Abstract
Abstract
The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been one of the most catastrophic diseases observed in recent years. It has reported nearly 550 million cases worldwide, with more than 6.35 million deaths. In Mexico, an increased incidence and mortality of this disease were observed, where the immune response has been involved in the magnitude and severity. A critical version of the disease is accompanied by hyperinflammatory responses, with cytokine and defective cellular responses. A detailed understanding of the role of molecules and cells in the immune response during COVID-19 disease may help to generate effective protection mechanisms, improving those we already have. Here we analyzed blood samples obtained from patients at the Hospital Regional de Alta Especialidad de Ixtapaluca (HRAEI), Mexico, which were classified according to living guidance for clinical management of COVID-19 by the World Health Organization: asymptomatic, mild, severe, and critical disease. We observed increased interleukin (IL)-6 levels and a T-CD8+ and T-CD4+ cell reduction correlated with the critical disease version. Importantly, here, we described a significant reduction of CD11b+CD45highCD14low monocytes during severe disease, which displayed a non-classical profile, expressing IL-10, transforming growth factor (TGF)-β, and indoleamine 2,3-dioxygenase (IDO)1 molecule. Moreover, CD11b+CD45highCD14low monocytes obtained from infected one-dose vaccinated patients (Pfizer® vaccine) who suffered minimal symptoms showed simultaneously a dual classical and no-classical profile expressing pro- and anti-inflammatory cytokines. These results suggest that blood monocytes expressing a dual pro- and anti-inflammatory profile might be a predictive marker for protection in the Mexican population during COVID-19 disease.
Key points
• Exacerbated immune response is associated with COVID-19 severe disease.
• Dual monocyte activation profile is crucial for predicting protection during COVID-19.
• Vaccination is crucial to induce the dual activation profile in monocytes.
Keywords: SARS-CoV-2, COVID-19, Immune response, T cells, Dual monocyte activation profile
Introduction
The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been the most recent unexpected health problem, affecting more than 520 million people worldwide (Dong et al. 2020). Since the pandemic began, the immune response has developed a protagonist role in understanding how the disease works and also in generating strategies helping to resolve the infection; effective and rapid capacity for the generation of vaccines in record time has been highlighted (Rudan et al. 2022).
In addition to the leading role of the immune response, one of the main characteristics observed during COVID-19 infection is the severity of the disease, which has a wide range. It was described that 80% of patients testing positive for COVID-19 develop a mild illness characterized by general and unspecific symptoms and 14% developed the severe disease, while 5% had the critical disease version, characterized by potential complications like a respiratory and multi-organ failure (Merad et al. 2022; Merad and Martin 2020). Some comorbidities associated with human development have been associated with the critical version of the disease, like diabetes, obesity, chronic obstructive pulmonary disease (COPD), digestive system disorders, and cancers (Seyed Hosseini et al. 2020). Also, patients with the critical COVID-19 disease showed a dysregulation in some characteristics associated with the immune response, such as reduction of peripheral T cells and increased levels of inflammatory cytokines, like IL-6, tumor necrosis factor (TNF)-α, IL-1β (Ragab et al. 2020), and C-reactive protein (C-PR) (Xie et al. 2020), and also increased levels in both C3a and C5a anaphylatoxins associated with complement (Kurtovic and Beeson 2021). Such deregulation of the inflammatory profile is associated with both severe lung damage and low oxygen saturation (Huang et al. 2020).
The role of lymphoid cells as well as myeloid cells during the COVID-19 disease has been emphasized; their polarization profile between pro- or anti-inflammatory could predict the disease version in either asymptomatic or critical patients. It has been reported that patients with COVID-19 acute respiratory distress syndrome (ARDS) have a defective antigen presentation in monocytes caused by interferon dysregulation. Also, the cytotoxic activity in both natural killer (NK) and T-CD8+ cells is deficient (Yao et al. 2021). During COVID-19, critical disease was described as an expansion of non-classical monocytes with increased platelet activation in the blood (Stephenson et al. 2021). On the other hand, in bronchoalveolar lavage fluid from patients with the critical disease, increased proinflammatory monocyte-derived macrophages were shown (Liao et al. 2020; Melms et al. 2021). However, in either asymptomatic or moderate cases, the presence of highly expanded CD8+ T cells was described (Liao et al. 2020). All this evidence suggests that exacerbated inflammatory immune response is associated with severe symptoms and mortality in COVID-19 positive patients, where monocytes and T-CD8+ cells have a significant role.
Based on the relevance of immune regulation in this disease, this study aimed to define whether the severity of the disease displayed during COVID-19 disease is associated with either increased or reduced lymphoid and myeloid immune cells in peripheral blood samples from Mexican patients positive for SARS-CoV-2 infection. According to living guidance for clinical management of COVID-19 from the World Health Organization published in November 2021, we had a categorical and ordinal classification of patients according to severity disease index in asymptomatic, mild disease, severe disease, and critical disease, to analyze cytokines, lymphoid, and myeloid immune response cells from the blood. We mainly observed that non-classical monocytes CD11b+CD45highCD14low were significantly reduced in patients with critical illness. In contrast, in patients who had access to a dose of the Pfizer® vaccine, these monocytes presented a dual profile, expressing simultaneously inflammatory cytokines such as IL-1β, IL-6, and TNF-α, as well as anti-inflammatory cytokines such as TGF-β, IL-10, and the IDO1 molecule. Therefore, our data strongly suggest that CD11b+CD45highCD14low non-classical monocytes are possibly involved in protection during SARS-CoV-2 virus infection, and maybe they will be helpful for the diagnosis and prediction for the severity and outcome of COVID-19 infection.
Materials and methods
Sample and experimental group classification
All procedures were approved in accordance with the ethical standards of the Committee of Research, Ethics and Biosafety of Hospital Regional de Alta Especialidad de Ixtapaluca (NR-22–2020) and with the 1964 Helsinki declaration. Our studied groups were male and female adults, including both medical staff and people requiring medical attention. Excepting the control group, all studied groups were positive (at some point) for COVID-19 by COVIDsure Multiplex Real-Time PCR Kit (Labsystems). Groups were classified according to Table 1. Sample collection was obtained from two different times. First, from April to May 2020, samples were obtained for CBA cytokine detection. Second, from December 2020 to April 2021, samples were obtained for ELISA cytokine detection, T cell, and monocyte analysis by flow cytometry. Also, monocytes were sorted and processed for RT-PCR analysis.
Table 1.
Classification and characteristics of studied groups
| Group | Group features | COVID-19 PCR test | Arbitrary value | Oxygen saturation (SpO2) | Supplemental oxygen | Additional information |
|---|---|---|---|---|---|---|
| N | Negative controls | Negative | 1 | > 90% | No | – |
| A+ | Asymptomatic | Positive | 2 | > 90% | No | – |
| MD | Mild disease | Positive | 3 | > 90% | No | Headache, myalgia, arthralgia, fever, cough, and diarrhea were described as symptoms. Patients did not required hospitalization |
| SD | Severe disease | Positive | 4 |
< 90% > 80% |
Yes | Patients required hospitalization, but after medical attention they survive |
| CD | Critical disease | Positive | 5 | < 70% | Respiratory ventilator was needed | Patients were managed at intensive care unit, and they died* |
| V+ | First vaccinated, subsequently infected | Positive | W** | > 90% | No | Patients were vaccinated with one Pfizer® dose; a few weeks later, they were positive for COVID-19 PCR test having low symptoms |
| +V | First infected, subsequently vaccinated | Negative | W** | > 90% | No | Patients had positive COVID-19 diagnosis from several months. At the time of both vaccination and blood sample collection, they were negative to COVID-19 PCR test |
*Particularly in this group, some patients had comorbidities like type 2 diabetes mellitus, hypertension, obesity, hyperglycemia, hypothyroidism, and kidney injury. **Without classification
Blood samples for cytokine detection
Blood samples were obtained in EDTA tubes (Vacutainer BD®). For plasma obtention, samples were centrifugated at 3000 rpm for 10 min; after that, the supernatant was collected in Eppendorf tubes. Samples were stored at −80 °C until used.
ELISA assays
We detected the macrophage inhibitor factor (MIF) from plasma samples using MIF Human ELISA Kit (ThermoFisher™). We also analyzed tumor necrosis factor alpha (TNF-α) by Human TNF-α Standard ABTS Elisa Development Kit (Peprotech). Both kits were used according to the manufacturer instructions.
Flow cytometry
Cytometric bead array (CBA)
The human Th1/Th2/Th17 CBA kit (BD®) was used according to manufacturer instructions with 50 µl of either plasma or serum to analyze cytokines.
Blood peripheral leucocyte immunofluorescence
A stain for both lymphocytes and myeloid cells was performed from peripheral blood. For each staining, 50 µL of blood was obtained, and the respective antibodies were added according to the concentrations recommended by the manufacturer, incubating for 15 min at room temperature. Subsequently, 150 µL of BD FACS™ Lysing 1× solution was added to the samples and incubated for 15 min at room temperature. Finally, the samples were washed, resuspended in FACS sheath buffer, and acquired in the Sony SH800S Cell Sorter cytometer. We obtained 20,000 representative events for either lymphocytes or myeloid cells. The used antibodies were anti-CD4-FITC, anti-CD8-PE, and anti-CD45 PerCP all from BD Biosciences®, and anti-CD33-FITC, anti-CD45-PE, anti-CD11b-APC, and anti-CD14-APC/Cy7 all from Biolegend®.
Myeloid cell sorting
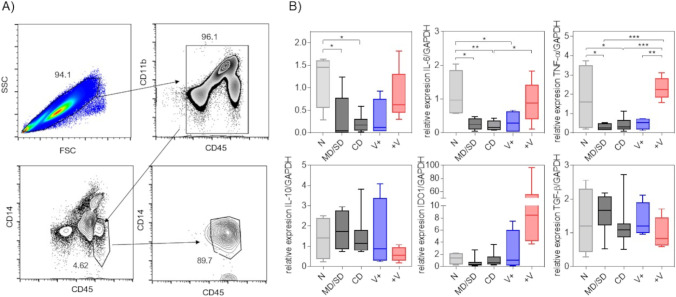
Total blood leucocytes were obtained from Ficoll gradient. Four milliliters from Ficoll-Paque Premium density gradient media (Cytiva Life Sciences™) was added in 1:1 dilution with FACS sheath fluid (BD®), and samples were centrifugated for 4000 rpm for 40 min. The middle phase with mononuclear cells was obtained, 2 ml of FACS sheath fluid (BD®) was added, and samples were centrifugated at 3000 rpm for 40 min. The supernatant was decanted, and samples were resuspended for immunofluorescence staining with anti-CD33-FITC, anti-CD45-PE, anti-CD11b-APC, and anti-CD14-APC/Cy7 antibodies. Cells were sorted using the Sony SH800S Cell Sorter cytometer with 90% purity, according to the analysis strategy described in Fig. 4A. All flow cytometry samples were analyzed with the FlowJo VX.6 (BD®) software.
Fig. 4.
Dual expression of pro-inflammatory and anti-inflammatory cytokines in CD11b+CD45highCD14low myeloid cells in patients infected and subsequently vaccinated with a dose of Pfizer. A Analysis strategy for sorting in non-classical monocytes, having a near 90% purity. B Non-classical monocytes were processed to mRNA and cDNA obtention, to later analyze the relative expression of IL-1β, IL-6, TNF-α, IL-10, IDO1, and TGF-β genes, having a relationship with the endogenous GADPH gene. For these experiments, groups MD and SD were pooled. Total n = 28. *p < 0.05, **p < 0.01, ***p < 0.001; one-way ANOVA and Tukey’s post-test
RNA extraction
The sorted monocytes were processed by TRIzol (Invitrogen™) and chloroform (Sigma-Aldrich™) RNA obtention. Samples were stored at −20 °C until used. Aurum™ total RNA mini Kit (Bio-Rad®) was used for high-quality DNA-free total RNA according to manufacturer instructions. Samples were stored at −80 °C until used.
Real-time PCR
Total RNA samples were used for cDNA synthesis with iScript reverse transcription supermix cDNA Synthesis Kit (Bio-RAD®) according to manufacturer instructions. Once cDNA was obtained, real-time PCR was developed for IL1B, IL-6, IL10, TGFβ, TNFα, and GAPDH genes. The primer used for IL1β was F: 5′-AAGCTGATGGCCCTAAACAG-3′, R: 5′-AGGTGCATCGTGCACATAAG-3′; IL6 was F: 5′-CCAGCTATGAACTCCTTCTC-3′, R: 5′-GCTTGTTCCTCACATCTCTC-3′; IL10 was F: 5′-TCTCCGAGATGCCTTCAGCAGA-3′, R: 5′-TCAGACAAGGCTTGGCAACCCA-3′; TGFβ was F: 5′-TACCTGAACCCGTGTTGCTCTC-3′, R: 5′-GTTGCTGAGGTATCGCCAGGAA-3′; TNFα was F: 5′-CTCTTCTGCCTGCTGCACTTTG-3′, R: 5′-ATGGGCTACAGGCTTGTCACTC-3′; and GAPDH was F: 5′-ACCCACTCCTCCACCTTTGA-3′, R: 5′-CTGTTGCTGTAGCCAAATTCGT-3′.
Statistical analysis
Statistical differences were performed by one-way ANOVA with Tukey’s multiple comparison post-test, considering significant *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 differences. We performed Spearman’s correlation coefficient expressed as an r value for association between numerical and categorical variables. Data and graphics were analyzed and developed in GraphPad Prism V.7 software.
Results
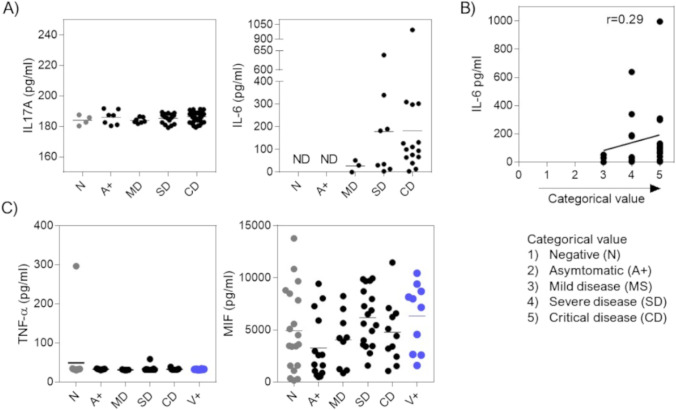
It has been described that SARS-CoV-2 viral infection triggers a systemic inflammatory response. Therefore, we decided to quantify serum cytokines in patients classified as in Table 1 from blood samples obtained at the Hospital Regional de Alta Especialidad de Ixtapaluca (HRAEI), Mexico. Our first option was to evaluate cytokine levels using the CBA technique, since this methodology allows to analyze several cytokines simultaneously with a small amount of either serum or plasma sample (50 μl). From a panel of seven cytokines, we only detected IL-17 and IL-6 by this technique. No significant differences in IL-17 levels were observed among the infected groups (Fig. 1A). On the other hand, IL-6 was not detected in the negative (N) neither the asymptomatic (A+) patient groups. Still, interestingly, in the symptomatic patient’s mild disease (MD), severe disease (SD), and critical disease (CD, Table 1), we observed a higher IL-6 concentration as the severity of the disease increased; the experimental group of MD patients had a minimum concentration of IL-6, while the hospitalized MD and SD groups showed an increased amount (Fig. 1A). As a higher concentration of IL-6 was observed when the severity of the disease increased, we decided to assign a categorical and ordinal value to the experimental groups depending on the severity of the disease index, where 1-value is a healthy patient (N), and 5-value is a patient with critical disease (CD, Table 1); therefore, the greater the categorical and ordinal value, the higher the quantitative value. Once we assigned this value, we performed a linear regression and a Spearman correlation test, yielding an r value of 0.29 (Fig. 1B). These data suggest that the increased IL-6 levels in blood correlates slightly with the increased severity of symptoms.
Fig. 1.
Quantification of systemic cytokines during COVID-19 disease, in patients classified according to living guidance for clinical management of COVID-19, from the World Health Organization. A By CBA (BD®), cytokines IL-17 and IL-6 were analyzed in serum or plasma. Representative data of 3 independent experiments, with at least 3 samples per group. B Spearman’s correlation test between the concentration of IL-6 and the severity COVID-19 disease index. C Analysis of proinflammatory cytokines TNF-α and MIF by ELISA; total data with at least 9 samples per group. Statistical differences were performed by one-way ANOVA with Tukey’s multiple comparison post-test, considering significant *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001
Although inflammatory cytokines such as TNF-α are included in our CBA kit, we could not detect this cytokine by this technique. Therefore, we evaluated TNF-α and monocyte-macrophage migration inhibitory factor (MIF) by ELISA. While we were conducting these experiments, the national vaccination plan in Mexico already had significant progress, which allowed us to have access to samples from patients who were vaccinated with the first dose of the Pfizer® vaccine, and, subsequently, it became infected with the SARS-CoV-2 virus (V+, Table 1). We observed that the TNF-α concentration is similar in all groups, consistently below 100 pg/ml (Fig. 1C). When we analyzed the MIF concentration, we observed a slight increase in the HG group; however, there were heterogenous concentrations ranging from 0 pg/ml to over 10,000 pg/ml in most groups, including healthy donors (Fig. 1C). Therefore, we did not find a correlation between either TNF-α or MIF with the severity of the disease.
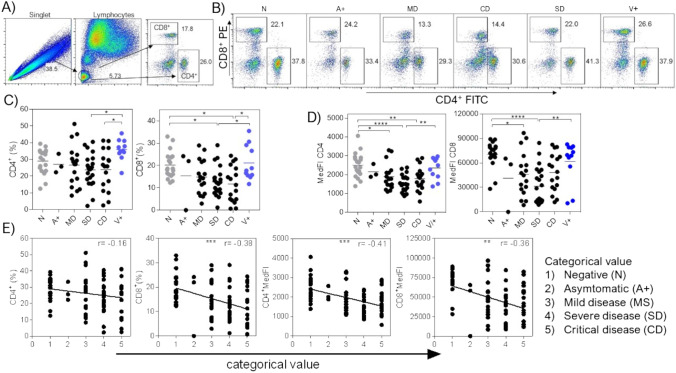
Subsequently, we decided to analyze some immune response cell populations that have a role in the resolution of viral infections. We first analyzed CD4+ and CD8+ lymphocytes in peripheral blood by flow cytometry. Based on the analysis strategy described in Fig. 2A, we exclude doublets; then, we select the region of small and slightly granular cells that are lymphocytes. Finally, we select the positive cells for CD4 and CD8 molecules. We first evaluated the percentage of CD4+ and CD8+ cells for each experimental group, observing that CD4+ lymphocytes decreased mainly in hospitalized SD and CD groups, which are groups with stronger symptoms (Fig. 2B and C). Also, we observed that CD8+ lymphocytes decreased in the SD and CD groups (Fig. 2B and C). Interestingly, we observed that the V+ group had similar percentages of CD4 and CD8 lymphocytes as the healthy control group (Fig. 2B and C). This finding suggests that vaccination confers a protective effect against infection since the reduction of CD4+ T lymphocytes caused by the infection is avoided, in addition to the fact that none of these patients had severe symptoms caused by infection. In addition to evaluating the percentage, we also evaluated the median fluorescence intensity (MedFI) of CD4 and CD8 cell populations, MedFI is the median statistics value of the cell population expressing either CD4 or CD8 molecules. Similar to the observed in the percentage, the MedFI of CD4 and CD8 cells decreased as the severity of the disease increased. In contrast, the MedFI in the healthy and the V+ group remained unchanged (Fig. 2D), suggesting a protective effect of vaccination over the reduction in CD4 and CD8 MedFI molecules. We again performed a linear regression and a Spearman correlation test to confirm whether the decreased percentage of CD4+ and CD8+ lymphocytes and the decrease in MedFI for each population of analyzed T lymphocytes correlate with the increased severity of the disease observed in patients positive for the COVID-19 disease. Again, a categorical and ordinal value was assigned to each experimental group, where the 1-value is a healthy patient (N), and the 5-value is a CD patient (Table 1). Therefore, the greater the categorical and ordinal value, the higher the quantitative value. We observed that if the severity of the disease increases, then a reduction in both percentage and MedFI of CD4+ and CD8+ cells exists. Therefore, we observed an inverse correlation, which is highlighted in T-CD8+ cell percentage (r = −0.38), the T-CD4+ cells MedFI (r = −0.41), and T-CD8+ cells MedFI (r = −0.36). Thus, these data suggest as the severity of the disease index increases in Mexican patients positive for the SARS-CoV-2 virus, there is a reduction in T-CD8+ cell percentage, as well as a reduction in the expression of both CD4 and CD8 molecules, which is prevented with a dose of the Pfizer® vaccine.
Fig. 2.
CD4+ and CD8+ lymphocytes decrease as the severity of the COVID-19 disease increases. A Flow cytometry analysis strategy to obtain the percentage and MedFI of CD4+ and CD8+ lymphocytes in peripheral blood samples. B Representative dot plots of the T-CD4+ and T-CD8+ lymphocyte populations by group, which were classified according to living guidance for clinical management of COVID-19, from the World Health Organization. C Graphs showing the percentage and D MedFI of CD4+ and CD8+ lymphocytes by group. E Spearman’s correlation between the percentage and the MedFI of T-CD4+ and T-CD8+ lymphocytes with the severity of the disease, where arbitrary values were given to the experimental groups; 1-value is for healthy control patients (N) and 5 is for critical disease patients (Table 1). Total data with at least 3 samples per group, n = 87. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; one-way ANOVA and Tukey’s post-test. Spearman’s correlation degree and r value are shown on each correlation plot
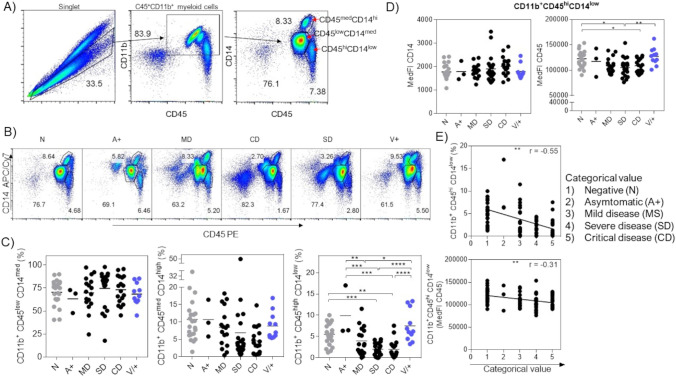
On the other hand, we analyzed cells of myeloid lineage from the same peripheral blood samples by flow cytometry. We first excluded doublets based on the analysis strategy described in Fig. 3A. We selected myeloid cells expressing CD11b and CD45 molecules and finally classified monocytes into three populations based on the CD14 and CD45 expression levels, obtaining CD11b+CD45lowCD14med, CD11b+CD45medCD14high, and CD11b+CD45highCD14low populations (Fig. 3A). When we analyzed the percentage of each population (Fig. 3B and C), we observed that CD11b+CD45lowCD14med myeloid cells have very similar percentages in all groups, with no significant differences (Fig. 3C, left). In the CD11b+CD45medCD14high population, we observed a tendency for a decreased percentage, mainly in the groups with severe disease SD and CD, but we did not observe significant differences (Fig. 3C, center). In contrast, we observed a decreased percentage in CD11b+CD45highCD14low monocytes, mainly in groups with severe disease SD and CD. Interestingly, the percentage in the V+ group is similar to the N group (Fig. 3C, right). Therefore, our data suggest that as the severity of the disease increases during infection with the SARS-CoV-2 virus, there is a decrease in CD11b+CD45highCD14low monocytes in the peripheral blood of Mexican patients. As these CD11b+CD45highCD14low monocytes have the most significant differences between the groups, we decided to evaluate the MedFI of CD14 and CD45 (Fig. 3D). We observed that the MedFI of CD14 did not change between groups (Fig. 3D, left), while the MedFI of CD45 decreased in the SD and CD groups (Fig. 3D, right). Again, we assessed whether a correlation between the decrease in the percentage of CD11b+CD45highCD14low cells or the CD45 MedFI, with the severity of the COVID-19 disease, exists, by a Spearman correlation (Fig. 3E). Again, each experimental group was assigned a categorical and ordinal value, where 1-value is a healthy patient (N) and 5-value is a CD patient (Table 1); therefore, the greater the severity of the value, the higher the quantitative value. We first performed a linear regression obtaining a Spearman r value of −0.55 for CD11b+CD45highCD14low cell percentage (Fig. 3E, upper), while we obtained an r value of −0.31 for the CD45 MedFI. These data suggest that the decrease in CD11b+CD45highCD14low monocyte percentage is involved with the greater severity of symptoms during COVID-19 disease. We highlight that the r value for the CD11b+CD45highCD14low monocyte population is greater than the r value of any previously analyzed variables in CD4 and CD8 T lymphocytes (Fig. 2).
Fig. 3.
Correlation between the decrease in CD11b+CD45highCD14low monocytes with the increased severity of the COVID-19 disease. A Analysis strategy for myeloid cells. B Dot plots of representative data for the CD11b+CD45lowCD14med, CD11b+CD45medCD14high, and CD11b+CD45highCD14low populations by group, which were classified according to living guidance for clinical management of COVID-19, from the World Health Organization. C Total percentage and D MedFI data of the different populations for myeloid cells. E Linear regression and Spearman’s correlation between the percentage and the MedFI of CD11b+CD45highCD14low with the severity of the disease. Total data with at least 3 samples per group, n = 87. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; one-way ANOVA and Tukey’s post-test. Correlation degree r2 and r value of Spearman’s are shown on each correlation plot
The reduction in CD11b+CD45highCD14low monocytes yielded significant differences associated with the increased susceptibility to infection observed in SD and CD patients, leading us to analyze the cytokine profile produced exclusively by this myeloid cell population, looking to confirm their predisposed either inflammatory or anti-inflammatory profile in the bloodstream. Therefore, we selected CD11b+CD45highCD14low cells sorting them according to the analysis strategy described (Fig. 4A). We excluded the doublets, selected the double-positive cells for CD11b and CD45, and selected the CD11b+CD45highCD14low myeloid population, obtaining a purity close to 90% in all samples (Fig. 4A). Subsequently, RNA extraction from these cells was performed, followed by the complementary DNA (cDNA) synthesis, to amplify pro-inflammatory and anti-inflammatory cytokine genes by real-time PCR (Fig. 4B). It is worth mentioning that, at this time, the Mexican government’s vaccination plan had greater coverage, allowing us to dispose of a group of patients who were first infected with SARS-CoV-2, having resolved the infection, and subsequently vaccinated with one dose of the Pfizer® vaccine (+V group, Table 1).
We observed that CD11b+CD45highCD14low cells from MD/SD patients showed low gene expression of the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α, even below the control group N (Fig. 4B). Interestingly, the group V+ also showed low gene expression for these cytokines (Fig. 4B). In contrast, the +V group had similar gene expression for IL-6 and TNF-α as the N control group (Fig. 4B). Conversely, anti-inflammatory gene expression for IL-10 and TGF-β cytokines had no differences between groups. In the IDO1 gene expression, we observed increased values specifically in the +V group, but we did not find significant differences due to a single highly high data (96 relative value). All these data strongly suggest that the CD11b+CD45highCD14low monocytes that decrease in the peripheral blood of Mexican patients positive for COVID-19 infection (no matter which symptoms group belongs to) have an activation profile towards the non-classical pathway because they maintain the expression of IL-10, IDO1, and TGF-β. In contrast, in patients who first resolved the natural infection and then were vaccinated (+V), these monocytes display a dual activation profile by expressing both classic and non-classic profiles of cytokines. Since they express IL-1β, IL-6, and TNF-α, as well as TGF-β, IL-10, and IDO1, for this reason, we propose that the dual activation profile in CD11b+CD45highCD14low monocytes is important to induce protection in Mexican patients during infection with the SARS-CoV-2 virus and also this dual profile could be useful for diagnosis and prediction for the severity of the disease during COVID-19 infection.
Discussion
Since SARS-CoV-2 infection was declared a pandemic, efforts worldwide have been improved to understand both the etiology and the natural process of disease development, which was essential to understand the role of the immune response in the outcome of this disease. All these processes have been involved in developing strategies to contain and control the disease, which stand out in a full range of vaccines (Fiolet et al. 2022; Folegatti et al. 2020; Sahin et al. 2020). It is necessary to continue understanding how the immune response is involved during COVID-19 disease, which will help us generate more effective protection mechanisms and improve those we already have.
According to living guidance for clinical management of COVID-19, from the World Health Organization published in November 2021, patients positive for COVID-19 infection are classified as an asymptomatic disease (A+) having no evidence of viral pneumonia or hypoxia; mild disease (MD) where no severe pneumonia is observed but fever, cough, dyspnea, and low oxygen saturation (SpO2) are observed; severe disease (SD) where severe pneumonia and respiratory distress or SpO2 below to 90% is observed; and, finally, critical disease (CD) where ARDS is observed. We used this classification, also including negative patients (N), patients with one Pfizer® vaccine dose subsequently infected (V+), and, finally, patients infected and subsequently vaccinated with one Pfizer® dose (+V) to describe whether the severity of COVID-19 disease is associated with either increased or reduced lymphoid and myeloid immune cells in peripheral blood samples from Mexican patients positive for SARS-CoV-2 infection. With all this classification, we observed some parameters associated with the disease severity having relevance as previously reported, like increased IL-6 levels (Zhou et al. 2020) and the reduction in both T-CD4 and T-CD8+ cells (Mehta et al. 2020; Zhou et al. 2020). As previously mentioned, immunopathology and hyperinflammation have been associated with severe COVID-19 cases. Increased IL-6, C-reactive protein, IL-1β, monocyte chemotactic protein-1 (MCP1), IFN-γ, and TNF-α cytokines have been described in severe COVID-19 patients (Huang et al. 2020). Postmortem analysis suggested the presence of activated macrophages and lymphocytes in lung tissues (Gustine and Jones 2021). However, the role of peripheral monocytes during COVID-19 disease is controversial. First, cytokines produced by mononuclear phagocytes could be induced directly by damage-associated molecular patterns (DAMPs) released by epithelial cells infected by SARS-CoV-2 or by pathogen-associated molecular patterns (PAMPs) recognized by innate receptors like TLRs, RIGs, C-lectin receptors, and MDA-5 receptors (Knoll et al. 2021). Second, it was described a reduction of CD14+ monocytes in peripheral blood of severe disease in COVID-19 patients, with interferon-stimulated gene signature, and without induction of inflammatory cytokines genes like IL-1β, TNF-α, or IL-6 (Knoll et al. 2021; Wilk et al. 2020). Conversely, it was reported that B cells, monocytes, and megakaryocytes in PBMC samples were increased mainly in severe disease patients from China (Ren et al. 2021). Also, CD14+CD16+ monocytes producing IL-6 and IL-10 were expanded in critical patients (Merad and Martin 2020; Zhang et al. 2021). It was suggested that these pro-inflammatory monocytes have a role in the lungs, inducing the extrinsic coagulation pathway and favoring fibrin deposition and blood clotting (Merad and Martin 2020). All these different observations could be explained by the ethnicity (nationality) of the patients in the study; the sample origin, and the methodology used for sample analysis. For example, our monocyte classification as CD11b+CD45highCD14low, is a tool previously suggested in the literature to differentiate the source of mononuclear renal phagocytes between tissue-resident and monocyte-derived cell monocytes (Mikulin et al. 2021), to distinguish the recruitment and differentiation of monocytes to M2 macrophages associated with bladder cancer (Kong et al. 2021), or also for monocyte recruitment in the spleen (Cole et al. 2021).
Blood monocyte reduction could be explained because these cells migrate to the lungs to perform their functions. It was described that mononuclear cells, mainly lymphocytes, were infiltrated in a patient who died from COVID-19 disease with severe symptoms (Xu et al. 2020); also, as previously mentioned, pro-inflammatory monocytes in the lungs could be involved in the hyper-coagulation observed in critical COVID-19 disease. Our results showed a worse prognosis for COVID-19 disease when a reduction in T-CD4+ and T-CD8+ lymphocytes and an increase in IL-6 levels were observed. However, our main finding is the correlation between the critical disease version and the reduction of no-classical monocytes. Our data strongly suggest that the best prognosis will occur when these no-classical monocytes persist in the blood. An extra point possibly induced by one Pfizer vaccine dose is that these no-classical monocytes have a dual profile, simultaneously expressing pro- and anti-inflammatory cytokines. A relevant finding was that a single dose of the Pfizer vaccine is enough to induce a phenotyping change in this myeloid population. Therefore, we propose that this switch can be used as a prognostic marker of Pfizer vaccine efficacy. It would be interesting to demonstrate in the future if other types of vaccines from either different brands or nature, such as recombinant vector vaccines, and even full doses or combined doses of vaccines, induce any modification in the myeloid cell populations or even also in other immune cell populations. Interestingly, we also observed that lymphoid T-CD4+ and T-CD8+ cells are not decreasing by one Pfizer vaccine dose, and we associated this response with protection because, at least during our research development, these patients did not show either adverse effects or symptomatology associated. Previously, a positive effect with either Pfizer or Moderna vaccine over patients with mild disease symptoms was observed with a robust and long-live humoral antigen-specific response (Turner et al. 2021). Also, it recently was shown that vaccination booster with Pfizer vaccine induces an increased T cell immune response along with high antibody titters (Seidel et al. 2022).
According to Our World in Data, Oxford University, the Mexican population fully vaccinated is 61% (https://ourworldindata.org/covid-vaccinations?country=MEX).
In Mexico, the official data from the public health ministry suggested 5.3 million cases and 324 thousand deaths by COVID-19 disease (covid19.who.int/table). Thus, an increased incidence and mortality have been observed in our country. This excessive mortality could be explained by comorbidities associated with the Mexican population; for example, the percentage of the population with obesity exceeds 36% (Barquera and Rivera 2020), and 11.5 million people were diagnosed with type 2 diabetes mellitus (Bello-Chavolla et al. 2017). Some reports described comorbidities involved with severe COVID-19 disease, including hypertension, heart disease, and chronic obstructive pulmonary disease. On the other hand, the Mexican population has a general index for infectious diseases where helminths and parasites persist as a health problem (Diaz et al. 2018). It was described that some infectious diseases predispose the immune system to failure favoring the host-parasite interaction (Gazzinelli-Guimaraes and Nutman 2018; Oyesola et al. 2022). It was suggested that monocytes from the infected COVID-19 patients have an attenuated response to Candida albicans fungus (Moser et al. 2021). It would be interesting to analyze whether frequent infectious diseases in the Mexican population have an impact on susceptibility to COVID-19 development.
Efforts are needed to increase the percentage of the fully vaccinated population in Mexico and the world, and also, it is necessary to understand how the immune response will induce long-term protection. We are currently in a race where new infectious diseases are emerging, where we must be alert, generating mechanisms that stimulate and promote the immune response, inducing protection for the world population. However, it is necessary for world governments to achieve balanced policies, where access to health for the entire population exists, with investment in basic and clinical research.
Finally, we and other authors strongly suggest that myeloid immune cell profile in blood could be a marker associated with either susceptibility or protection during SARS-CoV-2 infection (Ren et al. 2021; Stephenson et al. 2021; Unterman et al. 2022; Wilk et al. 2021). Most authors raise this conclusion using multiomics technique (single cell sequencing), having a full range of genes and cells to deduce the relevance of immune system. However, only a select number of laboratories worldwide have access to this multiomics technique. The use of most common and cheaper immunologic and molecular techniques could be a tool for a precise diagnosis and treatment in patients positive to COVID-19. Through conventional flow cytometry and quantitative PCR, we showed that CD11b+CD45highCD14low no-classical monocytes are involved in protection during SARS-CoV-2 virus infection. This finding may be useful for predicting the severity and outcome of COVID-19 infection.
Author contribution
Conception and experimental design: JEO, LIT, GAA, and EGJ. Experimental performance: JEO, EGJ, MSM, VHG, JSE, IJA, and JCB. Data analysis: EGJ, JEO, MRS, IJA, and MSM. Interpretation of the results: JEO, EGJ, FVP, and GAA. Paper writing: JEO, EGJ, and LIT. All the authors read and approved the manuscript. Funding resources: GAA, JEO, and LIT.
Funding
This work was supported by the grant S669 from Fundación Gonzalo Río Arronte (Mexico), the grants from Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT) de la Dirección General de Asuntos de Personal Académico (DGAPA)-UNAM IA209720 and IA205622, and the Programa de Apoyo a Profesores de Carrera (PAPCA) de la FES Iztacala FESI-PAPCA-2021–2022-24 to JEO.
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Ethical approval
All procedures were approved in accordance with the ethical standards of the Committee of Research, Ethics and Biosafety of Hospital Regional de Alta Especialidad de Ixtapaluca (NR-22–2020) and with the 1964 Helsinki declaration.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Barquera S, Rivera JA. Obesity in Mexico: rapid epidemiological transition and food industry interference in health policies. Lancet Diabetes Endocrinol. 2020;8(9):746–747. doi: 10.1016/S2213-8587(20)30269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello-Chavolla OY, Rojas-Martinez R, Aguilar-Salinas CA, Hernandez-Avila M. Epidemiology of diabetes mellitus in Mexico. Nutr Rev. 2017;75(suppl 1):4–12. doi: 10.1093/nutrit/nuw030. [DOI] [PubMed] [Google Scholar]
- Cole KE, Ly QP, Hollingsworth MA, Cox JL, Padussis JC, Foster JM, Vargas LM, Talmadge JE. Human splenic myeloid-derived suppressor cells: Phenotypic and clustering analysis. Cell Immunol. 2021;363:104317. doi: 10.1016/j.cellimm.2021.104317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz D, Vazquez-Polanco AM, Argueta-Donohue J, Stephens CR, Jimenez-Trejo F, Ceballos-Liceaga SE, Mantilla-Beniers N. Incidence of Intestinal Infectious Diseases due to Protozoa and Bacteria in Mexico: Analysis of National Surveillance Records from 2003 to 2012. Biomed Res Int. 2018;2018:2893012. doi: 10.1155/2018/2893012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28(2):202–221. doi: 10.1016/j.cmi.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, Dold C, Faust SN, Finn A, Flaxman AL, Hallis B, Heath P, Jenkin D, Lazarus R, Makinson R, Minassian AM, Pollock KM, Ramasamy M, Robinson H, Snape M, Tarrant R, Voysey M, Green C, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ, Oxford CVTG. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli-Guimaraes PH, Nutman TB (2018) Helminth parasites and immune regulation. F1000Res 7. 10.12688/f1000research.15596.1 [DOI] [PMC free article] [PubMed]
- Gustine JN, Jones D. Immunopathology of Hyperinflammation in COVID-19. Am J Pathol. 2021;191(1):4–17. doi: 10.1016/j.ajpath.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll R, Schultze JL, Schulte-Schrepping J. Monocytes and Macrophages in COVID-19. Front Immunol. 2021;12:720109. doi: 10.3389/fimmu.2021.720109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Zhu M, Wang Z, Xu Z, Shao J. Characteristics and clinical significance of CD163+/CD206+M2 mono-macrophage in the bladder cancer microenvironment. Turk J Biol. 2021;45(5):624–632. doi: 10.3906/biy-2104-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtovic L, Beeson JG. Complement Factors in COVID-19 Therapeutics and Vaccines. Trends Immunol. 2021;42(2):94–103. doi: 10.1016/j.it.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, Cheng L, Li J, Wang X, Wang F, Liu L, Amit I, Zhang S, Zhang Z. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26(6):842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, Hlh Across Speciality Collaboration UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melms JC, Biermann J, Huang H, Wang Y, Nair A, Tagore S, Katsyv I, Rendeiro AF, Amin AD, Schapiro D, Frangieh CJ, Luoma AM, Filliol A, Fang Y, Ravichandran H, Clausi MG, Alba GA, Rogava M, Chen SW, Ho P, Montoro DT, Kornberg AE, Han AS, Bakhoum MF, Anandasabapathy N, Suarez-Farinas M, Bakhoum SF, Bram Y, Borczuk A, Guo XV, Lefkowitch JH, Marboe C, Lagana SM, Del Portillo A, Tsai EJ, Zorn E, Markowitz GS, Schwabe RF, Schwartz RE, Elemento O, Saqi A, Hibshoosh H, Que J, Izar B. A molecular single-cell lung atlas of lethal COVID-19. Nature. 2021;595(7865):114–119. doi: 10.1038/s41586-021-03569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375(6585):1122–1127. doi: 10.1126/science.abm8108. [DOI] [PubMed] [Google Scholar]
- Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulin JA, Bates BL, Wilson TJ. A simplified method for separating renal MPCs using SLAMF9. Cytometry A. 2021;99(12):1209–1217. doi: 10.1002/cyto.a.24469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser D, Biere K, Han B, Hoerl M, Schelling G, Chouker A, Woehrle T. COVID-19 Impairs Immune Response to Candida albicans. Front Immunol. 2021;12:640644. doi: 10.3389/fimmu.2021.640644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyesola OO, Souza COS, Loke P. The Influence of Genetic and Environmental Factors and Their Interactions on Immune Response to Helminth Infections. Front Immunol. 2022;13:869163. doi: 10.3389/fimmu.2022.869163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 Cytokine Storm; What We Know So Far. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Wen W, Fan X, Hou W, Su B, Cai P, Li J, Liu Y, Tang F, Zhang F, Yang Y, He J, Ma W, He J, Wang P, Cao Q, Chen F, Chen Y, Cheng X, Deng G, Deng X, Ding W, Feng Y, Gan R, Guo C, Guo W, He S, Jiang C, Liang J, Li YM, Lin J, Ling Y, Liu H, Liu J, Liu N, Liu SQ, Luo M, Ma Q, Song Q, Sun W, Wang G, Wang F, Wang Y, Wen X, Wu Q, Xu G, Xie X, Xiong X, Xing X, Xu H, Yin C, Yu D, Yu K, Yuan J, Zhang B, Zhang P, Zhang T, Zhao J, Zhao P, Zhou J, Zhou W, Zhong S, Zhong X, Zhang S, Zhu L, Zhu P, Zou B, Zou J, Zuo Z, Bai F, Huang X, Zhou P, Jiang Q, Huang Z, Bei JX, Wei L, Bian XW, Liu X, Cheng T, Li X, Zhao P, Wang FS, Wang H, Su B, Zhang Z, Qu K, Wang X, Chen J, Jin R, Zhang Z. COVID-19 immune features revealed by a large-scale single-cell transcriptome atlas. Cell. 2021;184(23):5838. doi: 10.1016/j.cell.2021.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudan I, Adeloye D, Sheikh A. COVID-19: vaccines, efficacy and effects on variants. Curr Opin Pulm Med. 2022;28(3):180–191. doi: 10.1097/MCP.0000000000000868. [DOI] [PubMed] [Google Scholar]
- Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, Baum A, Pascal K, Quandt J, Maurus D, Brachtendorf S, Lorks V, Sikorski J, Hilker R, Becker D, Eller AK, Grutzner J, Boesler C, Rosenbaum C, Kuhnle MC, Luxemburger U, Kemmer-Bruck A, Langer D, Bexon M, Bolte S, Kariko K, Palanche T, Fischer B, Schultz A, Shi PY, Fontes-Garfias C, Perez JL, Swanson KA, Loschko J, Scully IL, Cutler M, Kalina W, Kyratsous CA, Cooper D, Dormitzer PR, Jansen KU, Tureci O. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- Seidel A, Zanoni M, Gross R, Krnavek D, Erdemci-Evin S, von Maltitz P, Albers DPJ, Conzelmann C, Liu S, Weil T, Mayer B, Hoffmann M, Pohlmann S, Beil A, Kroschel J, Kirchhoff F, Munch J, Muller JA. BNT162b2 booster after heterologous prime-boost vaccination induces potent neutralizing antibodies and T cell reactivity against SARS-CoV-2 Omicron BA.1 in young adults. Front Immunol. 2022;13:882918. doi: 10.3389/fimmu.2022.882918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyed Hosseini E, Riahi Kashani N, Nikzad H, Azadbakht J, Hassani Bafrani H, Haddad Kashani H. The novel coronavirus Disease-2019 (COVID-19): Mechanism of action, detection and recent therapeutic strategies. Virology. 2020;551:1–9. doi: 10.1016/j.virol.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson E, Reynolds G, Botting RA, Calero-Nieto FJ, Morgan MD, Tuong ZK, Bach K, Sungnak W, Worlock KB, Yoshida M, Kumasaka N, Kania K, Engelbert J, Olabi B, Spegarova JS, Wilson NK, Mende N, Jardine L, Gardner LCS, Goh I, Horsfall D, McGrath J, Webb S, Mather MW, Lindeboom RGH, Dann E, Huang N, Polanski K, Prigmore E, Gothe F, Scott J, Payne RP, Baker KF, Hanrath AT, Schim van der Loeff ICD, Barr AS, Sanchez-Gonzalez A, Bergamaschi L, Mescia F, Barnes JL, Kilich E, de Wilton A, Saigal A, Saleh A, Janes SM, Smith CM, Gopee N, Wilson C, Coupland P, Coxhead JM, Kiselev VY, van Dongen S, Bacardit J, King HW, Cambridge Institute of Therapeutic I, Infectious Disease-National Institute of Health Research C-BC, Rostron AJ, Simpson AJ, Hambleton S, Laurenti E, Lyons PA, Meyer KB, Nikolic MZ, Duncan CJA, Smith KGC, Teichmann SA, Clatworthy MR, Marioni JC, Gottgens B, Haniffa M (2021) Single-cell multi-omics analysis of the immune response in COVID-19. Nat Med 27(5):904–916. 10.1038/s41591-021-01329-2 [DOI] [PMC free article] [PubMed]
- Turner JS, Kim W, Kalaidina E, Goss CW, Rauseo AM, Schmitz AJ, Hansen L, Haile A, Klebert MK, Pusic I, O'Halloran JA, Presti RM, Ellebedy AH. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature. 2021;595(7867):421–425. doi: 10.1038/s41586-021-03647-4. [DOI] [PubMed] [Google Scholar]
- Unterman A, Sumida TS, Nouri N, Yan X, Zhao AY, Gasque V, Schupp JC, Asashima H, Liu Y, Cosme C, Jr, Deng W, Chen M, Raredon MSB, Hoehn KB, Wang G, Wang Z, DeIuliis G, Ravindra NG, Li N, Castaldi C, Wong P, Fournier J, Bermejo S, Sharma L, Casanovas-Massana A, Vogels CBF, Wyllie AL, Grubaugh ND, Melillo A, Meng H, Stein Y, Minasyan M, Mohanty S, Ruff WE, Cohen I, Raddassi K, Yale IRT, Niklason LE, Ko AI, Montgomery RR, Farhadian SF, Iwasaki A, Shaw AC, van Dijk D, Zhao H, Kleinstein SH, Hafler DA, Kaminski N, Dela Cruz CS. Single-cell multi-omics reveals dyssynchrony of the innate and adaptive immune system in progressive COVID-19. Nat Commun. 2022;13(1):440. doi: 10.1038/s41467-021-27716-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk AJ, Lee MJ, Wei B, Parks B, Pi R, Martinez-Colon GJ, Ranganath T, Zhao NQ, Taylor S, Becker W, Stanford C-B, Jimenez-Morales D, Blomkalns AL, O'Hara R, Ashley EA, Nadeau KC, Yang S, Holmes S, Rabinovitch M, Rogers AJ, Greenleaf WJ, Blish CA (2021) Multi-omic profiling reveals widespread dysregulation of innate immunity and hematopoiesis in COVID-19. J Exp Med 218(8). 10.1084/jem.20210582 [DOI] [PMC free article] [PubMed]
- Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martinez-Colon GJ, McKechnie JL, Ivison GT, Ranganath T, Vergara R, Hollis T, Simpson LJ, Grant P, Subramanian A, Rogers AJ, Blish CA. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med. 2020;26(7):1070–1076. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Ma W, Tang H, Liu D. Severe COVID-19: A Review of Recent Progress With a Look Toward the Future. Front Public Health. 2020;8:189. doi: 10.3389/fpubh.2020.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Bora SA, Parimon T, Zaman T, Friedman OA, Palatinus JA, Surapaneni NS, Matusov YP, Cerro Chiang G, Kassar AG, Patel N, Green CER, Aziz AW, Suri H, Suda J, Lopez AA, Martins GA, Stripp BR, Gharib SA, Goodridge HS, Chen P. Cell-Type-Specific Immune Dysregulation in Severely Ill COVID-19 Patients. Cell Rep. 2021;34(1):108590. doi: 10.1016/j.celrep.2020.108590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Guo R, Lei L, Liu H, Wang Y, Wang Y, Qian H, Dai T, Zhang T, Lai Y, Wang J, Liu Z, Chen T, He A, O'Dwyer M, Hu J. Frontline Science: COVID-19 infection induces readily detectable morphologic and inflammation-related phenotypic changes in peripheral blood monocytes. J Leukoc Biol. 2021;109(1):13–22. doi: 10.1002/JLB.4HI0720-470R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.