Abstract
Purpose of Review
Breast cancer screening is highly controversial and different agencies have widely varying guidelines. Yet it is currently used extensively in the USA and frequently the thought is “the more, the better.” The purpose of this review is to objectively assess the risks and benefits of screening mammography and consider whether there may be areas where it could be de-escalated.
Recent Findings
Over the past few years, there have been several meta-analyses that are concordant, and it is now agreed that the main benefit of screening mammography is about a 20% reduction in breast cancer mortality. This actually benefits about 5% of patients with mammographically detected tumors. We now appreciate that the main harm of screening is overdiagnosis, i.e. detection of a cancer that will not cause the patient any harm and would not have ever been detected without the screening. This currently represents about 20 to 30% of screening detected cancers. Finding extra cancers with more intense screening is not always good, because in this situation, the risk of overdiagnosis increases and the benefit decreases. In some groups, the risk of overdiagnosis approaches 75%.
Summary
Our goal should be not only to find more cancers, but to avoid finding cancers that would never have caused the patient any harm and lead to unnecessary treatment. The authors suggest some situations where it may be reasonable to de-escalate screening.
Keywords: Breast cancer screening, Overdiagnosis, Harms of breast cancer screening, De-escalation of breast cancer screening
Introduction
According to the American Cancer Society, roughly 287,500 women will be diagnosed with invasive breast cancer, and 43,250 women will die from it in 2022 in the USA [1]. As the most common non-skin cancer in women in the USA, breast cancer is diagnosed in 1 in 8 women. Due to its substantial health risk, population-based screening mammography programs have been widely introduced since the 1980s, and many studies have shown a reduction in breast cancer-related mortality ranging from 0 to 40% [2]. According to a systematic review by Myers et al., women of all ages at average risk experience a 20% reduction in breast cancer mortality thanks to screening mammography [3•].
Although screening mammography has improved the lives of countless women worldwide, it is not a perfect tool and has associated risks including overdiagnosis, false positive results, overtreatment, radiation exposure, and psychosocial burdens of stress and anxiety [4–6]. As a result, this topic has become heavily controversial and become the center for many recent debates in both the medical field and public media. Therefore, these risks pose the question of whether patients would benefit from the de-escalation of screening mammography and subsequent treatment. The purpose of this review is to discuss the major benefits and harms associated with screening mammograms, and to identify potential areas where de-escalation might be possible.
Epidemiologic Methodology Used for Evaluation of Screening Mammography
The epidemiologic methodology appropriate to evaluation of screening mammography has been extensively described and is widely accepted [7, 8]. In almost every hospital in the world with a mammography screening program, breast cancer patients diagnosed by mammography have a much better survival and require less treatment compared to patients who are diagnosed because of clinical breast symptoms. Therefore, the everyday experience of both physicians and patients is that mammography leads to earlier diagnosis and improved cure rates. But does this prove that screening mammography is beneficial? The answer, surprisingly, is no. There are a number of large biases that account for the majority of the perceived improvement in survival. The first is lead time bias. The lead time is the time between when a cancer can be detected mammographically and the time when it would otherwise be discovered clinically. We now know that this varies tremendously in different tumors from less than a year to over 30 years. However, if the average lead time in a group of patients diagnosed by mammography is 5 years, those patients will live 5 years longer than a group detected clinically without any change in the natural history of the disease. The second large bias is length time bias. Screening mammography is much more likely to detect slowly growing tumors with a long lead time than faster growing tumors [9, 10]. Thus, even when matched for tumor size, mammographically detected tumors will have more favorable biology and better survival [11]. In addition, there are other selection biases because patients who are healthy or have better socioeconomic resources are much more likely to have screening mammography and also independently more likely to receive better care and be cured.
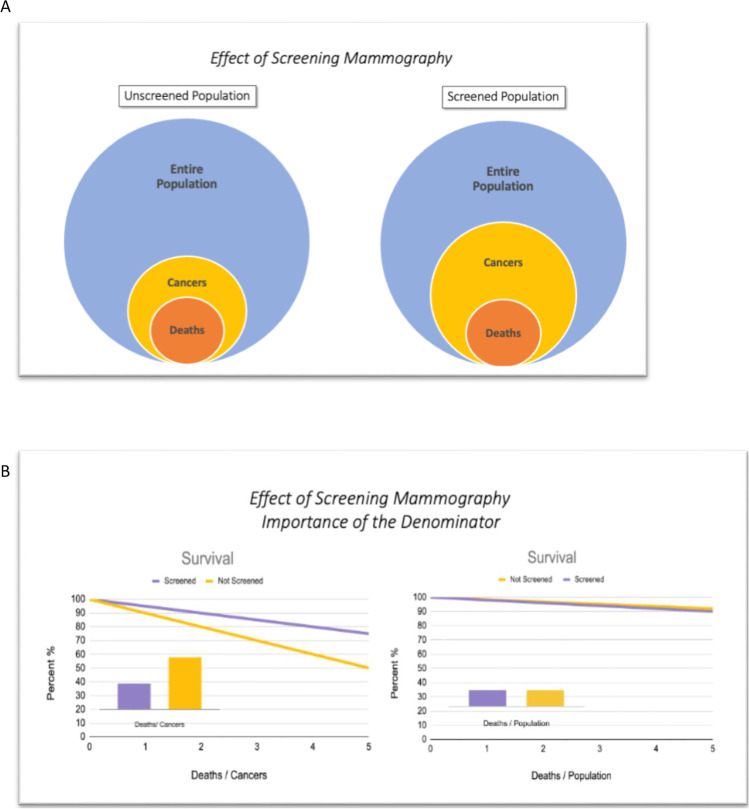
The only way to get around these biases is to change the denominator in the survival curves from the individual cancer patient to the population from which the patient is drawn. Figure 1 illustrates this concept. Screening always results in an increased number of cancers detected, both because of the lead time discussed above and also because of overdiagnosis which will be discussed below. As seen in the figure, even if there is no actual reduction in deaths within the population, the deaths per cancer patient will always be reduced with screening. The real question we want to know, however, is whether the deaths per patient in the population is reduced by screening. This benefit, if it exists, will be smaller and harder to detect. It requires large population-based studies, and there have only been about eight of these ever performed, all in the 1960s to 1980s. Much of the controversy, therefore, revolves around how to interpret these old studies. Yet, because screening mammography is now so engrained in our medical practice, there will probably never be another similar study performed.
Fig. 1.
Effect of screening mammography. A The screened group always has an increased number of cancers due to both lead time and overdiagnosis. B This illustration assumes no true reduction in cancer deaths but nevertheless the standard survival curves (deaths/cancer) show a large benefit for screening. Only large population-based studies (deaths/population) can determine whether there is really a benefit for screening
Benefits and Harms of Mammography
Mortality Benefit
There are multiple studies discussing the benefits and harms of mammography, but the single most cited benefit is breast cancer-related mortality reduction. According to Shepardson and Dean, three separate meta-analyses of the eight randomized controlled trials showed an 18–20% reduction in mortality among women who were invited to screen [2]. These findings were echoed by a systematic review by Myers et al. in 2015, who saw an overall 20% breast cancer-related mortality reduction in women of all ages at average risk based on pooled estimates of meta-analyses of RCTs: UK Independent Panel (RR 0.80 [95% CI, 0.73–0.89]), Canadian Task Force (RR 0.82 [95% CI, 0.74–0.94]), and Cochrane Database (RR, 0.81 [95% CI, 0.74–0.87]) [3•]. Pace and Keating performed a similar review and concluded that there was a 19% reduction in mortality [12]. Therefore, it is clear that early detection of breast cancer through population-based screening has had a positive contribution to the health of women in the USA and worldwide. A reduction of 20%, however, is actually quite small; it means 80% of women who would die without mammography will still die with it.
Benefit of Less Aggressive Treatment
An additional benefit not commonly discussed is that breast cancers found by screening mammography are smaller, less likely to metastasize to lymph nodes, and are more likely to be effectively treated with breast conservation therapy and without chemotherapy [13, 14]. These findings have a significant impact on a patient’s physical and mental health as less intensive treatments lead to less toxicity, faster recovery, and fewer complications. However, if the overdiagnosed cancers which currently are over-treated are excluded from the screening group, then the benefit for the remaining patients is much less apparent [15].
Harms
Multiple societies in the USA ranging from the US Preventative Services Task Force (USPSTF) [16•], National Comprehensive Cancer Network (NCCN) [17], American College of Obstetrics and Gynecology (ACOG) [18], American Cancer Society (ACS) [19], and American College of Radiology (ACR) [20] differ in their screening recommendations due to the limitations and harms of population-based screening mammography. Some of the most referenced harms are overdiagnosis, radiation exposure, pain during mammography, false positive results, and psychosocial distress.
Radiation Exposure
Both digital mammogram and DBT confer a low dose of radiation that are within FDA limits. A study conducted in 2018 demonstrated that the average radiation dose was 2.74 mGy for each breast in a 2-view study. There is an argument that the radiation from serial screenings may cause breast cancer. However, Miglioretti et al. modeled that screening women aged 40–75 annually might induce an average of 125 breast cancers but avoid 968 breast cancer deaths due to early detection [21]. In other words, 1 radiation-induced breast cancer could avert 8 breast cancer deaths. On the same tone, Hendrick suggests that a single 3-mGy digital mammogram has the same risk of causing cancer as 2 months of natural background radiation in the USA [22]. Therefore, the benefit-to-radiation risk is overall still in favor for screening mammography. However, physicians should weigh the risks individually with each patient when discussing screening.
False Positive Results
A second harm associated with screening mammography is potential for false positive test results. These lead to a potential cascade of additional diagnostic imaging, possible benign breast biopsies, psychological distress, and increased utilization of health care resources. In the USA, the 10-year false positive rate with annual screening has been reported at 61% [23]. And unfortunately, a false positive mammogram could take up to 2 years for a patient to be declared cancer-free [24]. Furthermore, research has shown that women experience psychological distress for at least 3 years after screening [25]. Luckily, there have been advances in screening technology to improve the sensitivity and specificity of mammography. Digital breast tomosynthesis is a 3-dimensional image acquisition platform that has helped address this issue by reducing recall rates by 15–17% when compared to conventional 2-D mammography [2, 26].
Overdiagnosis
Over the past decade, we have realized that overdiagnosis is the most significant harm of breast cancer screening. Since the intention of screening is to detect cancers early, inevitably some screen detected cancers will be found so early that they never become clinically symptomatic within a patient’s lifetime, and this is referred to as overdiagnosis [2, 27]. Cancers grow at variable rates and some progress slower than others, some remain static, and some may even regress [28]. Unfortunately, our ability to predict which cancers are indolent is very limited. Since we can never be certain which cancers are overdiagnosed, all screen detected cancer leads to interventions such as surgery, radiation, and adjuvant chemo and endocrine therapy which all contribute to a patient’s physical and psychosocial morbidity [29].
Estimating the frequency of overdiagnosis has turned out to be complicated and controversial, and studies have reported rates from 0 to 50% [27–29]. This wide divergence is due to varying methodology and whether are not DCIS is considered overdiagnosed. An important principle in reliably estimating overdiagnosis is the concept that overdiagnosis must be differentiated from early diagnosis by using a long follow-up period to accommodate for lead time [30]. There are two main types of evidence suggesting overdiagnosis: population studies and clinical trials.
The first suggestion of overdiagnosis was described by Esserman et al. in a 2009 study of breast cancer incidence in the Surveillance, Epidemiology, and End Results (SEER) database [31•]. With the introduction of screening mammography in the 1980s, the incidence of breast cancer in the USA quickly increased about 40%. If this were due to early detection alone, we would expect that after the lead time of the detected cancers, the incidence would decline back to baseline. For the next 40 years, the incidence has not dropped which far surpasses the reasonable lead times and suggests that much of the increase was due to overdiagnosis. Bleyer and Welch [32••] and Welch et al. [33••] studied this in more detail in a couple of classic articles in the New England Journal of Medicine. They found that small and early stage cancer increased markedly whereas large or late stage cancer declined only slightly. This suggests that a large percent of small tumors are not destined to become large tumors. They estimated that the rate of overdiagnosis was 22% for invasive cancers and 31% if DCIS is included. Similarly when screening mammography was introduced into Norway and Sweden, the breast cancer incidence doubled and the authors estimated that one third of invasive cancers were overdiagnosed [34].
There is also important evidence from the randomized trials of mammography screening. According to systematic reviews by the Independent UK Panel [29] and Nelson et al. [6], the most reliable estimates for overdiagnosis are drawn from three commonly cited randomized control trials: Malmo I (women aged 55–69) [35], and the Canadian National Breast Screening Study I (women aged 40–49) [36] and II (women aged 50–59) [37]. These three trials screened women for 5 years during the study and did not systematically invite them to screening at the end of the trial. At 15-year follow-up, the Malmo trial had a rate of overdiagnosis of 10% for invasive tumors. A recently published long-term follow-up of the 2 Canadian trials 25 years after enrollment indicated that among women age 40–49 the rate of overdiagnosis was 30% for invasive tumors and 40% if DCIS is included, and for women age 50–59 it was 20% for invasive tumors and 30% including DCIS [38•].
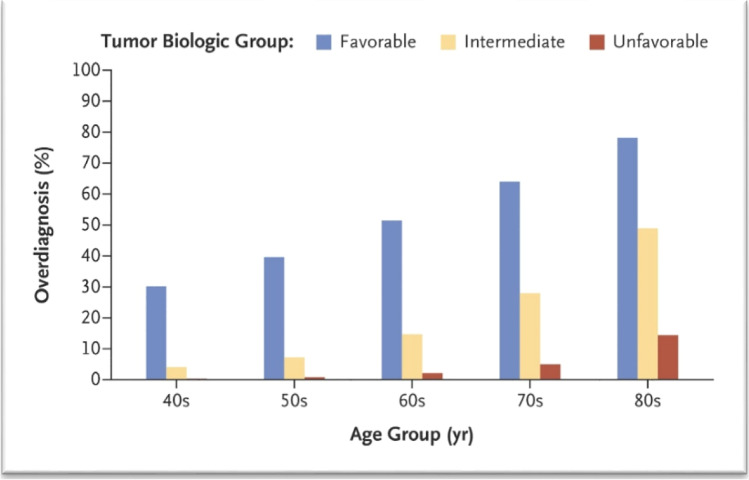
We now understand the mechanism for the overdiagnosis [39••]. There is much more variability in the lead time for different tumors than was previously appreciated; it ranges from <1 year to over 30 years. Overdiagnosis results when the lead time for a tumor exceeds the life expectancy. Two factors are critical: tumor biology and patient age. Figure 3 shows an estimate of the rate of overdiagnosis for different age groups by a measure of tumor biology based on grade and hormone receptor status. Overdiagnosis is most common in older women with biologically favorable tumors. This information may point to areas where we might consider de-escalation of mammography screening.
Fig. 3.
Percent overdiagnosis by patient age and tumor biology. Favorable tumors were low grade and ER and PR positive. (From New England Journal Medicine, Lannin DR and Wang S. Are small breast cancers good because they are small or small because they are good? 376(23):2286–2291.
Copyright © 2017 Massachusetts Medical Society. Reprinted with permission.)
Natural History of Current Invasive Cancers Found on Mammography
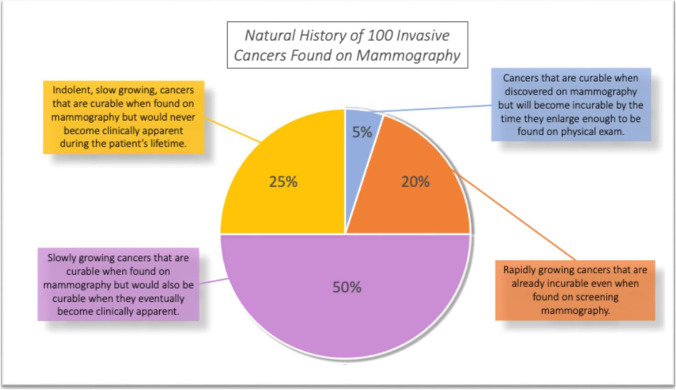
Figure 2 summarizes the natural history of current cancers found by mammography. The figure starts with current SEER data that have shown that over the past decade the mortality for invasive breast cancers has remained stable at about 20%. Then the data for mortality reduction from mammography and the data for overdiagnosis are overlayed on the 80% who currently survive. The results show that about half of current patients will have their tumor cured but would also be cured if they waited a few years until it became clinically apparent. These data match nicely the mortality from prior to the introduction of mammography in the 1980s [40]. Another 25%, the overdiagnosed cancers, will be cured but would never have even known they had cancer if it were not for mammography. About 20% will die of their cancer either with or without mammography; only 5% (a 20% reduction from 25%) will have their life actually saved by the mammogram. This 5% amounts to about 10,000 women in the USA annually, so it is clearly important. However, all 80% who are cured (about 200,000 women annually in the USA) think they have been cured because of the mammogram and it just is not so. Furthermore, treatments for breast cancer are improving dramatically and as the treatments get better, the value of early detection is reduced. In the future, at some point, the treatments may be good enough that we will no longer need screening.
Fig. 2.
Natural history of current mammography detected invasive cancers. Recent SEER data showing 20% mortality for invasive tumors were overlayed with the mammography mortality reduction of 20% and the overdiagnosis rate of 25%. Only 5% had a cancer that was cured because of the mammography. This represents a 20% (5/25) reduction in mortality
Potential Areas for De-escalation
De-escalating Screening Intervals
The US Preventive Services Task Force [16•] recommends biennial screening for women over 50, and the American Cancer Society [19] recommends biennial screening for women over 55. Nevertheless, many physicians still perform annual mammography. This would seem to be a possible area of de-escalation.
According to multiple systematic reviews, there are reasonable estimates gathered from observational studies, examining the association of screening intervals on the 10-year cumulative false-positive probability in women undergoing mammography screening starting at age 40 or 50 [3•, 6]. Based on the Breast Cancer Surveillance Consortium, the cumulative 10-year probability of receiving at least 1 false positive mammogram was 61% (95% CI, 59 to 63%) with annual screening and 42% (CI, 41 to 43%) with biennial screening starting at age 40. These rates were similar when beginning annual and biennial screening at age 50 [23].
Furthermore, they found the cumulative probability of receiving a biopsy due to a FPM after 10 years of screening starting at age 40 was higher with annual than biennial screening (7% vs 5%). Similarly, if screening began at age 50, then the cumulative probability for biopsy was 9.4% with annual and 6.4% with biennial screening. Overall, we see that increased frequency intervals lead to higher cumulative probabilities of unnecessary biopsy regardless of when screening begins. Furthermore, statistical modeling studies have found that biennial screening achieves 81% of the benefit of annual screening with only half of the number of false positive results [41]. It seems likely that many of the extra 19% of cancers found by annual screening are probably overdiagnosed cancers. Therefore, physicians should have a discussion with individual patients regarding whether to de-escalate from annual to biennial screening.
Stopping Mammography at Age 70
Much uncertainty exists regarding the benefits of screening mammogram in older women. Although data shows that increasing age is a risk factor for breast cancer, there are no randomized control trials investigating the mortality benefit of screening patients older than 74 years of age [29, 42–44]. As patients age, there are other competing causes of morbidity and mortality such as heart failure, hypertension, and diabetes to consider. Furthermore, a closer look at the small subset of women aged 70 to 74 years of age in the only RCT where they were included did not demonstrate a significant reduction in breast cancer mortality (relative risk = 1.12; 95% confidence interval, 0.73 to 1.72) [45]. Due to this lack of data, studies have used statistical modeling to assess benefits in screening women aged 70 to 79 as opposed to stopping at age 69 [41, 46, 47]. These statistical models suggest that screening women aged greater than 70 result in only 2 fewer deaths per 1000 women when compared to stopping at age 69 (6 vs 8 deaths per 1000 women). Unfortunately, the rates of false positives and overdiagnosis are particularly high in the oldest age groups [41]. As seen in Fig. 3, for women diagnosed with favorable tumors in their 70s, the chances are over 60% that it is an overdiagnosed tumor, and in the 80s it is over 75%. According to a meta-analysis by Lee et al., there is a significant lag-time to benefit time frame of 10 years after screening to even see a mortality benefit [48]. Therefore, screening is most appropriate in patients who are less than 70 years of age. Recommending screening beyond this age seems to put patients at a greater risk of harm without an added tangible benefit.
Atypical Ductal Hyperplasia
Atypical ductal hyperplasia (ADH) is considered a benign epithelial lesion and nonobligate precursor to invasive cancer that is found in 1.2 to 16% of breast biopsies [49]. Due to the risk for a simultaneous undiagnosed cancer, multiple studies have examined the upgrade rate to DCIS or invasive carcinoma with excision and results have varied widely from 4 to 54% [50, 51]. As a result, the NCCN guidelines have recommended complete surgical excision of all ADH lesions found on biopsy as the standard of care [52].
More recent studies have found lower upgrade rates of 5 to 20% thanks to advances in imaging and biopsy techniques. Therefore, efforts have been made to try and identify risk factors associated with upgrading ADH to identify a favorable subgroup that is potentially suited for observation rather than surgical excision. Racz and Degnim summarized their data suggesting that women who meet the following criteria have less than 5% chance of a missed invasive carcinoma: no mass lesion or discordance, removal of greater than or equal to 90% of calcifications at the time of core needle biopsy, involvement of ≤2 terminal duct lobules, and absence of necrosis [50].
More importantly, however, ADH is low grade by definition and when it is upgraded it is almost always to a low grade invasive or in situ cancer [49]. These are exactly the cancers most likely to be overdiagnosed. The important outcome in this situation should not be finding the cancer but determining whether there is any clinical benefit to finding it at the time of diagnosing ADH. More than likely, many of these small cancers would not progress, and if they do, they would have the same cure rate when they are diagnosed a few years later. We currently have very reasonable trials of observation for low grade DCIS and it does not make sense to excise all ADH.
Auxiliary Screening Modalities
Mammography has been the gold standard imaging tool for breast cancer screening due to its widespread availability, relatively low cost, and cancer detection capabilities. However, it is well known that mammographic sensitivity and specificity are reduced with increasing breast density, making it an imperfect tool [53]. Breast density not only hides underlying tumors from mammography but also increases breast cancer risk for women compared to women with fatty breasts [54]. Therefore, recent efforts have been made to study supplemental screening tools such as ultrasound and MRI.
In a systematic review by Melnikow et al., researchers examined the performance of supplemental breast ultrasound and MRI [55]. They found that supplemental US was able to detect additional cancers at a rate of 4.4 per 1000 examinations but at the cost of increased recall rates of 14%. Similarly, breast MRI detected 3.5 to 28.6 additional cancers per 1000 cases but recall rates were also elevated at 12–24%. No studies examined breast cancer outcomes. The authors were able to conclude that supplemental screening can find additional breast cancers but at the risk of increasing false positive results and the benefit is unclear. The USPSTF gave it a recommendation of “I” meaning current evidence is insufficient to assess the balance of benefits and harms.
This was a very valid conclusion. Finding extra cancers, in and of itself, is not necessarily a good thing. Some evidence suggests that the biological characteristics of cancers found by screening ultrasound are more compatible with overdiagnosis [56]. It is also possible that, even if not overdiagnosed, they may be found at subsequent routine mammography with equivalent survival rates. Certainly, we should remain skeptical and not be too enthusiastic about cancers picked up by supplemental screening until evidence shows actual patient benefit. The benefits and harms should be weighed carefully and discussed individually between patient and provider.
Conclusion
The optimum algorithm for breast cancer screening is somewhat controversial with varying recommendations by organizations such as the USPSTF, ACOG, and ACS. Although there is clearly a modest mortality benefit to screening mammography, there are many potential negative effects that should not be overlooked. The most serious of these is overdiagnosis which occurs in 20 to 25% of invasive cancers and 30 to 35% if DCIS is included. Overdiagnosis is most common in older women and those with low grade, biologically favorable tumors. Some de-escalation of mammography should be considered in situations where the risk of overdiagnosis is especially high or the tumors are especially likely to be favorable. Supplemental tests such as screening ultrasound and MRI will detect additional cancers but there is no data suggesting a mortality benefit and the risk of overdiagnosis is high.
Declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Breast Cancer Management: De-Escalating Breast Cancer Care
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Breast Cancer Statistics | How common is breast cancer? https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html. Accessed 4/1/2022.
- 2.Shepardson LB, Dean L. Current controversies in breast cancer screening. Semin Oncol. 2020;47(4):177–181. doi: 10.1053/j.seminoncol.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Myers ER, Moorman P, Gierisch JM, Havrilesky LJ, Grimm LJ, Ghate S, et al. Benefits and harms of breast cancer screening: a systematic review. JAMA. 2015;314(15):1615–1634. doi: 10.1001/jama.2015.13183. [DOI] [PubMed] [Google Scholar]
- 4.Seely JM, Alhassan T. Screening for breast cancer in 2018-what should we be doing today? Curr Oncol. 2018;25(Suppl 1):S115–S124. doi: 10.3747/co.25.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuller MS, Lee CI, Elmore JG. Breast cancer screening: an evidence-based update. Med Clin North Am. 2015;99(3):451–468. doi: 10.1016/j.mcna.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson HD, Pappas M, Cantor A, Griffin J, Daeges M, Humphrey L. Harms of breast cancer screening: systematic review to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164(4):256–67. doi: 10.7326/M15-0970. [DOI] [PubMed] [Google Scholar]
- 7.Schottenfeld D, Fraumeni JF. Cancer epidemiology and prevention. 3. Oxford; New York: Oxford University Press; 2006. [Google Scholar]
- 8.Fletcher GS, Fletcher RH. Clinical epidemiology: the essentials. Sixth edition. ed. Philadelphia: Wolters Kluwer, 2021
- 9.Hayse B, Hooley RJ, Killelea BK, Horowitz NR, Chagpar AB, Lannin DR. Breast cancer biology varies by method of detection and may contribute to overdiagnosis. Surgery. 2016;160(2):454–462. doi: 10.1016/j.surg.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 10.Drukker CA, Schmidt MK, Rutgers EJ, Cardoso F, Kerlikowske K, Esserman LJ, et al. Mammographic screening detects low-risk tumor biology breast cancers. Breast Cancer Res Treat. 2014;144(1):103–111. doi: 10.1007/s10549-013-2830-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joensuu H, Lehtimaki T, Holli K, Elomaa L, Turpeenniemi-Hujanen T, Kataja V, et al. Risk for distant recurrence of breast cancer detected by mammography screening or other methods. JAMA. 2004;292(9):1064–1073. doi: 10.1001/jama.292.9.1064. [DOI] [PubMed] [Google Scholar]
- 12.Pace LE, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA. 2014;311(13):1327–1335. doi: 10.1001/jama.2014.1398. [DOI] [PubMed] [Google Scholar]
- 13.Barth RJ, Gibson GR, Carney PA, Mott LA, Becher RD, Poplack SP. Detection of breast cancer on screening mammography allows patients to be treated with less-toxic therapy. Am J Roentgenol. 2005;184(1):324–329. doi: 10.2214/ajr.184.1.01840324. [DOI] [PubMed] [Google Scholar]
- 14.Elder K, Nickson C, Pattanasri M, Cooke S, Machalek D, Rose A, et al. Treatment intensity differences after early-stage breast cancer (ESBC) diagnosis depending on participation in a screening program. Ann Surg Oncol. 2018;25(9):2563–2572. doi: 10.1245/s10434-018-6469-7. [DOI] [PubMed] [Google Scholar]
- 15.Lannin DR. Treatment intensity for mammographically detected tumors: an alternative viewpoint. Ann Surg Oncol. 2018;25(9):2502–2505. doi: 10.1245/s10434-018-6641-0. [DOI] [PubMed] [Google Scholar]
- 16.• Siu AL, U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(4):279–96. 10.7326/M15-2886. Current recommendations. [DOI] [PubMed]
- 17.Bevers TB, Helvie M, Bonaccio E, Calhoun KE, Daly MB, Farrar WB, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(11):1362–89. doi: 10.6004/jnccn.2018.0083. [DOI] [PubMed] [Google Scholar]
- 18.Practice bulletin number 179: breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130(1):e1-e16. 10.1097/AOG.0000000000002158 [DOI] [PubMed]
- 19.Oeffinger KC, Fontham ETH, Etzioni R, Herzig A, Michaelson JS, Shih Y-CT, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–614. doi: 10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monticciolo DL, Newell MS, Hendrick RE, Helvie MA, Moy L, Monsees B, et al. Breast cancer screening for average-risk women: recommendations from the ACR Commission on Breast Imaging. J Am Coll Radiol. 2017;14(9):1137–1143. doi: 10.1016/j.jacr.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Miglioretti DL, Lange J, van den Broek JJ, Lee CI, van Ravesteyn NT, Ritley D, et al. Radiation-induced breast cancer incidence and mortality from digital mammography screening. Ann Intern Med. 2016;164(4):205–214. doi: 10.7326/M15-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hendrick RE. Radiation doses and cancer risks from breast imaging studies. Radiology. 2010;257(1):246–253. doi: 10.1148/radiol.10100570. [DOI] [PubMed] [Google Scholar]
- 23.Hubbard RA, Kerlikowske K, Flowers CI, Yankaskas BC, Zhu W, Miglioretti DL. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155(8):481–492. doi: 10.7326/0003-4819-155-8-201110180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lidbrink E, Elfving J, Frisell J, Jonsson E. Neglected aspects of false positive findings of mammography in breast cancer screening: analysis of false positive cases from the Stockholm trial. BMJ. 1996;312(7026):273–276. doi: 10.1136/bmj.312.7026.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brodersen J, Siersma VD. Long-term psychosocial consequences of false-positive screening mammography. Ann Fam Med. 2013;11(2):106–115. doi: 10.1370/afm.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hooley RJ, Durand MA, Philpotts LE. Advances in digital breast tomosynthesis. AJR Am J Roentgenol. 2017;208(2):256–266. doi: 10.2214/AJR.16.17127. [DOI] [PubMed] [Google Scholar]
- 27.Loberg M, Lousdal ML, Bretthauer M, Kalager M. Benefits and harms of mammography screening. Breast Cancer Res. 2015;17:63. doi: 10.1186/s13058-015-0525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neal CH, Helvie MA. Overdiagnosis and risks of breast cancer screening. Radiol Clin North Am. 2021;59(1):19–27. doi: 10.1016/j.rcl.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Independent UKPoBCS The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380(9855):1778–86. doi: 10.1016/S0140-6736(12)61611-0. [DOI] [PubMed] [Google Scholar]
- 30.Bell RJ. Screening mammography—early detection or over-diagnosis? Contribution from Australian data. Climacteric. 2014;17(Suppl 2):66–72. doi: 10.3109/13697137.2014.956718. [DOI] [PubMed] [Google Scholar]
- 31.Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302(15):1685–1692. doi: 10.1001/jama.2009.1498. [DOI] [PubMed] [Google Scholar]
- 32.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367(21):1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 33.Welch HG, Prorok PC, O'Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375(15):1438–1447. doi: 10.1056/NEJMoa1600249. [DOI] [PubMed] [Google Scholar]
- 34.Zahl P-H, Strand BH, Maehlen J. Incidence of breast cancer in Norway and Sweden during introduction of nationwide screening: prospective cohort study. BMJ. 2004;328(7445):921–924. doi: 10.1136/bmj.38044.666157.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zackrisson S, Andersson I, Janzon L, Manjer J, Garne JP. Rate of over-diagnosis of breast cancer 15 years after end of Malmö mammographic screening trial: follow-up study. BMJ. 2006;332(7543):689–692. doi: 10.1136/bmj.38764.572569.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller AB, To T, Baines CJ, Wall C. The Canadian National Breast Screening Study-1: breast cancer mortality after 11 to 16 years of follow-up. A randomized screening trial of mammography in women age 40 to 49 years. Ann Intern Med. 2002;137(5 Part 1):305–12. doi: 10.7326/0003-4819-137-5_part_1-200209030-00005. [DOI] [PubMed] [Google Scholar]
- 37.Miller AB, To T, Baines CJ, Wall C. Canadian National Breast Screening Study-2: 13-year results of a randomized trial in women aged 50–59 years. J Natl Cancer Inst. 2000;92(18):1490–1499. doi: 10.1093/jnci/92.18.1490. [DOI] [PubMed] [Google Scholar]
- 38.Baines CJ, To T, Miller AB. Revised estimates of overdiagnosis from the Canadian National Breast Screening Study. Prev Med. 2016;90:66–71. doi: 10.1016/j.ypmed.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 39.Lannin DR, Wang S. Are small breast cancers good because they are small or small because they are good? N Engl J Med. 2017;376(23):2286–2291. doi: 10.1056/NEJMsr1613680. [DOI] [PubMed] [Google Scholar]
- 40.Haagensen CD. Diseases of the breast. Philadelphia: Sauders; 1956. [Google Scholar]
- 41.Mandelblatt JS, Cronin KA, Bailey S, Berry DA, de Koning HJ, Draisma G, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151(10):738–747. doi: 10.7326/0003-4819-151-10-200911170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L, et al. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(10):727–37, W237–42. 10.7326/0003-4819-151-10-200911170-00009 [DOI] [PMC free article] [PubMed]
- 43.Gøtzsche PC, Jørgensen KJ. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2013;6:CD001877. doi: 10.1002/14651858.CD001877.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salzman B, Beldowski K. Paz Adl Cancer screening in older patients. AFP. 2016;93(8):659–667. [PubMed] [Google Scholar]
- 45.Nyström L, Andersson I, Bjurstam N, Frisell J, Nordenskjöld B, Rutqvist LE. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet. 2002;359(9310):909–919. doi: 10.1016/S0140-6736(02)08020-0. [DOI] [PubMed] [Google Scholar]
- 46.Schousboe JT, Kerlikowske K, Loh A, Cummings SR. Personalizing mammography by breast density and other risk factors for breast cancer: analysis of health benefits and cost-effectiveness. Ann Intern Med. 2011;155(1):10–20. doi: 10.7326/0003-4819-155-1-201107050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barratt A, Howard K, Irwig L, Salkeld G, Houssami N. Model of outcomes of screening mammography: information to support informed choices. BMJ. 2005;330(7497):936. doi: 10.1136/bmj.38398.469479.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O'Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441. doi: 10.1136/bmj.e8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gagnon N, Martel E, Cadrin-Chênevert A, Ledoux E, Racicot C, Villiard R. Upgrade rate of atypical ductal hyperplasia: ten years experience and predictive factors. J Surg Res. 2021;266:311–318. doi: 10.1016/j.jss.2021.03.063. [DOI] [PubMed] [Google Scholar]
- 50.Racz JM, Degnim AC. When does atypical ductal hyperplasia require surgical excision? Surg Oncol Clin N Am. 2018;27(1):23–32. doi: 10.1016/j.soc.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Schiaffino S, Calabrese M, Melani EF, Trimboli RM, Cozzi A, Carbonaro LA, et al. Upgrade rate of percutaneously diagnosed pure atypical ductal hyperplasia: systematic review and meta-analysis of 6458 lesions. Radiology. 2019;294(1):76–86. doi: 10.1148/radiol.2019190748. [DOI] [PubMed] [Google Scholar]
- 52.Racz JM, Carter JM, Degnim AC. Lobular neoplasia and atypical ductal hyperplasia on core biopsy: current surgical management recommendations. Ann Surg Oncol. 2017;24(10):2848–2854. doi: 10.1245/s10434-017-5978-0. [DOI] [PubMed] [Google Scholar]
- 53.Geisel J, Raghu M, Hooley R. The role of ultrasound in breast cancer screening: the case for and against ultrasound. Semin Ultrasound CT MRI. 2018;39(1):25–34. doi: 10.1053/j.sult.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 54.McCormack VA, dos Santos SI. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 55.Melnikow J, Fenton JJ, Whitlock EP, Miglioretti DL, Weyrich MS, Thompson JH, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164(4):268–78. doi: 10.7326/M15-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayse B, Lannin DR. Supplemental ultrasonography screening for women with dense breasts. Ann Intern Med. 2015;162(11):801–802. doi: 10.7326/L15-5061-2. [DOI] [PubMed] [Google Scholar]