Abstract
The gene encoding an outer membrane protein A (OmpA)-like, heat-modifiable Omp of Actinobacillus actinomycetemcomitans ATCC 43718 (strain Y4, serotype b) was cloned by a PCR cloning procedure. DNA sequence analysis revealed that the gene encodes a protein of 346 amino acid residues with a molecular mass of 36.9 kDa. The protein expressed by the cloned gene reacted with a monoclonal antibody to the previously described 29-kDa Omp (Omp29) of strain Y4. This monoclonal antibody reacted specifically with Omp29 of A. actinomycetemcomitans (serotype b), but not with any Omp of Escherichia coli, including OmpA. This protein exhibited characteristic heat modifiability on sodium dodecyl sulfate-polyacrylamide gels, showing an apparent molecular mass of 29 kDa when unheated and a mass of 34 kDa when heated. The N-terminal amino acid sequence of the protein expressed in E. coli perfectly matched those deduced from the purified Omp29 of strain Y4. The deduced amino acid sequence of the gene coding for Omp29 from serotype b matched completely (except for valine at position 321) that of a recently reported omp34 gene described for A. actinomycetemcomitans serotype c (NCTC 9710). Because of the conserved nature of the gene within these serotypes, we designated the gene described herein from serotype b as omp34.
Actinobacillus actinomycetemcomitans is strongly associated with localized juvenile periodontitis (LJP) and with rapidly progressive periodontitis (17, 26). Among the five currently recognized serotypes of A. actinomycetemcomitans (8), serotype b strains often predominate in periodontal lesions of LJP patients (26). These patients often exhibit increased levels in serum of immunoglobulin G (IgG) antibody to A. actinomycetemcomitans antigens, including serotype-specific lipopolysaccharide and outer membrane proteins (Omps) (3, 6, 14, 23, 25). Among many identified antigens from A. actinomycetemcomitans, the 29-kDa Omp is a prominent protein antigen to which IgG antibodies in the sera of many LJP patients react (23, 24). The N-terminal amino acid sequences of Omp29 from strain Y4 (22) strongly suggested that this protein belonged to the OmpA family of gram-negative bacteria, which includes Escherichia coli (13, 24), Salmonella typhimurium (7), Haemophilus influenzae type b (11), and Haemophilus ducreyi (19).
E. coli OmpA has been well characterized and is thought to be a multifunctional protein which displays both phage receptor (5, 16) and porin (12) activities. OmpA has also been associated with the structural integrity of the cell membrane (18) and invasion of brain microvascular endothelial cells (15). However, no definitive role for A. actinomycetemcomitans serotype b Omp has been elucidated.
In this study, we cloned and sequenced the gene encoding the OmpA-like protein from A. actinomycetemcomitans ATCC 43718 (serotype b), because of its prominence in disease, by utilizing a PCR cloning procedure. Molecular characterization of the cloned gene revealed that it encodes Omp29, a heat-modifiable Omp belonging to the OmpA family. Moreover, we demonstrated that Omp29 of A. actinomycetemcomitans ATCC 43718 (serotype b) is encoded by a gene (omp34) which encodes a virtually identical protein from A. actinomycetemcomitans serotype c (21).
Bacterial strains.
A. actinomycetemcomitans ATCC 43718 (strain Y4, serotype b) was cultured at 37°C in Trypticase soy broth supplemented with 0.6% yeast extract (Difco Laboratories, Detroit, Mich.) in humidified CO2. E. coli was cultured at 37°C in Luria-Bertani broth containing ampicillin (100 μg/ml) when needed. An OmpA-deficient mutant of E. coli (Bre51) was the kind gift of Ulf Henning, Max-Planck-Institut für Biologie, Tübingen, Germany.
MAb to A. actinomycetemcomitans Y4 Omp29.
A monoclonal antibody (MAb) to A. actinomycetemcomitans Y4 OmpA was developed as described elsewhere (9). Briefly, 8-week-old BALB/c mice were immunized with sonicated A. actinomycetemcomitans Y4 in Freund’s complete adjuvant subcutaneously in the scruff of the neck and then with incomplete adjuvant 2 weeks later. An intraperitoneal injection was administered 4 days later, after which the spleens were removed, and single-cell suspensions were then fused with the SP2/0 myeloma cell line. Hybridomas producing an IgG-class MAb specific to A. actinomycetemcomitans Y4 were initially screened in an enzyme-linked immunosorbent assay against A. actinomycetemcomitans sonicates. Positive hydridomas were subsequently evaluated for specificity to purified A. actinomycetemcomitans Omp29, the preparation of which has been described elsewhere (22). After cloning of the antibody-producing hybridomas, we obtained one hybridoma which produced IgG Omp29-specific MAb.
PCR cloning of the OmpA-like gene.
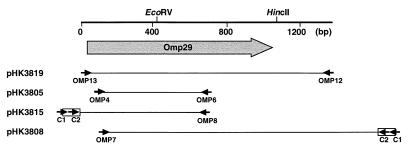
Since Omps belonging to the OmpA family exhibit a high level of homology among gram-negative bacteria, we first constructed mixed primers (Omp4, CCNCARGCNAAYACNTTY [5′→3′]; Omp6, YTGNCCRAANCGRTANGA [5′→3′]) in order to clone the 29-kDa Omp gene. The Omp4 primer was derived from the N-terminal sequence PQANTF, and the Omp6 primer was derived from SYRFGQ, corresponding to the highly conserved region of OmpA in E. coli, S. typhimurium, H. influenzae, and Serratia marcescens. After PCR amplification with primers Omp4 and Omp6 (Fig. 1 and 2), a 550-bp fragment was generated with strain Y4 chromosomal DNA as a template. The fragment was cloned into pGEM T-easy vector (Promega, Madison, Wis.) to generate pHK3805 and sequenced with an automated DNA sequencing system (ALFred; Pharmacia, Björkgatan, Sweden). The amino acid sequence derived from the DNA sequence of the PCR fragment revealed homology to OmpA-like proteins, including E. coli OmpA, H. influenzae P5, and H. ducreyi major Omp (MOMP). Therefore, it appeared that this fragment represented a partial sequence of the OmpA-like gene. We subsequently attempted to clone the flanking region of the cloned fragment covering the complete open reading frame (ORF) of the OmpA-like gene by using a Takara LA PCR in vitro cloning kit (Takara Biomedicals, Kusatsu, Japan). Briefly, the chromosomal DNA was digested with EcoRI and then ligated with an EcoRI cassette with its 5′ end dephosphorylated, which causes nick formation between the 3′ terminal of the chromosomal DNA and the 5′ terminal of the cassette. Following ligation, the first PCR was performed with the cassette primer C1 and either Omp7 for downstream cloning or Omp8 for upstream cloning (Fig. 1). A portion of the first PCR product was used as a template for the second PCR. The second PCR was performed with the cassette primer C2 (Fig. 1) and either Omp7 or Omp8. After the second PCR, we obtained DNA fragments in the respective samples: one was a 750-bp product amplified with Omp8 and C2, and one was an 1,800-bp product amplified with Omp7 and C2. The two DNA fragments were cloned into pGEM T-easy vector to generate pHK3808 and pHK3815 (Fig. 1), which were then sequenced. They were found to contain either side of the flanking region of the DNA fragment generated by PCR with Omp4 and Omp6. Comparison of overlapping deduced DNA sequences revealed the complete ORF.
FIG. 1.
Schematic representation of the gene encoding Omp29 and plasmids used in this study. The shaded arrow represents the ORF (Omp29) and the direction of transcription. The solid line shows the size of the inserted fragment of each plasmid, designated as the left side. Solid arrows and boxes represent the primers and cassette, respectively.
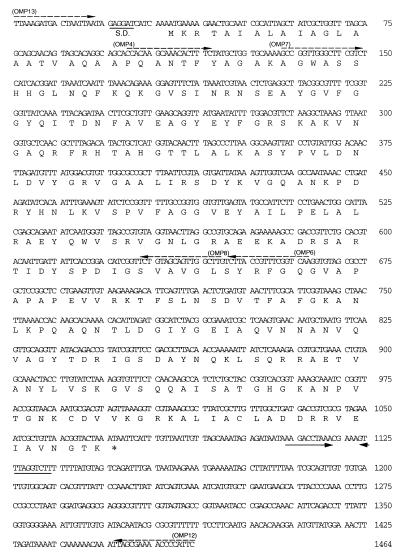
FIG. 2.
Nucleotide sequence of the gene encoding Omp29 from A. actinomycetemcomitans Y4. The ribosome-binding site (Shine-Dalgarno site [S.D.]) is underlined. The stop codon is marked by asterisks. Palindromic sequences are indicated by arrows. Dotted arrows represent the primers used in this study.
To determine whether this gene was indeed present in the chromosomal DNA, PCR was performed with the primers Omp12 and Omp13, which were outside the ORF, and Y4 chromosomal DNA as the template (Fig. 1). A single DNA fragment was amplified which had a molecular size perfectly matched to that calculated from the DNA sequencing. This fragment was cloned into pGEM T-easy vector to generate pHK3819. DNA sequencing of this fragment revealed one complete ORF containing the OmpA-like gene (Fig. 2). This ORF could potentially encode a protein of 346 amino acid residues with a molecular mass of 36.9 kDa. Cleavage of the signal sequence would result in a protein with a molecular mass of 34.9 kDa.
Identification of the cloned fragment as the gene encoding the 29-kDa Omp.
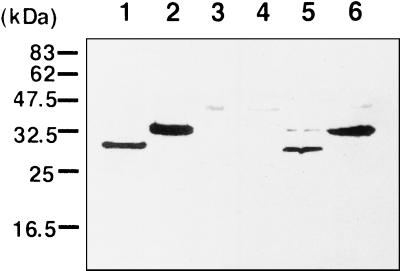
To determine whether the cloned fragment encoded the 29-kDa Omp, Western blot analysis was performed with the MAb to the 29-kDa Omp as the first antibody, followed by peroxidase-conjugated goat F (ab′)2 anti-mouse IgG (Cappel, West Chester, Pa.). This MAb reacted with purified Omp29 of Y4 (data not shown) and with Omp29 in the lysate of Y4, but not with other proteins in the lysate, suggesting that this MAb specifically reacted with Omp29. Figure 3 shows that the MAb specifically reacted with the 29-kDa Omp in the Y4 whole-cell lysate (Fig. 3, lanes 1 and 2). The MAb did not recognize any proteins (including OmpA) in the E. coli whole-cell lysate (Fig. 3, lanes 3 and 4). The MAb reacted with a 29-kDa protein with a mass similar to that estimated from the deduced amino acid sequence of Omp29 of Y4 in the whole-cell lysate of E. coli harboring pHK3819 (Fig. 3, lanes 5 and 6). The protein reacting with the MAb migrated as a 34-kDa protein when the sample was boiled for 15 min before running, showing that the protein encoded by the cloned fragment was heat modifiable. Omp29 of the Y4 strain has been reported to be a heat-modifiable protein (22). Also, Omp29 treated with sample buffer at elevated temperature (>50°C) migrates more slowly in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) than Omp29 incubated at ambient temperature (data not shown). The molecular mass of Omp29 was calculated to be 34 kDa when the protein was heated, but 29 kDa when unheated. These results strongly suggested that the cloned fragment was coding for a 29-kDa heat-modifiable Omp, and, therefore, we designated this gene as omp34.
FIG. 3.
Western blot of whole lysates of A. actinomycetemcomitans Y4 and E. coli strains. Lanes: 1 and 2, proteins from A. actinomycetemcomitans Y4; 3 and 4, proteins from E. coli XL-1 Blue; 5 and 6, proteins from E. coli XL-1 Blue harboring pHK3819. Whole lysates were solubilized in SDS sample buffer at room temperature (lanes 1, 3, and 5) or at 100°C for 15 min (lanes 2, 4, and 6) and then electrophoresed in 15% polyacrylamide gel. Western blotting was performed as described previously (20). Immunodetection was performed according to the manual supplied with Chemiluminescence Reagent Plus (NEN Life Science Products, Boston, Mass.).
Expression of rOmp29 in OmpA-deficient E. coli.
To define the location of recombinant Omp29 (rOmp29) in E. coli, pHK3819 was transformed into an OmpA-deficient E. coli mutant strain, Bre51 (18). We confirmed that Bre51 did not have OmpA in its outer membrane and that the MAb against Omp29 of Y4 did not recognize any proteins of the whole-cell lysate of Bre51. Membrane fractionation was performed as described previously (22). Briefly, the membrane proteins, obtained from E. coli lysate by ultracentrifugation (100,000 × g), were incubated for 30 min at room temperature in 1% sodium lauroyl sarcosinate. The insoluble materials were solubilized by incubation for 30 min at room temperature in 50 mM Tris-HCl (pH 8.0) containing 5 mM EDTA and 1% (wt/vol) octylglucoside. Following ultracentrifugation, insoluble material was solubilized by incubation for 30 min at room temperature in 20 mM sodium phosphate (pH 7.5) containing 1% SDS. Western blot analysis indicated that rOmp29 was found in the sarcosyl-insoluble, octylglucoside-insoluble, SDS-soluble fraction, consistent with an outer membrane localization (data not shown). The SDS-soluble fraction was then separated by SDS-PAGE and electroblotted onto a Trans-Blot membrane (polyvinylidene difluoride membrane; Bio-Rad Laboratories, Hercules, Calif.) by using 30 mM Tris-borate buffer containing 0.02% SDS (pH 8.5). The membrane was stained with 0.1% Coomassie brilliant blue R-250 and then destained with 50% methanol. The band corresponding to the 29-kDa protein was excised and then washed with distilled water, and the amino-terminal sequence was determined with a Shimazu gas-phase protein sequencer, PSQ-1. The N-terminal sequence of rOmp29 was APQANTFYAG, which was a complete (perfect) match with the N-terminal sequence obtained from Omp29 of strain Y4. This result indicated that rOmp29 was correctly processed in E. coli.
Relationship of Omp29 from A. actinomycetemcomitans to other members of the OmpA family.
The deduced (predicted) amino acid sequence of Omp29 from Y4 (serotype b) matched completely (except for valine at position 321, compared with alanine in the same position of strain NCTC 9710, serotype c) the sequence of Omp34 from A. actinomycetemcomitans NCTC 9710 (serotype c [21]). Western blot analysis using the MAb was performed with the whole-cell lysates of three serotype a strains, two serotype b strains, and three serotype c strains. The MAb reacted with protein bands of an approximate similar size (34 kDa when heated, 29 kDa when nonheated) in all samples, suggesting that Omp29 is commonly present among the different serotypes of A. actinomycetemcomitans. A protein structural homology search revealed that Omp29 exhibits varions degrees of homology with several members of the OmpA family, including OmpP5 of H. influenzae (64% identity [11]), MOMP of H. ducreyi (44% [19]), OmpA of E. coli (48% [2]), OmpA of S. typhimurium (47% [7]), and OmpA of Shigella dysenteriae (47% [4]).
One of the purported features of A. actinomycetemcomitans is putative invasion of gingival epithelium (10). Also, A. actinomycetemcomitans OmpA binds to laminin, which is one of the basement membrane components of endothelium or epithelium (1). Furthermore, it has been reported that E. coli OmpA contributes to invasion of brain microvascular endothelial cells (15). In addition, purified OmpA of E. coli and anti-OmpA antibodies inhibited the invasion by OmpA+ E. coli cells of brain microvascular endothelial cells. Also, there was 25- to 50-fold-less invasion of these cells by OmpA− E. coli cells than by OmpA+ E. coli cells. These findings suggest that Omp29 of A. actinomycetemcomitans may play an important role in binding to endothelium or epithelium and/or for potential invasion of host cells. Further studies would be helpful to elucidate the mechanism of Omp29-related pathogenicity.
Nucleotide sequence accession number.
The nucleotide sequence discussed in this study will appear in the DDBJ, EMBL, and GenBank databases under accession no. AB015936.
Acknowledgments
This work was supported by a grant-in-aid for Encouragement of Young Scientists (grant no. 10770119) from the Ministry of Education, Science, Sports and Culture of Japan and grants DE-03420 and DE-10041 from the National Institute of Dental Research. Part of this work was conducted at the Research Center for Molecular Medicine, Hiroshima University School of Medicine.
We thank Ulf Henning for the generous gift of strains of E. coli.
REFERENCES
- 1.Alugupalli K R, Kalfas S, Forsgren A. Laminin binding to a heat-modifiable outer membrane protein of A. actinomycetemcomitans. Oral Microbiol Immunol. 1996;5:326–331. doi: 10.1111/j.1399-302x.1996.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 2.Beck E, Bremer E. Nucleotide sequence of the OmpA gene coding the outer membrane protein II of Escherichia coli K-12. Nucleic Acids Res. 1980;8:3011–3024. doi: 10.1093/nar/8.13.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolstad A I, Kristoffersen T, Olsen I, Preus H R, Jensen H B, Vasstrand E N, Bakken B. Outer membrane proteins of Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus studied by SDS-PAGE and immunoblotting. Oral Microbiol Immunol. 1990;5:155–161. doi: 10.1111/j.1399-302x.1990.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 4.Braun G, Cole S T. The nucleotide sequence coding for major outer membrane protein OmpA of Shigella dysenteriae. Nucleic Acids Res. 1982;10:2367–2378. doi: 10.1093/nar/10.7.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Datta D B, Arden B, Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977;131:821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farida R, Wilson M, Ivanyi L. Serum IgG antibodies to lipopolysaccharides in various forms of periodontal disease in man. Arch Oral Biol. 1986;31:711–715. doi: 10.1016/0003-9969(86)90001-4. [DOI] [PubMed] [Google Scholar]
- 7.Freudl R, Cole S T. Cloning and molecular characterization of the OmpA gene from Salmonella typhimurium. Eur J Biochem. 1983;134:497–502. doi: 10.1111/j.1432-1033.1983.tb07594.x. [DOI] [PubMed] [Google Scholar]
- 8.Gmür R, McNabb H, van Steenbergen T J M, Baehni P, Mombelli A, van Winkelhoff A J, Guggenheim B. Seroclassification of hitherto nontypeable Actinobacillus actinomycetemcomitans strains: evidence for a new serotype e. Oral Microbiol Immunol. 1993;8:116–120. doi: 10.1111/j.1399-302x.1993.tb00556.x. [DOI] [PubMed] [Google Scholar]
- 9.Kawai T, Ito H-O, Sakado N, Okada H. A novel approach for detecting an immunodominant antigen of Porphyromonas gingivalis in diagnosis of adult periodontitis. Clin Diagn Lab Immunol. 1998;5:11–17. doi: 10.1128/cdli.5.1.11-17.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer D H, Fives-Taylor P M. The role of Actinobacillus actinomycetemcomitans in the pathogenesis of periodontal disease. Trends Microbiol. 1997;5:224–228. doi: 10.1016/S0966-842X(97)01055-X. [DOI] [PubMed] [Google Scholar]
- 11.Munson R S, Jr, Grass S, West R. Molecular cloning and sequence of the gene for outer membrane protein P5 of Haemophilus influenzae. Infect Immun. 1993;61:4017–4020. doi: 10.1128/iai.61.9.4017-4020.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikaido H. Proteins forming large channels from bacterial and mitochondrial outer membrane: porins and phage lambda receptor protein. Methods Enzymol. 1983;97:85–101. doi: 10.1016/0076-6879(83)97122-7. [DOI] [PubMed] [Google Scholar]
- 13.Nikaido H, Vaara M. Outer membrane. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. 1st ed. Vol. 1. Washington, D.C: American Society for Microbiology; 1987. pp. 7–22. [Google Scholar]
- 14.Page R C, Sims T J, Engel L D, Moncla B J, Bainbridge B, Stray J, Darveau R P. The immunodominant outer membrane antigen of Actinobacillus actinomycetemcomitans is located in the serotype-specific high-molecular-mass carbohydrate moiety of lipopolysaccharide. Infect Immun. 1991;59:3451–3462. doi: 10.1128/iai.59.10.3451-3462.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasadarao N V, Wass C A, Weiser J N, Stins M F, Huang S-H, Kim K S. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect Immun. 1996;64:146–153. doi: 10.1128/iai.64.1.146-153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schweizer M, Henning U. Action of a major outer cell envelope membrane protein in conjugation of Escherichia coli K-12. J Bacteriol. 1997;129:1651–1652. doi: 10.1128/jb.129.3.1651-1652.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slots J, Bragd L, Wikström M, Dalén G. The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J Clin Periodontol. 1986;13:570–577. doi: 10.1111/j.1600-051x.1986.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 18.Sonntag I, Schwartz H, Hirota Y, Henning U. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J Bacteriol. 1978;136:280–285. doi: 10.1128/jb.136.1.280-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spinola S M, Griffiths G E, Shanks K L, Blake M S. The major outer membrane protein of Haemophilus ducreyi is a member of the OmpA family of proteins. Infect Immun. 1993;61:1346–1351. doi: 10.1128/iai.61.4.1346-1351.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugai M, Koike H, Hong Y, Miyake Y, Nogami R, Suginaka H. Purification of a 51 kDa endo-β-N-acetylglucosaminidase from Staphylococcus aureus. FEMS Microbiol Lett. 1989;61:267–272. doi: 10.1016/0378-1097(89)90209-7. [DOI] [PubMed] [Google Scholar]
- 21.White P A, Nair S P, Kim M-J, Wilson M, Henderson B. Molecular characterization of an outer membrane protein of Actinobacillus actinomycetemcomitans belonging to the OmpA family. Infect Immun. 1998;66:369–372. doi: 10.1128/iai.66.1.369-372.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson M E. The heat-modifiable outer membrane protein of Actinobacillus actinomycetemcomitans: relationship to OmpA proteins. Infect Immun. 1991;59:2505–2507. doi: 10.1128/iai.59.7.2505-2507.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson M E. IgG antibody response of juvenile periodontitis patients to the 29 kilodalton outer membrane protein of Actinobacillus actinomycetemcomitans. J Periodontol. 1991;62:211–218. doi: 10.1902/jop.1991.62.3.211. [DOI] [PubMed] [Google Scholar]
- 24.Wilson M E. The OmpA protein of Actinobacillus actinomycetemcomitans: physicochemical and immunologic features. In: Genco R, Hamada S, Lehner T, McGhee J, Mergenhagen S, editors. Molecular pathogenesis of periodontal disease. Washington, D.C: American Society for Microbiology; 1994. pp. 363–372. [Google Scholar]
- 25.Wilson M E, Schifferle R E. Evidence that the serotype b antigenic determinant of Actinobacillus actinomycetemcomitans Y4 resides in the polysaccharide moiety of lipopolysaccharide. Infect Immun. 1991;59:1544–1551. doi: 10.1128/iai.59.4.1544-1551.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zambon J J. Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol. 1985;12:1–20. doi: 10.1111/j.1600-051x.1985.tb01348.x. [DOI] [PubMed] [Google Scholar]