Abstract
Several vaccines have been approved for the prevention of COVID-19. However, no head-to-head trials comparing their clinical efficacy have been performed. This network meta-analysis aims to identify those, among the competing existing vaccines, conferring the maximum protection against COVID-19. A literature search was done in Medline (via PubMed), Embase and Cochrane Library databases for phase 3 randomized controlled trials evaluating the efficacy of different COVID-19 vaccines. Search results were screened and eligible studies were included to perform a network meta-analysis in software ‘R’ version 4.1.2 using a random effect model. Cochrane’s ‘Risk of Bias tool (RoB2)’ was used for quality assessment. Raw data from the included studies was used for network meta-analysis. Assessment of inconsistency was not possible as no study compared two or more vaccines directly. A forest plot for indirect comparison of various COVID-19 vaccines was obtained. Rankogram and ‘P’ scores were obtained to rank the vaccines based on the indirect evidence of their comparative efficacy. A total of 17 randomized controlled trials evaluating the efficacy of 16 COVID-19 vaccines, were included in the network meta-analysis. A total of 361,386 participants was included in this network meta-analysis. Overall risk of bias among included studies was of ‘some concern’. All the COVID-19 vaccines had a statistically significant reduction of risk for contracting symptomatic SARS-CoV-2 in comparison to the placebo, however, the maximum protection (RR 0.05) was with BNT126b2. The indirect comparison also revealed BNT126b2 vaccine confers the highest protection against symptomatic SARS-CoV-2 infection in comparison to all others included, with a ‘P’ score of 0.9771 followed by mRNA-1273, rAD26 & rAD5 and NVX-CoV2373. The evidence generated from this network meta-analysis indicates the good efficacy of all the included vaccines in preventing symptomatic COVID-19 as compared to placebo. The BNT126b2 vaccine was found to provide the highest protection against symptomatic SARS-CoV-2 among all included followed by mRNA-1273, rAD26 & rAD5, NVX-CoV2373 and others.
Supplementary Information
The online version contains supplementary material available at 10.1007/s43440-022-00429-1.
Keywords: Coronavirus disease 2019, Vaccine, Efficacy, Network meta-analysis
Introduction
The severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection, first reported in China in December 2019, swiftly spread to other countries, resulting in the Coronavirus Disease-19 (COVID-19) pandemic [1]. Many countries implemented several measures in the past two years to slow the spread of COVID-19 and avoid fatalities, such as strict lockdowns, physical distancing and home/institutional isolation, yet the pandemic continues [2]. As of March 9, 2022, > 448 million cases of COVID-19 including > 6 million deaths, have been reported worldwide [3].
The lack of definitive medical therapy for COVID-19 led to a search for an effective and safe vaccine to prevent transmission and a substantial magnitude of research and funding were devoted to vaccine development [4, 5]. The first anti-COVID-19 vaccine was made available to the public in a matter of months in December 2020 [6]. Since then, several COVID-19 vaccines have been approved and many countries are undertaking mass vaccination drives. As of March 9, 2022, 35 COVID-19 vaccines have been approved across the globe. Sputnik V (Gamaelya), Spikevax (Moderna), Vaxzevria (Oxford/AstraZeneca), Comirnaty (Pfizer/BioNTech), Ad26.CoV2.S (Johnson & Johnson), and Covilo (Sinopharm) are among those COVID-19 vaccines that have received approval in most nations. There are also more than 190 vaccine candidates under different phases of clinical development. Moreover, eleven vaccines have been granted emergency approval status by WHO [7].
As of March 9, 2022, around 11 billion vaccine doses have been administered worldwide. The top four nations where the highest number of doses of COVID-19 vaccines have been given include China (3.17 billion), India (1.79 billion), the United States (0.56 billion), and Indonesia (0.36 billion) [8]. Most vaccines were given emergency use authorization by regulatory authorities [9].
Though all of these vaccines have been found to be efficacious, there is no direct evidence of their comparative efficacies due to a lack of head-to-head clinical trials between vaccines. Therefore, this network meta-analysis was performed to combine phase 3 data of all the published randomized controlled trials to determine the comparative efficacy of COVID-19 vaccines. It aims to answer the natural clinical question of which among the competing existing COVID-19 vaccines shows the maximum protection against symptomatic COVID-19 infection and to comment on their comparative efficacies. This network meta-analysis is expected to provide insight and a piece of valuable evidence-based information to the healthcare community on the same and help taking appropriately informed decisions by the stakeholders.
Methods
The protocol of this systematic review and network meta-analysis was registered in PROSPERO on February 28, 2022 (registration number CRD42022313196). Reporting of this network meta-analysis slightly differs from the protocol with an intent to include updated information and tool for analysis for improving the quality of the work. First, the literature search was performed in Medline, Scopus, Embase and Cochrane library to include recent studies as well. Second, the latest version of ‘Cochrane Risk of Bias tool’ was used for the assessment of the risk of bias for the included studies. Results of this systematic review and network meta-analysis is being reported following ‘Preferred reporting items for systematic review and meta-analysis (PRISMA)-NMA extension 2015 guidelines.
Search strategy
A comprehensive search was made in Medline (via PubMed), Embase and Cochrane Library databases using search terms ‘Covid-19’, ‘SARS-CoV-2’, ‘severe acute respiratory syndrome coronavirus 2’, in combination with ‘vaccines’ and ‘phase 3 clinical trial’. Two review authors (SK and VM) independently reviewed the studies for inclusion/exclusion. Any disagreement between the two was resolved by discussion and/or involving the third review author (DS). Details of the search strategy for PubMed are described in the supplementary material.
Eligibility criteria
Published original research articles of phase 3 randomized controlled trials reporting the efficacy of COVID-19 vaccine(s) in comparison to other COVID-19 vaccines or placebo in adult healthy or diseased participants with chronic stable comorbid conditions were eligible for inclusion. In this network meta-analysis adults are considered as individuals of age ≥ 18 years of age and/or those described as adults in the respective studies. Phase 3 randomized controlled clinical trials were chosen for this network meta-analysis as these trials involve a large enough number of participants and are considered to generate the most reliable evidence of efficacy for the intervention of interest. Articles published till September 05, 2022 were included in the study.
Study outcome(s)
The outcome measure was vaccine efficacy defined as the protection conferred by COVID-19 vaccines against laboratory (nucleic acid amplification i.e. polymerase chain reaction test)-confirmed symptomatic SARS-CoV-2 infection following completion of the dosing regimen. It was measured as a relative risk by using raw data i.e., no. of events (laboratory-confirmed symptomatic COVID-19) in the vaccine and placebo groups in the per-protocol population.
Data extraction and synthesis
Two independent review authors screened the search records based on their titles, abstracts, and full texts. Data were extracted from the included studies on the efficacy of the COVID-19 vaccine using a prespecified format. The data items extracted, include study-related parameters (study author, year of publication, study design, level of blinding, study duration), population-related parameters (age, number of participants receiving intervention, number of participants receiving control), intervention-related parameters (name of intervention/drug, type of intervention, dose/dosage, route of administration), comparator related parameters (name of control, route of administration), outcome-related parameters (primary efficacy outcome(s), number of participants achieving primary efficacy outcomes). Raw data from the included individual studies were used in this network meta-analysis.
Risk of bias and quality assessment
The risk of bias in the included studies was assessed by ‘The Cochrane Risk of Bias tool version 2 (RoB 2)’ by two independent reviewers. Any disagreement was resolved by discussion and/or involvement of a third author. RoB2 tool includes a randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome and selection of the reported results as components for an assessment of the quality of the included studies [10].
Assessment of inconsistency and heterogeneity
Assessment of inconsistency was not applicable as there was no randomized controlled trial comparing two COVID-19 vaccines as direct evidence. Heterogeneity was assessed using Cochrane Q statistics and I2 test.
Statistical analysis.
Network meta-analysis was performed according to PRISMA-NMA extension 2015. Raw data (number of events) from the included individual studies were used to estimate treatment effects (risk ratio). This was followed by a network meta-analysis with a random effect model by estimating indirect evidence between two interventions. The statistical analysis was done using software ‘R’ studio version 4.1.2 and ‘netmeta’ (v 2.1–0)’ package which is based on the frequentist method. The geometry of the treatment network was assessed by the ‘netgraph.netmeta’ function of netmeta package version 2.0–1 of ‘R’ software. ‘P-scores’ were used to rank vaccine efficacy which is based on point estimates and the standard errors and was calculated using ‘netrank {netmeta}’ function. ‘P-score’ (ranges from ‘0’ to ‘1’) indicates, the mean extent of certainty that a treatment is better than the competing treatments. In other words, it shows ‘how better an intervention is relative to all others (a higher score indicates a better rank)’. Rankogram and league table (which indicate the probability of each treatment being at each possible rank) and all pairwise comparisons (direct and indirect) respectively, were also obtained. The asymmetry of included studies (in terms of small and large) was assessed using comparison-adjusted funnel plot.
Results
A total of 460 items were found after a database search using relevant terms. After excluding non-relevant studies a total of 17 studies were included in the systematic review and network meta-analysis [Fig. 1]. These 17 studies [11–27] included 16 vaccines. Characteristics of the studies included are shown in Table 1 (Refer to Supplementary Table 1 for details). The inclusion criteria for the population were studies involving adult human participants. One of the studies of search results included individuals of age more than 16 years considering them as adults. The study was included in the network meta-analysis to avoid the loss of a sufficient magnitude of the sample. Sixteen COVID-19 vaccines were compared with a placebo in these included studies. None of the included studies compared two COVID-19 vaccines directly and therefore geometry of the network graph obtained is of star shape [Fig. 2]. A total of 361,386 participants was included in this network meta-analysis of which 204,390 received an intervention and 156,996 a placebo [Supplementary Table 1].
Fig. 1.
Prisma flow chart showing inclusion and exclusion of studies
Table 1.
Characteristics of various randomised controlled trials comparing efficacy of COVID-19 vaccines with that of placebo, included in network meta-analysis
| Authors’ details & study identifier | Name of vaccine | Sponsor/funding agency | Study design | No. of participants randomizeda | Dosage | Primary Efficacy outcome |
|---|---|---|---|---|---|---|
|
Ella R, et al. 2021 [11] |
BBV152 (Covaxin) | Bharat Biotech International and Indian Council of Medical Research | Randomized double blind placebo controlled |
Vaccine arm: 12899 Placebo arm: 12899 |
6 mcg/dose, Two IM injection 4 weeks apart | Preventing laboratory confirmed symptomatic COVID-19, occurring at least 14 days after the second dose |
| Polack FP, et al. 2020 [12] (NCT04368728) | BNT162b2 | BioNTech and Pfizer | Randomized observer blind placebo controlled |
Vaccine: 21720 Placebo: 21728 |
30 mcg/dose, Two IM injections, 21 days apart | Preventing laboratory confirmed symptomatic COVID-19 at least after 7 days after second dose |
| Sadoff J, et al. 2022 [13] (NCT04505722) | Ad26.COV2.S | Janssen Research and Development and others | Randomized double blind placebo controlled |
Vaccine arm: 21895 Placebo arm: 21888 |
5 × 1010 viral particles, single IM injection | Prevention against moderate to severe–critical COVID-19 disease at least 14 days after administration of vaccine |
| Heath PT, et al. 2021 [14] (EudraCT number, 2020–004123-16) | NVX-CoV2373 | Novavax | Randomized observer blind placebo controlled |
Vaccine arm: 7593 Placebo arm: 7594 |
5 mcg/doses, Two IM injection administered 21 days apart | Lab confirmed symptomatic infection with an onset at least 7 days after the second dose |
| Dunkle LM, et al. 2021 [15] (NCT04611802) | NVX-CoV2373 | Novavax and others | Randomized observer blind placebo controlled |
Vaccine arm: 19965 Placebo arm: 9984 |
5 mcg/doses, Two IM injection administered 21 days apart | Lab confirmed symptomatic infection with an onset at least 7 days after the second dose |
| El Sahly HM, et al. 2021 [16] (NCT04470427) | mRNA-1273 | Bio-medical Advanced Research and Development Authority and the National Institute of Allergy and Infectious Diseases | Randomized observer blind placebo controlled |
Vaccine arm: 15209 Placebo arm: 15206 |
100 mcg/dose, Two IM injections 28 days apart | Preventing laboratory-confirmed symptomatic COVID-19 at least after 14 days after the second dose |
| Al Kaabi N, et al. 2021 [17] (NCT04510207) | WIV04 | National Key Research and Development Project of China, Wuhan Institute of Biological Products Co. Ltd., the Beijing Institute of Biological Products Co, Ltd | Randomized double-blind alum controlled |
WIV04: 13470 HB02: 13470 Alum: 13471 |
5 mcg/dose, 2 intramuscular injections 21 days apart | Preventing laboratory-confirmed symptomatic COVID-19 at least 14 days after the second dose |
| Al Kaabi N, et al. 2021 [17] (NCT04510207) | HB02 | National Key Research and Development Project of China, Wuhan Institute of Biological Products Co. Ltd., the Beijing Institute of Biological Products Co, Ltd | Randomized double-blind alum controlled |
WIV04: 13470 HB02: 13470 Alum: 13471 |
4 mcg/dose 2 intramuscular injections 21 days apart | Preventing laboratory-confirmed symptomatic COVID-19 at least 14 days after the second dose |
| Falsey AR, et al. 2021 [18] (NCT04516746) | ChAdOx1 nCoV-19 | AstraZeneca and others | Randomized double-blind placebo-controlled |
Vaccine arm: 21635 Placebo arm: 10816 |
5 × 1010 viral particles/dose two intramuscular injections 4 weeks apart | Preventing laboratory-confirmed symptomatic COVID-19 at least after 15 days after the second dose |
| Tanriover MD, et al. 2021 [19] (NCT04582344) | CoronaVac | Turkish Health Institutes Association | Randomized double-blind placebo controlled |
Vaccine arm: 6650 Placebo arm: 3568 |
3 mcg/dose 2 intramuscular injections 14 days apart | Preventing laboratory-confirmed symptomatic COVID-19 at least after 14 days after second dose |
| Fadlyana E, et al. 2021 [20] (NCT04508075) | CoronaVac | PT Bio Farma | Randomized double-blind placebo controlled |
Vaccine arm: 810 Placebo arm: 810 |
3 mcg/dose, intramuscular injections 14 days apart | Preventing laboratory-confirmed symptomatic COVID-19 at least after 14 days after second dose |
| Halperin SA, et al. 2022 [21] (NCT04526990) | Ad5-nCoV | CanSino Biologics and the Beijing Institute of Biotechnology | Randomized double-blind placebo controlled |
Vaccine arm: 18493 Placebo arm: 18489 |
2.5 × 1010 viral particle/dose, single IM injection | Preventing laboratory-confirmed symptomatic COVID-19 at least after 28 days after a single dose |
| Bravo L, et al. 2022 [22] (NCT04672395) | SCB 2019 | Clover Biopharmaceuticals and the Coalition for Epidemic Preparedness Innovations | Randomized double-blind placebo controlled |
Vaccine arm15092 Placebo arm15082 |
30 mcg/dose, two IM injections 21 days apart | Preventing laboratory-confirmed symptomatic COVID-19 at least after 14 days after the second dose |
|
Logunov DY, et al. 2021 [23] |
rAd 26 & rAd 5 | Moscow City Health Department, Russian Direct Investment Fund, and Sberbank | Randomized double-blind placebo controlled |
Vaccine arm: 16501 Placebo arm: 5476 |
First and second doses; rAd26 and rAd5, 1011 particles per dose, IM, 0.5 mL, 21 days apart | Preventing laboratory-confirmed symptomatic COVID-19 at least 21 days after the first dose |
| Dai L, et al. 2022 [24] | ZF2001 | National Science and Technology Major Project and National Natural Science Foundation of China | Randomized double-blind placebo controlled |
Vaccine arm: 14,453 Placebo arm: 14,451 |
25 mcg ZF2001 vaccine, 3 IM doses each 30 days apart | Preventing laboratory-confirmed symptomatic Covid-19 at least after 7 days after third dose |
| Khairullin B, et al. 2022 [25] | QazCovid-in | Science Committee of the Ministry of Education and Science of Kazakhstan | Randomized single-blind placebo controlled |
Vaccine arm: 2400 Placebo arm: 600 |
5 mcg, 2 IM doses 21 days apart | Preventing laboratory-confirmed symptomatic Covid-19 at least after 14 days after the first dose |
| Hager KJ, et al. 2022 [26] | CoVLP + AS03 | Medicago | Randomized double-blind placebo controlled |
Vaccine arm: 12074 Placebo arm: 12067 |
3.75 mcg CoVLP + AS03, 2 IM doses 21 days apart | Preventing laboratory-confirmed symptomatic Covid-19 at least after 7 days after second dose |
| Kremsner PG, et al. 2022 [27] | CVnCoV SARS-CoV-2 | German Federal Ministry of Education and Research and CureVac | Randomized observer blind placebo controlled |
Vaccine arm: 19846 Placebo arm: 19834 |
12 μg of CVnCoV, 2 IM doses 28 days apart | Preventing laboratory-confirmed symptomatic Covid-19 at least after 15 days after second dose |
(Refer Supplementary Table 1 for details). All the included studies contained a statement on declaration of (conflict of) interest by authors
mcg microgram, IM intramuscular, COVID−19 Coronavirus Disease 2019
aNote: number of participants shown in the table are those who were randomly assigned one of the two arms and not those who received treatment or number used in the analysis
Fig. 2.

Network plot of studies included in the network meta-analysis. Line width represents inverse standard error of random effect model comparing two treatments. The numerals on the lines represent the number of studies between the respective two treatment arms
Forest plots for comparative efficacy of direct and indirect evidence of COVID-19 vaccines are shown in Figs. 3 & 4, respectively, and in the form of a league table [Supplementary Table 2]. The ranking of individual vaccines for the study outcome against all competing vaccines is shown as a ‘P’ score [Table 2] and a Rankogram plot [Supplementary Fig. 1]. The highest ‘P’ score is for BNT126b2 and the least for the CVnCoV SARS-Cov-2 vaccine.
Fig. 3.
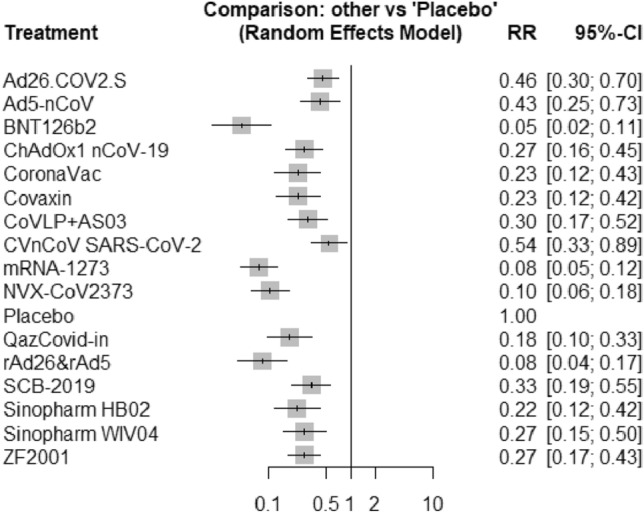
Forest plot of intervention (COVID-19 vaccines) in comparison to placebo in various clinical trials included in the network meta-analysis. RR relative risk, CI confidence interval
Fig. 4.

Forest plot of indirect comparison of different pairs of various COVID-19 vaccines through a network meta-analysis. RR relative risk, 95%-CI 95% confidence interval
Table 2.
‘P’ score of various vaccines: used to rank treatment in network meta-analysis
| Vaccine | ‘P’ score |
|---|---|
| BNT126b2 | 0.9771 |
| mRNA-1273 | 0.9087 |
| rAd26&rAd5 | 0.8808 |
| NVX-CoV2373 | 0.8345 |
| QazCovid-in | 0.6589 |
| Sinopharm HB02 | 0.5614 |
| CoronaVac | 0.5491 |
| Covaxin | 0.5487 |
| ZF2001 | 0.4532 |
| ChAdOx1nCoV-19 | 0.4512 |
| Sinopharm WIV04 | 0.4465 |
| CoVLP + AS03 | 0.3959 |
| SCB-2019 | 0.3406 |
| Ad5-nCoV | 0.2088 |
| Ad26.COV2.S | 0.1694 |
| CVnCoV SARS-CoV-2 | 0.1146 |
| Placebo | 0.0006 |
‘P’ score denotes the mean certainty of a vaccine to be better/worse relative to others
The Cochrane ‘Q’ statistics were found to be 2.73, which was not significant (p = 0.25). Variability between designs was shown to be ‘zero’ as there is no direct comparison between vaccines. I2 value was 26.8% denoting the absence of much heterogeneity among the studies [Supplementary Fig. 2].
RoB2 tool assesses the risk of bias under five major domains namely ‘bias arising from randomisation process (D1)’, ‘bias due to deviation from intended intervention (D2)’, ‘bias due to missing outcome data (D3)’, ‘bias in measurement of the outcome (D4)’ and ‘bias in selection of the reported results (D5)’. All of the studies used the per-protocol method for data analysis instead of intention-to-treat, therefore, had some concern under the D2 domain except for one study (Khairullin B, et al. 2022) which was of low risk under this domain. One study (Khairullin B, et al. 2022) has some concerns under D3, D4 and D5 domains. One study (Fadlyana E, et al. 2021) had some concerns under the D5 domain. Study by Hager KJ, et al. 2022 had some concerns under D1 domain. All studies had low risk under the remaining domains. The overall risk of bias assessment revealed ‘some concern’. [Supplementary Fig. 3].
Comparison adjusted funnel plot suggests a minimal small study effect [Supplementary Fig. 4]. However, because of a smaller number of included studies the power of the tests of asymmetry may decrease and, therefore, results should be interpreted cautiously.
Discussion
We performed this network meta-analysis in an effort to find the most efficacious vaccine against SARS-CoV-2 infection as none of the COVID-19 vaccines has been compared directly till now. Though two network meta-analyses on COVID-19 vaccines have been performed, in the current one we included the highest number of COVID-19 vaccines whose phase 3 clinical trial data have been published.
The added information from this network meta-analysis has been obtained at the cost of additional assumptions of transitivity and consistency. However, the characteristics of the included studies are sufficiently akin to allow the pooling of the data. The consistency of the network could not be assessed due to the lack of information on any direct study comparing two vaccines. Since there is no clinically relevant heterogeneity, it was not explored further.
To date, one recent meta-analysis reported the results of phase 3 clinical trials of five vaccines [28]. The overall vaccine efficacy to prevent SARS-CoV-2 infection obtained in this study was 89.1% (95% CI 85.6–92.6%) against the placebo. BNT162b2 mRNA COVID- 19 vaccine (Pfizer-BioNTech) and mRNA-1273 vaccine (Moderna) showed the highest summary vaccine efficacy (98.1% & 91.2% respectively). However, using the Network meta-analysis techniques, multiple treatment interventions can be compared indirectly without performing comparative clinical trials of different therapy interventions in a single statistical analysis, unlike the conventional meta-analysis, in which only two interventions can be compared that are directly reported/used in randomized clinical trials [29].
In one of the earlier network metanalyses, indirect comparison of seven COVID-19 was performed based on the results of phase 3 randomized controlled trials to find the vaccine with the highest probability of reducing symptomatic COVID-19. The vaccine with the highest probability of efficacy was BNT162b2-Corminarty vaccine with a P-score of 90.6%), followed by mRNA-1273-Spikevax, Gam-COVID-Vac-Sputnik-V, NVX-CoV2373-Novavax, Ad26.COV2.S-Janssen, ChAdOx1 nCoV-19-Vaxzevria, and CoronaVac (P-scores 88.8%, 76.5%, 50.7%, 37.7%, 28.5% and 25.1%, respectively) [30]. Similarly, in another network meta-analysis, published in November 2021, nine COVID-19 vaccines were compared indirectly, and the candidate vaccine with the greatest likelihood of being most effective against symptomatic SARS-CoV-2 infection was BNT162b2, followed by the mRNA-1273 vaccine, Gam-COVID-Vac, NVX-CoV23730, CoronaVac, BN02, WIV04, ChAdOx1 and Ad26.COV2.S (P-scores 0.952, 0.843, 0.782, 0.700, 0.570, 0.428, 0.327, 0.199 and 0. 0.198, respectively) [31].
In line with these previously published network meta-analyses, our results indicate BNT126b2 as a COVID-19 vaccine conferring the maximum reduction in the risk to contract symptomatic SARS-CoV-2 infection in comparison to placebo with a relative risk of 0.05 followed by mRNA1273 and rAd26 & rAd5, both of which had a relative risk of 0.08 against placebo. Further, the indirect evidence, generated by the present network meta-analysis, does not indicate a significant difference in the efficacy of BNT126b2 in comparison to that of mRNA-1273, rAd26 + rAd5 and NVX-CoV2373. On the other hand, the BNT126b2 vaccine was found to be of significantly greater efficacy compared to SCB-2019, ChAdOx1 nCoV-19, Sinopharm, Covaxin and Coronvac, to prevent symptomatic SARS-CoV-2 infection. BNT126b2 vaccine had the highest probability to hold the rank ‘1’ in the rankogram and also had the highest ‘P’ score. While interpreting the results, it is important to remember that risk of bias in all the included studies is of ‘some concern’, as stated earlier.
One of the possible explanations for results suggesting that mRNA technology-based COVID-19 vaccines could be the most efficacious against symptomatic SARS-CoV-2 infection is helping the immune cells remember the infection for a long time apart from generating a good neutralizing antibody response, leading to longer-lasting immunity against Coronavirus.
Thus the present piece of work through network meta-analysis shows BNT126b2, amongst all the included vaccines, to provide the highest magnitude of protection against contracting symptomatic SARS-CoV-2 infection followed by mRNA-1273, rAd26 + rAd5 and NVX-CoV2373 vaccines.
Strengths of the study
To the best of our knowledge, the highest number of COVID-19 vaccines and randomized controlled phase 3 trial data are used in this network meta-analysis to generate an evidence of the efficacy of COVID-19 vaccines amongst themselves in absence of any direct comparison. This network meta-analysis focused on one outcome only, at a time, to not compromise the quality of the analysis. There is no publication restriction on behalf of authors.
Limitations of the study
The assessment of consistency was not possible as there was no study to compare two or more vaccines directly. So, our network meta-analysis is based on indirect comparison only, amongst the included vaccines. Though the vaccines have been compared only on the basis of their efficacies, a number of other factors such as dose and frequency of administration, safety, and cost-effectiveness have an important role in the selection of vaccines. Most of the included studies have similar efficacy outcomes, however, they are different in terms of timelines of their conduction during the pandemic, the number of doses of vaccines and the follow-up period for assessment of efficacy. The grading for the certainty of evidence has not been performed so the conclusion from the study should be interpreted cautiously. This network meta-analysis included the efficacy outcome from included studies irrespective of these factors. Further, the efficacy of these vaccines against several new strains and variants of concern (delta, omicron, etc.) is either unknown or limited; hence any statement about comparability in relation to specific strains cannot be made.
Conclusion & recommendation
This network meta-analysis finds that BNT162b2 followed by mRNA-1273, rAd26&rAd5, and NVX-Cov2373 to confer the highest protection against symptomatic COVID-19 compared to others. Besides, they were not found significantly different from each other. In this light, a wise decision should be taken while choosing a particular vaccine against COVID-19 before considering the indirect evidence(s) generated through network meta-analyses.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Dr. SK: Conceptualization, Data extraction, data analysis, manuscript drafting, final approval of the manuscript. Dr. DS: Manuscript editing, reviewing scientific content, final approval of the manuscript. Dr. MB: Manuscript writing, reviewing scientific content, final approval of the manuscript. Dr. MKS: Conceptualization, Data extraction, reviewing scientific content, final approval of the manuscript. Dr. HS: Manuscript editing, reviewing scientific content, final approval of the manuscript. Dr. SRV: Manuscript editing, reviewing scientific content, final approval of the manuscript. Dr. VM: Conceptualization, Data extraction, manuscript writing, manuscript editing, final approval of the manuscript. All the authors originally contributed to this study and/or preparation of the manuscript and fulfil the authorship criteria.
Funding
No funding was received to conduct this network meta-analysis.
Availability of data and material
Data and supplementary material (if any) will be available with the corresponding author and may be accessed on reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interest
All authors declare that they do not have any conflict of interest to declare.
Ethical approval
Exempted/Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
References
- 1.Rashedi R, Samieefar N, Masoumi N, Mohseni S, Rezaei N. COVID-19 vaccines mix-and-match: the concept, the efficacy and the doubts. J Med Virol. 2022;94:1294–1299. doi: 10.1002/jmv.27463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen JM. Should the world collaborate imminently to develop neglected live-attenuated vaccines for COVID-19? J Med Virol. 2022;94:82–87. doi: 10.1002/jmv.27335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Coronavirus (COVID-19) Dashboard [Internet]. WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. covid19.who.int; [cited 2022 Mar 10]. Available from: https://covid19.who.int/.
- 4.Kumar S, Saurabh MK, Maharshi V. Efficacy and safety of potential vaccine candidates against coronavirus disease 2019: a systematic review. J Adv Pharm Technol Res. 2021;12:215–221. doi: 10.4103/japtr.JAPTR_229_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S, Saurabh MK, Maharshi V, Saikia D. A narrative review of anti-viral drugs used for COVID-19 pharmacotherapy. J Pharm Bioall Sci. 2021;13:163–171. doi: 10.4103/jpbs.JPBS_498_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bian L, Gao F, Zhang J, He Q, Mao Q, Xu M, Liang Z. Effects of SARS-CoV-2 variants on vaccine efficacy and response strategies. Expert Rev Vaccines. 2021;20:365–373. doi: 10.1080/14760584.2021.1903879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaccines – COVID19 Vaccine Tracker [Internet]. Vaccines – COVID19 Vaccine Tracker. covid19.trackvaccines.org; [cited 2022 Mar 10]. Available from: https://covid19.trackvaccines.org/vaccines/
- 8.Ritchie H, Mathieu E, Rodés-Guirao L, Appel C, Giattino C, Ortiz-Ospina E, Hasell J, Macdonald B, Beltekian D, Roser M. Coronavirus (COVID-19) Vaccinations - Our World in Data [Internet]. Our World in Data. ourworldindata.org; [cited 2022 Mar 11]. Available from: https://ourworldindata.org/covid-vaccinations?country=OWID_WRL.
- 9.Krause PR, Gruber MF. Emergency use authorization of Covid vaccines - safety and efficacy follow-up considerations. N Engl J Med. 2020;383:e107. doi: 10.1056/NEJMp2031373. [DOI] [PubMed] [Google Scholar]
- 10.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 11.Ella R, Reddy S, Blackwelder W, Potdar V, Yadav P, Sarangi V, et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet. 2021;398:2173–2184. doi: 10.1016/S0140-6736(21)02000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, ENSEMBLE study group et al. Final analysis of efficacy and safety of single-dose Ad26COV2S. N Engl J Med. 2022;386(9):847–860. doi: 10.1056/NEJMoa2117608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunkle LM, Kotloff KL, Gay CL, Áñez G, Adelglass JM, Barrat Hernández AQ, et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med. 2022;386:531–543. doi: 10.1056/NEJMoa2116185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021;385:1774–1785. doi: 10.1056/NEJMoa2113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 Infection in adults: a randomized clinical trial. JAMA. 2021;326:35–45. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 Vaccine. N Engl J Med. 2021;385:2348–2360. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2022;2021(398):213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fadlyana E, Rusmil K, Tarigan R, Rahmadi AR, Prodjosoewojo S, Sofiatin Y, et al. A phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-CoV-2 inactivated vaccine in healthy adults aged 18–59 years: an interim analysis in Indonesia. Vaccine. 2021;39:6520–6528. doi: 10.1016/j.vaccine.2021.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halperin SA, Ye L, MacKinnon-Cameron D, Smith B, Cahn PE, Ruiz-Palacios GM, et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet. 2022;2022(399):237–248. doi: 10.1016/S0140-6736(21)02753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bravo L, Smolenov I, Han HH, Li P, Hosain R, Rockhold F, et al. Efficacy of the adjuvanted subunit protein COVID-19 vaccine, SCB-2019: a phase 2 and 3 multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2022;399:461–472. doi: 10.1016/S0140-6736(22)00055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;2021(397):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai L, Gao L, Tao L, Hadinegoro SR, Erkin M, Ying Z, ZF2001 Global trial group et al. Efficacy and safety of the RBD-dimer-based Covid-19 vaccine ZF2001 in adults. N Engl J Med. 2022;386(22):2097–2111. doi: 10.1056/NEJMoa2202261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khairullin B, Zakarya K, Orynbayev M, Abduraimov Y, Kassenov M, Sarsenbayeva G, et al. Efficacy and safety of an inactivated whole-virion vaccine against COVID-19, QazCovid-in®, in healthy adults: a multicentre, randomised, single-blind, placebo-controlled phase 3 clinical trial with a 6-month follow-up. EClinicalMedicine. 2022;25(50):101526. doi: 10.1016/j.eclinm.2022.101526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hager KJ, Pérez Marc G, Gobeil P, Diaz RS, Heizer G, Llapur C, CoVLP study team et al. Efficacy and safety of a recombinant plant-based adjuvanted Covid-19 vaccine. N Engl J Med. 2022;386(22):2084–2096. doi: 10.1056/NEJMoa2201300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kremsner PG, Ahuad Guerrero RA, Arana-Arri E, Aroca Martinez GJ, Bonten M, Chandler R, HERALD study group et al. Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate in ten countries in Europe and Latin America (HERALD): a randomised, observer-blinded, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis. 2022;22(3):329–340. doi: 10.1016/S1473-3099(21)00677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng C, Shao W, Chen X, Zhang B, Wang G, Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. 2022;114:252–260. doi: 10.1016/j.ijid.2021.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med. 2017;12:103–111. doi: 10.1007/s11739-016-1583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korang SK, von Rohden E, Veroniki AA, Ong G, Ngalamika O, Siddiqui F, Juul S, Nielsen EE, Feinberg JB, Petersen JJ, Legart C, Kokogho A, Maagaard M, Klingenberg S, Thabane L, Bardach A, Ciapponi A, Thomsen AR, Jakobsen JC, Gluud C. Vaccines to prevent COVID-19: a living systematic review with trial sequential analysis and network meta-analysis of randomized clinical trials. PLoS ONE. 2022;17:e0260733. doi: 10.1371/journal.pone.0260733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rotshild V, Hirsh-Raccah B, Miskin I, Muszkat M, Matok I. Comparing the clinical efficacy of COVID-19 vaccines: a systematic review and network meta-analysis. Sci Rep. 2021;11:22777. doi: 10.1038/s41598-021-02321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and supplementary material (if any) will be available with the corresponding author and may be accessed on reasonable request.
Not applicable.


