthropological Records 11. Bernice P. Bishop Museum; 1970. pp. 61–72. [Google Scholar]
- 20.Kramer RT, et al. Strontium (87Sr/86Sr) isotope analysis of the Namu skeletal assemblage: A study of past human migration on Taumako, a Polynesian Outlier in the eastern Solomon Islands. Am. J. Phys. Anthropol. 2021;174:479–499. doi: 10.1002/ajpa.24179. [DOI] [PubMed] [Google Scholar]
- 21.Gosling AL, Buckley HR, Matisoo-Smith E, Merriman TR. Pacific populations, metabolic disease and ‘Just-So Stories’: A critique of the ‘Thrifty Genotype’ hypothesis in Oceania. Ann. Hum. Genet. 2015;79:470–480. doi: 10.1111/ahg.12132. [DOI] [PubMed] [Google Scholar]
- 22.Tsuchiya C, Tagini S, Cafa D, Nakazawa M. Socio-environmental and behavioral risk factors associated with obesity in the capital (Honiara), the Solomon Islands; case-control study. Obes. Med. 2017;7:34–42. [Google Scholar]
- 23.Buckley HR. Health and Disease in the Prehistoric Pacific Islands. Oxford, UK: British Archaeological Reports International Series 2792. British Archaeological Reports Ltd; 2016. [Google Scholar]
- 24.Buckley HR, Tayles N. Skeletal pathology in a prehistoric Pacific Island sample: Issues in lesion recording, quantification, and interpretation. Am. J. Phys. Anthropol. 2003;122:303–324. doi: 10.1002/ajpa.10259. [DOI] [PubMed] [Google Scholar]
- 25.Cooke KM, et al. Paleohistopathology of treponemal disease in human bone from Taumako, Solomon Islands (700–300ybp) Am. J. Biol. Anthropol. 2022;177:36. [Google Scholar]
- 26.Stantis C, Buckley HR, Commendador A, Dudgeon JV. Expanding on incremental dentin methodology to investigate childhood and infant feeding practices on Taumako (southeast Solomon Islands) J. Archaeol. Sci. 2021;126:105294. [Google Scholar]
- 27.Ebeling PR, et al. Secondary prevention of fragility fractures in Asia Pacific: An educational initiative. Osteoporos. Int. 2020;31:805–826. doi: 10.1007/s00198-019-05197-y. [DOI] [PubMed] [Google Scholar]
- 28.Khosla S, Farr JN, Tchkonia T, Kirkland JL. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 2020;16:263–275. doi: 10.1038/s41574-020-0335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medina-Gomez C, et al. Life-course genome-wide association study meta-analysis of total body BMD and assessment of age-specific effects. Am. J. Hum. Genet. 2018;102:88–102. doi: 10.1016/j.ajhg.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gourlay ML, Hammett-Stabler CA, Renner JB, Rubin JE. Associations between body composition, hormonal and lifestyle factors, bone turnover, and BMD. J. Bone Metab. 2014;21:61–68. doi: 10.11005/jbm.2014.21.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bianchi ML, Sawyer AJ, Bachrach LK. Rationale for bone health assessment in childhood and adolescence. In: Fung E, Bachrach L, Sawyer A, editors. Bone Health Assessment in Paediatrics. Springer; 2016. pp. 1–21. [Google Scholar]
- 32.Link TM. Metabolic bone disease. In: Cassar-Pullicino VN, Davies AM, editors. Measurements in Musculoskeletal Radiology. Springer; 2020. pp. 785–807. [Google Scholar]
- 33.Agarwal SC. What is normal bone health? A bioarchaeological perspective on meaningful measures and interpretations of bone strength, loss, and aging. Am. J. Hum. Biol. 2021;33:e23647. doi: 10.1002/ajhb.23647. [DOI] [PubMed] [Google Scholar]
- 34.Miszkiewicz JJ, Mahoney P. Human bone and dental histology in an archaeological context. In: Errickson D, Thompson T, editors. Human Remains: Another Dimension. The Application of Imaging to the Study of Human Remains. Elsevier Academic Press; 2017. pp. 29–43. [Google Scholar]
- 35.Crowder C, Stout SD. Bone Histology: An Anthropological Perspective. CRC Press; 2011. [Google Scholar]
- 36.Lassen NE, et al. Coupling of bone resorption and formation in real time: New knowledge gained from human Haversian BMUs. J. Bone Miner. Res. 2017;32:1395–1405. doi: 10.1002/jbmr.3091. [DOI] [PubMed] [Google Scholar]
- 37.Britz HM, Thomas CDL, Clement JG, Cooper DM. The relation of femoral osteon geometry to age, sex, height and weight. Bone. 2009;45:77–83. doi: 10.1016/j.bone.2009.03.654. [DOI] [PubMed] [Google Scholar]
- 38.Miszkiewicz JJ. Investigating histomorphometric relationships at the human femoral midshaft in a biomechanical context. J. Bone Miner. Metab. 2016;34:179–192. doi: 10.1007/s00774-015-0652-8. [DOI] [PubMed] [Google Scholar]
- 39.van Oers RF, Ruimerman R, van Rietbergen B, Hilbers PA, Huiskes R. Relating osteon diameter to strain. Bone. 2008;43:476–482. doi: 10.1016/j.bone.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Stout SD, Crowder C. Bone remodeling, histomorphology, and histomorphometry. In: Crowder C, Stout SD, editors. Bone Histology: An Anthropological Perspective. CRC Press; 2011. pp. 1–21. [Google Scholar]
- 41.Cardoso L, Fritton SP, Gailani G, Benalla M, Cowin SC. Advances in assessment of bone porosity, permeability and interstitial fluid flow. J. Biomech. 2013;46:253–265. doi: 10.1016/j.jbiomech.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooper DML, Kawalilak CE, Harrison K, Johnston BD, Johnston JD. Cortical bone porosity: What is it, why is it important, and how can we detect it? Curr. Osteoporos. Rep. 2016;14:187–198. doi: 10.1007/s11914-016-0319-y. [DOI] [PubMed] [Google Scholar]
- 43.Mulhern DM, Van Gerven DP. Patterns of femoral bone remodeling dynamics in a medieval Nubian population. Am. J. Phys. Anthropol. 1997;104:133–146. doi: 10.1002/(SICI)1096-8644(199709)104:1<133::AID-AJPA9>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 44.Robling AG, Stout SD. Histomorphology, geometry, and mechanical loading in past populations. In: Agarwal SC, Stout SD, editors. Bone Loss and Osteoporosis: An Anthropological Perspective. Springer; 2003. pp. 189–205. [Google Scholar]
- 45.Miszkiewicz JJ, Mahoney P. Ancient human bone microstructure in medieval England: Comparisons between two socio-economic groups. Anat. Rec. 2016;299:42–59. doi: 10.1002/ar.23285. [DOI] [PubMed] [Google Scholar]
- 46.Pfeiffer S. Variability in osteon size in recent human populations. Am. J. Phys. Anthropol. 1998;106:219–227. doi: 10.1002/(SICI)1096-8644(199806)106:2<219::AID-AJPA8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 47.Pfeiffer S, Crowder C, Harrington L, Brown M. Secondary osteon and Haversian canal dimensions as behavioral indicators. Am. J. Phys. Anthropol. 2006;131:460–468. doi: 10.1002/ajpa.20454. [DOI] [PubMed] [Google Scholar]
- 48.Streeter M, Stout S, Trinkaus E, Burr D. Bone remodeling rates in Pleistocene humans are not slower than the rates observed in modern populations: A reexamination of Abbott et al. (1996) Am. J. Phys. Anthropol. 2010;141:315–318. doi: 10.1002/ajpa.21192. [DOI] [PubMed] [Google Scholar]
- 49.Abbott S, Trinkaus E, Burr DB. Dynamic bone remodeling in later Pleistocene fossil hominids. Am. J. Phys. Anthropol. 1996;99:585–601. doi: 10.1002/(SICI)1096-8644(199604)99:4<585::AID-AJPA5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 50.Buckley H. Epidemiology of gout: Perspectives from the past. Curr. Rheumatol. Rev. 2011;7:106–113. [Google Scholar]
- 51.Buckley HR, Oxenham M. Bioarchaeology in the Pacific Islands: A temporal and geographical examination of nutritional and infectious disease. In: Oxenham M, Buckley H, editors. The Routledge Handbook of Bioarchaeology in Southeast Asia and the Pacific Islands. Routledge; 2016. pp. 363–388. [Google Scholar]
- 52.Foster A, et al. Possible diffuse idiopathic skeletal hyperostosis (DISH) in a 3000-year-old Pacific Island skeletal assemblage. J. Archaeol. Sci. Rep. 2018;18:408–419. [Google Scholar]
- 53.Buckley HR, et al. Scurvy in a tropical paradise? Evaluating the possibility of infant and adult vitamin C deficiency in the Lapita skeletal sample of Teouma, Vanuatu, Pacific islands. Int. J. Palaeopathol. 2014;5:72–85. doi: 10.1016/j.ijpp.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Robb KF, Buckley HR, Spriggs M, Bedford S. Cortical index of three prehistoric human Pacific Island samples. Int. J. Osteoarchaeol. 2012;22:284–293. [Google Scholar]
- 55.Stock JT, Shaw CN. Which measures of diaphyseal robusticity are robust? A comparison of external methods of quantifying the strength of long bone diaphyses to cross-sectional geometric properties. Am. J. Phys. Anthropol. 2007;134:412–423. doi: 10.1002/ajpa.20686. [DOI] [PubMed] [Google Scholar]
- 56.Cooke KM, Mahoney P, Miszkiewicz JJ. Secondary osteon variants and remodeling in human bone. Anat. Rec. 2022;305:1299–1315. doi: 10.1002/ar.24646. [DOI] [PubMed] [Google Scholar]
- 57.Buikstra JE, Ubelaker DH. Standards for Data Collection from Human Skeletal Remains. Colorado Historical Society; 1994. [Google Scholar]
- 58.Brickley M, McKinley J. Guidance to Standards for Recording Human Skeletal Remains. IFA Technical Paper 7. IFA; 2004. [Google Scholar]
- 59.DuBois LZ, Shattuck-Heidorn H. Challenging the binary: Gender/sex and the bio-logics of normalcy. Am. J. Hum. Biol. 2021;33:e23623. doi: 10.1002/ajhb.23623. [DOI] [PubMed] [Google Scholar]
- 60.Miszkiewicz JJ, Mahoney P. Histomorphometry and cortical robusticity of the adult human femur. J. Bone Miner. Metab. 2019;37:90–104. doi: 10.1007/s00774-017-0899-3. [DOI] [PubMed] [Google Scholar]
- 61.Goldman HM, Hampson NA, Guth JJ, Lin D, Jepsen KJ. Intracortical remodeling parameters are associated with measures of bone robustness. Anat. Rec. 2014;297:1817–1828. doi: 10.1002/ar.22962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burr DB, Ruff CB, Thompson DD. Patterns of skeletal histologic change through time: Comparison of an archaic Native American population with modern populations. Anat. Rec. 1990;226:307–313. doi: 10.1002/ar.1092260306. [DOI] [PubMed] [Google Scholar]
- 63.Thompson DD, Salter EM, Laughlin WS. Bone core analysis of Baffin Island skeletons. Arc. Anthropol. 1981;18:87–96. [Google Scholar]
- 64.Miszkiewicz JJ, Stewart TJ, Deter CA, Fahy GE, Mahoney P. Skeletal health in medieval societies: Insights from ancient bone collagen stable isotopes and dental histology. In: Miszkiewicz JJ, Brennan-Olsen S, Riancho JA, editors. Bone Health: A Reflection of the Social Mosaic. Springer Nature; 2019. pp. 17–34. [Google Scholar]
- 65.Bachrach LK. Skeletal Development in Childhood and Adolescence. Primer on the Metabolic Bone Diseases and Disorders of Bone Metabolism. Wiley; 2009. pp. 74–79. [Google Scholar]
- 66.Klein SL. The effects of hormones on sex differences in infection: From genes to behavior. Neurosci. Biobehav. Rev. 2000;24:627–638. doi: 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- 67.Stinson S. Sex differences in environmental sensitivity during growth and development. Am. J. Phys. Anthropol. 1985;28:123–147. [Google Scholar]
- 68.Furusawa T, Aswani S. Well-nourished women in a Solomon Islands society with a biased sex ratio. Pac. Health Dialog. 2011;17:77–81. [PubMed] [Google Scholar]
- 69.Kinaston R. Prehistoric Diet and Health in the Western Pacific Islands, PhD Thesis. University of Otago; 2010. [Google Scholar]
- 70.Ross RD, Sumner DR. Bone matrix maturation in a rat model of intra-cortical bone remodeling. Calcif. Tissue Int. 2017;101:193–203. doi: 10.1007/s00223-017-0270-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ruth EB. Bone studies. II. An experimental study of the Haversian-type vascular channels. Am. J. Anat. 1953;93:429–455. doi: 10.1002/aja.1000930306. [DOI] [PubMed] [Google Scholar]
- 72.Wang X, Ni Q. Determination of cortical bone porosity and pore size distribution using a low field pulsed NMR approach. J. Orthop. Res. 2003;21:312–319. doi: 10.1016/S0736-0266(02)00157-2. [DOI] [PubMed] [Google Scholar]
- 73.Currey JD. Some effects of ageing in human Haversian systems. J. Anat. 1964;98:69. [PMC free article] [PubMed] [Google Scholar]
- 74.Frost HM. Secondary osteon population densities: An algorithm for estimating the missing osteons. Am. J. Phys. Anthropol. 1987;30:239–254. [Google Scholar]
- 75.Zebaze RM, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: A cross-sectional study. Lancet. 2010;375:1729–1736. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]
- 76.Cave C, Oxenham M. Identification of the archaeological ‘invisible elderly’: An approach illustrated with an Anglo-Saxon example. Int. J. Osteoarchaeol. 2016;26:163–175. [Google Scholar]
- 77.McFadden C, Cave CM, Oxenham MF. Ageing the elderly: A new approach to the estimation of the age-at-death distribution from skeletal remains. Int. J. Osteoarchaeol. 2019;29:1072–1078. [Google Scholar]
- 78.Gowland RL. Elder abuse: Evaluating the potentials and problems of diagnosis in the archaeological record. Int. J. Osteoarchaeol. 2016;26:514–523. [Google Scholar]
- 79.Pearson OM, Lieberman DE. The aging of Wolff's “law”: Ontogeny and responses to mechanical loading in cortical bone. Am. J. Phys. Anthropol. 2004;125:63–99. doi: 10.1002/ajpa.20155. [DOI] [PubMed] [Google Scholar]
- 80.Davidson J. Cultural replacement on small islands: New evidence from polynesian outliers. Mankind. 1974;9:273–277. [Google Scholar]
- 81.Kirch PV. The Polynesian outliers: Continuity, change, and replacement. J. Pac. Hist. 1984;19:224–238. [Google Scholar]
- 82.Leach F, Davidson J, Davenport W. Social organization notes on the northern Santa Cruz Islands: The Duff Islands (Taumako) Baessler-Archiv. Neue. Folge. 1968;16:137–205. [Google Scholar]
- 83.Leach H. Did East Polynesians have a concept of luxury foods? World Archaeol. 2003;34:442–457. [Google Scholar]
- 84.Fyfe DM, Chandler NP, Wilson NHF. Alveolar bone status of some pre-seventeenth century inhabitants of Taumako, Solomon Islands. Int. J. Osteoarchaeol. 1993;3:29–35. [Google Scholar]
- 85.Scott RM, Buckley HR. Biocultural interpretations of trauma in two prehistoric Pacific Island populations from Papua New Guinea and the Solomon Islands. Am. J. Phys. Anthropol. 2010;142:509–518. doi: 10.1002/ajpa.21250. [DOI] [PubMed] [Google Scholar]
- 86.Pinhasi RD, et al. Optimal ancient DNA yields from the inner ear part of the human petrous bone. PLoS One. 2015;10:e0129102. doi: 10.1371/journal.pone.0129102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miszkiewicz, J. J. et al. Data for Taumako sample: Femur histology, femur morphometry, genetic sex. Figshare dataset: 10.6084/m9.figshare.16815295 (2022).
- 88.Dabney J, et al. Complete mitochondrial genome sequence of a Middle Pleistocene cave bear reconstructed from ultrashort DNA fragments. Proc. Natl. Acad. Sci. U.S.A. 2013;110:15758–15763. doi: 10.1073/pnas.1314445110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meyer M, Kircher M. Illumina sequencing library preparation for highly multiplexed target capture and sequencing. Cold Spring Harb. Protoc. 2010;2010:pdb.prot5448. doi: 10.1101/pdb.prot5448. [DOI] [PubMed] [Google Scholar]
- 90.Kircher M, Sawyer S, Meyer M. Double indexing overcomes inaccuracies in multiplex sequencing on the Illumina platform. Nucleic Acids Res. 2012;40:e3. doi: 10.1093/nar/gkr771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rohland N, Harney E, Mallick S, Nordenfelt S, Reich D. Partial uracil–DNA–glycosylase treatment for screening of ancient DNA. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015;370:20130624. doi: 10.1098/rstb.2013.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peltzer A, et al. EAGER: Efficient ancient genome reconstruction. Genome Biol. 2016;17:60. doi: 10.1186/s13059-016-0918-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jun G, Wing MK, Abecasis GR, Kang HM. An efficient and scalable analysis framework for variant extraction and refinement from population-scale DNA sequence data. Genome Res. 2015;25:918–925. doi: 10.1101/gr.176552.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fu Q, et al. DNA analysis of an early modern human from Tianyuan Cave, China. Proc. Natl. Acad. Sci. U.S.A. 2013;110:2223–2227. doi: 10.1073/pnas.1221359110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haak W, et al. Massive migration from the steppe was a source for Indo-European languages in Europe. Nature. 2015;522:207–211. doi: 10.1038/nature14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mathieson I, et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature. 2015;528:499–503. doi: 10.1038/nature16152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fu Q, et al. The genetic history of ice age Europe. Nature. 2016;534:200–205. doi: 10.1038/nature17993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mittnik A, Wang CC, Svoboda J, Krause J. A molecular approach to the sexing of the triple burial at the Upper Paleolithic Site of Dolní Věstonice. PLoS One. 2016;11:e0163019. doi: 10.1371/journal.pone.0163019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bell KL, et al. Super-osteons (remodeling clusters) in the cortex of the femoral shaft: Influence of age and gender. Anat. Rec. 2001;264:378–386. doi: 10.1002/ar.10014. [DOI] [PubMed] [Google Scholar]
- 100.Chan AH, Crowder CM, Rogers TL. Variation in cortical bone histology within the human femur and its impact on estimating age at death. Am. J. Phys. Anthropol. 2007;132:80–88. doi: 10.1002/ajpa.20465. [DOI] [PubMed] [Google Scholar]
- 101.Mays S, Elders J, Humphrey L, White W, Marshall P. Science and the Dead: A Guideline for the Destructive Sampling of Archaeological Human Remains for Scientific Analysis. English Heritage Publishing with the Advisory Panel on the Archaeology of Burials in England; 2013. [Google Scholar]
- 102.Richman EA, Ortner DJ, Schulter-Ellis FP. Differences in intracortical bone remodeling in three aboriginal American populations: Possible dietary factors. Calcif. Tissue Int. 1979;28:209–214. doi: 10.1007/BF02441238. [DOI] [PubMed] [Google Scholar]
- 103.Dempster DW, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013;28:2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ciani C, Doty SB, Fritton SP. An effective histological staining process to visualize bone interstitial fluid space using confocal microscopy. Bone. 2009;44:1015–1017. doi: 10.1016/j.bone.2009.01.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bell KL, et al. Regional differences in cortical porosity in the fractured femoral neck. Bone. 1999;24:57–64. doi: 10.1016/s8756-3282(98)00143-4. [DOI] [PubMed] [Google Scholar]
- 106.Schlecht SH, Pinto DC, Agnew AM, Stout SD. The effects of disuse on the mechanical properties of bone: What unloading tells us about the adaptive nature of skeletal tissue. Am. J. Phys. Anthropol. 2012;149:599–605. doi: 10.1002/ajpa.22150. [DOI] [PubMed] [Google Scholar]
- 107.Taylor R. Interpretation of the correlation coefficient: A basic review. J. Diagn. Med. Sonogr. 1990;6:35–39. [Google Scholar]
- 108.Tommasini SM, Nasser P, Jepsen KJ. Sexual dimorphism affects tibia size and shape but not tissue-level mechanical properties. Bone. 2007;40:498–505. doi: 10.1016/j.bone.2006.08.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available open access from Figshare (Miszkiewicz et al. 202287) 10.6084/m9.figshare.16815295.