Abstract
The mechanism of human T-lymphocyte activation by the pathogenic yeast Cryptococcus neoformans has not been established. Previous investigations have suggested that C. neoformans contains a mitogen for T lymphocytes, while other investigators have attributed lymphocyte proliferation in vitro to a recall antigen. Because of the potential importance of the mechanism of T-cell activation for our understanding of the immune response to C. neoformans, the present studies were performed to determine whether C. neoformans contains a mitogen for T lymphocytes. C. neoformans stimulates fetal blood lymphocytes to proliferate and stimulates proliferation of CD45RA+ cells from adults, indicating that it stimulates naive T cells. The T-cell response to C. neoformans was dependent upon the presence of accessory cells. However, allogeneic cells were sufficient for accessory cell function, indicating that the response was not major histocompatibility complex restricted. The percentage of T cells in the cell cycle was higher than that with the recall antigen tetanus toxoid but lower than that with the mitogenic lectin phytohemagglutinin A or the superantigen Staphylococcus enterotoxin B. Precursor frequency analysis established that 1 in 7,750 ± 2,270 T cells proliferated in response to the cryptococcal cell wall and membrane. Compared to the case for most mitogens or superantigens, the proliferative response is late and the number of T cells that enter the cell cycle and the precursor frequency are low, indicating that the mitogenic effect is modest. However, the mitogenic effect of C. neoformans should be considered when interpreting the immune response to C. neoformans, since even weak mitogens can have profound effects on host defense.
Cryptococcus neoformans is a yeast that causes one of the most common invasive and fatal fungal infections in AIDS patients. Despite the importance of this pathogen, our knowledge of the immune response to C. neoformans is incomplete. Since AIDS patients have defective T-cell function, it appears that T lymphocytes may play an essential role in the host defense against this pathogen. Studies of T cells in mice have suggested that clonal expansion of lymphocytes affords protection (1, 5, 9, 10), and by correlation, antigen-driven clonal expansion may be protective in humans. However, it has been proposed that T cells are activated by additional mechanisms in response to C. neoformans.
Previous studies have determined that lymphocytes from almost all healthy adults proliferate in response to C. neoformans (8, 15, 18, 23). This suggests one of two possibilities. First, it is possible that the majority of adults have undergone natural immunization against C. neoformans. However, infections of nonimmunocompromised adults with C. neoformans are rare (4), and infection is often required for development of immunity to microbial pathogens. Further, fetal umbilical cord blood lymphocytes proliferate in response to the cell wall of C. neoformans (16), and since the fetus is unlikely to have been exposed to C. neoformans, this has led to the suggestion that C. neoformans is capable of stimulating an innate T-lymphocyte response as a mitogen (8, 16). This would have important implications for our understanding of the host defense against C. neoformans, since mitogens have profound effects on T-cell responses.
To determine whether lymphocyte proliferation in response to C. neoformans was due to a mitogen, proliferation of naive lymphocytes obtained from fetal blood or of CD45RA-enriched cells in response to C. neoformans and cryptococcal cell wall and membrane (CCW/M) was examined. The requirement for accessory cells (AC) was determined, and major histocompatibility complex (MHC) restriction was assessed by lymphocyte proliferation with allogeneic or autologous AC. Finally, the percentage of T cells in the cell cycle was assessed by flow cytometric analysis of DNA content, and the precursor frequency of proliferating lymphocytes was determined by limiting-dilution analysis.
MATERIALS AND METHODS
Preparation of C. neoformans and CCW/M.
C. neoformans CAP 67 (ATCC 52817, acapsular mutant) was obtained from the American Type Culture Collection (Rockville, Md.). The organisms were maintained on Sabouraud dextrose slants (Difco, Detroit, Mich.) and passaged to fresh slants every month as previously described (19).
CCW/M was prepared as previously described (16). Briefly, strain CAP 67 was grown for 96 h, autoclaved, washed three times, and resuspended at 5 × 108 organisms/ml in phosphate-buffered saline. C. neoformans was disrupted by rotating the organisms with 0.5-mm-diameter glass beads in a bead mill for 30 cycles of 1 min on and 1 min off. These disrupted organisms were centrifuged at 200 × g for 5 min to remove the larger particles. The supernatant was removed and stored at −70°C until it was used. The protein concentration was determined by the bicinchoninic acid protein assay (Pierce, Rockford, Ill.). Bovine serum albumin was used to prepare the standard curve.
Isolation of mononuclear cells.
Peripheral blood was obtained by venipuncture from healthy adults. Blood was anticoagulated by adding heparin (10 U/ml) (Organon Teknika-Cappel, Scarborough, Ontario, Canada). Fetal umbilical cord blood was collected in 8-ml heparinized tubes (Becton Dickinson, Rutherford, N.J.). Peripheral blood mononuclear cells (PBMC) or fetal blood mononuclear cells (FBMC) were isolated by centrifugation (800 × g, 20 min) over a Ficoll-Hypaque density gradient (C-six Diagnostics Inc., Mequon, Wis.). PBMC or FBMC were harvested and washed three times in Hanks’ balanced salt solution (Gibco, Burlington, Ontario, Canada) and then resuspended in medium containing RPMI 1640 (Gibco), 5% human AB serum (BioWhittaker, Walkersville, Md.), and 100 U of penicillin per ml, 100 μg of streptomycin per ml, 0.25 μg of amphotericin B per ml, 2 mM l-glutamine, 1 mM sodium pyruvate, and 0.1 mM nonessential amino acids (all from Gibco). Viable cells were then counted by trypan blue exclusion as visualized by light microscopy.
To isolate T lymphocytes, PBMC were allowed to adhere to plastic petri plates (Corning Glass Works, Corning, N.Y.) for 1 h at 37°C. The nonadherent cells were collected and rosetted to 2-aminoethylisothiouronium bromide (AET)-treated sheep erythrocytes (SRBC) (Cedarlane, Hornby, Ontario, Canada) as previously described (22, 24) with minor modifications. Briefly, 20 ml of AET solution was added to 5 ml of washed SRBC and left for 15 min at 37°C. A 5% solution of AET-treated SRBC was added to nonadherent cells (<10 × 106 cells/ml) at a 1:2 ratio. The suspension was incubated at 37°C for 10 min, centrifuged (500 × g, 10 min), and refrigerated overnight. The cells that were adherent to SRBC were collected by gradient centrifugation, and the SRBC were lysed with NH4Cl buffer. The cells were incubated in a nylon wool column, and nonadherent cells were collected. These cells were >95% CD3+. Cells that were adherent to plastic after a 1-h incubation were irradiated (3,000 rads) and used as a source of AC.
To obtain CD45RA- and CD45RO-enriched cells, nonadherent cells were depleted of CD45RO or CD45RA cells by immunomagnetic separation. Briefly, nonadherent cells were incubated with either anti-CD45RA (L48) or anti-CD45RO (UCHL-1) antibody (Becton Dickinson) for 30 min at 4°C under gentle agitation. The cells were washed three times in phosphate-buffered saline containing 2% fetal calf serum (Gibco). M-450-conjugated goat antimouse antibody (Dynabeads; Dynal, Oslo, Norway) was added at a bead-to-target cell ratio of 3:1 and left for 30 min at 4°C under gentle agitation. The labeled cells were removed by using a magnet (Dynal). The enriched populations contained <3% of the cells of the reciprocal subset. Irradiated adherent cells (3,000 rads; 105) were added to the enriched cells as a source of AC.
Lymphocyte proliferation assays.
C. neoformans (2 × 105 organisms/well) or CCW/M (1.5 μg/ml) was added to 2 × 105 PBMC or FBMC and incubated for 7 days in 96-well round-bottom plates (Falcon; Becton Dickinson). Eighteen hours before the end of the incubation, 1 μCi of [3H]thymidine ([3H]TdR) (ICN, Montreal, Quebec, Canada) was added. Cells were harvested on glass filters, and counts per minute were determined with a liquid scintillation counter. The bacterial mitogens staphylococcal enterotoxin B (SEB) and toxic shock syndrome toxin 1 (Toxin Technologies, Sarasota, Fla.) were used at 1 μg/ml, and the mitogenic lectin phytohemagglutinin A (PHA) (Sigma, St. Louis, Mo.) was used at 10 μg/ml. Tetanus toxoid (10−2 Lf units) (Connaught, Willowdale, Ontario, Canada) was used as a recall antigen.
Preparation of T lymphocytes and AC to determine MHC restriction.
PBMC were stimulated with CCW/M (1.5 μg/ml) for 10 days. On day 10, the T lymphocytes were enriched by depletion of CD14+ and CD19+ cells by immunomagnetic depletion. Briefly, M-450-conjugated mouse anti-human CD14 or CD19 antibody (Dynabeads; Dynal) was added at a bead-to-target cell ratio of 3:1 and left for 30 min at 4°C under gentle agitation. The labeled cells were removed by using a magnet (Dynal). The depleted populations contained <2% of the contaminating cells. The T cells were allowed to rest for 4 or 5 days (2 × 105/well) and then restimulated with CCW/M (1.5 μg/ml) in the presence of freshly isolated, irradiated (3,000 rads) allogeneic or autologous PBMC (1 × 105/well) as a source of AC.
HLA typing was performed to ensure that the donors were allogeneic. Three different donor combinations were used. The first combination of donors that was used in experiments was DRB1 1104/0701–DRB3 0202–DRB4 01XX–DQB1 0301/0303 and DRB1 0301/0801–DRB3 0101–DQB1 0201/0402. The second combination of donors used was DRB1 1501/1101–DRB3 0202–DRB4 0101–DQB1 0602/0301 and DRB1 0301/0801–DRB3 0101–DQB1 0201/0401, and the third combination of donors used was DRB1 1104/0701–DRB3 0202–DRB4 01XX–DQB1 0301/0303 and DRB1 1502/0406–DRB4 01XX–DRB5 0101–DQB1 0502/0302.
Analysis of the percentage of T cells in the cell cycle.
For analysis of the percentage of T cells in the cell cycle, PBMC were stimulated with PHA, SEB, tetanus toxoid, C. neoformans, or CCW/M. At the optimal time determined by [3H]TdR incorporation, cells were harvested and labeled with fluorescein isothiocyanate-conjugated anti-CD3. Cells were treated with 75% ethanol for 1 h. RNase (1 mg/ml; Sigma) was added, immediately followed by propidium iodide (5 μg/ml; Sigma). Cells were then analyzed for DNA content and expression of surface phenotype by flow cytometry with CellQuest software (Becton Dickinson).
Precursor frequency.
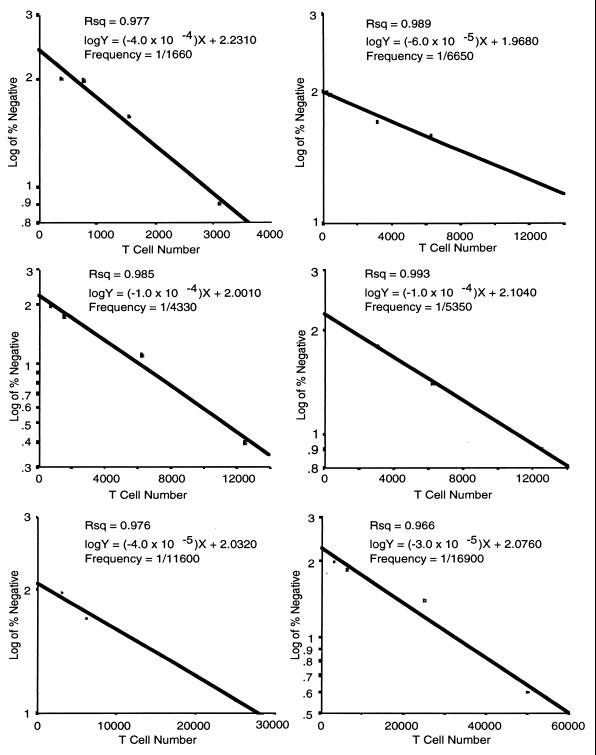
The precursor frequency of responding lymphocytes was determined as previously described (6, 7, 25). Briefly, T cells were enriched by nonadherence to plastic and passaged through a nylon wool column. Progressively fewer T cells were stimulated with CCW/M (1.5 μg/ml) in the presence of irradiated PBMC (3,000 rads; 105/well) as a source of AC. Twenty-four stimulated and unstimulated wells were used at each cell concentration. The mean counts per minute ± standard deviation was calculated for the unstimulated wells. Each stimulated well was scored as positive if its result was greater than three standard deviations above the mean for the unstimulated wells and negative if its result was less than three standard deviations above the mean for the unstimulated wells. The number of cells in the well was plotted against the log of the fraction of negative wells in each group, and a regression line was calculated. The precursor frequency was determined by Poisson’s formula where 37% of the wells were negative.
Statistics.
Values are expressed as means ± standard errors of the means (SEMs). Each experiment was performed with different donors on different days. Statistical analysis was performed by using the Fisher least-squares difference, when allowed by the F value (analysis of variance) (Statview 512+; Brain Power Inc., Calabasas, Calif.). For these tests, a P of <0.05 was considered significant.
RESULTS
Fetal lymphocyte proliferation in response to C. neoformans.
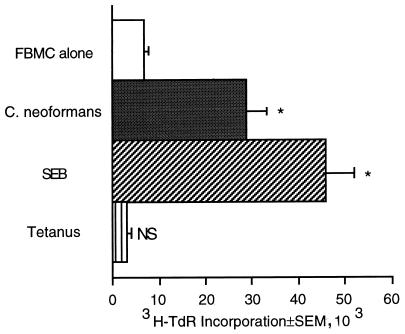
We have previously shown that FBMC proliferate in response to CCW/M (16), suggesting that CCW/M has mitogenic properties. Experiments were performed to determine whether fetal lymphocytes would also respond to whole cryptococcal organisms. FBMC proliferated in response to C. neoformans CAP 67 (Fig. 1). As a control, FBMC were stimulated with the bacterial mitogen SEB to confirm that they were capable of proliferating. FBMC did not proliferate in response to tetanus toxoid, which is a recall antigen requiring prior immunization. Thus, both whole organisms and the cell wall of C. neoformans stimulated fetal lymphocytes to proliferate, suggesting that C. neoformans contains a mitogen.
FIG. 1.
FBMC proliferate in response to C. neoformans. FBMC (2 × 105/well) were cultured for 7 days with medium alone, C. neoformans (2 × 105/well), SEB (1 μg/ml), or tetanus toxoid (1:10 Lf). This experiment was repeated four times with similar, statistically significant results. ∗, P < 0.05 compared to the value for FBMC alone; NS, not significant compared to FBMC alone.
Naive and memory T cells proliferate in response to C. neoformans and CCW/M.
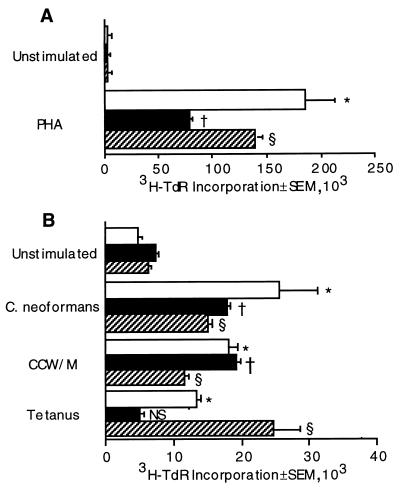
Prior to antigen stimulation and clonal expansion, T cells express the RA isoform of the CD45 molecule. Following antigenic stimulation and clonal expansion, T cells express the RO isoform of the CD45 molecule (21). To determine which subset of cells is capable of proliferating in response to C. neoformans or CCW/M, PBMC were depleted of either CD45RA or CD45RO cells. The CD45RA- and CD45RO-enriched cells both proliferated in response to C. neoformans or CCW/M (Fig. 2B). The recall antigen tetanus toxoid was capable of inducing only CD45RO cells to proliferate, while PHA, a T-cell mitogen, and SEB, a superantigen, caused substantial proliferation in both the CD45RA and CD45RO populations (Fig. 2).
FIG. 2.
Naive (CD45RA+) and memory (CD45RO+) T cells respond to C. neoformans and CCW/M. Unseparated PBMC (□) or CD45RA (■)- or CD45RO (▨)-enriched cells were stimulated for 3 days (A) or 7 days (B). Irradiated AC (105/well) were added to CD45RA- and CD45RO-enriched cells. The cells were stimulated with PHA (10 μg/ml), whole C. neoformans (2 × 105/well), CCW/M (1.5 μg/ml), or tetanus toxoid. This experiment was repeated three times with similar, statistically significant results. ∗, P < 0.05 compared to the value for unstimulated PBMC; †, P < 0.05 compared to the value for unstimulated CD45RA-enriched cells; §, P < 0.01 compared to the value for unstimulated CD45RO-enriched cells; NS, not significant compared to the value for unstimulated CD45RA-enriched cells.
Requirement for AC.
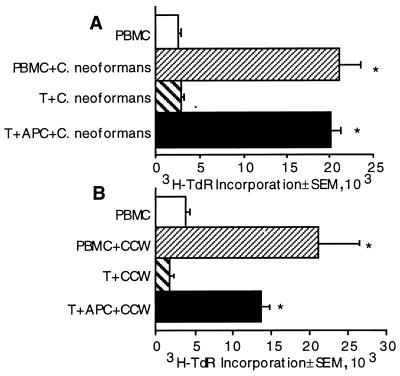
It was possible that C. neoformans contains a peptide mimic for a T-cell growth factor. If this were the case, T-cell proliferation would be independent of AC. By contrast, both mitogens and recall antigens require AC signals to stimulate T lymphocytes to proliferate. To determine whether the T-cell response to C. neoformans and CCW/M requires AC, purified T cells were stimulated with C. neoformans and CCW/M in the presence or absence of AC. T cells were incapable of responding to C. neoformans or CCW/M in the absence of AC (Fig. 3) but were capable of significant proliferation following the addition of irradiated loosely adherent cells as a source of AC. Thus, T cells require AC for presentation of C. neoformans.
FIG. 3.
T cells require AC to proliferate in response to C. neoformans (A) and CCW/M (B). PBMC or T cells with or without AC were cultured with medium, C. neoformans (2 × 105/well), or CCW/M (1.5 μg/ml) for 7 days. The experiment was repeated three times for C. neoformans and CCW/M with similar, statistically significant results. ∗, P < 0.05 compared to the value for the corresponding unstimulated group.
T-lymphocyte proliferation in response to CCW/M is not MHC restricted.
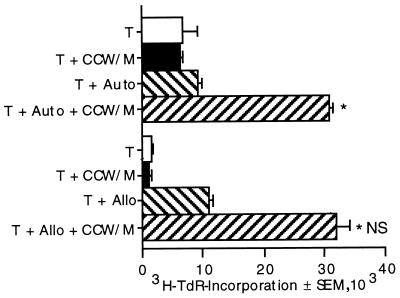
Having established that T-lymphocyte proliferation in response to C. neoformans was AC dependent, we performed experiments to determine whether the response was MHC restricted. T cells are capable of responding to recall antigens when presented by autologous AC but not when presented by allogeneic AC. By contrast, allogeneic cells are sufficient to provide the accessory requirements for mitogens. The primary response to CCW/M or C. neoformans peaks after 7 days in culture, and because of the mixed lymphocyte reaction to allogeneic AC, we were unable to detect lymphocyte proliferation when allogeneic cells were used as the source of AC. To circumvent this problem, the responding T cells were expanded prior to restimulation, which peaks on day 4 to 5 and would allow the response to be detected prior to the mixed lymphocyte reaction. The peak response to autologous AC occurred on day 4 (Fig. 4). The peak response to allogeneic cells occurred on day 5. A small mixed lymphocyte reaction occurred with allogeneic cells, but the response to CCW/M was easily detectable. Thus, there was significant T-lymphocyte proliferation in the presence of allogeneic cells, indicating that the T-cell response to C. neoformans was not MHC restricted.
FIG. 4.
Autologous or allogeneic cells can provide the AC for lymphocyte proliferation to CCW/M. Responding T cells were expanded by stimulating them with CCW/M for 10 days. The CD14 and CD19 cells were depleted to enrich the T-cell population prior to restimulation with CCW/M and autologous (Auto) or allogeneic (Allo) AC for 4 or 5 days, respectively. The experiment was repeated twice with similar, statistically significant results. ∗, P < 0.05 compared to the value for corresponding unstimulated T cells and AC; NS, not significant compared to the value for T cells plus autologous AC plus CCW/M.
Percentage of T cells that are activated and enter the cell cycle in response to C. neoformans and CCW/M.
The percentage of T cells that proliferate in response to mitogens is usually greater than the percentage of T cells that proliferate in response to recall antigens, and the number of responding T cells is a major determinant of immunomodulatory effects. To determine the percentage of cells that entered S phase in response to C. neoformans and CCW/M, DNA labeling and cell cycle analysis were performed after stimulation. The percentages of T cells in the cell cycle after stimulation with C. neoformans and CCW/M were greater than those after stimulation with a recall antigen (Table 1). However, the responses were less than those seen with a mitogen and a superantigen.
TABLE 1.
Percentages of T cells in S and G2/M phases of the cell cycle in response to C. neoformans and CCW/Ma
| Stimulus | % of cells in S + G2/Mb
|
|
|---|---|---|
| Unstimulated | Stimulated | |
| PHA | 1.0 ± 0.3 | 42.7 ± 1.5c |
| SEB | 0.5 ± 0.3 | 34.1 ± 4.4c |
| Tetanus toxoid | 1.7 ± 1.0 | 3.8 ± 1.1c |
| C. neoformans | 1.7 ± 1.0 | 11.4 ± 1.7cd |
| CCW/M | 1.7 ± 1.0 | 12.5 ± 1.6cd |
PBMC were cultured in the presence of medium alone, PHA (10 μg/ml), SEB (1 μg/ml), tetanus toxoid (1:10 Lf), C. neoformans (2 × 105/well), or CCW/M (1.5 μg/ml). The percentage of CD3+ cells in the S and G2/M phases of the cell cycle was determined by using propidium iodide stain on the day of maximum [3H]TdR incorporation for each stimulus. Cultures stimulated with PHA were studied on day 3, cultures stimulated with SEB were studied on day 5, and cultures stimulated with tetanus toxoid, C. neoformans, or CCW/M were studied on day 7.
Means ± SEMs from four experiments.
P < 0.05 compared to the value for the corresponding unstimulated group.
P < 0.05 compared to the value for PBMC with tetanus toxoid.
Precursor frequency of responding lymphocytes.
The percentage of responding T cells is frequently evaluated by determining the precursor frequency. This determines the minimum number of T cells to mount a proliferative response. The assumption is that one T cell is sufficient to proliferate and therefore determines the number of T cells among which there is one responding T cell. With limiting dilutions and Poisson’s formula, this technique determines the minimum number of T cells that must be present to contain at least one proliferating cell. Mitogens that are commonly used in experimental systems stimulate 1 in 5 to 100 lymphocytes, while recall antigens stimulate 1 in 104 to 106 lymphocytes (6, 7, 25). The precursor frequencies that were observed among subjects were as high as 1 in 1,660 and as low as 1 in 16,940. The mean precursor frequency for response to C. neoformans was 1 in 7,750 ± 2,270 (Fig. 5). Thus, the precursor frequency is intermediate between those for mitogens and recall antigens.
FIG. 5.
Precursor frequency analysis of the T-lymphocyte response to CCW/M. Various numbers of T lymphocytes were incubated with irradiated PBMC (105/well) with or without CCW/M (1.5 μg/ml) for 7 days. In semilogarithmic plots, the frequency of responding T cells is determined by the number of cells present when 37% of the wells are negative. Twenty-four wells were used for each cell concentration. This experiment was repeated six times. Each graph represents the precursor frequency for one of six subjects.
DISCUSSION
We have made the following three observations: (i) C. neoformans possesses a mitogen, since it stimulates naive T cells to proliferate; (ii) the T-cell response is AC dependent but not MHC restricted; and (iii) although the number of T cells that proliferate in response to C. neoformans is greater than the number of T cells that respond to recall antigens, it is less than the number of T cells that respond to mitogens that are commonly used in experimental systems.
The mechanism of lymphocyte activation by microbial pathogens involves two distinct pathways. Recall antigens are taken up by antigen-processing cells, processed by lysosomal digestion, and placed in the binding groove of an MHC molecule. The MHC-peptide complex is then transported to the surface of the antigen-presenting cell, where it is available for recognition by T cells bearing histocompatible T-cell receptors. By contrast, mitogens bind to the surfaces of T cells and AC, resulting in receptor ligation, signaling, and entry of the T cells into the cell cycle.
There are a number of pieces of evidence that C. neoformans possesses a mitogen. Prior to antigen stimulation and clonal expansion, naive T cells express the RA isoform of the CD45 molecule. Following antigenic stimulation and clonal expansion, T cells express the RO isoform of the CD45 molecule (2, 14). Since in vitro lymphocyte proliferation in response to recall antigens can be detected only in T cells that have undergone prior clonal expansion, only CD45RO+ cells proliferate (21). By contrast, mitogens and superantigens stimulate a large percentage of T cells regardless of whether they have undergone prior expansion, and therefore both CD45RO+ and CD45RA+ cells proliferate (21). The present studies demonstrate that CD45RA cells proliferate in response to C. neoformans, supporting the experiments with fetal lymphocytes and indicating that naive lymphocytes proliferate in response to C. neoformans.
The AC requirements of mitogens and recall antigens are distinct. In response to recall antigens, T cells recognize the combination of the epitope and histotope, and therefore the response is MHC restricted. By contrast, in response to mitogens, receptor ligation on the T cells does not recognize the unique sequences of the epitope-histotope combination, and the response is not MHC restricted. The present experiments indicate that histoincompatible AC are sufficient to provide accessory signals and that the response is not MHC restricted, indicating that C. neoformans possesses a mitogen.
The present studies provide strong evidence that C. neoformans contains a mitogen, based on its ability to stimulate naive lymphocytes to proliferate and on the ability of allogeneic cells to function as AC. However, by some criteria CCW/M is a relatively weak mitogen. We found that the percentage of T cells that were in S phase after stimulation was only three times that after stimulation with a recall antigen and one-quarter of that after stimulation with the mitogenic lectin or bacterial exotoxin that was tested. We also found that the precursor frequency was only slightly greater than that reported for recall antigens and was significantly less than that for mitogenic lectins and bacterial exotoxins. The fact that a smaller percentage of T cells responded to C. neoformans means that the effect may be subtle in in vitro assays. In this regard, it is interesting that prior experiments with a murine model have demonstrated that murine cells have low levels of proliferation in response to C. neoformans (3). Whether this modest mitogenic response correlates with the intensity of cytokine production and other T-cell effector functions will have to be determined by further study.
It is clear that in addition to a mitogen, C. neoformans possesses T-cell antigens. It is likely that both effects are important, and the mitogenic effect may have profound effects on antigen responses and host defense. However, the presence of both mechanisms of activation raises questions about which is the predominant effect during immune responses to C. neoformans. The following observations may provide some insight in this regard. In mice, it is clear that antigen-specific, protective responses occur. Naive mice have low levels of [3H]TdR incorporation in response to C. neoformans (3), and thus, in mice the effect of the mitogen on lymphocyte proliferation is minor. In humans, it is possible that both mechanisms contribute to lymphocyte proliferation in vitro. When naive lymphocytes or allogeneic AC are present, then the only possible mechanism of lymphocyte activation is via a mitogen. When efficient antigen presentation is present, then a recall antigen may predominate. In this regard, it is interesting that the autologous response peaked 1 day prior to the response of allogeneic cells, suggesting that antigen-specific expansion had also occurred, and more lymphocytes were responding to the antigen than to the mitogen.
The observation that fewer T cells are activated and proliferate in response to C. neoformans than in response to mitogens that are commonly used in experimental systems raises interesting questions about the mechanism of T-cell activation by C. neoformans. The mechanism of the mitogenic activation was not addressed in these studies; however, some insights may be gained. It is possible that C. neoformans ligates a receptor that is present on all T cells but stimulates suboptimal AC ligands or cytokines that are required for T-cell proliferation, thus limiting proliferation. However, suboptimal AC or T-cell growth factor signals can produce nonlinear precursor frequencies, and since we observed linear relationships, this is less likely. Alternately, C. neoformans may bind to a surface molecule that is expressed by only a subset of T cells. The nature of this interaction will be determined by future studies.
There are a number of possible mechanisms by which the mitogenic effect could contribute to the immunopathogenesis of cryptococcal infections. Since the mitogenic effect involves T-cell proliferation, this requires the production of T-cell growth factors (17). It is possible that these T-cell growth factors could be utilized by the T cells that are responding in an antigen-specific manner, thus enhancing the antigen-specific response. Alternately, although we have no data to suggest that the mitogenic response would have an adverse effect on antigen-specific lymphocyte proliferation, in other systems, mitogenic T-cell activation suppresses subsequent antigen-specific T-cell responses (11, 12). T-cell activation is also important in the progression of AIDS. It is possible that mitogen-induced activation would augment the growth of human immunodeficiency virus and contribute to the pathogenesis of the disease.
The mitogenic activation of T cells may also have an important consequence for anticryptococcal effector mechanisms. Mitogen-activated T cells might produce proinflammatory cytokines that could recruit or activate anticryptococcal effector cells, including macrophages and NK cells. Additionally, since T lymphocytes are effector cells for Cryptococcus (13, 20), it is possible that the mitogenic effect could act directly on T lymphocytes to enhance anticryptococcal activity.
In summary, we have demonstrated that C. neoformans possesses a mitogen for human T lymphocytes in addition to potent recall antigens that elicit a complex immune response. This mitogenic effect may have important consequences for the immune response to C. neoformans and should be considered when evaluating T-cell responses to this organism.
ACKNOWLEDGMENTS
We thank Barbara Herbut and the Tissue Typing Laboratory of the Foothills Hospital for performing the HLA typing and the nurses of Labor and Delivery at the Foothills Hospital for obtaining umbilical cord blood. We also thank Laurie Robertson for technical assistance and Jennifer Bickell for clerical assistance.
This work was supported by grants from the Medical Research Council of Canada/National Health Research and Development Program for AIDS and the Canadian Foundation for AIDS Research. R.M.S. is supported by a studentship from the National Health Research Development Program, and C.H.M. is a Scholar of the Alberta Heritage Foundation for Medical Research.
REFERENCES
- 1.Abrahams J, Gilleran T G. Studies on actively acquired resistance to experimental cryptococcosis in mice. J Immunol. 1960;85:629–635. [Google Scholar]
- 2.Byrne J A, Butler J L, Cooper M D. Differential activation requirements for virgin and memory T cells. J Immunol. 1988;141:3249–3257. [PubMed] [Google Scholar]
- 3.Collins H L, Bancroft G J. Encapsulation of Cryptococcus neoformans impairs antigen-specific T-cell responses. Infect Immun. 1991;59:3883–3888. doi: 10.1128/iai.59.11.3883-3888.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Currie B P, Casadevall A. Estimation of the prevalence of cryptococcal infection among patients infected with the human immunodeficiency virus in New York City. Clin Infect Dis. 1994;19:1029–1033. doi: 10.1093/clinids/19.6.1029. [DOI] [PubMed] [Google Scholar]
- 5.Diamond R D. Effects of stimulation and suppression of cell-mediated immunity on experimental cryptococcosis. Infect Immun. 1977;17:187–194. doi: 10.1128/iai.17.1.187-194.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford D, Burger D. Precursor frequency of antigen-specific T cells: effects of sensitization in vivo and in vitro. Cell Immunol. 1983;79:334–344. doi: 10.1016/0008-8749(83)90075-8. [DOI] [PubMed] [Google Scholar]
- 7.Gebel H M, Scott J R, Parvin C A, Rodey G E. In vitro immunization to KLH. II. Limiting dilution analysis of antigen-reactive cells in primary and secondary culture. J Immunol. 1983;130:29–32. [PubMed] [Google Scholar]
- 8.Graybill J R, Alford R H. Cell-mediated immunity in cryptococcosis. Cell Immunol. 1974;14:12–21. doi: 10.1016/0008-8749(74)90164-6. [DOI] [PubMed] [Google Scholar]
- 9.Hill J O, Aguirre K M. CD4+ T cell-dependent acquired state of immunity that protects the brain against Cryptococcus neoformans. J Immunol. 1994;152:2344–2350. [PubMed] [Google Scholar]
- 10.Huffnagle G B, Yates J L, Lipscomb M F. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J Exp Med. 1991;173:793–800. doi: 10.1084/jem.173.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ignatowicz L, Kappler J, Marrack P. The effects of chronic infection with a superantigen-producing virus. J Exp Med. 1992;175:917–923. doi: 10.1084/jem.175.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee W T, Vitetta E S. Memory T cells are anergic to the superantigen staphylococcal enterotoxin B. J Exp Med. 1992;176:575–579. doi: 10.1084/jem.176.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levitz S M, Dupont M P. Phenotypic and functional characterization of human lymphocytes activated by interleukin-2 to directly inhibit growth of Cryptococcus neoformans in vitro. J Clin Invest. 1993;91:1490–1498. doi: 10.1172/JCI116354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merkenschlager M, Terry L, Edwards R, Beverley P C. Limiting dilution analysis of proliferative responses in human lymphocyte populations defined by the monoclonal antibody UCHL1: implications for differential CD45 expression in T cell memory formation. Eur J Immunol. 1988;18:1653–1661. doi: 10.1002/eji.1830181102. [DOI] [PubMed] [Google Scholar]
- 15.Miller G P G, Puck J. In vitro human lymphocyte responses to Cryptococcus neoformans. Evidence for primary and secondary responses in normals and infected subjects. J Immunol. 1984;133:166–172. [PubMed] [Google Scholar]
- 16.Mody C H, Sims K L, Wood C J, Syme R M, Spurrell J C, Sexton M M. Proteins in the cell wall/membrane of Cryptococcus neoformans stimulate both adult and fetal cord blood lymphocyte to proliferate. Infect Immun. 1996;64:4811–4819. doi: 10.1128/iai.64.11.4811-4819.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mody C H, Spurrell J C L, Wood C J. Interleukin 15 (IL-15) induces antimicrobial activity after release by Cryptococcus neoformans-stimulated monocytes. J Infect Dis. 1998;178:803–814. doi: 10.1086/515381. [DOI] [PubMed] [Google Scholar]
- 18.Mody C H, Syme R M. Effect of polysaccharide capsule and methods of preparation on human lymphocyte proliferation in response to Cryptococcus neoformans. Infect Immun. 1993;61:464–469. doi: 10.1128/iai.61.2.464-469.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mody C H, Toews G B, Lipscomb M F. Cyclosporin A inhibits the growth of Cryptococcus neoformans in a murine model. Infect Immun. 1988;56:7–12. doi: 10.1128/iai.56.1.7-12.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy J W, Hidore M R, Wong S C. Direct interactions of human lymphocytes with the yeast-like organism, Cryptococcus neoformans. J Clin Invest. 1993;91:1553–1566. doi: 10.1172/JCI116361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plebanski M, Saunders M, Burtles S, Crowe S, Hooper D C. Primary and secondary human in vitro T-cell responses to soluble antigens are mediated by subsets bearing CD45 isoforms. Immunology. 1992;75:86–91. [PMC free article] [PubMed] [Google Scholar]
- 22.Saxon A J, Robins R A. Single step separation of human T and B cells using AET treated srbc rosettes. J Immunol Methods. 1976;12:285–288. doi: 10.1016/0022-1759(76)90050-8. [DOI] [PubMed] [Google Scholar]
- 23.Schimpff S C, Bennett J E. Abnormalities in cell-mediated immunity in patients with Cryptococcus neoformans infection. J Allergy Clin Immunol. 1975;55:430–441. doi: 10.1016/0091-6749(75)90082-2. [DOI] [PubMed] [Google Scholar]
- 24.Syme R M, Wong H, Wood C J, Mody C H. Both CD4+ and CD8+ human lymphocytes are activated and proliferate to Cryptococcus neoformans. Immunology. 1997;92:194–200. doi: 10.1046/j.1365-2567.1997.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.vanOers M H J, Pinkster J, Zeijlemaker W P. Quantification of antigen-reactive cells among human T lymphocytes. Eur J Immunol. 1978;8:477–484. doi: 10.1002/eji.1830080706. [DOI] [PubMed] [Google Scholar]